Abstract
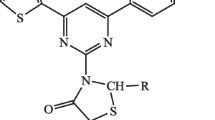
A novel series of fifteen pyrimidine derivatives was prepared from pyrazolobenzothiazine-based chalcones by refluxing with guanidine hydrochloride. The starting materials 4-(3,4-dimethyl-5,5-dioxidobenzo[4,3-c][1,2]thiazin-2(4-H)yl)phenyl)ethanone (2) or 4-(3,4-dimethyl-5,5-dioxidobenzo[4,3-c][1,2]thiazin-2(4-H)yl)benzaldehyde (3) were obtained by N-arylation of 3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (1) with 4-fluoroacetophenone or 4-fluorobenzaldehyde, respectively, using phase transfer catalyst, hexadecyl-tri-n-butylphsophonium bromide. The N-arylated product (2) or (3) was reacted in MeONa/MeOH with diversified aromatic aldehydes or ketones to furnish two series of new chalcones 4 and 5. Refluxing of 4 or 5 with guanidine hydrochloride in KOH(aq) and H2O2/EtOH yielded the 2-(4-(2-amino-6-arylpyrimidin-4-yl)phenyl)3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine-5,5-dioxide (6). The structures of chalcones (4 or 5) and corresponding pyrimidines (6) were confirmed with spectral data and elemental analysis. Several chalcones as well as pyrimidines showed marked activity against E. coli and S. aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrimidine and its derivatives are most important nitrogen based heterocycles which play a vital role in many life processes. The ring system is present in nucleic acids and their derivatives (willardiine, tingitanine) (Bell and Foster, 1962), several vitamins (vitamin B1) (Jansen and Donath, 1926), antibiotics (bacimethrin, sparsomycin, bleomycin) (Tanaka et al., 1961), alkaloids (heteromines, crambescins, manzacidins, variolins, meridianins, psammopemmins) (Berlinck et al., 1993; Lin et al., 1997), toxins (Banker et al., 2000; Ohtani et al., 1992), coenzymes, uric acid, and purines. Many synthetic members of the group are also important as drugs including barbituric acid derivatives and chemotherapeutic agents including sulfadiazine (Petersen and Schmidt 2003), Gleevec (imatinib mesilate) (Nadal and Olavarria, 2004), and Xeloda (capecitabine) (Blum, 2001). Trimethoprim, Iclaprim, and metronidazole are well known synthetic antibacterial remedies based on pyrimidine scaffold (Joffe et al., 1989). Some pyrimidine derivatives are recently reported as inhibitors of CDK (Chu et al., 2006; Moravec et al., 2003), MK2 (Argiriadi et al., 2010), CB2 (Sullivan et al., 1998), VEGFR (Munchhof et al., 2004), and Adenosine A1/A2a/A3 (Baraldi et al., 2001; Chang et al., 2004) (Fig. 1).
The current investigations reveal that pyrimidine analogs exhibit potential biological activities such as anticancer (Baraldi et al., 2002), antiviral (Chern et al., 2004), antimycobacterial (Ballell et al., 2007), anti-inflammatory and analgesic (Sondhi et al., 2005), antiallergic (Ban et al., 1998), and anti-HIV (Malik et al., 2006). Pyrrolo-pyrimidine nucleoside derivatives act as potential anti-HCV (Hepatitis C Virus) agents (Chamakura et al., 2007; Coelmont et al., 2006).
On the other hand, 1,2-benzothiazine-1,1-dioxides are also known as potentially biologically active molecules e.g., 1,2-benzothiazine-3-carboxamide-1,1-dioxide derivatives belonging to oxicams, i.e., piroxicam, meloxicam, ampiroxicam, and isoxicam are well known as analgesic and anti-inflammatory compounds (Lee et al., 2008) (Fig. 2).
Moreover, benzothiazine derivatives are known as potent calpain I inhibitors (Xu, 2007) while its 3-aryl-quinazolin-4-one derivatives showed marked antimicrobial (Ahmad et al., 2011) activity. We have already reported N′-arylmethylidene-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-2(4H)-yl)acetohydrazides as potent anti-oxidant and anti-bacterial agents (Ahmad et al., 2010).
Keeping in view the long-lasting interest of the synthetic community in pyrimidines as well as 1,2-benzothiazine-1,1-dioxides as potential drugs, we planned to synthesize both the heterocyclic moieties in a single nucleus and study their synergic effect which may result some biologically more potent molecules.
Results and discussion
Chemistry
3,4-Dimethyl-2,4-dihydropyrazolo[4,3-c][1,2]benzothiazine-5,5-dioxide 1 was synthesized by our own method (Ahmad et al., 2010) starting from commercially available sodium saccharin. N-arylation of 1 was carried out with 4-fluoroacetophenone or 4-fluorobenzaldehyde in the presence of phase transfer catalyst hexadecyl-tri-n-butylphosphonium bromide yielding 4-(3,4-dimethyl-5,5-dioxidobenzo[4,3-c][1,2]thiazin-2(4-H)yl)phenyl)ethanone 2 or 4-(3,4-dimethyl-5,5-dioxidobenzo[4,3-c][1,2]thiazin-2(4-H)yl)benzaldehyde 3, respectively. Further reaction of 2 or 3 with corresponding aromatic aldehyde or acetophenone (Scheme 1) gave two series of chalcones i.e., 4a–k and 5a–f, respectively (Table 1). For this reaction, stronger base NaOMe, in MeOH instead of NaOH was used.
Each chalcone was treated with guanidine hydrochloride in the presence of 50% aqueous KOH solution in absolute ethanol followed by portion wise addition of 30% H2O2 solution at reflux temperature (Varga et al., 2003). This crucial step resulted in a novel series of pyrimidines (6a–o) by ring closure (Table 1). Spectral data IR, 1H- and 13C-NMR, and MS of all the synthesized compounds were recorded and found in full agreement with the proposed structures. The elemental analysis results were within ±0.4% of the theoretical values.
Antibacterial studies
Bioassay of synthesized compounds summarized in Table 2 indicated that bioactivity of pyrimidines was somewhat greater than their corresponding chalcones. It seems that pyrimidine ring may have enhanced the activity against pathogens. Moreover, it was observed that all the compounds were active against E. coli (gram negative) but only two compounds, i.e., pyrimidines 6e and 6h showed activity against both pathogens. The results indicated that compound 6h showed high activity against both pathogens which may be attributed by 2-MeO-phenyl group of the compound which was also higher than its corresponding chalcone 4g. However, interestingly, compound 6j showing highest activity against E. coli was inactive against S. aureus. It may be considered that two methoxy functionalities at 3 and 4 positions of 6-phenyl group enhanced its activity against E. coli but these groups inactivated the compound against S. aureus. Bromo-chalcones 4c, 4f, and 5b showed marked activity against E. coli, while bromo-pyrimidine 6e exhibited significant activity against both pathogens. The results are summarized in Table 2.
Conclusion
We have synthesized series of pyrazolobenzothiazine based chalcones and their pyrimidine derivatives which were found to possess anti-bacterial activity. It was observed that all the chalcones as well as pyrimidines except 4j and 5d showed activity against gram negative bacteria i.e., E. coli. On the other hand, no activity was observed against gram positive bacteria i.e., S. aureus except two pyrimidines i.e., 6e and 6h. Compound 6h containing 2-methoxyphenyl group at position 6 exhibited highest activity against both pathogens. Bromo derivatives showed more activity against the pathogens, in general. Moreover, pyrimidines showed more activity than chalcones and could be a suitable template for further manipulation leading to novel anti-bacterial agents. The new moieties may also possess other biological activities of the parent ring systems.
Experimental
General
All the chemicals were purchased from E. Merck, Sigma Aldrich or Wako and used without purification. However, solvents were purified through distillation. 1H NMR spectra were recorded on a Bruker DPX-400 instrument at 400 MHz. Chemical shifts are reported in ppm referenced to the residual solvent signal. Mass spectra were recorded on Agilent 5973N instrument using EI mode. Melting points were recorded on a Gallenkamp melting point apparatus and are uncorrected. Elemental analysis was carried out using a Perkin Elmer 2400-CHN Analyser. X-ray crystallography was carried out on Bruker Nonius Kappa CCD diffractometer with graphite monochromated Mo-Kα radiation and the data were corrected for Lorentz and polarization effects and for absorption using multi-scan method [25, 26].
Synthesis of 4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)ethanone (2)
A mixture of 3,4-dimethyl-2,4-dihydropyrazolo[4,3-c][1,2]benzothiazine 5,5-dioxide (1) (6.25 g; 25.0 mmol), 4-fluoroacetophenone (4.14 g; 30.0 mmol), anhydrous K2CO3 (4.15 g; 30.0 mmol), and hexadecyl-n-tributylphosphonium bromide (1.27 g; 2.5 mmol) was refluxed in DMF (100 mL) for a period of 2 h under nitrogen atmosphere. The precipitates formed after adding ice cold water were collected, dried, and recrystallized from EtOH. Pale yellow crystals. Yield: 6.88 g; (75%). mp 230–232 °C. 1H NMR (400 MHz, CDCl3) δ: 2.50 (3H, s, CH3), 2.68 (3H, s, COCH3), 3.13 (3H, s, NCH3), 7.57–7.61 (1H, m, ArH), 7.64–7.67 (2H, m, ArH), 7.69–7.73 (1H, m, ArH), 7.97 (2H, d, J = 7.7 Hz, ArH), 8.10 (1H, m., ArH), 8.11–8.13 (1H, m, ArH). 13C NMR: 10.9, 26.7, 40.0, 124.2, 124.4, 124.7, 124.9, 125.2 (2C), 127.9, 129.2, 129.6, 132.5, 132.9, 133.5, 136.4, 139.5, 142.9, 196.9. MS m/z: 390.09 (M + Na)+. Anal. calc. for C19H17N3O3S; C, 62.11; H, 4.66; N, 11.44; Found: C, 62.10; H, 4.67; N, 11.43.
General procedure for the synthesis of 3-aryl-1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H) yl)phenyl)prop-2-en-1-ones (4a–k)
All chalcones were prepared according to the literature procedure (Furniss et al., 1989). A mixture of 4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)acetophenone (2) (20.0 mmol), corresponding aromatic aldehyde (20.0 mmol), MeONa (20.0 mmol) in MeOH (100 mL) was stirred at room temperature for a period of 2–4 h. The resulted precipitates were collected and washed with MeOH followed by cold water. The products were purified by flash chromatography by eluting with MeOH/CHCl3 (1:4).
1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-3-(4-fluorophenyl)prop-2-en-1-one (4a)
Yellowish white powder; 1H NMR (400 MHz, CDCl3) δ: 2.51 (3H, s, CH3), 3.13 (3H, s, NCH3), 7.14 (2H, t, J = 8.5 Hz, ArH), 7.49 (1H, d, J = 15.7 Hz, Hα), 7.54–7.62 (2H, m, ArH), 7.66 (1H, d, J = 8.0 Hz. ArH), 7.70 (4H, d, J = 8.5 Hz, ArH), 7.83 (1H, d, J = 15.7 Hz, Hβ), 7.97 (1H, d, J = 7.8 Hz, ArH), 8.12 (1H, d, J = 7.4 Hz, ArH), 8.19 (1H, d, J = 8.5 Hz, ArH). 13C NMR: 10.9, 40.0, 116.2, 116.4, 121.3, 124.2, 124.5, 124.9, 125.2, 127.9, 129.3, 129.6, 129.7, 130.5, 130.6, 130.9, 132.5, 133.0, 133.6, 137.5, 139.5, 142.7, 144.3, 162.9, 165.4, 189.0. MS m/z: 496.11 (M + Na)+.
3-(4-chlorophenyl)-1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c] [1,2]thiazine-2(4H)-yl)phenyl)prop-2-en-1-one (4b)
Pale yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.52 (3H, s, CH3), 3.14 (3H, s, NCH3), 7.43 (2H, m, ArH), 7.54 (1H, d, J = 15.8 Hz, Hα), 7.60–7.63 (3H, m, ArH), 7.71 (3H, d, J = 8.6 Hz, ArH), 7.82 (1H, d, J = 15.8 Hz, Hβ), 7.98 (1H, d, J = 7.8 Hz, ArH), 8.12 (1H, d, J = 7.7 Hz, ArH), 8.19 (2H, m, J = 8.6 Hz, ArH). 13C NMR: 10.9, 40.0, 121.9, 124.2, 124.5, 127.9, 129.2, 129.4, 129.6, 129.8, 130.1, 130.5, 131.0, 131.6, 132.1, 132.5, 132.9, 133.1, 133.5, 135.5, 136.8, 137.4, 139.5, 142.7, 144.1, 188.9. MS m/z: 512.08 (M + Na)+.
3-(3-chlorophenyl)-1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)prop-2-en-1-one (4c)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.53 (3H, s, CH3), 3.15 (3H, s, NCH3), 7.42 (1H, m, ArH), 7.52 (1H, d, J = 15.8 Hz, Hα), 7.57–7.60 (2H, m, ArH), 7.64 (1H, d, J = 15.8 Hz, Hβ), 7.69–7.73 (3H, m, ArH), 7.76 (2H, d, J = 5.1 Hz, ArH), 7.99 (1H, d, J = 6.7 Hz, ArH), 8.13 (1H, d, J = 7.0 Hz, ArH), 8.20–8.23 (2H, m, ArH). 13C NMR: 10.6, 40.2, 121.7, 124.0, 124.5, 127.4, 128.8, 129.4, 129.7, 129.9, 130.2, 130.5, 131.4, 131.7, 132.3, 132.6, 133.1, 133.4, 133.7, 135.3, 136.1, 138.4, 139.8, 141.6, 143.2, 188.6. MS m/z: 512.08 (M + Na)+.
3-(2,4-dichlorophenyl)-1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)prop-2-en-1-one (4d)
Yellow amorphous solid; 1H NMR (400 MHz, CDCl3) δ: 2.52 (3H, s, CH3), 3.15 (3H, s, NCH3), 7.51 (1H, m, ArH), 7.57 (1H, d, J = 15.6 Hz, Hα), 7.58 (2H, m, ArH), 7.64 (1H, d, J = 15.6 Hz, Hβ), 7.70 (3H, t, J = 8.0 Hz, ArH), 7.76–7.80 (1H, m, ArH), 7.95–8.01 (1H, m, ArH), 8.10–8.15 (1H, m, ArH), 8.18 (1H, J = 5.1 Hz, ArH). 13C NMR: 10.8, 40.2, 121.9, 124.2, 124.5, 127.9, 129.2, 129.4, 129.6, 129.8, 130.1, 130.5, 131.0, 131.6, 132.1, 132.5, 132.9, 133.1, 133.5, 135.5, 136.8, 137.4, 140.5, 143.7, 144.9, 187.6. MS m/z: 546.04 (M + Na)+.
3-(4-bromophenyl)-1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)prop-2-en-1-one (4e)
Yellow powder; Yield: 1H NMR (400 MHz, CDCl3) δ: 2.53 (3H, s, CH3), 3.15 (3H, s, NCH3), 7.32 (1H, d, J = 15.7 Hz, Hα,), 7.36 (1H, d, J = 1.9 Hz, ArH), 7.49 (1H, m, ArH), 7.55 (1H, d, J = 3.5 Hz, ArH), 7.62 (1H, d, J = 15.7 Hz, Hβ), 7.68–7.73 (4H, m, ArH), 7.79 (1H, m, ArH), 7.99 (1H, d, J = 6.5 Hz, ArH), 8.12–8.17 (2H, m, ArH), 8.22 (1H, m, ArH). 13C NMR: 10.8, 40.1, 121.7, 124.0, 124.5, 127.4, 128.8, 129.4, 129.7, 129.9, 130.2, 130.5, 131.4, 131.7, 132.3, 132.6, 132.9, 133.2, 133.9, 135.5, 136.5, 138.4, 139.2, 142.3, 144.0, 187.6. MS m/z: 556.03 (M + Na)+.
3-(3-bromophenyl)-1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)prop-2-en-1-one (4f)
Yellow crystals; 1H NMR (400 MHz, CDCl3) δ: 2.53 (3H, s, CH3), 3.15 (3H, s, NCH3), 7.31–7.33 (1H, m, ArH), 7.36–7.39 (1H, m, ArH), 7.49 (1H, d, J = 16.9 Hz, Hα), 7.55–7.58 (1H, m, ArH), 7.62 (1H, d, J = 16.9 Hz, Hβ), 7.69–7.72 (4H, m, ArH), 7.79 (1H, m., ArH), 7.99 (1H, d, J = 6.5 Hz, ArH), 8.12–8.14 (1H, m, ArH), 8.18 (1H, d, J = 6.3 Hz, ArH), 8.22 (1H, d, J = 5.5 Hz, ArH). 13C NMR: 10.8, 40.1, 121.7, 124.0, 124.5, 127.4, 128.8, 129.4, 129.7, 129.9, 130.2, 130.5, 131.4, 131.7, 132.3, 132.6, 132.9, 133.2, 133.9, 135.5, 136.5, 138.4, 139.2, 142.3, 144.0, 187.6. MS m/z: 556.03 (M + Na)+.
1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-3-(2-methoxyphenyl)prop-2-en-1-one (4g)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.49 (3H, s, CH3), 3.03 (3H, s, NCH3), 3.90 (3H, s, OCH3), 6.91 (1H, d, J = 16.7 Hz, Hα), 7.37 (2H, m, ArH), 7.61 (1H, d, J = 16.7 Hz, Hβ), 7.65 (1H, m, ArH), 7.70–7.73 (1H, m, ArH), 7.78 (1H, d, J = 6.1 Hz, ArH), 7.82 (2H, d, J = 2.7 Hz, ArH), 7.89 (1H, m, ArH), 7.97 (1H, d, J = 5.8 Hz, ArH), 8.03–8.06 (1H, m, ArH), 8.10–8.14 (2H, m, ArH). 13C NMR: 10.8, 40.1, 60.8 121.2, 121.8, 124.6, 124.8, 126.8, 128.4, 129.7, 130.5, 130.8, 131.1, 131.4, 131.7, 132.3, 132.6, 132.9, 133.2, 133.9, 135.5, 136.2, 138.4, 139.3, 141.9, 142.9, 189.7. MS m/z: 508.13 (M + Na)+.
3-(3,4-dimethoxyphenyl)-1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)prop-2-en-1-one (4h)
Yellow solid; Yield; 59%; mp 261–262 °C. 1H NMR (400 MHz, CDCl3) δ: 2.52 (3H, s, CH3), 3.14 (3H, s, NCH3), 3.93 (3H, s, OCH3), 3.95 (3H, s, OCH3), 6.87–6.91 (2H, m, ArH), 7.40 (1H, d, J = 5.5 Hz, ArH), 7.44 (1H, d, J = 15.6 Hz, Hα), 7.58–7.62 (1H, m, ArH), 7.71 (3H, d, J = 5.6 Hz, ArH), 7.78 (1H, d, J = 15.6 Hz, Hβ), 7.99 (1H, d, J = 7.8 Hz, ArH), 8.12 (1H, d, J = 7.7 Hz, ArH), 8.19 (2H, d, J = 8.5 Hz, ArH). 13C NMR: 10.9, 40.0, 56.2, 61.0, 105.8, 120.9, 121.6, 124.6, 124.8, 124.8, 125.2, 127.9, 129.2, 129.8, 130.1, 132.4, 132.9, 133.5, 133.9, 135.5, 136.2, 137.6, 139.3, 140.6, 142.5, 145.8, 153.5 189.4. MS m/z: 538.14 (M + Na)+.
1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-3-(2,3,4-trimethoxyphenyl)prop-2-en-1-one (4i)
Pale yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.67 (3H, s, CH3), 3.13 (3H, s, NCH3), 3.91 (3H, s, OCH3), 3.93 (3H, s, OCH3), 3.98 (3H, s, OCH3), 6.75 (1H, d, J = 15.8 Hz, Hα), 7.42 (1H, d, J = 15.8 Hz, Hβ), 7.58 (2H, d, J = 7.2 Hz, ArH), 7.65–7.69 (2H, m, ArH), 7.96–7.99 (1H, m, ArH), 8.13 (3H, d, J = 8.7 Hz, ArH), 8.18 (2H, d, J = 8.5 Hz, ArH). 13C NMR: 10.9, 40.0, 56.1, 60.9, 61.4, 107.6, 120.8, 121.7, 124.1, 124.5, 127.9, 129.2, 129.4, 129.6, 129.7, 131.5, 131.9, 132.5, 132.9, 133.5, 136.3, 138.0, 139.4, 141.0, 142.3, 142.5, 152.9, 156.1, 189.7. MS m/z: 568.15 (M + Na)+.
1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (4j)
Yellow amorphous solid; 1H NMR (400 MHz, CDCl3) δ: 2.47 (3H, s, CH3), 3.13 (3H, s, NCH3), 3.91 (3H, s, OCH3), 3.94 (6H, s, 2xOCH3), 6.75 (2H, d, J = 8.8 Hz, ArH), 7.42 (1H, d, J = 15.8 Hz, Hα), 7.58 (1H, d, J = 15.8 Hz, Hβ), 7.65–7.68 (2H, m, ArH), 7.94–7.98 (1H, m, ArH), 8.13 (3H, d, J = 8.7 Hz, ArH), 8.18 (2H, d, J = 8.5 Hz, ArH). 13C NMR: 10.9, 40.0, 56.1, 60.9, 61.4, 107.6, 120.8, 121.7, 124.1, 124.5, 127.9, 129.2, 129.4, 129.6, 129.7, 132.5, 132.9, 133.5, 133.9, 134.5, 136.3, 138.0, 139.4, 141.0, 142.4, 142.5, 152.9, 156.1, 189.7. MS m/z: 568.15 (M + Na)+.
1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-3-(4-nitrophenyl)prop-2-en-1-one (4k)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.53 (3H, s, CH3), 3.14 (3H, s, NCH3), 7.59 (1H, d, J = 15.6 Hz, Hα), 7.66 (1H, d, J = 15.6 Hz, Hβ), 7.72 (2H, d, J = 1.7 Hz, ArH), 7.76–7.79 (1H, m, ArH), 7.81 (1H, d, J = 5.2 Hz, ArH), 7.85-7.88 (1H, m, ArH), 7.98 (1H, d, J = 7.7 Hz, ArH), 8.12 (2H, d, J = 6.8 Hz, ArH), 8.20 (2H, m, ArH), 8.24 (1H, m, ArH), 8.31 (1H, d, J = 8.7 Hz, ArH). 13C NMR: 10.9, 40.0, 121.9, 124.1, 124.6, 127.6, 128.7, 129.3, 129.6, 129.9, 130.2, 130.4, 131.5, 131.8, 132.3, 132.6, 132.9, 133.2, 133.9, 135.5, 136.5, 138.4, 139.2, 143.3, 145.4, 188.5. MS m/z: 523.1 (M + Na)+.
Synthesis of 4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)benzaldehyde (3)
A mixture of 3,4-dimethyl-2,4-dihydropyrazolo[4,3-c][1,2]benzothiazine 5,5-dioxide (1) (6.25 g, 25.0 mmol), 4-fluorobenzaldehyde (3.72 g, 30.0 mmol), anhydrous K2CO3 (4.15 g, 30.0 mmol), and hexadecyl-n-tributylphosphonium bromide (1.27 g, 2.5 mmol) was refluxed in DMF (100 mL) for a period of 2 h under nitrogen atmosphere. The precipitates formed after adding ice cold water were collected, dried, and recrystallized from EtOH. Yellow crystals. Yield: 7.15 g, (81%); mp 230–232 °C. 1H NMR (400 MHz, CDCl3) δ: 2.52 (3H, s, CH3), 3.13 (3H, s, NCH3), 7.57–7.60 (1H, m, ArH), 7.68–7.72 (1H, m, ArH), 7.73–7.76 (2H, m, ArH), 7.97 (1H, d, J = 7.7 Hz, ArH), 8.06–8.09 (2H, m, ArH), 8.11 (1H, d, J = 7.7 Hz, ArH), 10.10 (1H, s, CHO). 13C NMR: 11.0, 40.0, 124.2, 124.7, 124.9(2C), 125.5, 127.8, 129.3(2C), 130.8, 132.6, 133.0, 133.6, 135.4, 139.7, 143.9, 190.9; MS m/z: 376.1 (M + Na) + . Anal. calc. for C18H15N3O3S; C, 61.18; H, 4.28; N, 11.89; Found: C, 61.18; H, 4.28; N, 11.88.
General procedure for the synthesis of 1-aryl-3-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H) yl)phenyl)prop-2-en-1-ones (5a–f)
A mixture of 1-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)benzaldehyde (3) (20.0 mmol), corresponding acetophenone (20.0 mmol), MeONa (20.0 mmol) in MeOH (100 mL) was stirred at room temperature for a period of 2–4 h. The resulted ppt were collected and washed with MeOH followed by cold water. The products were purified by flash chromatography by eluting with CHCl3/MeOH (4:1).
1-(4-chlorophenyl)-3-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)prop-2-en-1-one (5a)
Yellow amorphous solid; 1H NMR (400 MHz, CDCl3) δ: 2.48 (3H, s, CH3), 3.12 (3H, s, NCH3), 7.50 (2H, d, J = 8.6 Hz, ArH), 7.52 (1H, d, J = 14.2 Hz, Hα), 7.60 (2H, d, J = 8.3 Hz, ArH), 7.64 (1H, d, J = 14.2 Hz, Hβ), 7.74–7.89 (4H, m, ArH), 7.94–8.02 (3H, m, ArH), 8.10 (1H, d, J = 7.7 Hz, ArH). 13C NMR: 10.8, 40.0, 122.5, 124.2, 124.8, 125.0, 127.9, 129.0, 129.4, 129.9, 130.1, 130.5, 131.0, 131.6, 132.4, 132.9, 133.1, 133.4, 133.5, 134.5, 136.2, 137.4, 139.2, 140.8, 143.6, 188.7. MS m/z: 512.08 (M + Na)+.
1-(4-bromophenyl)-3-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)prop-2-en-1-one (5b)
Pale yellow crystals; 1H NMR (400 MHz, CDCl3) δ: 2.50 (3H, s, CH3), 3.14 (3H, s, NCH3), 7.48–7.59 (4H, m, ArH), 7.62 (1H, d, J = 14.4 Hz, Hα), 7.68–7.73 (3H, m, ArH), 7.82 (1H, d, J = 14.5 Hz, Hβ), 7.87 (1H, d, J = 7.1 Hz, ArH), 7.96–8.03 (3H, m, ArH), 8.11 (1H, d, J = 7.7 Hz, ArH). 13C NMR: 10.8, 40.0, 122.8, 124.1, 124.7, 125.0, 128.0, 128.7, 129.1, 129.3, 130.5, 130.8, 132.4, 132.9, 133.0, 133.4, 134.8, 137.9, 138.4, 139.0, 139.2, 140.6, 142.3, 143.1, 144.0, 190.1. MS m/z: 556.03 (M + Na)+.
3-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (5c)
Yellow amorphous solid; 1H NMR (400 MHz, CDCl3) δ: 2.48 (3H, s, CH3), 3.13 (3H, s, NCH3), 3.90 (3H, s, OCH3), 7.01 (1H, d, J = 15.2 Hz, Hα), 7.57–7.61 (2H, m, ArH), 7.64–7.67 (2H, m, ArH), 7.74 (1H, d, J = 15.2 Hz, Hβ), 7.75–7.79 (1H, m, ArH), 7.82 (2H, d, J = 2.7 Hz, ArH), 7.86–7.89 (1H, m, ArH), 7.97 (1H, d, J = 6.8 Hz, ArH), 8.05–8.14 (3H, m, ArH). 13C NMR: 10.8, 40.1, 57.8, 121.2, 121.8, 124.6, 124.8, 126.8, 128.4, 129.7, 130.5, 130.8, 131.1, 131.4, 131.7, 132.3, 132.6, 132.9, 133.2, 133.9, 135.5, 136.2, 138.4, 139.3, 141.9, 142.9, 189.7. MS m/z: 508.13 (M + Na)+.
3-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-1-phenylprop-2-en-1-one (5d)
Yellowish white solid; 1H NMR (400 MHz, CDCl3) δ: 2.49 (3H, s, CH3), 3.13 (3H, s, NCH3), 7.52-7.57 (2H, m, ArH), 7.59–7.62 (4H, m, ArH), 7.65 (1H, d, J = 15. 7 Hz, Hα), 7.70 (1H, t, J = 8.2 Hz, ArH), 7.81 (2H, d, J = 8.5 Hz, ArH), 7.86 (1H, d, J = 15.7 Hz, Hβ), 7.97 (1H, d, J = 7.7 Hz, ArH), 8.06 (2H, d, J = 7.2 Hz, ArH), 8.11 (1H, d, J = 7.7 Hz, ArH). 13C NMR: 10.8, 40.0, 123.1, 124.2, 124.7, 124.9, 125.1, 125.2, 128.8, 128.5, 128.7(2C), 129.1, 130.5, 130.8, 132.4(2C), 132.9, 133.1, 133.4, 134.8, 138.0, 139.2, 140.6, 143.1, 190.1. MS m/z: 478.12 (M + Na)+.
3-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-1-(p-tolyl)prop-2-en-1-one (5e)
Pale yellow crystalline solid; 1H NMR (400 MHz, CDCl3) δ: 2.46 (3H, s, CH3), 2.49 (3H, s, CH3), 3.14 (3H, s, NCH3), 7.34 (1H, d, J = 14.8 Hz, Hα), 7.60 (1H, J = 14.8 Hz, Hβ), 7.63 (2H, d, J = 5.4 Hz, ArH), 7.70 (2H, t, ArH), 7.79–7.84 (4H, m, ArH), 7.98 (3H, d, J = 8.0 Hz, ArH), 8.11 (1H, d, J = 7.7 Hz, ArH). 13C NMR: 10.8, 21.7, 40.1, 121.2, 121.8, 124.6, 124.8, 126.8, 128.4, 129.7, 130.5, 130.8, 131.1, 131.4, 131.7, 132.3, 132.6, 132.9, 133.2, 133.9, 135.5, 136.2, 138.4, 139.3, 141.9, 142.9, 189.7. MS m/z: 492.14 (M + Na)+.
3-(4-(3,4-dimethyl-5,5-dioxidobenzo[e]pyrazolo[4,3-c][1,2]thiazin-2(4H)-yl)phenyl)-1-mesitylprop-2-en-1-one (5f)
Yellow crystals; 1H NMR (400 MHz, CDCl3) δ: 2.22 (6H, s, 2xCH3), 2.34 (3H, s, CH3), 2.47 (3H, s, CH3), 3.12 (3H, s, NCH3), 6.94 (2H, d, J = 6.2 Hz, ArH), 6.98 (1H, d, J = 16.2 Hz, Hα), 7.19–7.22 (1H, m, ArH), 7.56 (1H, d, J = 16.2 Hz, Hβ), 7.58–7.61 (2H, m, ArH), 7.65–7.70 (3H, m, ArH), 7.96 (1H, d, J = 7.8 Hz, ArH), 8.08 (1H, m, ArH). 13C NMR: 10.8, 19.4, 21.2, 40.0, 56.1, 107.6, 120.8, 121.7, 124.1, 124.8, 125.0, 128.5, 129.4, 129.6, 129.7, 131.4, 131.9, 132.5, 132.9, 133.5, 134.1, 138.0, 139.4, 141.0, 142.4, 144.8, 152.9, 156.1, 188.9. MS m/z: 520.17 (M + Na)+.
General procedure for the synthesis of 2-(4-(2-amino-6-arylpyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine-5,5-dioxides (6a–o)
All compounds were prepared according to the literature procedure (Varga et al., 2003). A mixture of corresponding chalcone (9.1 mmol), guanidine hydrochloride (13.6 mmol) and 50% aqueous KOH solution (4.0 mL) was stirred at reflux temperature for a period of 1 h in EtOH (20.0 mL) followed by portion wise addition of 30% H2O2 (30.3 mmol, 3.1 mL) over 1 h under the same conditions. The precipitates thus formed were thoroughly washed with EtOH and then with pure water. Recrystallization from a suitable solvent resulted pure compounds.
2-(4-(2-amino-6-(4-fluorophenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6a)
Yellowish white powder; 1H NMR (400 MHz, CDCl3) δ: 2.50 (3H, s, CH3), 3.15 (3H, s, NCH3), 5.21 (2H, br. s, NH2), 7.21 (2H, t, J = 8.5 Hz, ArH), 7.45–7.48 (1H, m, ArH), 7.57–7.61 (1H, m, ArH), 7.66–7.75 (3H, m, ArH), 7.99 (1H, d, J = 7.8 Hz, ArH), 8.07–8.16 (3H, m, ArH), 8.25 (2H, d, J = 8.3 Hz, ArH). 13C NMR: 10.8, 40.0, 103.8, 115.7, 124.2, 124.8 (2C), 125.0, 128.2 (2C), 129.1, 129.4, 132.4, 132.9, 133.0, 133.5, 136.4, 137.5, 137.6, 139.1, 143.2, 153.5, 162.0, 162.9, 163.8, 164.6, 165.5. MS m/z: 535.14 (M + Na)+.
2-(4-(2-amino-6-(4-chlorophenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6b)
Pale yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.48 (3H, s, CH3), 3.09 (3H, s, NCH3), 5.25 (2H, br. s, NH2), 7.38 (2H, m, ArH), 7.56–7.60 (3H, m, ArH), 7.67 (2H, d, J = 8.7 Hz, ArH), 7.87 (2H, d, J = 7.6 Hz, ArH), 7.93 (1H, d, J = 8.0 Hz, ArH), 8.07 (2H, d, J = 8.0 Hz, ArH), 8.12–8.16 (1H, m, ArH). 13C NMR: 10.8, 40.0, 104.0, 104.5, 105.4, 124.2, 124.4, 124.8, 125.0, 127.8, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.0, 133.3, 134.3, 136.4, 138.8, 137.6, 139.1, 143.9, 163.5, 163.9, 165.7. MS m/z: 551.10 (M + Na)+.
2-(4-(2-amino-6-(3-chlorophenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6c)
Light brown amorphous powder; 1H NMR (400 MHz, CDCl3) δ: 2.50 (3H, s, CH3), 3.14 (3H, s, N CH3), 5.27 (2H, br. s, NH2), 7.04 (1H, m, ArH), 7.29–7.40 (4H, m, ArH), 7.48 (1H, s, ArH), 7.67 (3H, d, J = 7.3 Hz, ArH), 7.98 (2H, d, J = 7.3 Hz, ArH), 8.11 (1H, d, J = 2.4 Hz, ArH), 8.24 (1H, d, J = 8.5 Hz, ArH). 13C NMR: 10.8, 39.9, 48.8, 49.0, 49.2, 49.4, 49.7, 121.9, 124.2, 124.6, 124.8, 125.5, 127.7, 129.3, 129.7, 129.8, 130.2, 130.6, 132.4, 133.1, 133.8, 135.8, 136.4, 137.5, 163.3, 163.9, 165.5. MS m/z: 551.10 (M + Na)+.
2-(4-(2-amino-6-(2,4-dichlorophenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6d)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.47 (3H, s, CH3), 3.09 (3H, s, NCH3), 5.25 (2H, br. s, NH2), 7.35–7.38 (2H, m, ArH), 7.56–7.60 (2H, m, ArH), 7.67 (3H, d, J = 8.7 Hz,,ArH), 7.77 (1H, d, J = 7.7 Hz, ArH), 7.93 (1H, d, J = 8.0 Hz, ArH), 8.07 (1H, d, J = 9.0 Hz, ArH), 8.15–8.18 (2H, m, J = 8.7 Hz, ArH). 13C NMR: 10.8, 40.0, 104.0, 104.5, 105.4, 124.2, 124.4, 124.8, 125.0, 127.8, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.0, 133.3, 134.3, 136.4, 138.8, 137.6, 139.1, 143.9, 163.5, 163.9, 165.7. MS m/z: 585.06 (M + Na)+.
2-(4-(2-amino-6-(4-bromophenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6e)
Dirty white crystals; 1H NMR (400 MHz, CDCl3) δ: 2.50 (3H, s, CH3), 3.14 (3H, s, NCH3), 5.29 (2H, br. s, NH2), 7.49 (1H, s, ArH), 7.54–7.64 (3H, m, ArH), 7.69 (4H, d, J = 5.6 Hz, ArH), 7.99 (3H, d, J = 8.2 Hz, ArH), 8.13 (1H, d, J = 7.7 Hz, ArH), 8.25 (1H, d, J = 8.4 Hz, ArH). 13C NMR: 10.9, 40.0, 124.2, 124.4, 124.9, 125.2, 127.9, 129.2, 129.6, 132.5, 132.9, 133.5, 136.4(2C), 136.7, 136.9, 137.2, 137.5, 137.7(2C), 137.9, 139.5, 142.9(2C), 162.3, 162.5, 164.3. MS m/z: 595.05 (M + Na)+.
2-(4-(2-amino-6-(3-bromophenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6f)
White amorphous powder; 1H NMR (400 MHz, CDCl3) δ: 2.50 (3H, s, CH3), 3.15 (3H, s, NCH3), 5.32 (2H, br. s, NH2), 7.42 (2H, d, J = 7.8 Hz, ArH), 7.49 (1H, s, ArH), 7.65 (3H, d, J = 7.5 Hz, ArH), 8.00 (3H, t, J = 7.2 Hz, ArH), 8.10–8.13 (1H, m, ArH), 8.24–8.27 (2H, m, ArH). 13C NMR: 10.9, 40.0, 124.2, 124.4, 124.9, 125.4, 127.8, 129.3, 129.8, 132.5, 132.9, 133.5, 134.4(2C), 136.7, 136.9, 137.2, 137.5, 137.4(2C), 137.9, 139.5, 142.2(2C), 162.3, 162.5, 164.3. MS m/z: 571.06 (M-H)+.
2-(4-(2-amino-6-(4-methoxyphenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6g)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.48 (3H, s, CH3), 3.13 (3H, s, NCH3), 3.63 (3H, s, OCH3), 5.24 (2H, br. s, NH2), 6.82 (1H, d, J = 8.8 Hz, ArH), 7.55–7.60 (2H, m, ArH), 7.64–7.72 (4H, m, ArH), 7.74 (1H, s, ArH), 7.97 (1H, d, J = 7.8 Hz, ArH), 8.02 (1H, d, J = 7.7 Hz, ArH), 8.14 (2H, d, J = 8.2 Hz, ArH), 8.27(1H, d, J = 8.1 Hz, ArH). 13C NMR: 10.8, 40.0, 60.0, 104.0, 104.5, 105.4, 118.2, 120.7, 124.8, 125.0, 126.9, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.0, 133.5, 136.4, 138.8, 137.6, 139.1, 140.9, 153.6, 157.8, 163.2, 164.5. MS m/z: 525.17 (M + H)+.
2-(4-(2-amino-6-(2-methoxyphenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6h)
Dark yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.49 (3H, s, CH3), 3.18 (3H, s, NCH3), 3.93 (3H, s, OCH3), 5.24 (2H, br. s, NH2), 6.87 (1H, d, J = 8.6 Hz, ArH), 7.35–7.40 (2H, m, ArH), 7.64–7.68 (4H, m, ArH), 7.72 (1H, s, ArH), 7.95 (1H, d, J = 6.8 Hz, ArH), 8.10 (1H, d, J = 8.0 Hz, ArH), 8.22 (2H, d, J = 8.1 Hz, ArH), 8.29(1H, d, J = 7.7 Hz, ArH). 13C NMR: 10.8, 40.0, 60.0, 104.0, 104.5, 105.4, 118.2, 120.7, 124.8, 125.0, 127.8, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.0, 133.5, 136.4, 138.8, 137.6, 139.1, 140.9, 153.5, 158.2, 163.5, 164.8. MS m/z: 526.17 (M + H)+.
2-(4-(2-amino-6-(3,4-dimethoxyphenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6i)
Pale yellow crystals; 1H NMR (400 MHz, CDCl3) δ: 2.49 (3H, s, CH3), 3.14 (3H, s, NCH3), 3.97 (3H, s, OCH3), 4.03 (3H, s, OCH3), 5.25 (2H, br. s, NH2), 6.99 (1H, d, J = 8.4 Hz, ArH), 7.47 (1H, s, ArH), 7.64–7.74 (6H, m, ArH), 7.98 (1H, d, J = 7.7 Hz, ArH), 8.13 (1H, d, J = 7.5 Hz, ArH), 8.23 (2H, d, J = 8.5 Hz, ArH). 13C NMR: 10.2, 40.1, 56.1, 56.4, 101.5, 109.0, 111.5, 124.2, 124.4, 124.8, 125.0, 127.8, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.0, 133.5, 136.4, 136.8, 137.6, 139.1, 143.9, 153.5, 163.5, 164.8, 166.2. MS m/z: 555.18 (M + H)+.
2-(4-(2-amino-6-(2,3,4-trimethoxyphenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6j)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.48 (3H, s, CH3), 3.13 (3H, s, NCH3), 3.87 (3H,s, OCH3), 3.93 (3H, s, OCH3), 3.95 (3H, s, OCH3), 5.24 (2H, br. s, NH2), 6.82 (1H, d, J = 8.8 Hz, ArH), 7.55–7.60 (1H, m, ArH), 7.64–7.72 (4H, m, ArH), 7.74 (1H, s, ArH), 7.97 (1H, d, J = 7.8 Hz, ArH), 8.12 (1H, d, J = 7.7 Hz, ArH), 8.22 (2H, d, J = 8.2 Hz, ArH). 13C NMR: 10.8, 40.0, 56.1, 56.4, 61.0, 104.0, 104.5, 105.4, 124.2, 124.4, 124.8, 125.0, 127.8, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.0, 133.5, 136.4, 136.8, 137.6, 139.1, 140.9, 153.5, 163.5, 163.8, 167.0. MS m/z: 585.19 (M + H)+.
2-(4-(2-amino-6-(3,4,5-trimethoxyphenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6k)
Light brown powder; 1H NMR (400 MHz, CDCl3) δ: 2.49 (3H, s, CH3), 3.14 (3H, s, NCH3), 3.87 (3H, s, OCH3), 3.94 (3H, s, OCH3), 4.00 (3H, s, OCH3), 5.24 (2H, s, NH2), 6.49 (1H, m, ArH), 7.33 (1H, d, J = 7.0 Hz, ArH), 7.44 (1H, d, J = 7.0 Hz, ArH), 7.56–7.59 (2H, m, ArH), 7.65–7.74 (3H, m, ArH), 7.98 (1H, d, J = 7.7 Hz, ArH), 8.13 (2H, d, J = 7.4 Hz, ArH). 13C NMR: 10.8, 40.0, 56.1, 56.4, 61.0, 104.0, 104.5, 105.4, 124.2, 124.4, 124.8, 125.0, 127.8, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.0, 133.5, 136.4, 138.8, 137.6, 139.1, 140.9, 153.5, 163.5, 164.8, 166.2. MS m/z: 607.17 ((M + Na)+ .
2-(4-(2-amino-6-(4-nitrophenyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6l)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.51 (3H, s, CH3), 3.15 (3H, s, NCH3), 5.49 (2H, br. s, NH2), 7.57 (1H, m, ArH), 7.64 (1H, s, ArH), 7.69–7.75 (3H, m, ArH), 7.91–8.03 (4H, m, ArH), 8.13 (2H, d, J = 7.2 Hz, ArH), 8.27–8.37 (2H, m, ArH). 13C NMR: 10.8, 40.0, 104.0, 104.5, 105.4, 121.9, 124.2, 124.6, 124.8, 125.5, 127.7, 129.3, 129.7, 129.8, 130.2, 130.6, 132.4, 133.1, 133.8, 134.8, 135.4, 136.5, 141.9, 147.8, 163.3, 163.8, 165,6. MS m/z: 562.14 ((M + Na)+.
2-(4-(2-amino-6-phenylpyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6m)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.49 (3H, s, CH3), 3.14 (3H, s, NCH3), 5.22 (2H, br. s,, NH2), 7.51–7.56 (4H, m, ArH), 7.58 (1H, t, J = 7.6 Hz, ArH), 7.66–7.72 (3H, m, ArH), 7.98 (1H, d, J = 7.7 Hz, ArH), 8.08–8.10 (2H, m, ArH), 8.13 (1H, d, J = 7.7 Hz, ArH), 8.25 (2H, d, J = 8.5 Hz, ArH). 13C NMR: 10.5, 40.0, 104.2, 124.2, 124.8, 124.9, 125.2, 127.1, 128.0, 128.2, 128.7, 129.02, 129.4, 130.6, 130.9 132.4, 132.9, 133.2, 133.5, 134.0, 137.5, 137.6, 139.1, 140.8, 163.6, 164.7, 166.6. MS m/z: 517.14 (M + Na)+.
2-(4-(2-amino-6-(p-tolyl)pyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6n)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.46 (3H, s, CH3), 2.49 (3H, s, CH3), 3.14 (3H, s, CH3), 5.25 (2H, br. s, NH2), 7.34 (2H, d, J = 8.0 Hz, ArH), 7.56–7.61 (2H, m, ArH), 7.64 (2H, d, J = 3.8 Hz, ArH), 7.79 (1H, s, ArH), 7.83 (2H, d, J = 3.8 Hz, ArH), 7.98 (3H, d, J = 7.9 Hz, ArH), 8.12 (1H, d, J = 7.6 Hz, ArH). 13C NMR: 10.7, 20.4, 40.0, 104.0, 104.5, 105.4, 124.2, 124.4, 124.8, 125.0, 127.8, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.2, 133.5, 136.4, 138.8, 137.6, 139.1, 140.9, 153.5, 163.8, 164.6, 166.5. MS m/z: 509.18 (M + H)+.
2-(4-(2-amino-6-mesitylpyrimidin-4-yl)phenyl)-3,4-dimethyl-2,4-dihydrobenzo[e]pyrazolo[4,3-c][1,2]thiazine 5,5-dioxide (6o)
Yellow powder; 1H NMR (400 MHz, CDCl3) δ: 2.20 (6 H, s, 2xCH3), 2.37 (3H, s, CH3), 2.45 (3H, s, CH3), 3.12 (3H, s, NCH3), 6.12 (2H, br. s, NH2), 7.07–7.26 (3H, m, ArH), 7.53 (2H, d, J = 8.3 Hz, ArH), 7.69 (2H, t, J = 7.6 Hz, ArH), 7.94–7.99 (2H, m, ArH), 8.10 (2H, d, J = 7.7 Hz, ArH). 13C NMR: 10.7, 19.3 (3C), 40.0, 104.0, 104.5, 105.4, 124.2, 124.4, 124.8, 125.0, 127.8, 128.2, 129.2, 129.6, 130.4, 132.4, 132.9, 133.0, 133.5, 136.4, 138.8, 137.6, 139.1, 140.9, 153.5, 163.5, 164.8, 166.2. MS m/z: 536.20 (M+).
Anti-bacterial testing
Anti-bacterial assays were performed by the hole-plate method (Baldwin et al., 1989; Baldwin et al., 1987; Smith et al., 1967) with the test organisms Staphylococcus aureus N.C.T.C. 6571 and E. coli X580. Solutions (100 μl) of the compounds to be tested (2 mg/mL) were loaded into wells in bioassay plates and incubated overnight at 37 °C. The diameters of the resultant inhibition zones were measured, and amounts of product were estimated by reference to standards prepared with Cephalosporin C. The results are summarized in Table 2.
References
Ahmad M, Siddiqui HL, Zia-ur-Rehman M, Parvez M (2010) Anti-oxidant and anti-bacterial activities of novel N’-arylmethylidene-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-2(4H)-yl) acetohydrazides. Eur J Med Chem 45:698–704
Ahmad N, Zia-ur-Rehman M, Siddiqui HL, Fasih Ullah M, Parvez M (2011) Microwave assisted synthesis and structure–activity relationship of 4-hydroxy-N’-[1-phenylethylidene]-2H/2-methyl-1,2-benzothiazine-3-carbohydrazide 1,1-dioxides as anti-microbial agents. Eur J Med Chem 46(6):2368–2377
Argiriadi MA, Ericsson AM, Harris CM, Banach DL, Borhani DW, Calderwood DJ, Demers MD, DiMauro J, Dixon RW, Hardman J et al (2010) 2,4-Diaminopyrimidine MK2 inhibitors. Part I: observation of an unexpected inhibitor binding mode. Bioorg Med Chem Lett 20(1):330–333
Baldwin JE, Pratt AJ, Moloney MG (1987) The synthesis of aryl substituted analogues of phenoxyacetyl-l-cysteinyl-d-valine and phenylacetyl-l-cysteinyl-d-valine. Application to the photoaffinity labelling of isopenicillin N synthetase. Tetrahedron 43:2565–2575
Baldwin JE, Coates JB, Halpern J, Moloney MG, Pratt AJ (1989) Photoaffinity labelling of isopenicillin N synthetase by laser-flash photolysis. Biochem J 261:197–204
Ballell L, Robert AF, Chung GAC, Young RJ (2007) New mercaptopyrazolo[3,4-d]pyrimidine derivatives as anti-mycobacterial agents. Bioorg Med Chem Lett 17:1736–1740
Ban M, Taguchi H, Katsushima T, Aoki S, Watanabe A (1998) Novel antiallergic agents. Part I: synthesis and pharmacology of pyrimidine amide derivatives. Bioorg Med Chem Lett 6(7):1057–1067
Banker R, Teltsch B, Sukenik A, Carmeli S (2000) 7-epicylindrospermopsin, a toxic minor metabolite of the cyanobacterium aphanizomenon ovalisporum from Lake Kinneret. Israel J Nat Prod 63:387–389
Baraldi PG, Cacciari B, Romagnoli R, Klotz K-N, Spalluto G, Varani K, Gessi S, Merighi S, Borea PA (2001) Pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine derivatives as adenosine receptor ligands: a starting point for searching A2B adenosine receptor antagonists. Drug Dev Res 53(2–3):225–235
Baraldi PG, Pavani MG, MdC Nunez, Brigidi P, Vitali B, Gambari R, Romagnolia R (2002) Antimicrobial and antitumor activity of N-heteroimmine-1,2,3-dithiazoles and their transformation in triazolo-, imidazo-, and pyrazolopirimidines. Bioorg Med Chem 10:449–456
Bell EA, Foster RG (1962) Structure of lathyrine. Nature 194(4823):91–92
Berlinck RGS, Braekman JC, Daloze D, Bruno I, Riccio R, Ferri S, Spampinato S, Speroni E (1993) Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca2+ channel blocker activity of crambescin-816. J Nat Prod 56:1007–1015
Blum JL (2001) The role of capecitabine, an oral, enzymatically activated fluoropyrimidine, in the treatment of metastatic breast cancer. Oncologist 6:56–64
Chamakura VNSV, Ramasamy KS, Girardet JL, Gunic E, Lai V, Zhong W, An H, Hong Z (2007) Synthesis of pyrrolo[2,3-d]pyrimidine nucleoside derivatives as potential anti-HCV agents. Bioorg Chem 35(1):25–34
Chang LCW, Brussee J, Ijzerman AP (2004) Non-xanthine antagonists for the adenosine A1 receptor. Chem Biodiver 1(11):1591–1626
Chern J-H, Shia K-S, Hsu T-A, Tai C-L, Lee C–C, Lee Y-C, Chang C-S, Tseng S-N, Shih S-R (2004) Design, synthesis, and structure–activity relationships of pyrazolo[3,4-d]pyrimidines: a novel class of potent enterovirus inhibitors. Bioorg Med Chem Lett 14(10):2519–2525
Chu X-J, DePinto W, Bartkovitz D, So S–S, Vu BT, Packman K, Lukacs C, Ding Q, Jiang N, Wang K et al (2006) Discovery of [4-Amino-2-(1-methanesulfonylpiperidin-4-ylamino)pyrimidin-5-yl](2,3-difluoro-6- methoxyphenyl)methanone (R547), a potent and selective cyclin-dependent kinase inhibitor with significant in vivo antitumor activity. J Med Chem 49:6549–6560
Coelmont L, Paeshuyse J, Windisch MP, Clercq ED, Bartenschlager R, Neyts J (2006) Ribavirin antagonizes the in vitro anti-hepatitis C virus activity of 2′-C-methylcytidine, the active component of valopicitabine. Antimicrob Agents Chemother 50(10):3444–3446
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (1989) Vogel’s textbook of practical organic chemistry, 5th edn. Longman, New York, pp 1034–1035
Jansen BCP, Donath WF (1926) On the isolation of antiberiberi vitamin. Proc Kon Ned Akad Wet 29:1390–1400
Joffe AM, Farley JD, Linden D, Goldsand G (1989) Trimethoprim-sulfamethoxazole-associated aseptic meningitis: case reports and review of the literature. Am J Med 87:332–338
Lee HR, Kim WH, Park AY, Kang JA, Chun P, Bae JH, Jeong LS, Moon HR (2008) Synthesis of pyrimidine analog of fluoroneplanocin A as potential anti-HCV agent. Nucleic Acids Symp Ser (Oxf). 52:607–608
Lin Y-L, Huang R-L, Chang C-M, Kuo Y-H (1997) Two new puriniums and three new pyrimidines from Heterostemma brownii. J Nat Prod 60:982–985
Malik V, Singh P, Kumar S (2006) Unique chlorine effect in regioselective one-pot synthesis of 1-alkyl-/allyl-3-(o-chlorobenzyl) uracils: anti-HIV activity of selected uracil derivatives. Tetrahedron 62(25):5944–5951
Moravec J, Krytof V, Hanu J, Havlíek L, Moravcová D, Fuksová K, Kuzma M, Lenobel R, Otyepka M, Strnad M (2003) 2,6,8,9-Tetrasubstituted purines as new CDK1 inhibitors. Bioorg Med Chem Lett 13(18):2993–2996
Munchhof MJ, Beebe JS, Casavant JM, Cooper BA, Doty JL, Higdon RC, Hillerman SM, Soderstrom C, Knauth EA, Marx MA et al (2004) Design and SAR of thienopyrimidine and thienopyridine inhibitors of VEGFR-2 kinase activity. Bioorg Med Chem Lett 14(1):21–24
Nadal E, Olavarria E (2004) Imatinib mesylate (Gleevec/Glivec) a molecular-targeted therapy for chronic myeloid leukaemia and other malignancies. Int J Clin Pract 58:511–516
Ohtani I, Moore RE, Runnegar MTC (1992) Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J Am Chem Soc 114:7941–7942
Petersen E, Schmidt DR (2003) Sulfadiazine and pyrimethamine in the postnatal treatment of congenital toxoplasmosis: what are the options? Expert Rev Anti Infect Ther 1:175–182
Smith B, Warren SC, Newton GGF, Abraham EP (1967) Biosynthesis of penicillin N and Cephalosporin C-Antibiotic production and other features of the metabolism of a Cephalosporium sp. Biochem J 103:877–890
Sondhi SM, Singh N, Johar M, Kumar A (2005) Synthesis, anti-inflammatory and analgesic activities, evaluation of some mono-, bi- and tricyclic pyrimidine derivatives. Bioorg Med Chem 13:6158–6166
Sullivan RW, Bigam CG, Erdman PE, Palanki MSS, Anderson DW, Goldman ME, Ransone LJ, Suto MJ (1998) 2-Chloro-4-(trifluoromethyl)pyrimidine-5-N-(3′,5′-bis(trifluoromethyl)phenyl-carboxamic: a potent inhibitor of NF-KB-and AP-1-mediated gene expression identified using solution-phase combinatorial chemistry. J Med Chem 41:413–419
Tanaka F, Takeuchi S, Tanaka N, Yonehara H, Umezawa H, Sumiki YJ (1961) Bacimethrin, a new antibiotic produced by B. megatherium. Antibiot A 14:161–162
Varga L, Nagy T, Kovesdi I, Benet-Buchholz J, Dorman G, Urge L, Darvas F (2003) Solution-phase parallel synthesis of 4,6-diaryl-pyrimidine-2-ylamines and 2-amino-5,5-disubstituted-3,5-dihydro-imidazol-4-ones via a rearrangement. Tetrahedron 59:655–662
Xu J (2007) Synthesis of novel sulfonamide-based calpain inhibitors and their potential as anti-tumor agents [M, Sc.]. The University of Tennessee, Tennessee, USA
Acknowledgments
The authors are thankful to the Higher Education Commission, Pakistan for grant of scholarship to M. H. Bukhari and University of the Punjab, Lahore for research facilities. We are also thankful to the Department of Chemistry, University of Oxford, UK for spectral and X-rays studies. Special thanks are because of Wendy Sobey of the Department of Chemistry Research Laboratories, University of Oxford, UK for antibacterial testing and valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bukhari, M.H., Siddiqui, H.L., Ahmad, M. et al. Synthesis and anti-bacterial activities of some novel pyrazolobenzothiazine-based chalcones and their pyrimidine derivatives. Med Chem Res 21, 2885–2895 (2012). https://doi.org/10.1007/s00044-011-9820-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9820-0