Abstract
Foragers of many ant species use pheromone trails to guide nestmates to food sources. During foraging, individual workers can also learn the route to a food source. Foragers of the mass-recruiting ant Lasius niger use both pheromone trails and memory to locate a food source. As a result, an experienced forager can have a conflict between social information (trail pheromones) and private information (route memory) at trail bifurcations. We tested decision making in L. niger foragers facing such an informational conflict in situations where both the strength of the pheromone trail and the number of previous visits to the food source varied. Foragers quickly learned the branch at a T bifurcation that leads to a food source, with 74.6% choosing correctly after one previous visit and 95.3% after three visits. Pheromone trails had a weaker effect on choice behaviour of naïve ants, with only 61.6% and 70.2% choosing the branch that had been marked by one or 20 foragers versus an unmarked branch. When there was a conflict between private and social information, memory overrides pheromone after just one previous visit to a food source. Most ants, 82–100%, chose the branch where they had collected food during previous foraging trips, with the proportion depending on the number of previous trips (1 v. 3) but not on the strength of the pheromone trail (1 v. 20). In addition, the presence of a pheromone trail at one branch in a bifurcation had no effect on the time it took an experienced ant to choose the correct branch (the branch without pheromone). These results suggest that private information (navigational memory) dominates over social information (chemical tail) in orientation decisions during foraging activities in experienced L. niger foragers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social insect foragers exploiting renewable or large food sources often direct nestmates to the location of the food source (von Frisch 1967; Hölldobler and Wilson 1990; Jarau and Hrncir 2009). Many ants (Hölldobler and Wilson 1990), termites (Wilson 1971) and stingless bees (Lindauer and Kerr 1960; Barth et al. 2008; Jarau 2009) use pheromone trails to do this. The use of trail pheromones in ants is remarkably diverse and sophisticated, involving multicomponent pheromone blends (Hölldobler 1995) and different pheromones deposited at different locations on the trail having different functions (Robinson et al. 2005; Jackson and Ratnieks 2006; Ratnieks 2008). During foraging, workers also acquire navigational memories when travelling between food sources and the nest (reviewed in von Frisch 1923; Collett and Collett 2002; Collett et al. 2003). The ability of ant and bee foragers to learn the route leading to one or several food sources allows foragers to make repeated visits, a phenomenon called site fidelity (‘Ortstreue’) (Ribbands 1949; Hölldobler 1976; Traniello 1977; Rosengren and Fortelius 1986, Quinet and Pasteels 1996; Beverly et al. 2009). Ants can remember food locations for weeks or even months (Lasius fuliginosus, Quinet and Pasteels 1996; Formica spp., Rosengren and Fortelius 1986; Salo and Rosengren 2001).
Experienced foragers leaving the nest can thus make use of both social information (pheromone trails) and private information (navigational memory) to locate a food source. As described in Rosengren and Fortelius (1986), experienced ant foragers will face situations in which the two types of information are in conflict, such as at a trail bifurcation with trail pheromone on both branches. In wood ants, for example, trail pheromone guides foragers lacking knowledge on food source locations, whereas private navigational information is more important for experienced foragers who know a specific food location (Rosengren and Fortelius 1986; Salo and Rosengren 2001). Several studies have investigated the relative importance of social chemical information versus private navigational information by creating conflicts between the two information sources (Linepithema humile = Iridomyrmex humilis, Lasius niger, Aron et al. 1993; Pogonomyrmex sp., Hölldobler 1976; Formica sp., Rosengren and Fortelius 1986; Paraponera clavata, Harrison et al. 1989). These studies show that foragers of some species rely more on the chemical trail and others more on memory.
However, with the exception of Aron et al. (1993), experiments have not systematically investigated the effect of factors such as the strength of the pheromone trail, the number of previous visits to a food source, and the quality of the food or the distance to the food source. It is, therefore, not clear how flexibly foragers can choose among different information sources. In vertebrates, the study of information use strategies (e.g. private vs. social) and the factors affecting decision making is an active and rapidly expanding field of research (reviewed in Laland 2004; Kendal et al. 2005; Seppänen et al. 2007). Social insects, including ants, are ideal model systems for investigating decision-making strategies in foraging. One area of special relevance concerns the prioritisation of alternative information sources, given that social insect foragers will often have access to multiple, and potentially conflicting, information sources (Hölldobler 1999; Grüter et al. 2008). For example, does a foraging ant prioritise social information when its private information is subject to a high degree of uncertainty, as will occur if the ant has made only a few previous visits to a particular feeding location? Or should it prioritise social information when the reliability or strength of the social information is high, as will occur if a trail or branch is well marked with pheromone? Various strategies are possible and the result may vary among species depending on the type of food that is collected, the temporal and spatial distribution of food, colony size, worker size or predation pressure (Carroll and Janzen 1973; Beckers et al. 1989; Aron et al. 1993; see Laland 2004 and Kendal et al. 2005 for a discussion of different information use strategies in vertebrates).
Foragers of the common garden ant L. niger use both pheromone trails and private navigational information acquired during previous foraging trips to locate food sources (Beckers et al. 1990, 1992, 1993; Aron et al. 1993; Evison et al. 2008). A preliminary study on L. niger showed that a substantial proportion of foragers uses private navigational information even if it conflicts with the information provided by a pheromone trail (Aron et al. 1993). Aron et al. (1993) is an important study because, to our knowledge, it is the only study in social insects in which the quality of both types of information were experimentally manipulated. However, the sample size of this study is too small to draw solid conclusions about the effect of these manipulations on decision making in experienced L. niger foragers. Furthermore, their findings raise another question—whether informational conflict affects decision-making speed. In this study, we investigated the extent to which the number of previous foraging trips (variation in private information) and the strength of the pheromone trail (variation in social information) affect decision making in L. niger at a trail bifurcation, when acting as either a sole or a competing information source. We then investigated whether the presence of an informational conflict (private information vs. social information) at a trail bifurcation confuses ants and affects the decision-making process. Our results show that even small amounts of private information (memory) override chemical information, and that the time it takes an experienced ant to make a decision is not affected by the presence of a competing pheromone trail.
Methods
Study species
We studied eight L. niger colonies collected on the University of Sussex campus. Experimental colonies were housed in plastic foraging boxes (40 × 30 × 20 cm high) containing a wooden nestbox (15 × 15 × 2 cm high). The bottom of each plastic box was covered with a layer of plaster of Paris. The colonies were queenless with 700–1,500 workers and small amounts of brood. Queenless colonies forage, make trails and are frequently used in foraging experiments (e.g. Dussutour et al. 2005; Evison et al. 2008). We fed the colonies three times per week with a food mixture made from honey, raw egg and agar (see Hölldobler and Wilson 1990, p. 632) and once per week with dead mealworms, Tenebrio molitor. Colonies were given water ad libitum.
Experimental setup
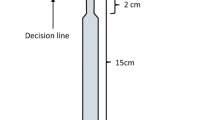
Colonies were deprived of the food mixture for 4–6 days prior to testing to ensure that foragers were motivated to forage and recruit nestmates to a sucrose syrup feeder. The foraging box was connected to a T-shaped foraging trail via a paper (white photo copier paper) bridge. The stem of the trail was 15 cm long and each branch was 11 cm long (Fig. 1). The experiments were performed in a room that had both natural light coming from windows and artificial room lights. The walls and the ceiling were bright. The room contained different kinds of lab equipment and furniture and so provided visual landmarks of different sizes and colours.
Schematic of the experimental setup used to investigate the effect of private information (memory from previous foraging trips to a feeder on the same side branch) and social information (pheromone trail) on branch choice by outgoing Lasius niger forager ants at a trail bifurcation: a trail marking phase. Foragers feed at a drop of 1.8 M sucrose solution and deposit trail pheromone on the experimental branch (EB) when returning to the nest. One part of the EB was masked with another piece of paper. b Testing phase. After a trail of a certain strength had been formed, we moved the food source either to X or Y on one of the branches. Ants collecting sugar solution were marked, and the number of visits recorded. To determine an ant’s decision, the piece of paper that masked the hatched part was removed and the EB (grey) was transferred to the junction. An ant was considered to have made a decision when she crossed one of the decision lines. This procedure was used for experiments 2–5
Experiment 1: memory and trail choice
We studied six colonies. A 1.8 M sucrose solution (50% w/w; commercial table sugar) was offered at the end of one of the T branches (X or Y). This high-quality food source guaranteed that ants were motivated to collect the offered solution. Foraging ants were marked individually with acrylic colour dots on the abdomen when they were feeding for the first time. To investigate the effect of private information, we tested their choice at the bifurcation after 0, one, two, three and four training visits. (It took ants c. 2–4 min to make one round trip from feeder to nest to feeder.).
When a marked forager returned from the nest we put a fresh piece of paper (without any pheromone) on the branches of the T-shaped trail (Fig. 1b). In order to exclude any transfer of location information during physical contacts between ants walking from the nest to the bifurcation and returning ants, one of the experimenters put a small piece of paper in the middle of the trail where two ants might meet to form two corridors, one for each ant, thereby preventing physical contact. As a result, focal foragers could use only their own memory, in choosing which branch to take. We considered an ant as having chosen a particular branch after she first crossed either the left or right ‘decision line’ (Fig. 1b).
We tested 62 foragers that had previously found food on the left branch and 97 on the right. In addition, we also recorded which branch ants chose before we offered any food (naïve ants, 0 visits). Of 98 naïve ants, 51 went right (52%) and 47 left (48%; χ 2 = 0.16, df = 1, p = 0.69), thereby confirming that there was no underlying preference. Each ant was tested once and then removed from the colony.
Experiment 2: pheromones and trail branch choice
We used 8 colonies to investigate the effect of pheromone trail strength on branch choice. To establish a pheromone trail of a particular strength, we placed a 1.8 M sucrose solution near the bifurcation (see Fig. 1a). The trail between the food source and the nest was covered with a piece of paper (experimental branch (EB); 10 × 2 cm) (Fig. 1a). One part of the EB was masked with another piece of paper (4 × 2 cm) (Fig. 1a). This guaranteed that only the unmasked part of the EB was marked with any trail pheromone being deposited.
The EB was removed after the trail had been established. The time allowed for trail formation was up to 30 min but was usually less than 15 min. When trail formation was complete we removed the food source and all the ants on the trail. The EB was then transferred to the end of the T-shaped trail (Fig. 1b). The paper that masked one part of the EB was also removed, thereby making two new arms, only one of which was marked with pheromone (Fig. 1b). For the next 20 min we recorded the decisions of foragers leaving the nest and searching for food. The maximum duration of an experimental trial was, therefore, 50 min and this was also the maximum duration in all other experiments in this paper. Previous research has shown that pheromone trails persist this long in L. niger (Beckers et al. 1993; Evison et al. 2008).
We tested the attractiveness of a branch marked by 1, 5 or 20 ant passages versus an unmarked branch. A passage was defined as one ant performing at least one pheromone deposition while walking along the EB either to or from the nest. A forager L. niger does not lay a continuous pheromone trail. Rather, its walk is interrupted for a fraction of a second and curves its abdomen vertically to the ground to deposit trail pheromone in a highly stereotyped manner (see Beckers et al. 1992 for more details). An experimenter can easily see this behaviour. One such behaviour was considered a single pheromone deposition. We counted both passages and depositions.
Experiment 3: conflicting information
Seven colonies were used to investigate the relative importance of strong or weak memory versus strong or weak pheromone trail on trail choice decisions by foragers at trail bifurcations. We tested four different situations: (1) strong experience (three training visits) versus a strong pheromone trail (≥20 passages or ≥40 depositions), (2) strong experience versus a weak pheromone trail (one passage), (3) weak experience (one visit) versus a strong pheromone trail and (4) weak experience versus a weak pheromone trail. Each colony was tested in all four combinations. Pheromone trails and experience treatments were established as described above. We tested a total of 176 ants, 79 encountered food on the left branch (X) (Fig. 1) and 97 on the right (Y) (Fig. 1).
Experiment 4: control for intra-nest communication
We prevented direct contacts between foragers returning to the nest and focal foragers that were about to be tested as described above. However, it is possible (but has not been shown) that active foragers might also exchange location information inside the nest as in honeybees using waggle dances (Apis mellifera) (von Frisch 1967). If this occurred, it would confound the results of the conflicting information experiment (previous section) because we could no longer distinguish private information use and the use location information communicated inside the nest. To eliminate this possibility, we did an experiment that repeated the weak experience versus strong trail combination in experiment 3. We worked with only one forager at a time so that no other ant in the colony had information about the food location. Six different colonies were used and food was offered once on each side for each colony (21 foragers in total, one to three per colony and side).
Experiment 5: time taken to make a decision
Ants walking on the EB (Fig. 1b) often spent a considerable amount of time walking back and forth, before finally crossing one of the decision lines. To investigate this further, we measured the time ants spent on the EB before crossing a decision line. Measurements were made on ants that had visited the food source once before testing (X or Y?) (Fig. 1) and then encountered a strong pheromone trail in the opposing direction (combination 3). The time spent walking on the EB until crossing the decision line near the food source was determined from video.
Statistical analysis
We mainly used generalised linear mixed-effect models (GLMM) with a binomial response variable in R 2.9 (R Development Core Team 2009). R fitted the models with the lmer function (Bates 2007). We included colony as a random effect to control for the non-independence of data points from the same colony (Bolker et al. 2009; Zuur et al. 2009). For model selection we used the protocol proposed by Zuur et al. (2009). We first explored the optimal structure of the random components (comparing random intercept models with random intercept and slope models). We then explored the significance of the fixed effects. For most hypotheses we had only one fixed effect, either number of visits (0 to four) or trail strength (one, five or 20 passages). For the conflicting information experiments we included both pheromone strength (strong, weak) and memory strength (strong, weak) as fixed effects. Wald tests were used to test the significance of the fixed effects (Bolker et al. 2009; Zuur et al. 2009).
Results
Experiment 1: memory and trail choice
Figure 2 shows that ants rapidly learned the branch leading to food. Even a single visit led to a considerable bias towards the side that had food on the previous trip (74.6%; Fig. 2a). There was a highly significant difference between naïve ants, (0 previous visits), 52%, and those with just one previous visit, 74.6% (Wald Z test: z = 3.06, p = 0.002). Most foragers, 95.3%, had learned the location of the food source after three trips (Fig. 2a). The difference between one visit and three previous visits is also significant (z = 2.46, p = 0.014). As shown in Fig. 2a, there was no further increase from three to four visits.
Experiments 1 and 2. The effect of previous foraging experience and trail pheromone strength at a particular branch on trail choice at a T bifurcation. a Proportion of ants choosing the branch with syrup after 0–4 previous visits. b Proportion of naïve ants choosing the branch with syrup when marked with pheromone by 1, 5 or 20 forager ants (ant passages). The hatched line shows the random expectation, 50%. Numbers above the bars indicate the number of ants tested. Asterisks indicate that bars are statistically different
Experiment 2: trail strength and trail choice
Ants made an average of 3.09 (N = 122; range, 1–8) depositions per passage on the exposed part of the EB. Pheromone that had been laid by just one ant led to a significant increase in the probability of the marked trail being chosen by a naïve ant, from 50% for random choice to 61.6% (χ 2 = 6.73, df = 1, p = 0.009). However, increasing the strength of the pheromone trail had relatively little additional effect (Fig. 2b). A trail with pheromone from 20 ant passages resulted in 70.2% correct choices, some 8.6% higher than a trail of one passage. However, this difference was not significant (z = 1.61, p = 0.107, N = 333).
Experiment 3: conflicting information
In all 4 combinations ants relied more strongly on memory than on the pheromone trail (Fig. 3). After one visit, 84.4% chose an unmarked branch on the side that they had found food on their previous trip versus a branch marked with trail pheromone (situation 3 and 4) (Fig. 3). This was actually 10.2% higher than when the alternative branch had zero pheromone (74.6%, one visit) (Fig. 2a). However, this difference was not statistically significant (z = −1.49, p = 0.14). A GLMM analysis investigating all four combinations showed no effect of pheromone strength (strong vs. weak) but a significant role of the number of visits (pheromone strength: z = 0.39, p = 0.69; one versus three visits: z = 2.51, p = 0.012; the interaction between both terms was not significant and removed for the final model: z = 0.004, p = 0.99).
Experiment 3. Branch choice at a trail bifurcation in four combinations of conflicting private and social information. The left bars show the proportions of ants choosing the branch where they had fed previously. The right bars show the proportions choosing the other branch, which was marked with pheromone; (weak experience = one previous visit; strong experience = three previous visits, strong trail ≥20 ant passages or ≥40 depositions; weak trail = one passage)
Experiment 4: control for intra-nest communication
When testing ants individually in combination (3) of experiment 3 (weak experience versus strong pheromone) private information overrides a strong pheromone signal; 90.5% of all ants chose the branch where they found food previously (19 out of 21 ants; χ 2 = 13.76, df = 1, p < 0.001 when compared with the random expectation of equal choice). This confirms that experienced ants rely on private information. This value of 90.5% is again higher than when testing memory alone (74.6%, experiment 1). However, the difference was not statistically significant (z = −1.48, p = 0.14). It should be noted that the conditions in experiment 1 were slightly different. Experiment 1 was performed some weeks earlier and there were more ants on the trail, hence more disturbances by other ants and the experimenters manipulating the trail. This may have affected the behaviour of ants.
Experiment 5: time taken to make a decision
The time needed to take a correct decision, that is to cross the decision line near the food source, did not depend on whether there was a conflict between private information and a strong pheromone trail (situation (3); median = 5.9 s (1st and 3rd Quartile: 3.54s, 13.2 s)) or no pheromone at all (5.7 s (4.4, 13.5 s); z = −0.6, p = 0.55) (Fig. 4).
Experiment 5. The time taken by ants between stepping onto the piece of paper on the branches of the T bifurcation (grey part in Fig. 1b) and crossing the decision line. All ants had a low level of experience (one previous visit to the food source). The grey piece of paper was either unmarked (no pheromone) or strongly marked with trail pheromone (≥20 passages or ≥40 depositions) was on the opposite branch to the ant’s previous experience of feeding side. The box plots show medians, quartiles, 5th and 95th percentiles and the number of tested ants
Discussion
Our results clearly show that when an informational conflict occurs, private navigational information (memory) overrides social information (trail pheromone). The effect of memory is very strong, and even a single previous visit has a large effect. Thus, 84% of ants chose a branch unmarked with trail pheromone in the direction that they had previously collected food just one time versus an alternative branch marked with pheromone (combinations 3 and 4) (Fig. 3c, d). This increased to 96.5% for ants that had made three previous trips to a feeder consistently on one branch (combinations 1 and 2) (Fig. 3a, b). We found no significant effect of pheromone strength on trail choice. This contrasts to the Argentine ant L. humile where the proportion of experienced foragers choosing a marked branch at a trail bifurcation depends on the strength of the pheromone trail in situations of conflicting private and social information (Aron et al. 1993).
When memory and the pheromone trail were tested separately on branch choice we found that memory had a much stronger effect than trail pheromone (Fig. 2). Even though a small amount of pheromone, the amount deposited by one ant, did have a significant effect on branch choice (62% of correct choices versus the random expectation of 50%), increasing trail strength had a relatively small effect on branch choice (70.2% of correct choices with 20 passages). Hence, a branch that was marked by many more foragers did not have a significantly greater proportion of ants choosing it versus an unmarked branch than a branch marked by only one ant. Aron et al. (1993) found a much stronger effect of the chemical trail (93% correct choice) (see Fig. 1) but did not provide information about trail strengths. Our results are similar to what has been found in Pharaoh’s ants (Monomorium pharaonis) where a strong pheromone trail leads to a preference of 70 or 78% for the marked branch at a Y bifurcation (Jeanson et al. 2003) depending on substrate. It has been argued that ‘wrong’ choices at bifurcations can be beneficial if they lead to the discovery of new or better food sources (Deneubourg et al. 1983; Detrain and Deneubourg 2008). The same logic, however, would presumably also apply to mistakes made by ants relying on memory. Hence, the ‘adaptive error’ argument does not explain why ants relying on pheromone information make more mistakes than ants relying on memory.
Why did our ants preferentially use private information versus pheromone information? L. niger often forages on relatively long-lasting food sources like honeydew producing aphid clusters (Völkl et al. 1999). Thus, foraging sources may be stable, and therefore predictable, over days or even weeks. The foragers we studied learned fast, choosing the correct branch 95% of the time after only three previous visits to the food source. Hence, private navigational information seems to be more reliable than pheromone information. Furthermore, ants following a trail must continually keep their antennae close to the ground, which is likely to reduce walking speed. L. niger foragers familiar with a route increased their travelling velocity by about 50% after five visits (Mailleux et al. 2005; see also Le Breton and Fourcassié (2004) for the effects of experience on walking speed in L. niger).
These results are not in agreement with the conclusion of Aron et al. (1993) that chemical communication dominates foraging in L. niger. This difference may be due to the conditions under which foraging experiments with L. niger are often performed. Usually, colonies are given access to one or a few ad libitum food sources after several days of starvation and recruitment is studied during a short (<1.5 h) initial phase immediately after the food is located (Aron et al. 1993; Beckers et al. 1990, 1992, 1993; Mailleux et al. 2005; this study). This is different from the natural situation, discussed above, where individual ants may collect food for days or even weeks at the same locations. Hence, while a colony is simultaneously exploiting multiple carbohydrate food sources, the majority of individual foragers may show attachment to particular locations and use route memories to relocate these. This also seems to be the case in other ant species including L. fuliginosus (Quinet and Pasteels 1996), Formica wood ants (Salo and Rosengren 2001) and also honeybees (Ribbands 1949; Grüter et al. 2008; Grüter and Farina 2009). Trail pheromone probably marks the entire active foraging system and may have a major role in helping naïve ants to locate profitable food sources. In addition, trail pheromone could help experienced foragers to travel faster along trails, or to mark out the colony’s territory as occurs in the closely related species Lasius neoniger (Traniello 1980). On the other hand, home range markings in L. niger (Devigne and Detrain 2002) and trail pheromones in L. fuliginosus (Hangartner 1967) do not seem to be colony specific. More research is needed to investigate the roles of pheromone trails on experienced foragers.
During experiment 3 we had noticed that ants sometimes made U-turns directly after the bifurcation or that they spent some time on the T before crossing the decision line, so we specifically tested whether a conflict between the pheromone trail and private navigational information somehow confused the ants and made the decision-making process slower. However, we found no difference in the time taken to make a decision by individual ants faced with conflicting private and social information versus ants only having private information. This is further evidence that ants with navigational information seem to be little affected by the presence of pheromones at trail bifurcations in our experimental setup.
Our results raise the question of the role of trail pheromones in L. niger, and in particular whether they may play a more important role under other circumstances. One possibility is that increasing trail complexity affects decision-making strategies, such as if the route to a food source is difficult to learn because it has many bifurcations, or if there is a lack of visual contrast in daytime foraging or if foraging occurs at night. This would correspond to a copy when uncertain strategy (Laland 2004). Similarly, if a food source is not particularly good or is far away, ants might be more affected by a strong pheromone trail (corresponding to the copy when dissatisfied strategy; Laland 2004). Research on factors affecting the adaptive use of social and private information has revealed considerable behavioural plasticity in vertebrates (reviewed in Laland 2004; Kendal et al. 2005). Social insects are ideal models to test the circumstances that favour the use of social or private information (see e.g. Leadbeater and Chittka 2007, 2009; Grüter et al. 2008). Our results also highlight the need to combine laboratory experiments with field studies. We will only be able to understand the behaviour of foragers in the light of information on foraging ecology, including the spatiotemporal availability of food sources, the proportion of foragers with field experience, their constancy to natural food sources and the role of competition with other ant colonies of the same or other species.
References
Aron S, Beckers R, Deneubourg JL, Pasteels JM (1993) Memory and chemical communication in the orientation of two mass-recruiting ant species. Insectes Soc 40:369–380
Barth FG, Hrncir M, Jarau S (2008) Signals and cues in the recruitment behavior of stingless bees (Meliponini). J Comp Physiol A 194:313–327
Bates D (2007) lme4: Linear mixed-effects models using S4 classes. R package version 0.99875-7.
Beckers R, Goss S, Deneubourg JL, Pasteels JM (1989) Colony size, communication and ant foraging strategy. Psyche 96:239–256
Beckers R, Deneubourg JL, Goss S, Pasteels JM (1990) Collective decision making through food recruitment. Insectes Soc 37:258–267
Beckers R, Deneubourg JL, Goss S (1992) Trail laying behaviour during food recruitment in the ant Lasius niger (L.). Insectes Soc 39:59–72
Beckers R, Deneubourg JL, Goss S (1993) Modulation of trail laying in the ant Lasius niger (Hymenoptera: Formicidae) and its role in the collective selection of a food source. J Insect Behav 6:751–759
Beverly BD, McLendon H, Nacu S, Holmes S, Gordon DM (2009) How site fidelity leads to individual differences in the foraging activity of harvester ants. Behav Ecol 20:633–638
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. TREE 24:127–135
Carroll CR, Janzen DH (1973) Ecology of foraging by ants. Annu Rev Ecol Syst 4:231–257
Collett TS, Collett M (2002) Memory use in insect visual navigation. Nat Rev Neurosci 3:542–552
Collett TS, Graham P, Durier V (2003) Route learning by insects. Curr Opin Neurobiol 13:718–725
Deneubourg JL, Pasteels JM, Verhaeghe JC (1983) Probabilistic behaviour in ants: a strategy of errors? J Theor Biol 105:259–271
Detrain C, Deneubourg JL (2008) Collective decision-making and foraging patterns in ants and honeybees. Adv Insect Physiol 35:123–173
Devigne C, Detrain C (2002) Collective exploration and area marking in the ant Lasius niger. Insectes Soc 49:357–362
Dussutour A, Deneubourg JL, Fourcassié V (2005) Temporal organization of bi-directional traffic in the ant Lasius niger (L.). J Exp Biol 208:2903–2912
Evison SEF, Petchey OL, Beckerman AP, Ratnieks FLW (2008) Combined use of pheromone trails and visual landmarks by the common garden ant Lasius niger. Behav Ecol Sociobiol 63:261–267
Grüter C, Farina WM (2009) The honeybee waggle dance: can we follow the steps? TREE 24:242–247
Grüter C, Balbuena MS, Farina WM (2008) Informational conflicts created by the waggle dance. Proc R Soc B 275:1321–1327
Hangartner W (1967) Spezifität und Inaktivierung des Spurpheromons von Lasius fuliginosus Latr. Und Orientierung der Arbeiterinnen im Duftfeld. Z vergl Physiol 57:103–136
Harrison JF, Fewell JH, Stiller TM, Breed MD (1989) Effects of experience on use of orientation cues in the giant tropical ant. Anim Behav 37:869–871
Hölldobler B (1976) Recruitment behavior, home range orientation and territoriality in harvester ants, Pogonomyrmex. Behav Ecol Sociobiol 1:3–44
Hölldobler B (1995) The chemistry of social regulation: multicomponent signals in ant societies. PNAS 92:19–22
Hölldobler B (1999) Multimodal signals in ant communication. J Comp Physiol A 184:129–141
Hölldobler B, Wilson EO (1990) The ants. The Belknap Press of Harvard University, Cambridge
Jackson DE, Ratnieks FLW (2006) Communication in ants. Curr Biol 16:R570–R574
Jarau S (2009) Chemical communication during food exploitation in stingless bees. In: Jarau S, Hrncir M (eds) Food Exploitation by social insects: ecological, behavioral, and theoretical approaches. CRC University Press, Taylor and Francis Group, Boca Raton
Jarau S, Hrncir M (2009) Food exploitation by social insects: ecological, behavioral, and theoretical approaches. CRC University Press, Taylor and Francis Group, Boca Raton
Jeanson R, Ratnieks FLW, Deneubourg JL (2003) Pheromone trail decay rates on different substrates in the Pharao’s ant, Monomorium pharaonis. Physiol Entomol 28:192–198
Kendal RL, Coolen I, van Bergen Y, Laland KN (2005) Trade-offs in the adaptive use of social and asocial learning. Adv Stud Behav 35:333–379
Laland KN (2004) Social learning strategies. Learn Behav 32:4–14
Le Breton J, Fourcassié V (2004) Information transfer during recruitment in the ant Lasius niger L. (Hymenoptera: Formicidae). Behav Ecol Sociobiol 55:242–250
Leadbeater E, Chittka L (2007) Social learning in insects—from miniature brains to consensus building. Curr Biol 17:R703–R713
Leadbeater E, Chittka L (2009) Bumble-bees learn the value of social cues through experience. Biol Letters 5:310–312
Lindauer M, Kerr WE (1960) Communication between the workers of stingless bees. Bee World 41:29–71
Mailleux AC, Detrain C, Deneubourg JL (2005) Triggering and persistence of trail-laying in foragers of the ant Lasius niger. J Insect Physiol 51:297–304
Quinet Y, Pasteels JM (1996) Spatial specialization of the foragers and foraging strategy in Lasius fuliginosus (Latreille) (Hymenoptera, Formicidae). Insectes Soc 43:333–346
Ratnieks FLW (2008) Biomimicry: further insights from ant colonies? In: Liò P, Yoneki E, Crowcroft J, Verma DC (eds) Bio-inspired computing and communication. Springer, Berlin, pp 50–58
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ribbands CR (1949) The foraging method of individual honey-bees. J Anim Ecol 18:47–66
Robinson EJH, Jackson DE, Holcombe M, Ratnieks FLW (2005) ‘No entry’ signal in ant foraging. Nature 438:442
Rosengren R, Fortelius W (1986) Ortstreue in foraging ants of the Formica rufa group—hierarchy of orienting cues and long-term memory. Insectes Soc 33:306–337
Salo O, Rosengren R (2001) Memory of location and site recognition in the Ant Formica uralensis (Hymenoptera: Formicidae). Ethology 107:737–752
Seppänen JT, Forsman JT, Mönkkönen M, Thomson RL (2007) Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88:1622–1633
Traniello JFA (1977) Recruitment behavior, orientation, and the organization of foraging in the carpenter ant Camponotus pennsylvanicus DeGeer (Hymenoptera: Formicidae). Behav Ecol Sociobiol 2:61–79
Traniello JFA (1980) Colony specificity in the trail pheromone of an ant. Naturwissenschaften 67:361–362
Völkl W, Woodring J, Fischer M, Lorenz MW, Hoffmann KH (1999) Ant-aphid mutualisms: the impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 118:483–491
von Frisch K (1923) Über die Sprache der Bienen. Zool Jb Physiol 40:1–186
von Frisch K (1967) The dance language and orientation of bees. Harvard University Press, Cambridge
Wilson EO (1971) The insect societies. Harvard University Press, Cambridge
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York, p 574
Acknowledgements
We thank Thomas Durance and Lucy Taylor for help with data collection. C.G. was supported by a postdoctoral fellowship from the Swiss National Science Foundation (SNSF grant PBBEP3-123648). T.C. was supported by a PhD studentship from the BBSRC.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Traniello
Rights and permissions
About this article
Cite this article
Grüter, C., Czaczkes, T.J. & Ratnieks, F.L.W. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav Ecol Sociobiol 65, 141–148 (2011). https://doi.org/10.1007/s00265-010-1020-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1020-2