Abstract
For social animals, decision-making is influenced by both social information provided by the group, and private information based on the individual’s personal experience. Social insects make excellent study systems for understanding how social and private information is used by individuals to influence their navigational route choice, and thereby influence the collective decision-making strategy of the group. Using colonies of the Australian meat ant, Iridomyrmex purpureus, we demonstrate that when individual workers are trained to a rewarding arm in a Y maze, the trained ants use private information (memory) in route choice when social information (trail pheromone) is experimentally removed and have no preference when private information and social information are in direct conflict with each other. Additional experience did not provide a strong training effect, such that ants returning after their first training trip tended to choose the path they had been trained on (private information) and subsequent trips did not have a significant additional effect on this initial preference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals, including ants, can increase their probability of successfully travelling from one point to the other using a range of navigational information including memory of landmarks (Collett and Collett 2002; Cheng et al. 2012), the position of the sun, moon or stars (Wehner 1984; Warrant and Dacke 2016), magnetic fields (Gould 1984; Banks and Srygley 2003; Buehlmann et al. 2012), the number of steps taken (Wolf 2011) chemosensory (Aron et al. 1989; Evison et al. 2008), and/or visual cues (Graham and Cheng 2009). Some animals can also incorporate ‘social information’ provided by other animals to make their own trips more efficient or reliable. Social information can be passed from one individual to another either deliberately (e.g. the pheromone trails of ants) or incidentally (e.g. game trails; see Perna and Latty 2014). Social information can provide reassurance that an individual is on the correct path thereby reducing cognitive load (Czaczkes et al. 2011), provide information about the shortest route to a resource (Goss et al. 1989), warn of predator risks (Abbott and Dukas 2009), and provide information about food quality (Beckers et al. 1990; Seeley et al. 1991, 2000; Reid et al. 2012; Latty and Beekman 2013).
Social information can be relatively cheap to collect, as the individual need not explore the environment to select a route (Laland 2004). However the costs of collecting social information can vary depending on a wide variety of factors such as the distribution of resources in the environment, the risk of predation, the rate of environmental change, the strategies of other individuals and the time it takes to collect information (Grüter and Leadbeater 2014). For example, if resources are common and easy to locate, then the cost of exploration might be relatively low compared to the potential cost of trail-following to a resource which might rapidly become overcrowded and depleted.
Social and private information can act synergistically when they are in agreement, providing the animal with reassurance that it is heading in the appropriate direction. For example, trained Lasius niger workers will travel faster along a memorised route when pheromone is present; the pheromone appears to improve individual confidence in the selected route (Czaczkes et al. 2011). Trouble can arise, however, when socially acquired information conflicts with the routing information stored in an individual’s memory. For example, an ant forager leaving the nest can choose between pheromone trails laid by nestmates (social information) or her personal route memory associated with a particularly rich food source in the opposite direction. Similarly, foraging honey bees often choose between prioritising their own memory of previously located food sources, or abandoning these sites in favour of foraging at a novel location communicated to them by the waggle dances of other foragers (Grüter and Ratnieks 2011). These conflict scenarios allow us to determine in which situations an organism will prioritise socially acquired information over individually acquired information and vice versa. Social insects vary in their use of social and private information with some species prioritising social (Iridomyrmex humilis and L. niger, Aron et al. 1993; Apis mellifera; Grüter and Ratnieks 2011), and others prioritising private (e.g. Paraponera clavata, Harrison et al. 1989; Dechaume-Moncharmont et al. 2005; L. niger; Grüter et al. 2011; for a review see; Grüter and Leadbeater 2014). Experience and memory can influence the use of social and private information; for example, Argentine ants are more likely to ignore trail information in favour of memory if they have made > 4 trips to a rewarding food source (Aron et al. 1993). Similarly, honey bees will disregard directional information encoded in the waggle dance if they remember the route to a particular flower (Grüter et al. 2008).
Here, we study the social navigation strategy of the Australian meat ant (Iridomyrmex purpureus) when faced with conflicts between individually acquired and socially available information. Studying social navigation under natural field conditions allows ants to take advantage of the wide variety of informational cues that are normally denied them in lab studies, such as the complex field and sky panorama, changing light intensities, and environmental weather variations, and also allows the use of complete colonies, rather than colony fragments. A recent study conducted by Card et al. (2016) showed that meat ants were more likely to prioritise private over social information when navigating back to their nest. Another field study on social navigation in Acromyrmex lobicornis also demonstrated that private information was more influential than social information. However, if social information was experimentally increased most individuals would switch to social information, increasing the flexibility of colony decisions based on broader environmental fluctuations (Elizalde and Farji-Brener 2012). Our work examines the relative importance of private and social information at the individual level for recruitment purposes, and further examines the influence of knowledgeable individuals on naïve foragers.
Some studies of social navigation remove focal ants immediately after they make a decision about which path to choose. This is done to prevent focal ants from influencing the decision making of following ants by, for example, laying pheromones. However, we chose not to remove returning ants so as not to disrupt their natural pheromone deposition behaviour. Pheromone deposition by ‘trained’ ants is part of the colony’s behavioural repertoire and thus may play a key role in dictating how social and private information are integrated as part of a colony-level social navigation strategy. Further, we use the opportunity to examine the impact of multiple journeys upon the behaviour of ants, rather than a binary examination of trained vs. untrained, as the level of experience may significantly contribute to individual and group behaviour. Rather than removing ants after they cross a decision line, we use statistical techniques to investigate the interplay between social and private information.
Methods
Study species
Iridomyrmex purpureus is an ecologically dominant ant species found in savannah woodlands and grasslands throughout Australia (Shattuck 2000). They feed on a variety of foods including seeds (Campbell and Clarke 2006), small invertebrates (Mobbs et al. 1978) and the honeydew secreted by sap sucking insects (Greaves and Hughes 1974). Mature I. purpureus colonies can contain hundreds of thousands of individuals inside large, distinct nest mounds which the ants clear of vegetation and cover with small sticks and rocks (Greaves and Hughes 1974). Workers of this species build distinct trunk trails between their food sources and their nests, often clearing the trails of vegetation so that they resemble roads through the undergrowth (Greaves and Hughes 1974).
General experimental setup
Colonies of I. purpureus were studied on the grounds of the Western Sydney University Hawkesbury campus in Richmond, New South Wales, Australia, from October 2015 through to February 2016. The field site was within a large remnant patch of Cumberland plains woodland, dominated primarily by open stands of Eucalyptus trees. Colonies tended to be located in bright sunny areas such as beside the dirt track and in forest clearings.
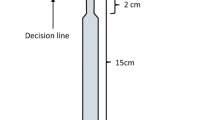
Our general experimental design consisted of Y decision mazes constructed from Corflute™ plastic sheets. On each of the arms of the Y, a clean piece of paper covered the Corflute™ as a substrate for the deposition of pheromone. The Y maze was elevated off the ground on plastic containers to prevent ants from bypassing the maze and climbing directly to the food source (Fig. 1). We started the training phase of experiments by placing the base of the Y onto the edge of a colony. The 0.5 M sucrose syrup food source was then moved in stages from the junction of the Y to the final location at the end of the training arm as follows. When five ants were seen feeding at the junction of the Y, the feeder was then moved halfway up the arm that served as the training arm for that replicate. After 5 min, the feeder was then moved to its final location at the end of the training arm. The training arm was chosen randomly to be on the right or left, to remove any underlying turning bias. Experiments commenced once 30 ants had been trained to the feeder at its final location. Each of the 30 colonies was tested once.
Experiment 1: social vs. private information
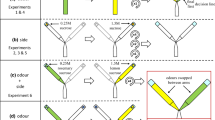
Using the general setup described above, we examined the choice of trained ants under three treatments: pheromone removal, social–private conflict, or a control. To distinguish trained ants from untrained ants (who had never visited the feeder during the training phase), we marked all ants that arrived at the feeder during the training phase with a paint mark on the gaster (Reeves acrylic paints). The experiment began once 30 ants were paint-marked as ‘trained’ (so had visited the feeder at least once) at which time the feeder was removed and new ants were blocked from entering the Y maze. We then applied one of the three treatments described below. Once the treatment was set up, food sources were placed at the end of each arm of the Y maze. Ants were then allowed to return to the Y maze. To assess the number of ants travelling on each maze arm, we filmed the behaviour of ants for 5 min after the treatment began.
During the pheromone removal treatment (n = 11 colonies), the pieces of paper lining the maze floor were removed and replaced with new clean papers to remove any pheromone signal. If ants are capable of using route memory, then we expect trained individuals to travel down the previously trained arm, while untrained ants would have an equal chance of going down either arm. During the social–private conflict treatment (n = 9 colonies), the pieces of paper lining the floor of each maze arm were swapped so that the pheromone trail led to the previously untrained arm. This treatment created an informational conflict such that trained ants needed to decide whether to follow the trail (social information) or their own internal route memory produced during training. Finally, during the control treatment (n = 10 colonies), the pieces of paper were lifted and replaced in the same configuration. The control treatment replicated the same disturbance to the apparatus as in the previous two experiments, but with no change in the direction of the pheromone trail.
For each treatment, we recorded the choice (training arm or non-training arm) for the first 20 ants to cross the decision line two-thirds of the way up each arm, measured from the Y junction. It is possible that an ant’s decision is influenced by the decision of ants that go before it, particularly if those ants deposit pheromones. Thus, for each of the first 20 ants (with the exception of the very first ant), we also recorded the decisions of all previous ants.
Statistical analysis
To assess the effect of experience and social information on arm choice, we used a mixed effects logistic regression model with focal ant choice as a dependent variable (right arm coded as 1 and left arm as 0, regardless of which arm was the training arm). As independent variables we used ant experience (untrained vs. trained, binary indicators), the identity of the training arm in the focal replicate (− 1 for left, 1 for right), the number of previous ants choosing the left arm (number left), and the number of previous ants choosing the right arm (number right). The effect of training was encoded as an interaction term and a random intercept term was also included, based on a unique identifier for each experiment. This analysis allows us to tease apart the relative role of social and private information, including the influence of the decisions of previous ants.
In our model, the treatment type was encoded as an interaction with each term, such that we identified a separate effect for training and social influences for each treatment. This encoding can be described in two ways: (1) absolute encoding, where we identify effects for each of control, pheromone-removal and social–private conflict treatments; and (2) relative encoding, where we identify a baseline effect for the control group and then identify effects for the other two treatments relative to this baseline. These two models are fundamentally mathematically equivalent. We present results from the first to describe effect sizes for each treatment. The second is used to test whether these effect sizes are significantly different between treatments.
In Table 1, we present the count data of the absolute numbers of ants (trained and untrained) to choose the training side in the control, pheromone removal, and social–private conflict treatments, as well as the number of individuals choosing the correct arm as a percentage.
Experiment 2: how does experience affect information use?
We wanted to determine if workers which had made more visits to the feeder during training (i.e. were more experienced) were more likely to ignore conflicting social information. We used the same setup described above to train ants toward one arm of the feeder (n = 11 colonies). To quantify experience, we marked every ant at the feeder with a small dot of acrylic paint on its gaster. The colour of the paint corresponded to the number of times the ant had been observed at the feeder, meaning that ants who visited multiple times were marked with a new paint colour each time. Ants were marked at 5-min intervals. This interval was chosen because the high density of foraging ants prevented us from maintaining a full record of every ant’s trip count simultaneously. To determine the appropriate timing intervals for marking individuals, feeding times were recorded from foraging trips pooled across all treatments in experiment 1, as treatment had no significant impact on feeding time (two-way ANOVA, p = 0.099). Trained ants fed for 2:20 min ± 7 s (n = 50), and untrained ants fed for 2:59 min ± 8 s (n = 50), and the travel distance from the nest to the food source was roughly 1 min, thus, we can be confident that our 5-min interval was appropriate for keeping track of the number of visits of each ant, while being unlikely to result in false trips being recorded for ants which were stationary for long periods. Once at least five ants had each experienced at least three return trips or 40 min had elapsed, the food source was removed, ants were blocked from the Y maze, and the paper on each arm was moved to the other arm (as in the social–private conflict treatment). New food sources were placed at the end of each arm and ants were allowed access to the Y maze.
Statistical analysis
A further analysis was carried out in the same manner as Experiment 1, that is, we recorded the choice (training arm or non-training arm) for the first 20 ants to cross the decision line. We used a mixed effects logistic regression model with focal ant choice as a dependent variable (right arm coded as 1 and left arm as 0). As independent variables we used ant experience (marked, marked > once, not marked), the identity of the training arm in the focal replicate (− 1 for left, 1 for right), the number of previous ants choosing the left arm (number left), and the number of previous ants choosing the right arm (number right). The effect of multiple training trips was encoded as an interaction term between the independent variables and the identity of the training arm, to assess the degree to which multiple training trips influenced the decisions of experienced ants. A random intercept term was also included, based on a unique identifier for each experiment. Note that ants which visited the feeder at least once were all labelled as ‘marked’, while those that visited the feeder more than once were labelled as both ‘marked’ and ‘marked > once’. Therefore, ‘marked > once’ represents the marginal effect of additional experience beyond the first visit to the feeder.
Results
Experiment 1: social vs. private information
Summary statistics
During the control treatment (trails left in original position), 90% of trained ants chose the training side, compared to 83% of untrained ants (Table 1).
During the pheromone removal treatment, 80% of trained ants chose the side to which they were trained, compared to 68% of the untrained ants choosing the training side (Table 1).
In the social–private conflict treatment, 57% of trained ants chose the training side, while only 33% of untrained ants chose the training arm (Table 1).
Regression model results
The result of the absolute coding regression model for the control showed that both the trained and untrained ants were significantly attracted to the training arm (p < 0.001 and p = 0.002, respectively; Fig. 2). For the pheromone removal, trained ants showed a significant attraction to the training arm (p < 0.7001), whereas untrained ants showed no significant attraction (p = 0.163; Fig. 2). For the social–private conflict, trained ants showed no significant attraction (p = 0.283), whereas untrained ants were significantly attracted to the non-training arm containing the newly relocated pheromone trail (p = 0.017; Fig. 2; for full absolute coding regression statistics, see Table S1). In each case, the choices of previous ants had no significant effect on decisions made by each ant (p > 0.2 across all treatments).
Without pheromone, trained ants rely more on their experience, while untrained ants show no preference. When pheromone and experience are in conflict, trained ants have no preference, while untrained ants rely on public information. Results of mixed effects logistic regression model of Y-maze arm choice of foragers in each treatment in Experiment 1. Stars denote groups in which a significant difference was found in route choice (p < 0.05, Table S1). Positive values indicate a preference for the training arm, negative values indicate a preference for the other arm. Figure created in R version 3.3.3
In the relative coding regression model, there was no significant difference in the preference of trained ants for the training arm between the control and pheromone removal treatments (p = 0.095), that is, trained ants were attracted to the training arm to a similar degree when presented with the control, or the pheromone removal treatments. Untrained ants were marginally more attracted to the training arm in the pheromone removal treatment than in the control treatment (p = 0.049). Comparing the control with the social–private conflict treatment, there was a significant difference between the preferences of trained ants and untrained ants for the training arm (p = 0.001, and p < 0.001, respectively; for full relative coding regression statistics, see Table S1)—both trained ants and untrained ants were more attracted to the training arm during the social–private conflict treatment than they were during the control.
Experiment 2: how does experience affect information use?
Our secondary analysis shows that there is no significant additional effect of extra training trips to the feeder (p = 0.484), once the first trip is accounted for (p = 0.008; Fig. 3; Table S2). Ants returning after their first training trip have a significant preference for the training arm (i.e. their private information), but subsequent trips do not show a significant additional preference for the training arm. The number of ants returning for 3 or more training trips was low, so we grouped all return trips into either singly returning ants (n = 66) or multiply returning ants (n = 19). The number of trips on the training arm did not have a significant effect on the behaviour of following ants. These results suggest that trained ants rely on their private information for the first return journey, and subsequent journeys provide no additional fidelity to the use of private information—the training arm does not become more attractive when compared to the first return journey, regardless of the number of times the ant returns to that arm. This supports the findings of Experiment 1, where the number of journeys was not accounted for and the time period suggests the possibility of multiple return visits. Here we find that there is no significant preference of trained ants for the training arm in the social–private conflict treatment.
A single training trip (training side: marked) is enough to create a foraging preference. Results of mixed effects logistic regression model of Y-maze arm choice of foragers in each treatment in Experiment 2, separated into level of experience of the training arm. Positive values indicate a preference for the training arm, negative values indicate a preference for the other arm. All ants that visited the feeder at least once are labelled as ‘marked’. Ants that visited the feeder more than once are labelled as both ‘marked’ and ‘marked > once’ effects, and thus ‘marked > once’ represents the marginal effect of extra experience beyond the first visit. Figure created in R version 3.3.3
Discussion
When faced with a conflict between trail-encoded social information and private route memory, our results show an impact of training, and utilisation of private route memory by trained individuals, however, this result was inconsistent. In our first experiment, our regression model showed that untrained ants tended to follow the pheromone (i.e. the social information) while trained ants had no significant preference for the social or private information. However, in our second experiment, we found that a single training trip was all that was required to induce a preference, demonstrating a preference of trained ants for their private information when social and private information are in conflict, with additional training trips having no additional effect on preference (though the number of ants returning for many multiple trips may be too low to allow us to make a strong statement on this). These results do not show a strong impact of memory on routing decisions in meat ants, but does indicate a slight preference for private information after an initial experience; one previous trip was sufficient to negate the effect of trail pheromones in trained ants, while untrained ants showed a significant preference for the pheromone in both the control and social–private conflict scenarios. Our study provides an interesting insight into the development of trail following behaviour and would complement future studies that examine the longer term development of trail foraging in this species.
A prior experiment on meat ants (conducted independently of our study) found that trained meat ants were also more likely to prioritise memory in a navigational context (Card et al. 2016). The researchers examined the role of memory and pheromone trails in the navigation of meat ants when displaced from their foraging trails. In this instance, individual memories are prioritised. Our study examined the role of memory and pheromone during recruitment and multiple visits to a food source, and when combined with Card et al.’s (2016) work, indicate that memory has a strong impact on the routing decisions of these ants in several behavioural contexts. Our results also agree with experimental findings on L. niger (Grüter et al. 2011) and on the modelling results of Letendre and Moses (2013). However, while L. niger and I. purpureus preferentially use private route memory, the Argentine ant Linepithema humile appears to prioritise trail-based information (Aron et al. 1993). Resource stability is likely to play a role by influencing the potential accuracy of both memory and pheromones. For long-lasting, renewable resources that are fixed in space, either private or social information will lead a forager to a food source. When a resource disappears, or a new one is discovered, then neither source of information is correct. Prioritising private memory information allows the handful of foragers in possession of new, accurate information to override the defunct social information. They can then update and correct the social information by laying their own pheromone trail to the new location, which is reinforced through positive feedback of trail laying by their nestmates.
Although no longterm data exists, the foraging environment for meat ants is thought to be quite stable, as their main source of carbohydrate is the sugary secretions of sap-sucking insects located in trees (Greaves and Hughes 1974; van Wilgenburg and Elgar 2007). However, meat ants also scavenge and hunt prey items for protein, which are far more ephemeral resources. It is possible that the foraging system is optimised for the retrieval of protein, rather than carbohydrate, or has evolved towards a compromise method for obtaining both resources. Future research would do well to determine how the persistence and stability of a resource influences the tendency of meat ants to prioritise route memory over pheromone trails.
Alternatively, the prioritising of memory might be due to the instability and impermanence of the trail networks. The decay rate of pheromones is related to temperature such that at high temperatures, ants can no longer follow trails (Van Oudenhove et al. 2011). Ruano et al. (2000) found that ant species known to be active at high soil temperatures tend not to use trail pheromones as a recruitment signal. Meat ants live in arid zones where temperatures can be high; indeed, meat ants seem to prefer to build nests in sunny, open areas. In a field study, Andrew et al. (2013) found meat ants walking across soil surfaces that exceeded 57 °C. Under such hot conditions, trail pheromones likely evaporate too quickly to be of use. In the face of such ephemeral trail pheromones, it might make sense for meat ants to prioritise memory-based information. Few studies before ours have examined the use of social and private information in ants under natural field conditions, so little is known about which ecological correlates drive the relative use of each information type. Field-based studies allow social insects to utilise informational cues available only under natural field conditions, including, but not limited to, patterns of polarised light, the field panorama and its complexity, fine-scale environmental weather variations, other chemosensory cues, and further work can build upon these findings to understand which of these variables influence the use of private and social information.
Trail laying ant species are often conceptualised as being heavily reliant on trail following to organise their foraging efforts. Recent work has shown that this view is overly simplistic, with ants using and integrating navigational information from a variety of sources (for examples see Collett 1996; Collett and Collett 2002; Reid et al. 2011; Buehlmann et al. 2012; Ramsch et al. 2012; von Thienen et al. 2016). It now appears that at least some ant species use trail pheromones in much the same way as humans use GPS systems; inexperienced individuals rely heavily on social information provided by the GPS/trail, while experienced individuals can override social directions if they conflict with their own knowledge of the environment. The ability to selectively use or ignore social information may be a key feature of many, if not all, trail-laying ant species.
References
Abbott KR, Dukas R (2009) Honeybees consider flower danger in their waggle dance. Anim Behav 78:633–635
Andrew NR, Hart RA, Jung M-P, Hemmings Z, Terblanche JS (2013) Can temperate insects take the heat? A case study of the physiological and behavioural responses in a common ant, Iridomyrmex purpureus (Formicidae), with potential climate change. J Insect Physiol 59:870–880
Aron S, Pasteels JM, Deneubourg JL (1989) Trail-laying behaviour during exploratory recruitment in the Argentine ant, Iridomyrmex humilis (Mayr). Biol Behav 14:207–217
Aron S, Beckers R, Deneubourg J-L, Pasteels J (1993) Memory and chemical communication in the orientation of two mass-recruiting ant species. Insectes Sociaux 40:369–380
Banks AN, Srygley RB (2003) Orientation by magnetic field in leaf-cutter ants, Atta colombica (Hymenoptera: Formicidae). Ethology 109:835–846
Beckers R, Deneubourg J, Goss S, Pasteels J (1990) Collective decision making through food recruitment. Insectes Sociaux 37:258–267
Buehlmann C, Hansson BS, Knaden M (2012) Desert ants learn vibration and magnetic landmarks. PLoS One 7:e33117
Campbell ML, Clarke PJ (2006) Seed dynamics of resprouting shrubs in grassy woodlands: seed rain, predators and seed loss constrain recruitment potential. Austral Ecol 31:1016–1026
Card A, McDermott C, Narendra A (2016) Multiple orientation cues in an Australian trunk-trail-forming ant, Iridomyrmex purpureus. Aust J Zool 64:227–232
Cheng K, Middleton EJT, Wehner R (2012) Vector-based and landmark-guided navigation in desert ants of the same species inhabiting landmark-free and landmark-rich environments. J Exp Biol 215:3169–3174
Collett T (1996) Insect navigation en route to the goal: multiple strategies for the use of landmarks. J Exp Biol 199:227–235
Collett TS, Collett M (2002) Memory use in insect visual navigation. Nat Rev Neurosci 3:542–552
Czaczkes TJ, Grüter C, Jones SM, Ratnieks FL (2011) Synergy between social and private information increases foraging efficiency in ants. Biol Lett 7:521–524
Dechaume-Moncharmont F-X, Dornhaus A, Houston AI, McNamara JM, Collins EJ, Franks NR (2005) The hidden cost of information in collective foraging. Proc R Soc Lond B Biol Sci 272:1689–1695
Elizalde L, Farji-Brener A (2012) To be or not to be faithful: flexible fidelity to foraging trails in the leaf-cutting ant Acromyrmex lobicornis. Ecol Entomol 37:370–376
Evison SE, Petchey OL, Beckerman AP, Ratnieks FL (2008) Combined use of pheromone trails and visual landmarks by the common garden ant Lasius niger. Behav Ecol Sociobiol 63:261–267
Goss S, Aron S, Deneubourg J, Pasteels J (1989) Self-organized shortcuts in the Argentine ant. Naturwissenschaften 76:579–581
Gould JL (1984) Magnetic field sensitivity in animals. Annu Rev Physiol 46:585–598
Graham P, Cheng K (2009) Ants use the panoramic skyline as a visual cue during navigation. Curr Biol 19:R935–R937
Greaves T, Hughes R (1974) The population biology of the meat ant. Aust J Entomol 13:329–351
Grüter C, Leadbeater E (2014) Insights from insects about adaptive social information use. Trends Ecol Evol 29:177–184
Grüter C, Ratnieks FLW (2011) Honeybee foragers increase the use of waggle dance information when private information becomes unrewarding. Anim Behav 81:949–954
Grüter C, Balbuena MS, Farina WM (2008) Informational conflicts created by the waggle dance. Proc R Soc Lond B Biol Sci 275:1321–1327
Grüter C, Czaczkes T, Ratnieks FW (2011) Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav Ecol Sociobiol 65:141–148
Harrison JF, Fewell JH, Stiller TM, Breed MD (1989) Effects of experience on use of orientation cues in the giant tropical ant. Anim Behav 37:869–871
Laland KN (2004) Social learning strategies. Anim Learn Behav 32:4–14
Latty T, Beekman M (2013) Keeping track of changes: the performance of ant colonies in dynamic environments. Anim Behav 85:637–643
Letendre K, Moses ME (2013) Synergy in ant foraging strategies: memory and communication alone and in combination. In: Proceedings of the 15th annual conference on genetic and evolutionary computation. ACM, pp 41–48
Mobbs CJ, Tedder G, Wade AM, Williams R (1978) A note on food and foraging in relation to temperature in the meat ant Iridomyrmex purpureus form viridiaeneus. Aust J Entomol 17:193–197
Perna A, Latty T (2014) Animal transportation networks. J R Soc Interface 11:20140334
Ramsch K, Reid CR, Beekman M, Middendorf M (2012) A mathematical model of foraging in a dynamic environment by trail-laying Argentine ants. J Theor Biol 306:32–45
Reid CR, Sumpter DJT, Beekman M (2011) Optimisation in a natural system: Argentine ants solve the Towers of Hanoi. J Exp Biol 214:50–58
Reid CR, Latty T, Beekman M (2012) Making a trail: informed Argentine ants lead colony to the best food by U-turning coupled with enhanced pheromone laying. Anim Behav 84:1579–1587
Ruano F, Tinaut A, Soler, José J (2000) High surface temperatures select for individual foraging in ants. Behav Ecol 11:396–404
Seeley TD, Camazine S, Sneyd J (1991) Collective decision-making in honey bees: how colonies choose among nectar sources. Behav Ecol Sociobiol 28:277–290
Seeley TD, Mikheyev AS, Pagano GJ (2000) Dancing bees tune both duration and rate of waggle-run production in relation to nectar-source profitability. J Comp Physiol A 186:813–819
Shattuck S (2000) Australian ants: their biology and identification. CSIRO Publishing, Clayton
van Wilgenburg E, Elgar MA (2007) Colony structure and spatial distribution of food resources in the polydomous meat ant Iridomyrmex purpureus. Insectes Sociaux 54:5–10
Van Oudenhove L, Billoir E, Boulay R, Bernstein C, Cerdá X (2011) Temperature limits trail following behaviour through pheromone decay in ants. Naturwissenschaften 98:1009–1017
von Thienen W, Metzler D, Witte V (2016) How memory and motivation modulate the responses to trail pheromones in three ant species. Behav Ecol Sociobiol 70:393
Warrant E, Dacke M (2016) Visual navigation in nocturnal insects. Physiology 31:182–192
Wehner R (1984) Astronavigation in insects. Annu Rev Entomol 29:277–298
Wolf H (2011) Odometry and insect navigation. J Exp Biol 214:1629–1641
Acknowledgements
We would like to thank Michael Duncan for logistical support.
Funding
This research was funded by a Branco Weiss Society Science grant and an Australian Research Council discovery grant (to TL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40_2018_656_MOESM1_ESM.docx
Supplementary Table 1. Results of the absolute and relative coding regression analysis for Experiment 1; bold indicates significant values (p < 0.05) (DOCX 19 KB)
40_2018_656_MOESM2_ESM.docx
Supplementary Table 2. Results of mixed effects logistic regression model for Experiment 2; bold indicates significant values (p < 0.05) (DOCX 14 KB)
Rights and permissions
About this article
Cite this article
Middleton, E.J.T., Reid, C.R., Mann, R.P. et al. Social and private information influence the decision making of Australian meat ants (Iridomyrmex purpureus). Insect. Soc. 65, 649–656 (2018). https://doi.org/10.1007/s00040-018-0656-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-018-0656-1