Abstract
A series of Schiff base compounds were synthesized by the reaction of different 3,5-dihalosalicylaldehyde (halo atoms equal to Cl, Br and I) with polymethylenediamines of varying chain length. The Schiff bases were characterized using FT-IR, UV–Vis, 1H NMR, 13C NMR and mass spectroscopic techniques, and elemental analyses (CHN), and crystal structure of some compounds was determined by X-ray crystallography. The in vitro biological screening effects of the synthesized compounds were tested against different microbial kinds. The results revealed that all compounds were biologically active.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compounds containing an azomethine group (–CH=N–) are known as Schiff bases. Their preparation, chemical and physical properties have been studied by many research groups. Schiff bases have different applications in various fields such as electrochemistry, bioinorganic, catalysis, metallic deactivator, separation processes, organic syntheses and environmental chemistry [1,2,3,4].
Due to the presence of donor atoms such as nitrogen, oxygen and sulfur in Schiff bases, these compounds are structurally similar to biological systems. In recent years, a considerable number of tetradentate salen-type Schiff base compounds, especially those have a N2O2 donor sets, can be used as ligands to obtain metal complexes that can serve as more or less successful models of biological compounds [5,6,7] and as catalyst in organic reactions [8, 9]. These compounds have been broadly examined for their antibacterial [10,11,12], antifungal [13,14,15], anticancer [16], antituberular [17, 18], antiviral [19], anti-inflammatory [20, 21], anticonvulsant [22, 23], antimalarial [24] and antitumor [25] activity. Structure–activity relationship (SAR) of Schiff bases has been studied by a lot of researchers [26]. On the basis of these studies, unsubstituted salicylaldehyde has only low activity. In some cases, the effect of substituents is dramatic. The presence of functional groups such as halogen, hydroxyl or nitro may cause higher activity, but the effects are not easily predictable nor can they be extrapolated from one microbe to another [27, 28].
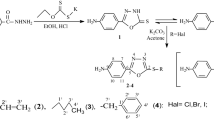
In the current work, we synthesized nine new Schiff bases derived from various primary diamines and 3,5-dichlorosalicylaldehyde, 3,5-dibromosalicylaldehyde and 3,5-diiodosalicylaldehyde (Fig 1). The compounds were characterized using FT-IR, UV–Vis, 1H NMR, 13C NMR, mass spectroscopic technique, elemental analyses (CHN) and single-crystal X-ray diffraction. The in vitro biological activities of the compounds were evaluated against Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa.
Experimental
Materials and physical measurements
All chemicals were of reagent grade and used without further purification. Melting points were determined in open capillaries and are uncorrected. The 1H (400 MHz) and 13C NMR (100 MHz) spectra were recorded at ambient temperature by a BRUKER AVANCE 400 MHz spectrometer using CDCl3 as solvent. The chemical shift values (δ) are given in ppm. Infrared spectra were recorded using KBr disks on an FT-IR Prestige21 spectrophotometer in the range of 4000–400 cm−1. The mass spectra were obtained on an Agilent Technologies 5975C instrument. The C, H and N microanalyses were obtained with a Thermo Finnigan Flash EA 1112 elemental analyzer. Electronic spectra were recorded on a UV–Vis Cary 50 instrument. The X-ray data for the compounds were collected at room temperature on a Bruker Smart APEX CCD diffractometer (Mo Kα = 0.71073 Å). Full spheres of reciprocal lattice were scanned by 0.3° steps in omega with a crystal-to-detector distance of 5 cm. Cell refinement and data reduction were performed with the help of the SAINT program [29]. Corrections for absorption were made with the multiscan method and SADABS program [29]. The structures were solved with direct methods using SHELXS, and structure refinement on F2 was carried out with the SHELXL-2014/7 program. All non-hydrogen atoms were refined using anisotropic displacement parameters [30]. All calculations were done with PLATON [31].
General procedure for the synthesis of Schiff bases
To a stirred ethanolic (25 mL) solution of 3,5-dihalosalicylaldehyde (2 mmol) was added an ethanolic (25 mL) solution of the diamine (1 mmol). The reaction mixture was refluxed for 1–3 h at 80–90 °C in a water bath. The resulting solution was cooled to room temperature, and the resulting precipitate was collected by suction filtration and washed with cold ethanol (3 × 10 mL) to afford the desired Schiff base. Single crystals of the isen, bspn and isbn suitable for X-ray diffraction experiments were obtained by slow evaporation of the hot ethanolic solution of the compounds.
6,6′((1E,1′E)(ethane1,2diylbis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenol) or csen was prepared using ethane-1,2-diamine and 3,5-dichlorosalicylaldehyde: Yield 373.6 mg (92%). m.p.: 216 °C. UV–Vis (λmax, nm): 429, 335, 262. IR (KBr): 3061m, 2912m, 2850m, 1635s, 1446s, 1362m, 1275m, 1207m, 1176m, 1042m, 854m, 731m, 696m, 563m, cm−1. 1H NMR (400 MHz, CDCl3, δ): 13.99 (br, 2H, –OH), 8.31 (s, 2H, H–C=N), 7.43 (d, 2H, J = 2.4, H-6), 7.17 (d, 2H, J = 2.4, H-4), 4.03 (s, 4H, =N–CH2–C). 13C NMR (100 MHz, CDCl3, δ): 166.21, 157.79, 132.27, 129.85, 122.70, 121.55, 119.26, 57.70. MS (m/z): calculated for [C16H12Cl4N2O2]+ 406.08, observed 406.0. Combustion analysis for C16H12Cl4N2O2: Calculated. C 47.32, H 2.98, N 6.90; found C 47.21, H 2.92, N 6.25.
6,6′((1E,1′E)(propane1,3diylbis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenol) or cspn was prepared using propane-1,3-diamine and 3,5-dichlorosalicylaldehyde: Yield 365.5 mg (87%). m.p.: 186 °C. UV–Vis (λmax, nm): 429, 333, 262. IR (KBr): 3071m, 2947m, 2858m, 1631s, 1458s, 1363m, 1292m, 1219s, 1182s, 1066m, 1001m, 974m, 869m, 731m, 569m, 482m, cm−1. 1H NMR (400 MHz, CDCl3, δ): 14.37 (br, 2H, –OH), 8.34 (s, 2H, H–C=N), 7.44 (d, 2H, J = 2.4, H-6), 7.18 (d, 2H, J = 2.4, H-4), 3.81 (t, 4H, J = 6.4, =N–CH2–C), 2.17 (q, 2H, J = 6.4, =N–C–CH2). 13C NMR (100 MHz, CDCl3, δ): 164.44, 157.37, 131.93, 129.04, 122.55, 121.61, 118.87, 55.02, 30.82. MS (m/z): calculated for [C17H14Cl4N2O2]+ 420.11, observed 420.0. Combustion analysis for C17H14Cl4N2O2: Calculated. C 48.60, H 3.36, N 6.67; found C 48.83, H 3.28, N 6.76.
6,6′((1E,1′E)(butane1,4diylbis(azanylylidene))bis(methanylylidene))bis(2,4-dichlorophenol) or csbn was prepared using ethane-1,2-diamine and 3,5-dibromosalicylaldehyde: Yield 356.0 mg (82%). m.p.: 216 °C. UV–Vis (λmax, nm): 428, 334, 264. IR (KBr): 3068m, 2914m, 2852m, 1636s, 1462s, 1355m, 1288, 1211m, 1161m, 1035m, 957m, 740m, 686m, 555m, cm−1. 1H NMR (400 MHz, CDCl3, δ): 14.58 (br, 2H, –OH), 8.28 (s, 2H, H–C=N), 7.43 (d, 2H, J = 2.4, H-6), 7.16 (d, 2H, J = 2.4, H-4), 3.71 (t, 4H, J = 6.0, =N–CH2–C), 1.85 (q, 2H, J = 6.0, =N–C–CH2). 13C NMR (100 MHz, CDCl3, δ): 167.34, 157.65, 134.54, 129.48, 127.34, 119.48, 117.65, 53.34, 27.34. MS (m/z): calculated for [C18H16Cl4N2O2]+ 434.14, observed 434.0. Combustion analysis for C18H16Cl4N2O2: Calculated. C 49.80, H 3.71, N 6.45; found C 49.98, H 3.66, N 6.43.
6,6′((1E,1′E)(ethane1,2diylbis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenol) or bsen was prepared using ethane-1,2-diamine and 3,5-dibromosalicylaldehyde: Yield 467.1 mg (80%). m.p.: 238 °C. UV–Vis (λmax, nm): 431, 335, 264. IR (KBr): 3099m, 3054m, 2939m, 1623s, 1596m, 1531m, 1508m, 1434m, 1251s, 978m, 854m, 822m, 748m, 569m, 513w, cm−1. 1H NMR (400 MHz, CDCl3, δ): 14.15 (br, 2H, –OH), 8.27 (s, 2H, H–C=N), 7.72 (d, 2H, J = 2.4, H-6), 7.34 (d, 2H, J = 2.4, H-4), 4.00 (s, 4H, =N–CH2–C). 13C NMR (100 MHz, CDCl3, δ): 166.84, 162.02, 138.23, 134.33, 118.90, 114.34, 106.60, 55.60. MS (m/z): calculated for [C16H12Br4N2O2]+ 583.90, observed 583.80. Combustion analysis for C16H12Br4N2O2: Calculated. C 32.91, H 2.07, N 4.80; found C 32.21, H 1.99, N 4.50.
6,6′((1E,1′E)(propane1,3diylbis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenol) or bspn was prepared using propane-1,3-diamine and 3,5-dibromosalicylaldehyde: Yield 466.4 mg (78%). m.p.: 160 °C. UV–Vis (λmax, nm): 430, 335, 259. IR (KBr): 3064m, 2943m, 2850m, 1632s, 1560m, 1514m, 1444s, 1360m, 1286m, 1221m, 1167m, 1067m, 1036m, 999m, 872w, 849m, 740m, 687m, 561w, 474w, 419w, cm−1. 1H NMR (400 MHz, CDCl3, δ): 14.52 (br, 2H, –OH), 8.29 (s, 2H, H–C=N), 7.73 (d, 2H, J = 2.4, H-6), 7.33 (d, 2H, J = 2.4, H-4), 3.79 (t, 4H, J = 6.4, =N–CH2–C), 2.16 (q, 2H, J = 6.4, =N–C–CH2). 13C NMR (100 MHz, CDCl3, δ): 166.16, 163.53, 138.28, 134.38, 118.53, 115.17, 105.41, 52.35, 30.51. MS (m/z): calculated for [C17H14Br4N2O2]+ 597.93, observed 597.90. Combustion analysis for C17H14Br4N2O2: Calculated. C 34.15, H 2.36, N 4.69; found C 34.04, H 2.28, N 4.57.
6,6′((1E,1′E)(butane1,4diylbis(azanylylidene))bis(methanylylidene))bis(2,4-dibromophenol) or bsbn was prepared using butane-1,4-diamine and 3,5-dibromosalicylaldehyde: Yield 428.4 mg (70%). m.p.: 207 °C. UV–Vis (λmax, nm): 429, 336, 261. IR (KBr): 3064m, 2945m, 2916m, 2846m, 1632s, 1560m, 1514m, 1452s, 1366m, 1286m, 1196m, 1163m, 1043m, 874m, 686m, 474w, cm−1. 1H NMR (400 MHz, CDCl3, δ): 14.74 (br, 2H, –OH), 8.24 (s, 2H, H–C=N), 7.72 (d, 2H, J = 2.4, H-6), 7.34 (d, 2H, J = 2.4, H-4), 3.71 (t, 4H, J = 6.0, =N–CH2–C), 1.85 (q, 2H, J = 6.0, =N–C–CH2). 13C NMR (100 MHz, CDCl3, δ): 165.92, 164.89, 138.52, 134.54, 117.77, 115.90, 104.22, 53.46, 27.34. MS (m/z): calculated for [C18H16Br4N2O2]+ 611.95, observed 611.9. Combustion analysis for C18H16Br4N2O2: Calculated. C 55.33, H 2.67, N 4.58; found C 54.97, H 2.61, N 4.47.
6,6′((1E,1′E)(ethane1,2diylbis(azanylylidene))bis(methanylylidene))bis(2,4-diiodophenol) or isen was prepared using ethane-1,2-diamine and 3,5-diiodosalicylaldehyde: Yield 578.9 mg (75%). m.p.: 236 °C. UV–Vis (λmax, nm): 439, 343, 260. IR (KBr): 3053m, 2881m, 1630s, 1580m, 1458m, 1437s, 1352m, 1285m, 1209m, 1153m, 1032m, 966m, 868m, 741m, 552m, cm−1. 1H NMR (400 MHz, CDCl3, δ): 14.49 (br, 2H, –OH), 8.48 (s, 2H, H–C=N), 8.04 (d, 2H, J = 2.0, H-6), 7.72 (d, 2H, J = 2.0, H-4), 4.01 (s, 4H, =N–CH2–C). 13C NMR (100 MHz, CDCl3, δ): 166.66, 164.66, 148.99, 141.15, 118.73, 92.51, 77.69, 55.43. MS (m/z): calculated for [C16H12I4N2O2]+ 771.90, observed 771.9. Combustion analysis for C16H12I4N2O2: Calculated. C 24.90, H 1.57, N 3.63; found C 24.99, H 1.69, N 3.55.
6,6′((1E,1′E)(propane1,3diylbis(azanylylidene))bis(methanylylidene))bis(2,4-diiodophenol) or ispn was prepared using propane-1,3-diamine and 3,5-diiodosalicylaldehyde: Yield 534.4 mg (68%). m.p.: 176 °C. UV–Vis (λmax, nm): 437, 343, 258. IR (KBr): 3049m, 2292m, 2851m, 1624s, 1582m, 1456m, 1435s, 1357m, 1319m, 1285m, 1271m, 1215m, 1155s, 1095m, 1060m, 987m, 964m, 871m, 850m, 738m, 657m, 547m, cm−1. 1H NMR (400 MHz, CDCl3, δ): 14.40 (br, 2H, –OH), 8.19 (s, 2H, H–C=N), 8.08 (d, 2H, J = 2.0, H-6), 7.52 (d, 2H, J = 2.0, H-4), 3.79 (t, 4H, J = 6.4, =N–CH2–C), 2.16 (q, 2H, J = 6.4, =N–C–CH2). 13C NMR (100 MHz, CDCl3, δ): 166.07, 165.94, 149.01, 141.26, 118.21, 93.76, 76.33, 52.19, 30.52. MS (m/z): calculated for [C17H14I4N2O2]+ 785.93, observed 785.90. Combustion analysis for C17H14I4N2O2: Calculated. C 25.98, H 1.80, N 3.56; found C 26.09, H 1.91, N 3.45.
6,6′((1E,1′E)(butane1,4diylbis(azanylylidene))bis(methanylylidene))bis(2,4-diiodophenol) or isbn was prepared using butane-1,4-diamine and 3,5-dibromosalicylaldehyde: Yield 480.0 mg (60%). m.p.: 196 °C. UV–Vis (λmax, nm): 434, 343, 261. IR (KBr): 3053w, 2940m, 2856w, 1634s, 1580m, 1477m, 1439m, 1352m, 1219m, 1182m, 1128m, 1041m, 1016m, 912m, 866m, 759m, 657m, cm−1. 1H NMR (400 MHz, CDCl3, δ): 14.72 (br, 2H, –OH), 8.13 (s, 2H, H–C=N), 8.06 (d, 2H, J = 2.0, H-6), 7.50 (d, 2H, J = 2.0, H-4), 3.69 (t, 4H, J = 6.8, =N–CH2–C), 1.83 (q, 2H, J = 6.8, =N–C–CH2). 13C NMR (100 MHz, CDCl3, δ): 167.24, 165.66, 149.19, 141.45, 117.68, 94.88, 75.39, 53.40, 27.37. MS (m/z): calculated for [C18H16I4N2O2]+ 799.96, observed 799.9. Combustion analysis for C18H16I4N2O2: Calculated. C 27.03, H 2.02, N 3.50; found C 27.14, H 2.03, N 3.63.
Biological activity evaluation
The in vitro antibacterial activity of the synthesized Schiff bases was investigated against the standard strains of two Gram-positive (Staphylococcus aureus PTCC1431, Bacillus cereus PTCC1015) and two Gram-negative (Escherichia coli PTCC1394, Pseudomonas aeruginosa PTCC1074) bacteria. In order to compare the results, ampicillin (10 mg/disk) and erythromycin (15 mg/disk) were used as standard antibacterial drugs. The compounds were dissolved in DMSO at 50 μg mL−1 concentration. Bacteria culture Petri dishes treated with the test compounds were incubated for 24 h at 37 °C. Inhibition zone (mm) around the specimens was used to indicate antibacterial activity of each of compounds. Each test was performed in triplicate.
Results and discussion
All the Schiff base compounds are stable at room temperature and are non-hygroscopic. The compounds are insoluble in water, ethanol and methanol but are soluble in DMSO.
FT-IR spectra
The infrared spectrum provided valuable information for identifying the nature of the functional groups. For example, FT-IR spectrum of csen is shown in Fig. 2. The IR spectra of the Schiff bases show strong intensity absorption bands at 1623–1636 cm−1 assigned to C=N stretching mode of the azomethine [32]. The presence of aromatic rings has been identified by their characteristic ring vibrations at 1500–1400, 1100–1050 and 900–700 cm−1 regions [33,34,35]. In all compounds, the absence of characteristic bands of unreacted carbonyl groups ν(C=O) and primary amine ν(NH2) confirms the formation of the proposed Schiff base structure. A band in the 1250–1290 cm−1 due to C–O phenolic group was also observed in these compounds [36].
UV–Vis spectra
The UV–visible absorption spectra were obtained in DMSO (10−4 M) at room temperature. Earlier papers emphasis that the electronic absorption bands of the salicylaldehyde-type Schiff base comprise of three bands [37]. The compounds exhibit three main peaks in the about 261, 337 and 432 nm. The 250–300 nm bands are due to the π → π* transitions of the aromatic rings. It is shown in Fig. 3. The bands at the 300–350 nm range involve π → π* transitions of the C=N chromophore group. The longer wavelength bands over ca. 400 nm can be assigned to the intramolecular charge transfer interactions (n → π* transition) of the non-bonding electrons present on the nitrogen of the azomethine group in the Schiff base compounds [38, 39].
NMR spectra
The 1H NMR spectra of the Schiff base compounds were obtained in CDCl3 at room temperature. The synthesized compounds showed signals of all protons in their expected regions, and the integration curve were equivalent to the total number of the protons deduced from their proposed structure. The chemical shift for the OH protons in the Schiff base compounds is observed in 13.99–14.74 ppm. The signals at δ = 8.13–8.48 ppm are assigned to the –C(H)=N– proton of azomethine [40, 41]. It is known that orthophenolic hydrogen bonding shifts the resonance signal of a proton to lower field (higher frequency). Comparing the 1H NMR data of the OH protons of salicyl part of the compounds, it can be said that the strongest intramolecular hydrogen bond (OH···N) is formed in bsbn, and the weakest one in csen. It is interesting that 13.99 ppm value of the csen is lower than those of the other phenolic Schiff bases. This difference means that the phenolic OH proton of csen has less acidic character and, consequently, csen has weaker intramolecular hydrogen bonding according to the others. The aromatic protons of the Schiff base compounds appeared at δ = 7.16–8.08 ppm. The H-4 signal was observed as a doublet, due to coupling with the H-6, and also the H-6 signal was observed as a doublet, due to coupling with the H-4. The multiples of the aliphatic protons were appeared within the range of 1.83–4.03 ppm.
The 13C NMR spectra of the Schiff base compounds are consistent with the number of carbons in their structures. The peaks observed at 166.22, 167.35, 164.44, 166.85, 166.17, 165.93, 166.66, 166.07 and 167.24 ppm are ascribed to the imine carbon atoms of csen, cspn, csbn, bsen, bspn, bsbn, isen, ispn and isbn, respectively. The existence of these peaks in the spectra of Schiff base compounds supports the presence of the Schiff base in them.
The chemical shift values of the different types of protons and carbons in the csen are reported in Figs. 4 and 5, respectively.
Mass Spectra
The formulation of the Schiff base compounds is deduced from analytical data, 1H and 13C NMR and further supported by mass spectroscopy. The electron impact mass spectra of Schiff base compounds are recorded and investigated at 70 eV of electron energy. The mass spectra of the studied Schiff bases are characterized by low to high relative intensity molecular ion peaks. The mass spectra of the compounds showed molecular ion peaks at m/e 404, 418, 432, 580, 594, 608, 772, 786 and 800 which corresponding to their molecular formula C16H12Cl4N2O2 (csen), C17H14Cl4N2O2 (cspn), C18H16Cl4N2O2 (csbn), C16H12Br4N2O2 (bsen), C17H14Br4N2O2 (bspn), C18H16Br4N2O2 (bsbn), C16H12I4N2O2 (isen), C17H14I4N2O2 (ispn) and C18H16I4N2O2 (isbn), respectively. It is obvious that the molecular ion peaks are in good agreement with their suggested empirical formula as indicated from elemental analyses (Table 1). The low intensities of the molecular ion peaks in some of compounds, [M]+, are indicative of the ease of fragmentation of these compounds, and this may reflect of halo atoms present in structures [42]. The mass spectrum of csen is shown in Fig. 6.
Crystal structure description
The solid-state structures of isbn, bspn and isen were determined by X-ray diffraction. The crystal data and refinement parameters are summarized in Table 1, and ORTEP plots of isbn, bspn and isen are shown in Fig. 1. Selected bond lengths and angles are summarized in Table 2. The asymmetric unit of isbn comprises a potentially tetradentate Schiff base ligand. Pair of the intramolecular O–H…N hydrogen bonds make S(6) ring. The compound crystallizes in monoclinic system with P2(1) space group. The absolute structure parameter (Flack parameter) was determined successfully. The crystal packing of the compound shows two-dimensional network of connected molecules through the intermolecular C–I…π interactions parallel to ab-plane (Fig. 7). The asymmetric unit of bspn comprises half of a potentially tetradentate Schiff base ligand. Pair of the intramolecular O–H…N hydrogen bonds make S(6) ring. The intermolecular C–H…O interactions link neighboring molecules along the a-axis. Similar to bspn compound, the asymmetric unit of the isen compound comprises half of a potentially tetradentate Schiff base ligand. The crystal is twinned non-merohedrally with a refined ration of 0.829(7)/0.171(7).
Antimicrobial activity
The in vitro antibacterial activities of the synthesized Schiff base compounds and known drugs such as ampicillin and erythromycin were screened in DMSO against more than medically important Gram-positive and Gram-negative bacteria to increase the chance of antibiotic principles in the tested materials. The microorganisms used in this work include Staphylococcus aureus and Bacillus cereus (as Gram-positive bacteria) and Escherichia coli and Pseudomonas aeruginosa (as Gram-negative bacteria).
The antibacterial activities of the compounds are represented by size of the diameter of inhibition zone in mm and for all Schiff bases are shown in Table 3.
The compounds were tested at the concentrations of 50 μg mL−1 in DMSO. Antimicrobial activity depends on the nature of bacterial strain, the solvent and chelating ability of the Schiff base. It is believed that Schiff bases act by forming a chelate with the bacterial strain. This may involve hydrogen bonding through the azomethine group with the active centers of cell constituents thus resulting in an interference with normal cell process.
In general, compounds having chloro, bromo or iodo substituent in their structure are having significant antibacterial and antifungal activity. In all nine compounds, the central ligand is salicylaldehyde with different side chains. In csen, cspn and csbn, it is 3,5-dichloro, in bsen, bspn and bsbn, it is 3,5-dibromo, and in isen, ispn and isbn, it is 3,5-diiodo. The diffusion capacity of the compounds and the effect of them against bacteria vary with their structure.
It is observed that the compounds with Br and I substituents have better antimicrobial activity according to the others (as reported in previous articles [43, 44]).
It is evident from Table 3 that all Schiff bases described here were found to be biologically active and showed significantly enhanced antibacterial activity against all of the bacterial species. From the bactericidal activity, it is apparent that the compounds were more toxic toward Gram-positive strains than Gram-negative strains. This difference may be attributed to the fact that the cell wall in Gram-positive bacteria is of a single layer, whereas the Gram-negative cell wall is a multilayered structure.
For E. coli, the cspn and bsbn showed maximum activity followed by csen and bspn. Against P. aeruginosa, csen and csbn showed maximum activity, and minimum inhibition was observed for cspn. For S. aureus, isen showed maximum inhibition. The bspn showed excellent activity against B. cereus.
Conclusion
In this paper, a series of symmetrical Schiff bases containing the N2O2 unit derived from condensation reaction of various diaminoalkyls with substituted salicylaldehydes have been synthesized. These compounds physically and chemically characterized through elemental analysis (CHN), FT-IR, UV–Vis, 1H NMR, 13C NMR and mass spectroscopic. The crystal structures of isen, bspn and isbn were determined by X-ray diffraction at room temperature. They have been crystallized in monoclinic, orthorhombic and monoclinic, respectively. The antimicrobial activity results showed that most of the synthesized Schiff base compounds possess a good antibacterial activity against both Gram-negative and Gram-positive bacteria tested. The structures of the Schiff bases determine an intensification of the antimicrobial activity.
Supplementary data
Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre. CCDCs 1563977, 1563978 and 1563979 and contain the supplementary crystallographic data for bspn, isen and isbn. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving. html or from the CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK; Fax: (+44) 01223-336-033; E-mail: deposit@ ccdc.cam.ac.
References
M.J. O’Donnell, Acc. Chem. Res. 37, 506 (2004)
S. Rana, S.K. Mittal, N. Singh, J. Singh, C.E. Banks, Sens. Actuators B Chem. 239, 17 (2016)
G. Yuan, Y. Tian, J. Liu, H. Tu, J. Liao, J. Yang, Y. Yang, D. Wang, N. Liu, Chem. Eng. J. 326, 691 (2017)
A.A. Isse, A. Gennaro, E. Vianello, Electrochim. Acta 42, 2065 (1997)
P. Taylor, Synth. React. Inorg. Met. Chem. 32, 1729 (2002)
R.K. Parashar, R.C. Sharma, A. Kumar, G. Mohan, Inorg. Chim. Acta 151, 201 (1988)
S. Chandra, S. Gautam, H.K. Rajor, R. Bhatia, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 137, 749 (2015)
H. Khojasteh, V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork, J. Iran. Chem. Soc. 14, 1139 (2017)
M. Hatefi Ardakani, M. Moghadam, S. Saeednia, Z. Pakdin-Parizi, J. Iran. Chem. Soc. 13, 631 (2016)
T. Jeewoth, H. Li Kam Wah, M.G. Bhowon, D. Ghoorohoo, K. Babooram, Synth. React. Inorg. Met. Chem. 30, 1023 (2000)
L. Shi, H.-M. Ge, S.-H. Tan, H.-Q. Li, Y.-C. Song, H.-L. Zhu, R.-X. Tan, Eur. J. Med. Chem. 42, 558 (2007)
H.G. Aslan, S. Akkoç, Z. Kökbudak, L. Aydın, J. Iran. Chem. Soc. 14, 2263 (2017)
Z. Guo, R. Xing, S. Liu, Z. Zhong, X. Ji, L. Wang, P. Li, Carbohydr. Res. 342, 1329 (2007)
M.M. Ali, M. Jesmin, J. Natl. Sci. Found. Sri Lanka 38, 145 (2010)
H. Khanmohammadi, M. Salehifard, M.H. Abnosi, J. Iran. Chem. Soc. 6, 300 (2009)
K. Neelima, S. Poonia, M. Siddiqui, D. Arshad, Kumar. Spectrochim. Acta A Mol. Biomol. Spectrosc. 155, 146 (2016)
T. Aboul-Fadl, F.A.S. Bin-Jubair, O. Aboul-Wafa, Eur. J. Med. Chem. 45, 4578 (2010)
M.J. Hearn, M.H. Cynamon, M.F. Chen, R. Coppins, J. Davis, H. Joo-On Kang, A. Noble, B. Tu-Sekine, M.S. Terrot, D. Trombino, M. Thai, E.R. Webster, R. Wilson, Eur. J. Med. Chem. 44, 4169 (2009)
K.S. Kumar, S. Ganguly, R. Veerasamy, E. De Clercq, Eur. J. Med. Chem. 45, 5474 (2010)
M.S. Alam, J.H. Choi, D.U. Lee, Bioorg. Med. Chem. 20, 4103 (2012)
A. Noureen, S. Saleem, T. Fatima, H.M. Siddiqi, B. Mirza, Pak. J. Pharm. Sci. 26, 113 (2013)
P. Paneerselvam, T. Raj, M.P.S. Ishar, B. Singh, V.D. Sharma, B.A. Rather, Indian J. Pharm. Sci. 72, 375 (2010)
M. Verma, S.N. Pandeya, K.N. Singh, J.P. Stables, Acta Pharm. 54, 49 (2004)
S.E. Harpstrite, S.D. Collins, A. Oksman, D.E. Goldberg, V. Sharma, Med. Chem. 4, 392 (2008)
C. Liang, J. Xia, D. Lei, X. Li, Q. Yao, J. Gao, Eur. J. Med. Chem. 74, 742 (2013)
Z. Asadi, M. Asadi, F. Dehghani Firuzabadi, R. Yousefi, M. Jamshidi, J. Iran. Chem. Soc. 11, 423 (2014)
S.P. Xu, L. Shi, Y. Pei, Y. Yang, G. Xu, H.L. Zhu, J. Coord. Chem. 63, 3463 (2010)
H. Kargar, Transit. Met. Chem. 39, 811 (2014)
Bruker and W. AXS Programs: SMART, version 5.625; SAINT, version 6.45; SADABS, version 2.10; XPREP, version 6.14. Bruker AXS Inc.: Madison, (2003)
G.M. Sheldrick, Acta Crystallogr. A 64, 112 (2008)
S. Al, Acta Crystallogr D 65, 148 (2009)
B. Ambrozini, E. Dockal, É. Cavalheiro, J. Therm. Anal. 115, 979 (2014)
D. Gürbüz, A. Cinarli, A. Tavman, A.S. Birteksz, Chin. J. Chem. 30, 970 (2012)
Y. Cui, X. Dong, Y. Li, Z. Li, W. Chen, Eur. J. Med. Chem. 58, 323 (2012)
M. Yıldız, Ö. Karpuz, C.T. Zeyrek, B. Boyacıoğlu, H. Dal, N. Demir, N. Yıldırım, H. Ünver, J. Mol. Struct. 1094, 148 (2015)
M. Asadi, H. Sepehrpour, K. Mohammadi, J. Serbian Chem. Soc. 76, 63 (2011)
H.H. Hammud, A. Ghannoum, M.S. Masoud, Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 63, 255 (2006)
A. Cinarli, D. Gürbüz, A. Tavman, A. Seher Birteksöz, Bull. Chem. Soc. Ethiop. 25, 407 (2011)
E.G. Bakirdere, M.F. Fellah, M. Kaya, J. Serb. Chem. Soc. 81, 509 (2016)
M. Malathy, Int. J. Sci. Technol. 2, 157 (2014)
F. Bagheri, A. Olyaei, J. Serb. Chem. Soc. 81, 1111 (2016)
A. Golcu, M. Tumer, H. Demirelli, R.A. Wheatley, Inorg. Chim. Acta. 358, 1785 (2005)
A.N. Kursunlu, E. Guler, F. Sevgi, B. Ozkalp, J. Mol. Struct. 1048, 476 (2013)
S.P. Xu, L. Shi, P.C. Lv, X.L. Li, H.L. Zhu, J. Coord. Chem. 62, 3198 (2009)
Acknowledgements
The support of this work by Payame Noor University Council of Research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ardakani, A.A., Kargar, H., Feizi, N. et al. Synthesis, characterization, crystal structures and antibacterial activities of some Schiff bases with N2O2 donor sets. J IRAN CHEM SOC 15, 1495–1504 (2018). https://doi.org/10.1007/s13738-018-1347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1347-6