Abstract
Ant communities are structured, in part, by competition between related and unrelated ant species for territories and food resources. In eastern deciduous forests of the United States, a single ant genus (Aphaenogaster) appears ecologically dominant with high abundance and opportunistic foraging. However, Aphaenogaster ants are not particularly behaviorally aggressive toward co-occurring ants, making it unclear as to how they might sustain dominance. We offered myrmecochorous seeds and termite carrion at bait stations and quantified ant aggression, food selection and recruitment. We conducted the experiments throughout the natural seed-release window to determine how the abundance of low- and high-quality food items impacted behavior. We found evidence that Aphaenogaster ants dominate the retrieval of both seeds and insect carrion (dead termites). Aphaenogaster foraging dominance did not appear driven by superior fighting or recruitment abilities but simply by having more foragers on the ground, essentially achieving control of different types of food resources through numerical dominance. Moreover, though they are the dominant effective seed dispersers in the system, A. picea exhibited a much greater affinity for termites than seeds, and the desirability of termites decreased in the presence of seeds. Overall, our results suggest that high numbers of foragers—as opposed to aggressive territoriality—can be an effective ecological strategy for sustaining ecological dominance through resource acquisition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ant ecology is anchored in understanding interspecific competition (Adams 2016; Cerda et al. 2013; Hölldobler and Wilson 1990; Parr and Gibb 2009). Fighting workers can be observed in aggressive behaviors such as biting, stinging and formic acid attacks, and foraging workers also can be seen securing resources through stealing, fast discovery and retrieval, or simply overwhelming resources with recruited co-workers. In some cases, these behaviors may shape ant communities through interspecific territoriality, but ant populations also are self-limiting which suggests a substantial role for intraspecific competition (Adams 2016; Gibb and Johansson 2011; Parr and Gibb 2009). Moreover, colony establishment, such as queen dispersal patterns, is increasingly recognized as important in shaping ant community structure (Andersen 2008; King and Tschinkel 2016).
Interspecific behavioral dominance hierarchies are used to explain patterns of ant species co-existence and, in combination with numerical dominance, to explain ecological dominance (the ecological impact of a species) among co-occurring species (Hölldobler and Wilson 1990). Given that ants exhibit direct (e.g., aggression) and indirect competition (e.g., foraging effectiveness), a simplifying assumption is that greater ability in one axis corresponds with decreased ability in the other (“Dominance-discovery trade-off”, Fellers 1987). However, empirical evidence suggests that a clear trade-off in these abilities is not ubiquitous in ant species and many species, particularly non-native ants, excel in both dominance and discovery (Adler et al. 2007; Parr and Gibb 2011). Stuble et al. (2017) suggest that the use of behavioral hierarchies, specifically dominance hierarchies, as a proxy for ecological dominance is spurious as behavioral hierarchies effectively ignore resource acquisition and cannot quantitatively assess differences in resource acquisition, colony abundance, or forager abundance. We focus on understanding the ability of species to occupy and gather resources as a determinant of ecological dominance, and behavioral interactions at bait stations act as a descriptor that matters if aggressive behaviors affect resource acquisition.
Aphaenogaster species in eastern North American deciduous forests are overwhelmingly the most abundant ant on the forest floors (King et al. 2013; Lubertazzi 2012); yet, despite their numbers, when confronted with more aggressive ant species, Aphaenogaster ants generally are displaced from bait stations and nest sites (Lubertazzi 2012; Stuble et al. 2012; Warren II et al. 2012, 2015a). Hence, Aphaenogaster food retrieval success (discovering and retrieving food) may be due to numerical abundance rather than aggression/retrieval dominance. Another possibility is that foraging workers mass recruit additional foragers from the colony using pheromone trails to the food resource (Bonabeau et al. 1998; Hölldobler and Wilson 1990), although Aphaenogaster species are not generally considered effective recruiters (Lubertazzi 2012). As such, Aphaenogaster’s relative dominance in the forest floor is likely a function of rapid discovery and retrieval of food items, potentially facilitated by the high density of foragers, so that they follow the classic dominance-discovery trade-off as subordinate ants adept at quickly finding and retrieving food (Fellers 1987; Lubertazzi 2012; Stuble et al. 2012).
Aphaenogaster ants are the key elaiosome-bearing myrmecochorous plant (ant-dispersed) disperser in eastern deciduous forests, generally retrieving ~ 75% of seeds offered at bait stations and effectively dispersing them (Ness et al. 2009; Warren II and Giladi 2014; Warren II et al. 2010, 2014). Ant-mediated seed dispersal is a cosmopolitan species interaction (sensu stricto) in which a chemically attractive seed appendage (elaiosome) prompts dispersal by omnivorous foraging ants (e.g., Beattie and Hughes 2002; Gorb and Gorb 2003; Rico-Gray and Oliveira 2007). Seed attractiveness for the ants generally increases with elaiosome size (Bas et al. 2009; Garrido et al. 2002; Gorb and Gorb 1995; Hughes and Westoby 1992; Warren II et al. 2014). Resource acquisition may be affected by food type. Aphaenogaster ants are generalist scavengers with diets ranging from invertebrates to fungi to seed elaiosomes, but invertebrates appear to make up a majority of their diet (Buczkowski and Bennett 2007; Lubertazzi 2012). Some elaiosomes contain key nutrients for ants (Fischer et al. 2008; Gammans et al. 2005; Lanza et al. 1992) so that their retrieval and consumption may supplement ant diets (Clark and King 2012; Gammans et al. 2005), but Aphaenogaster ants occur and thrive in the absence of myrmecochorous plants (Mitchell et al. 2002; Warren II et al. 2019) whereas they depend on invertebrate carrion (Buczkowski and Bennett 2007; Lubertazzi 2012). Whereas Aphaenogaster ants typically monopolize seed retrieval in eastern North American forests (Lubertazzi 2012; Ness et al. 2009), their effectiveness in scavenging other food items is not as well studied.
Our objective was to determine whether Aphaenogaster ants are dominant in acquiring seeds and carrion, and if such dominance is behavioral (e.g., aggression) or ecological (e.g., resource discovery). We expected, given the higher food quality and desirability of termite carrion (Buczkowski and Bennett 2008; Warren II et al. 2015a), that Aphaenogaster ant aggression would be higher at termite-bait stations. Alternately, Aphaenogaster ant dominance for either food item might just reflect forager density. We also examined whether mixing seeds and termites influenced retrieval; given that termites are a preferred food item for ants, we predicted that seed removal would be higher when mixed with termites. Alternately, if seeds are a poorer food choice, their presence may diminish termite removal at mixed bait stations. Given that there is some indication that there may be seasonality in ant preference for seeds, most notably that ants may prefer seeds in early spring when other food sources are less available (Carroll and Janzen 1973), we examined seed and termite carrion retrieval through the spring and early summer season.
Methods
Study species and site descriptions
Aphaenogaster congeners are the most common ants in understory habitats in eastern North American (N.A.) forests (King et al. 2013; Lubertazzi 2012), and they are the main dispersers of myrmecochorous seeds in these habitats (ca. 75% of those removed, Ness et al. 2009; Warren II et al. 2010, 2014). Aphaenogaster ants generally forage individually 60–120 cm away from their nests, although they are capable of weak recruitment of a small number of nestmates (typically less than ten workers) to large food resources (Lubertazzi 2012). Nests are usually located under or in logs and under rocks, though sometimes small nests may be found in a twig or folded leaf (King et al. 2013; Lubertazzi 2012). In our study area, the local Aphaenogaster species is A. picea (Wheeler 1908). Specifically, our study took place in Betty’s Creek watershed (35.286593, − 83.291421), at 780 m elevation, in the Cowee Mountains of Jackson County, North Carolina, USA, within the Southern Appalachian Mountain region. Previous work at this site indicated a high level of ant-mediated seed dispersal (Warren et al. 2014). The site is a rich cove forest where myrmecochorous plant diversity is particularly high. Some common myrmecochore plants in this region are Sanguinaria canadensis L., Asarum arifolium Michx., Trillium spp. Hepatica acutiloba DC. and Viola spp. We used seeds of A. arifolium, which are one of the largest myrmecochorous seeds and elaiosomes in eastern North American deciduous forests and generally are released in June/early July in the study area (Radford et al. 1968; Warren II et al. 2014). Seeds were collected from approximately a dozen local A. arifolium plants (at a site approximately 25 km from the study site) in June 2011 and frozen (− 18 °C) for use in 2012. Freezing myrmecochorous seeds does not diminish their attractiveness to seed-dispersing ants (Clark and King 2012; Ness and Morin 2008). Reticulitermes flavipes (“eastern subterranean termite”) is the most common and widespread termite in eastern North America (King et al. 2013; Maynard et al. 2015). In 2011, we collected several logs that had been colonized by R. flavipes in northern Georgia (where termites are much more common) and removed workers which were then immediately frozen (− 18 °C) for use as insect carrion in 2012.
Bait stations and indices
Bait stations are a common approach for examining interspecific behavior interactions among foraging ant species and to measure resource acquisition, food preference, and seed removal dynamics (Cerda et al. 2013; Warren II and Giladi 2014). We used 4 × 4 cm polystyrene weighing dish secured into the soil with a 2-cm nail for bait stations and two sets of bait stations, each set containing three bait stations: ten A. arifolium seeds (hereafter, “seed”), ten termite corpses (hereafter, “termite”) and a mix of five seeds and five corpses (hereafter, “mixed”). The sets were placed 200 m apart in the leaf litter of the forest understory with each station within each set 1 m apart (in a straight line with the ‘mixed’ bait station in the middle). The locations for the bait stations were chosen as representative of eastern deciduous hardwood forest habitat as they were characterized by a thick leaf litter layer and a lush understory layer beneath mature hardwood tree species such as oak (Quercus spp.), hickory (Carya spp.), tulip popular (Liriodendron tulipifera L.) and yellow birch (Betula alleghaniensis Britt.). The respective baits were added to the stations once each week at 9:00 h, on days without precipitation, for 7 weeks starting March 15, 2012 and ending June 18, 2012. A digital camcorder (Samsung SMX-F50BN) was positioned at each bait station and recorded all activity between 9:00 and 11:00 h each week. The bait timing and interval length were chosen to match peak ant-foraging activity, and most seed removal by ants occurs within the first 1–2 h of seed release (Gorb and Gorb 2003; Ness et al. 2009; Warren II et al. 2014).
We reviewed video from each bait station from each week (2 h · 6 bait stations · 7 weeks; 84 h total) to score ant behavior and bait removal. For ant behavior, we used an index modified from Roulston et al. (2003) and Suarez et al. (1999) in which ant interactions were recorded as (1) ignore—ant makes contact with another ant but exhibits no further reactions, (2) avoid—ant runs away or actively avoids other ant after contact, or (3) attack—ant initiates aggressive physical attack after contact such as biting or stinging. All individual behaviors were recorded for each ant at bait stations. For bait removal, we used the seed removal index developed by Culver and Beattie (1978) to score ant interest in seeds or termite corpses as (1) ignore—ant pays no attention to the bait, (2) antennate—the ant investigates the bait with antennae, (3) inspect—ant more thoroughly examines the bait with mandibles, (4) pickup—ant moves bait within the bait station, and (5) removal—ant removes the bait from the bait station.
We collected and identified a subsample of A. picea and non-A. picea ants from the bait stations; however, given that A. picea was the target species for the study, and because non-A. picea ants were not always discernable from one another in the video footage, we lumped non-A. picea ants together for analysis.
Data analysis
We used two clusters (blocks) of bait stations, which provided little spatial replication (n = 2); however, our study focus was replication across time to account for seasonal effects on ant foraging. As such, we essentially considered the bait station blocks equivalent. We tested that assumption using cumulative link mixed models (CLMM) for the ordinal dependent variables: ant behavior index and bait removal index. We evaluated the indices as functions of block, and we included species (A. picea, other ant species) and a block:species interaction term to test for species-specific block effects. The CLMM models were fit with the adaptive Gauss–Hermite quadrature approximation with ten quadrature points using the ordinal package (Christensen 2019) in the R statistical program (R Development Core Team Version 3.5.1 2019).
We evaluated ant behavior at the bait stations using a CLMM with the ant behavior index as a function of bait type (seeds, termites, mixed), ant taxa (A. picea, other ant species), week (1:7) and species interaction (inter or intraspecific). Because the measurements were taken repeatedly across time at the same bait stations, we included bait station as a random effect to account for autocorrelation. We also included interaction terms for week × bait type, week × species and week × interaction type to investigate seasonality effects on behavior. Because the interpretation of ordinal coefficients is not as straightforward as linear or logistic regression, we converted the CLMM coefficient parameters to odds ratios ± 95% confidence intervals (which are not necessarily symmetric).
We investigated foraging ability by measuring the time it took for ants to find the baits once placed at the bait station (discovery time). We evaluated discovery time as a function of bait type and ant taxa using a generalized linear mixed model (GLMM) assuming Poisson-distributed error in the lme4 package (Bates et al. 2015) in the R statistical program. We included bait station as a random effect, and we included an individual-level random effect for each row to account for overdispersion (\(\phi\) > 2.0). We also included a bait station × ant taxa interaction term to account for species-level differences in bait preference. The model was fit using analysis of deviance (ANODEV) with a χ2 test.
We looked at foraging efficiency to evaluate whether bait type influenced ant the likelihood that an ant visiting a bait station would remove bait. We calculated foraging efficiency as baits (seed or termite) removed per ant forager (baits removed · ant foragers−1). We evaluated foraging efficiency as a function of bait type (seed, termite, mixed) and ant taxa (A. picea or other ant species) assuming a binomial error distribution (binomial proportion) in a generalized linear model (GLM) in the R Statistical Package. We included a bait type × taxa interaction term to account for species-level differences in termite and seed bait removal. The model was not over dispersed (\(\phi\) < 2.0), and it was fit using ANODEV with a χ2 test.
Given that poor forage, or relatively poor forage, might lessen ant interest in a food resource, we investigated whether poor forage (seeds) mixed with preferred forage (termites) interfered with the ant interest in the preferred food. We tested for interference using CLMMs for seed and termite removal as ordinal functions of bait type (seed, termite, mixed) and ant taxa (A. picea, other species) with bait station as a random effect. We also included a bait type × ant taxa interaction term.
Given that ant interest in forage may change with season, we used Box–Jenkins autoregressive moving average (ARMA) models to analyze weekly trends in A. picea total bait removal and the removal index for termites and seeds using the nlme package (Pinheiro et al. 2019) in the R statistical program. We used generalized least squares with maximum likelihood to fit the models. The GLS model assumes that errors are correlated and may have unequal variances without assuming linearity in the data.
Results
The bait stations were visited 343 times by A. picea ants, and 272 times by other ants, including Crematogaster ashmeadi Mayr, Camponotus chromaiodes Bolton, Camponotus pennsylvanicus (DeGeer), Lasius alienus (Foerster), Prenolepis imparis (Say) and Tapinoma sessile (Say). These are all common, native species of ants found in eastern temperate forests of this region of the US.
Station (block) effects
The ant behavior index did not differ between station blocks (p value = 0.653) or by ant taxa between station blocks (p value = 0.241). Similarly, the bait removal index did not differ between station blocks (p value = 0.458) or by ant taxa between station blocks (p value = 0.199).
Ant behavior at food bait stations
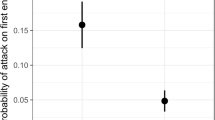
During the bait station trials, 51 behavioral interactions between ants were observed, which indicated that the ants interacted with one another approximately 0.6 times · h out of 7.3 ant visits · h. Hence, most bait station visits involved individual ants or disinterested groups of ants. Of the bait station species interactions, 36% were intraspecific and 65% interspecific. The ants generally ignored one another after contact (47% of the time) or one made explicit attempts to avoid the other ant (38%) and occasionally one aggressively attacked the other (15%). Ant aggression was less likely in intraspecific than interspecific interactions (0.6, CI 0.1–0.31; z value = −3.404, p value < 0.001; Fig. 1) with a mean aggression index (± SE) of 1.21 ± 0.12 for h interactions compared to 1.94 ± 0.11 for interspecific. The other parameters (bait type [z value = −0.908, p value = 0.363], ant taxa [z value = 0.405, p value = 0.684] and week [z value = 0.226, p value = 0.820]) did not impact the levels of ant aggression. None of the interaction terms were significant, and they were removed from the model. When aggressive attacks occurred, 88% were interspecific, and 38% of the attacks were A. picea foragers attacking other ant species (L. alienus and T. sessile) or another A. picea forager, whereas 62% of the attacks were other species (T. sessile and C. pennsylvanicus) attacking A. picea. Aphaenogaster picea attacks were rote and mechanical with the worker often continuing to strike at the air after the other ant retreated. Otherwise, A. picea often ran off the bait station trays when attacked by other ants.
Odds ratios (exponent of ordinal regression coefficients) for ant behavior at bait stations. The odds ratios (± 95% CI) indicate the degree of probability of one parameter against the other so that an odds ratio = 1 indicates equal probability of either. Ant aggression at the bait stations was similar whether the bait was termites or seeds, whether the foragers were A. picea or other ants, and it did not vary by week. The only influence on ant behavior was that intraspecific aggression was less likely at the bait stations than interspecific aggression
Seed and termite-bait removal
Across the bait stations, at which 210 termites and 210 seeds total were offered between May 5 and June 18, ants removed 95% of the termites and 12% of the seeds. Aphaenogaster picea workers discovered bait stations in half the time (19.6 ± 3.9 min) of all other ant workers combined (41.5.0 ± 6.3 min) (Fig. 2; df = 1, χ2 = 7.697, p value = 0.005]. Discovery time did not differ by bait type (df = 1, χ2 = 0.577, p value = 0.749) or by an interaction between bait type and ant taxa.
Mean time to bait discovery (± SE) for Aphaenogaster picea foragers and other co-occurring ant taxa (including Crematogaster ashmeadi, Camponotus chromaiodes, Camponotus pennsylvanicus, Lasius alienus, Prenolepis imparis and Tapinoma sessile). The total number of station visits by foraging ants is given for each category
Ants overwhelmingly preferred to remove termites (83.3 ± 2.1% removed weekly) as compared to seeds (9.8 ± 1.7% removed weekly). Overall, A. picea foragers were more efficient (bait removed · ant foragers−1) at removing baits (60.2 ± 8.5% of baits · week−1) than other ant taxa (39.0 ± 7.7% of baits · week−1); i.e., when an A. picea forager arrived at a bait station they were more likely to leave with bait than other ant taxa. The interaction term indicated that A. picea foragers were particularly efficient at removing termite corpses as compared to other ant taxa (Fig. 3; df = 2, χ2 = 6.624, p value = 0.036].
Interaction plot for the bait foraging efficiency (baits removed · ant foragers−1) of A. picea and other co-occurring ants at seed (Asarum arifolium), termite (Reticulitermes flavipes) and mixed (A. arifolium + R. flavipes) bait stations. Aphaenogaster picea and other ant taxa were equally efficient at removing baits from seed and mixed bait stations, but A. picea removed significantly more termites than did other ants. Connecting lines are shown only to aid visual interpretation and do not imply interpolation
Seed bait removal was equally likely in seed and mixed bait stations (1.40, CI 0.83–2.35; z value = 1.273, p value = 0.203; Fig. 4), and seed removal was equally likely by A. picea or other ant taxa (1.22, CI 0.75–1.96; z value = 0.800, p value = 0.432; Fig. 4). The interaction term was not significant, and it was removed from the model. Termite bait removal was more likely in termite than mixed bait stations (6.99, CI 1.77–7.63; z value = 1.943, p value = 0.005; Fig. 4), and seed removal was less likely by ant taxa other than A. picea (0.21, CI 0.09–0.52; z value = −3.408, p value < 0.001; Fig. 4). The interaction term was not significant, and it was removed from the model.
Odds ratios for the bait removal index at seed or mixed bait stations and termite and mixed bait stations. Ant interest in seeds did not depend on whether the forager was A. picea or another ant taxon, and it did not vary between single and mixed seed bait stations; however, A. picea exhibited higher termite-bait interest and removal compared to other ant taxa, and ant interest in termites and their removal was higher at single than mixed bait stations
Seeds removal by A. picea increased between May 5 and June 18 (Fig. 5a; coef. = 1.094, SE = 0.430, t value = 2.540, p value = 0.051) and termite removal by A. picea increased during the same time period as well (Fig. 5a; coef. = 1.957, SE = 0.589, t value = 3.321, p value = 0.020). At the same time, A. picea interest (mean removal index) in seeds essentially remained the same between May 5 and June 18 (Fig. 5b; coef. = −0.018, SE = 0.100, t value = −0.183, p value = 0.863), whereas A. picea interest in termites decreased during the same time period (Fig. 5b; coef. = −0.067, SE = 0.016, t value = −4.054, p value = 0.009).
Total weekly seed and termite baits removed from all bait stations by Aphaenogaster picea (a) and weekly A. picea mean removal index for seed and termite baits (b). The total weekly removal refers to the actual items removed whereas the removal index includes ant forager interest in the baits (index; ignore = 1; antennate = 2; inspect = 3, pickup = 4 and removal = 5)
Discussion
Aphaenogaster ants dominate seed retrieval in eastern deciduous forests of North America (Lubertazzi 2012; Ness et al. 2009). Our data suggest they may also dominate foraging of insect carrion on the forest floor (indeed, they preferred insect carrion to seeds). The key finding here is that A. picea ants required neither aggression nor recruiting to dominate bait stations; their domination appeared simply a result of superior forager numbers. Aphaenogaster picea foragers generally ignored other ants or, if they interacted, they tended to retreat with few overtly aggressive behaviors. At the same time, they were the first to find bait stations, and the most efficient at removing bait—particularly carrion (dead termites). Concomitantly, we found myrmecochorous seeds a poor forage item relative to termites. Ants removed eight times more termites than seeds, and A. picea removed a greater number of termites than any other ant species. When placed with termites, seeds decreased the attractiveness of termites for A. picea rather than termites improving the attractiveness of seeds. Moreover, we found no evidence that seeds are more desirable in early spring when competing forage is assumed to be less common whereas carrion was more desirable. Thus, though insect carrion and elaiosomes may be chemically and possibly nutritionally similar, the ants (A. picea in particular) clearly preferred insect carrion when given a choice.
Aphaenogaster species generally are considered relatively docile ants with little interspecific aggression and relatively low co-worker recruitment toward dominating baits through numbers (Lubertazzi 2012), however, they appear to compensate with relatively high forager numbers and very fast bait detection and retrieval—supporting the discovery-dominance trade-off hypothesis (Fellers 1987). Aphaenogaster congeners consistently find food baits faster than co-occurring ant species (Fellers 1987; Stuble et al. 2012). Still, the discovery-dominance dichotomy is not absolute with Aphaenogaster; specifically, though uncommon, we observed A. picea workers aggressively attacking and even killing other ants in this study and in prior work (Warren II et al. 2018), and several authors have questioned the consistency of the trade-off across ants and systems (Parr and Gibb 2011). The important implication from this study, however, is that this manner of foraging, coupled with their consistently high abundance of foragers, suggests that they dominate resource acquisition relative to co-occurring ants. We did not find a difference in aggressive behaviors between A. picea ants and the other ant taxa in this study, though we did find that A. picea found the baits much faster. We found no evidence that, once discovered, the Aphaenogaster ants recruited more workers as other ant taxa do. This characteristic makes Aphaenogaster ants effective dispersers for myrmecochorous plants—based more on a “get there first with the most” resource acquisition strategy than any special chemical characteristics of the elaiosome.
Seeds appear to be an adequate substitute for preferred food items (Clark and King 2012; Warren II et al. 2019), but termites are clearly a particularly desirable food item for ants when given a choice (Buczkowski and Bennett 2007; Warren II et al. 2015a). Given that ant aggressiveness typically increases with larger and higher value food resources (Adler et al. 2007), we expected higher interference behaviors at termite-bait stations, but there was no such effect, suggesting that either that there is no difference in quality or that interspecific aggression is not connected with competition in A. picea. One possibility, however, is that our daytime sampling regime underestimated behaviorally dominant ants that may be more likely to forage nocturnally (Fellers 1987; Stuble et al. 2012), but Aphaenogaster ants, including A. picea, are well established as the dominant ant and dominant myrmecochorous seed dispersers in eastern deciduous forest understories (King et al. 2013; Lubertazzi 2012; Ness et al. 2009).
Myrmecochorous plants appear to compete for ants through seed elaiosome size with the larger elaiosomes predictably more desirable for ant dispersers than smaller ones (Leal et al. 2014; Warren II et al. 2014). Moreover, eastern deciduous plants appear to stagger the release of seeds by size so that the least competitive seeds with the smallest elaiosomes are released in earliest spring to avoid competition with the larger myrmecochorous seed release in late spring to summer (Warren II et al. 2014). For this study, we used A. arifolium seeds, which are some of the largest myrmecochorous seeds/elaiosomes available in the study system and which are naturally released in the summer (Warren II et al. 2014). When paired at the same bait stations, all ants decidedly preferred termites to the high-quality seeds/elaiosomes, and overall ant interest decreased slightly in the seeds and significantly in the termites as the season progressed (removal increased because of a greater abundance of ants offsetting declining interest). One possibility is that the release of myrmecochorous seeds in the natural background during the study may have affected the experiment, which would have included the less desirable small seeds of Hepatica spp. in March and the more desirable seeds of Trillium spp. in June. We tested how desirable and undesirable foods may influence ant interest in baits. In the mixed stations, the presence of termites did not affect Aphaenogaster seed removal, suggesting that a preferred food item did not increase or diminish retrieval of the less preferred one. However, for Aphaenogaster, the co-occurrence of seeds reduced termite removal, suggesting that the presence of seeds somehow diminished the bait station desirability (or possibly the recruitment of more foragers). These results suggest that myrmecochorous seeds may distract ant foragers from more desirable foods (Warren II et al. 2015b, 2019). Given that undesirable foods interfered with bait removal, and that ant interest in bait station seeds diminished with time even though more desirable seeds were naturally released in the background, it is unlikely that natural seed set in the study area interfered with the study.
Our results suggest that Aphaenogaster species are the dominant myrmecochorous seed dispersers in eastern deciduous forests because of their foraging proficiency rather than a symbiotic relationship with seeds. Aphaenogaster appears to dominate through sheer numbers without showing any behaviors that enhance interference competition through aggression or appreciable nestmate recruitment at baits. Even though Aphaenogaster ants are the key myrmecochorous seed dispersers in eastern deciduous forests (Lubertazzi 2012; Ness et al. 2009; Warren II and Giladi 2014), they show no particular affinity for even the highest quality seeds/elaiosomes, much preferring insect carrion instead. Based on our results, Aphaenogaster may be regarded as the key insect-carrion scavengers in this system, at least relative to other ant species, with their dominance in seed retrieval more a function of forager abundance and their skill at discovery and retrieval, rather than a result of a specific preference for seeds. Somewhat bizarrely then, despite the importance of ant-mediated seed dispersal for the population viability of many understory herb species—and hence a significant proportion of the floristic diversity in eastern U.S. deciduous forests—seed retrieval by Aphaenogaster appears more opportunistic than the result of a specialist niche or a co-evolved symbiosis with the plants. These results add to accumulating evidence that the ant–plant relationship in this system very likely is not a mutualism but instead is closer to commensal or even facultatively parasitic. Nevertheless, Aphaenogaster ants are keystone species in shaping the dispersal and recruitment of myrmecochorous plants and carrion scavenging through their dominance in resource acquisition.
Data accessibility
The data generated and analyzed for the current study is available in the SUNY Buffalo State Digital Commons (https://digitalcommons.buffalostate.edu/biology_data/9/).
References
Adams ES (2016) Territoriality in ants (Hymenoptera: Formicidae): a review. Myrmecol News 23:101–118
Adler FR, LeBrun EG, Feener DJ Jr (2007) Maintaining diversity in an ant community: modeling, extending, and testing the dominance-discovery trade-off. Am Nat 169:323–333
Andersen AN (2008) Not enough niches: non-equilibrial processes promoting species coexistence in diverse ant communities. Austral Ecol 33:211–220
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bas JM, Oliveras J, Gomez C (2009) Myrmecochory and short-term seed fate in Rhamnus alaternus: Ant species and seed characteristics. Acta Oecologica 35:380–384
Beattie AJ, Hughes L (2002) Ant–plant interactions. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions: an evolutionary approach. Blackwell Science, Oxford, pp 211–235
Bonabeau E, Theraulaz G, Deneubourg JL (1998) Group and mass recruitment in ant colonies: the influence of contact rates. J Theor Biol 195:157–166
Buczkowski G, Bennett G (2007) Protein marking reveals predation on termites by the woodland ant, Aphaenogaster rudis. Insectes Soc 54:219–224
Buczkowski G, Bennett G (2008) Behavioral interactions between Aphaenogaster rudis (Hymenoptera: Formicidae) and Reticulitermes flavipes (Isoptera: Rhinotermitidae): the importance of physical barriers. J Insect Behav 21:296–305
Carroll CR, Janzen DH (1973) The ecology of foraging by ants. Annu Rev Ecol Syst 4:231–258
Cerda X, Arnan X, Retana J (2013) Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology? Myrmecol News 18:131–147
Christensen RHB (2019) Regression models for ordinal data. R package version 2019.12-10. https://CRAN.R-project.org/package=ordinal
Clark RE, King JR (2012) The ant, Aphaenogaster picea, benefits from plant elaiosomes when insect prey is scarce. Environ Entomol 41:1405–1408
Culver DC, Beattie AJ (1978) Myrmecochory in Viola: dynamics of seed-ant interactions in some West Virginia species. J Ecol 66:53–72
Fellers JH (1987) Interference and exploitations in a guild of woodland ants. Ecology 68:1466–1478
Fischer RC, Richter A, Hadacek F, Mayer V (2008) Chemical differences between seeds and elaiosomes indicate an adaptation to nutritional needs of ants. Oecologia 155:539–547
Gammans N, Bullock JJ, Schonrogge K (2005) Ant benefits in a seed dispersal mutualism. Oecologia 146:43–49
Garrido JL, Rey PJ, Cerda X, Herrera CM (2002) Geographical variation in diaspore traits of an ant-dispersed plant (Helleborus foetidus): are ant community composition and diaspore traits correlated? J Ecol 90:446–455
Gibb H, Johansson T (2011) Field tests of interspecific competition in ant assemblages: revisiting the dominant red wood ants. J Anim Ecol 80:548–557
Gorb SN, Gorb EV (1995) Removal rates of seeds of five myrmecochorous plants by the ant Formica polyctena (Hymenoptera: Formicidae). Oikos 73:367–374
Gorb EV, Gorb SN (2003) Seed dispersal by ants in a deciduous forest ecosystem. Kluwer, Dordrecht
Hölldobler B, Wilson EO (1990) The ants. Belknap, Cambridge
Hughes L, Westoby M (1992) Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 73:1300–1312
King JR, Tschinkel WR (2016) Experimental evidence that dispersal drives ant community assembly in human-altered ecosystems. Ecology 97:236–249
King JR, Warren RJ II, Bradford MA (2013) Social insects dominate eastern US temperate hardwood forest macroinvertebrate communities in warmer regions. PLoS ONE 8:e75843
Lanza J, Schmitt MA, Awad AB (1992) Comparative chemistry of elaisomes of three species of Trillium. J Chem Ecol 18:209–221
Leal LC, Lima M, de Oliveira AF, Andersen AN, Leal IR (2014) Myrmecochores can target high-quality disperser ants: variation in elaiosomes traits and ant preferences for myrmecochorous Euphorbiaceae in Brazilian Caatinga. Oecologia 174:493–500
Lubertazzi D (2012) The biology and natural history of Aphaenogaster rudis. Psyche 2012:752815
Maynard DS, Crowther TW, King JR, Warren RJ II, Bradford MA (2015) Temperate forest termites: ecology, biogeography, and ecosystem impacts. Ecol Entomol 40:199–210
Mitchell CE, Turner MG, Pearson SM (2002) Effects of historical land use and forest patch size on myrmecochores and ant communities. Ecol Appl 12:1364–1377
Ness JH, Morin DF (2008) Forest edges and landscape history shape interactions between plants, seed-dispersing ants and seed predators. Biol Conserv 141:838–847
Ness JH, Morin DF, Giladi I (2009) Uncommon specialization in a mutualism between a temperate herbaceous plant guild and an ant: Are Aphaenogaster ants keystone mutualists? Oikos 12:1793–1804
Parr CL, Gibb H (2009) Competition and the role of dominant ants. In: Lach L, Parr CL, Abbott KL (eds) Ant ecology. Oxford University Press, Oxford, pp 77–96
Parr CL, Gibb H (2011) The discovery–dominance trade-off is the exception, rather than the rule. J Anim Ecol 81:233–241
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2019) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-143. https://CRAN.R-project.org/package=nlme
R Development Core Team Version 3.5.1 (2019) R: a language and environment for statistical computing, 3rd.5.0 edn. R Foundation for Statistical Computing, Vienna
Radford AE, Ahles HE, Bell CR (1968) Manual of the vascular flora of the Carolinas. The University of North Carolina Press, Chapel Hill
Rico-Gray V, Oliveira P (2007) The ecology and evolution of ant–plant Interactions. The University of Chicago Press, Chicago
Roulston TH, Buczkowski G, Silverman J (2003) Nestmate discrimination in ants: effect of bioassay on aggressive behavior. Insectes Soc 50:151–159
Stuble KL, Rodriguez-Cabal MA, McCormick MK, Juric I, Dunn RR, Sanders NJ (2012) Tradeoffs, competition, and coexistence in eastern deciduous forest ant communities. Oecologia 171:981–992
Stuble KL, Juric I, Cerda X, Sanders NJ (2017) Dominance hierarchies are a dominant paradigm in ant ecology (Hymenoptera: Formicidae), but should they be? And what is a dominance hierarchy anyways? Myrmecol News 24:71–81
Suarez AV, Tsutsui ND, Holway DA, Case TJ (1999) Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol Invasions 1:43–53
Warren RJ II, Giladi I (2014) Ant-mediated seed dispersal: a few ant species (Hymenoptera: Formicidae) benefit many plants. Myrmecol News 20:129–140
Warren RJ II, Giladi I, Bradford MA (2010) Ant-mediated seed dispersal does not facilitate niche expansion. J Ecol 98:1178–1185
Warren RJ II, Giladi I, Bradford MA (2012) Environmental heterogeneity and interspecific interactions influence occupancy be key seed-dispersing ants. Environ Entomol 41:463–468
Warren RJ II, Giladi I, Bradford MA (2014) Competition as a mechanism structuring mutualisms. J Ecol 102:486–495
Warren RJ II, McMillan A, King JR, Chick L, Bradford MA (2015a) Forest invader replaces predation but not dispersal services by a keystone species. Biol Invasions 23:3153–3162
Warren RJ II et al (2015b) Cryptic indirect effects of exurban edges on a woodland community. Ecosphere 6:218
Warren R II, Reed K, Mathew A, Krupp K, Goodman M, Archibald K, Spiering DJ (2018) Release from intraspecific competition promotes dominance of a non-native invader. Biol Invasions. https://doi.org/10.1007/s10530-018-1868-z
Warren RJ II, Elliott KJ, Giladi I, King JR, Bradford MA (2019) Field experiments show contradictory short- and long-term myrmecochorous plant impacts on seed-dispersing ants. Ecol Entomol. https://doi.org/10.1111/een.12666
Acknowledgements
This research was supported by the Yale School of Forestry and Environmental Studies. The authors thank Itamar Giladi for a friendly review of an early draft of the mansucript and two anonymous reviewers for helpful comments on the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warren, R.J., King, J.R. & Bradford, M.A. Disentangling resource acquisition from interspecific behavioral aggression to understand the ecological dominance of a common, widespread temperate forest ant. Insect. Soc. 67, 179–187 (2020). https://doi.org/10.1007/s00040-020-00750-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-020-00750-z