Abstract
Ongoing climate change is expected to affect the diversity and activity of aquatic microbes, which play a key role in plant litter decomposition in forest streams. We used a before-after control-impact (BACI) design to study the effects of warming on a forest stream reach. The stream reach was divided by a longitudinal barrier, and during 1 year (ambient year) both stream halves were at ambient temperature, while in the second year (warmed year) the temperature in one stream half was increased by ca. 3 °C above ambient temperature (experimental half). Fine-mesh bags containing oak (Quercus robur L.) leaves were immersed in both stream halves for up to 60 days in spring and autumn of the ambient and warmed years. We assessed leaf-associated microbial diversity by denaturing gradient gel electrophoresis and identification of fungal conidial morphotypes and microbial activity by quantifying leaf mass loss and productivity of fungi and bacteria. In the ambient year, no differences were found in leaf decomposition rates and microbial productivities either between seasons or stream halves. In the warmed year, phosphorus concentration in the stream water, leaf decomposition rates, and productivity of bacteria were higher in spring than in autumn. They did not differ between stream halves, except for leaf decomposition, which was higher in the experimental half in spring. Fungal and bacterial communities differed between seasons in both years. Seasonal changes in stream water variables had a greater impact on the activity and diversity of microbial decomposers than a warming regime simulating a predicted global warming scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to ongoing climate change, global mean air temperature has increased by ca. 0.75 °C since the late nineteenth century and is predicted to increase by 1.5–4.6 °C by the year 2100 [1]. Such increases are likely to be mirrored in stream water temperatures, leading to alterations in flow regimes, disturbance intensity and frequency, water chemistry, and species interactions, which can strongly compromise the services provided by these ecosystems [2]. In particular, small-forest streams, where water temperature is typically low throughout the year, are expected to be highly vulnerable to temperature increases [3]. In these streams, the decomposition of organic matter provided by the riparian vegetation constitutes the main source of nutrients and energy to aquatic biota [4, 5]. Plant litter decomposition is a dynamic process that links riparian vegetation, environmental conditions, and aquatic biota, especially microbes and invertebrate detritivores [4, 5]. Aquatic fungi and bacteria are the basis of detritus food webs, converting plant litter into a more palatable food resource for detritivores, a process known as microbial conditioning [4, 5]. Aquatic hyphomycetes macerate the leaf matrix by the activities of extracellular enzymes [6], convert organic matter into fungal biomass (mycelium and conidia; [7]), and mineralize it [8, 9] increasing litter quality for detritivores [10, 11].
Temperature controls most physiological activities of aquatic hyphomycete species and their interactions, due to the tight coupling between species during litter decomposition [12, 13]. Therefore, thermal variations have the potential to alter microbial colonization of submerged leaf litter and, thus, interfere with the flow of carbon and energy to higher trophic levels which may impact the functioning of small-forest streams. Previous studies conducted in laboratory microcosms suggest that an increase in temperature by 5–10 °C stimulates litter decomposition mediated by aquatic microbes [14–20]. This stimulation is associated with enhanced fungal respiration rates, biomass accumulation, and reproduction on decomposing leaf litter [17–20]. On the other hand, temperature increases of the same order of magnitude did not affect fungal activity when control temperature was already high (18 °C) and similar to that attained in streams of Northwest Portugal in spring/summer [15]. Increases in temperature often lead to shifts in the structure of stream-dwelling microbial communities [14–18]. Thus, fungal species typical of warmer waters became dominant on decomposing plant litter under warming treatments [e.g., 15, 16]. Moreover, the effects of warming appear to be strongly modulated by the environmental context (such as differences in litter quality [16, 17, 21], eutrophication [18], and metal pollution [19]).
Approaches using microcosms have provided valuable information on the impacts of increased temperature on stream biota and ecological processes. However, the complex array of interactions in natural ecosystems cannot be fully simulated in microcosms [22, 23]. Correlative studies using elevation [24, 25], latitudinal [26–28], geothermal [29], and seasonal gradients [30, 31] provided more realistic information about the potential effects of future temperature on freshwater ecosystems. Nevertheless, it is impossible to unravel the effects of biogeography and temperature because community composition varies with factors other than differences in temperature (e.g., nutrient concentrations, hydrology, and dispersal constraints) [32]. In addition, the temperature range prevailing in these correlative studies [e.g., 28, 29] is often wider (>10 °C) than the temperature increase expected from global warming in this century [1].
A more realistic approach for assessing the effects of global warming is based on in-stream manipulation of water temperature, which allows experiments in field conditions preserving the natural diurnal and seasonal variation of abiotic factors [33–35]. In this study, we took advantage of a stream manipulation in a second-order forest stream [35–38] to assess the effects of warming on stream-dwelling microbial decomposers and litter decomposition. The stream reach was divided longitudinally in half, and a before-after control-impact (BACI) design was followed. During 1 year, both stream halves were at ambient temperature, while in the second year one half was maintained at ambient temperature and the other half was warmed ca. 3 °C above ambient [35]. This temperature increase represents a realistic climate change scenario as predicted for the next 30 years for air temperature in Portugal [39]. Oak (Quercus robur L.) leaves were incubated in both stream halves in spring and autumn before (ambient year) and during warming (warmed year), and microbial activity and diversity were assessed. Our expectations were as follows: (i) differences in temperature due to season and warming will induce shifts in the structure of stream-dwelling microbial decomposer communities; (ii) microbial activity will be stimulated at warmer temperatures; and (iii) increased microbial activities at higher temperatures will result in higher leaf decomposition rates.
Methods
Study Site and Experiment Setup
Decomposition experiments were conducted in a second-order forest stream reach (Candal stream, Lousã mountain, central Portugal; 40° 4ʹ 44ʺ N, 8° 12ʹ 10ʺ W; 620 m asl). This stream drains an area (0.8 km2) of schist bedrock, covered by deciduous mixed forest dominated by chestnut (Castanea sativa Mill.) and oak (Q. robur), and of low human activity [40]. Oak leaves were selected for the study because oak trees shed most of their leaves later than chestnut trees [41] and oak leaves decompose slower than chestnut leaves [40], and thus in spring there are more oak than chestnut leaves on the stream bed [42]. The study reach was divided in half with a longitudinal barrier of schist stones (ca. 22 m long), roughly cemented and driven into the sediment down to the bedrock to prevent any transfer of water between stream halves (ca. 0.5 m width). In the first year (ambient year—2010), stream water was at ambient temperature in both stream halves. In the second year (warmed year—2011), the temperature in one stream half was increased by ca. 3 °C (experimental half) above the ambient water temperature in the control half. Warming was achieved with two stainless steel tanks (260 L) that received water, by gravity, from the main channel. One tank was equipped with electric water heaters (n = 30; 2 kW) operating with a continuous supply of energy, while the other tank had no heating power. During the warmed year, the experimental stream half received water from the heating tank (ca. 3 °C above ambient temperature) while the control half received water from the unheated control tank. The target increase in water temperature was 3 °C, based on predictions for air temperature in Portugal by the end of this century [39] and on the relationship between water and air temperature reported for similar streams [43]. However, due to electrical shutoffs during thunderstorms, the difference in temperature between stream halves was 3 °C in spring 2011 and 2.3 °C in autumn 2011. A detailed description of the electrical and hydraulic systems can be found in Canhoto et al. [35].
Litter decomposition experiments were performed in both stream halves in spring and autumn, before (ambient year—2010) and during warming (warmed year—2011). Batches of 2.24–2.53 g of air-dried oak leaves, collected just after abscission (Lousã Mountains, central Portugal, autumn 2006), were enclosed in fine-mesh bags (10 × 12 cm, 0.5-mm pore size) and deployed in the stream (n = 15/half). Fine-mesh litter bags were used to exclude macroinvertebrates. On day 0, five extra litter bags, prepared as above, were taken to the stream, submerged for ca. 10 min, returned to the laboratory, oven-dried at 105 °C for 24 h, and weighed to establish a conversion factor for initial air-dry mass to initial oven-dry mass. Replicate bags (n = 3) were retrieved after 11, 20, 31, 45, and 60 days of immersion.
Physical and Chemical Stream Water Characterization
Weekly, electrical conductivity (LF 330, WTW, Weilheim, Germany), pH (pH 3110, WTW, Weilheim, Germany), and dissolved oxygen (Oxi 3210, WTW, Weilheim, Germany) were recorded in situ in both stream halves. Discharge was determined volumetrically at the outflows of the tanks that feed each stream half [44]. Temperature was measured hourly with submerged data loggers (Hobo Pendant UA-001-08, Onset Computer Corp., MA, USA), yielding a total of 1440 measurements (24 measurements per day × 60 days). Daily averages were used for data presentation and analyses. At each sampling date, 300 mL of water was collected from each stream half, filtered through glass fiber filters (47 mm diameter, pore size 0.7 μm; Millipore APFF04700, Millipore Corp., Billerica, MA, USA), transported to the laboratory in a cool box and frozen until analyzed for nutrient concentrations. Nitrate concentration was determined by ion chromatography (Dionex DX-120, Sunnyvale, CA, USA), and soluble reactive phosphorus (SRP) concentration was determined by the ascorbic acid method [45]. Alkalinity was determined by titration with sulfuric acid to an endpoint of pH 4.2 [45].
Litter Bag Processing and Leaf Dry Mass Remaining
In the laboratory, oak leaves were rinsed with tap water to remove sediment. From each replicate bag, leaf disks were cut with a 1.2-cm diameter cork borer; one set of four leaf disks was lyophilized for DNA extraction, one set of five leaf disks was immediately used to induce fungal sporulation, two sets of six leaf disks were used to determine fungal production, and three sets of four leaf disks were used to determine bacterial production (see below). The dry mass of the leaf disks used in the microbial analyzes was estimated from an additional set of 15 leaf disks that was oven-dried at 105 °C for 24 h and weighed. The remaining leaves were dried and weighed to the nearest 0.01 g. Leaf dry mass remaining (g) was determined as the sum of the mass of the remaining leaves and the mass of the leaf disks.
Microbial Diversity
DNA was extracted from one quarter each of four lyophilized leaf disks from each replicate bag (UltraClean® Soil DNA isolation kit, MoBio Laboratories, Solana Beach, CA, USA), according to the manufacturer’s instructions, except that the lysis step was conducted in the FastPrep FP120 instrument (velocity 5.0, duration 30”) (Qbiogene, Heidelberg, Germany). The ITS2 region of fungal ribosomal DNA and the V3 region of bacterial 16S rDNA were amplified with the primer pairs ITS3GC/ITS4 [46] and 338GC/518 [47], respectively. Briefly, 1 μL (1–10 ng μL−1) of DNA extract was mixed with 0.5 μL of each primer (0.4 μM final concentration), 12.5 μL of GoTaq® Green Master Mix (Promega Corporation, WI, USA), and 10.5 μL of water supplied with the GoTaq® Green Master Mix in 0.2 mL PCR tubes. PCR reactions were carried out in a Doppio thermocycler (VWR International, PA, USA) as follows: (i) initial denaturation for 2 min at 95 °C; (ii) 36 cycles of denaturation for 30 s at 95 °C; annealing for 30 s at 55 °C and extension for 1 min at 72 °C; and (iii) final elongation for 5 min at 72 °C. The PCR products were run on a 2 % agarose gel at 80 V for 45 min to check for the presence of the desired band.
The denaturing gradient gel electrophoresis (DGGE) analyses, which separate sequences of similar length but with different nucleotidic compositions [47], were performed with a DCode™ Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Samples with ca. 750 ng of fungal DNA (pooled from three replicates) from amplification products of 380–400 bp (ITS3GC/ITS4) or of bacterial DNA (pooled from three replicates) from amplification products of 200 bp (338GC/518) were loaded on 8 % (w/v) polyacrylamide gel in 1× TAE with a denaturing gradient from 30 to 70 % or from 45 to 75 %, respectively (100 % denaturant corresponds to 40 % formamide and 7 M urea). The gels were run at 55 V and 56 °C for 16 h and stained with 1× of GelStar (Lonza, Switzerland) for 10 min. The gel images were captured under UV light in a gel documentation system (GenoSmart, VWR International, PA, USA). A marker was prepared by mixing equal amounts of DNA of four aquatic hyphomycete species (AT, Articulospora tetracladia Ingold UMB-748.11; TC, Tricladium chaetocladium Ingold UMB-523.10; TE, Tetrachaetum elegans Ingold UMB-522.10; and TS, Tricladium splendens Ingold UMB-767.11) or DNA of five bacterial species (BC, Bacillus cereus Frankland & Frankland; PV, Proteus vulgaris Hauser; SA, Staphylococcus aureus Rosenbach; PP, Pseudomonas putida (Trevisan) Migula; and EC, Escherichia coli (Migula) Castellani & Chalmers). DGGE bands appearing at the same position on the gel were considered as the same operational taxonomic unit (OTU).
Fungal Conidial Production
One set of five leaf discs was used to induce conidial production by aquatic hyphomycetes. Leaf discs were incubated in 100-mL Erlenmeyer flasks with 25 mL of filtered stream water from the corresponding stream half (47 mm diameter, pore size 0.7 μm; Millipore APFF04700, Millipore Corporation, MA, USA). The flasks were incubated over 48 h in an orbital shaker (100 rpm) in a room acclimated at 15 °C and with a 12-h light/12-h dark photoperiod. The conidial suspensions were poured into 50-mL Falcon tubes, fixed with 2 mL of 37 % formalin, and the final volume adjusted to 35 mL with distilled water. When preparing filters for conidium identification and counting, 150 μL Triton X-100 (0.5 %) was added to the suspension, mixed with a magnetic stirring bar to ensure uniform distribution of conidia, and 1–5 mL of the suspension were filtered through membrane filters (2.5 mm diameter, pore size 5 μm; Millipore SMWP, Millipore Corporation, MA, USA). Filters were stained with 0.05 % trypan blue in 60 % lactic acid, and conidia were identified and counted under a compound microscope at ×320 magnification [48, 49].
Microbial Productivity
Fungal production was determined from rates of [1-14C]acetate incorporation into ergosterol [50, 51], using a conversion factor of 19.3 μg of fungal biomass per nanomole of acetate incorporated [50]. Two sets of six leaf disks from each replicate bag were transferred to 25-mL Erlenmeyer flasks containing 4 mL of filtered and sterilized stream water and incubated for 1 h at the temperature corresponding to the respective stream half, before the assay. In one set, microorganisms were killed by adding 200 μL of 37 % formaldehyde 30 min before the incubation with [1-14C]acetate to determine the background level of radioactivity. The reaction started by the addition of sodium [1-14C]acetate to a final concentration of 2.5 mM (specific activity, 48 MBq mmol−1, Amersham). Incubations were carried out for 2 h at the respective temperature on a shaker (80 rpm), and the uptake stopped by adding 200 μL of 37 % formaldehyde. The flask contents were filtered through glass microfiber filters (GF/C, Whatman), and leaf disks washed twice with 4 mL of cold deionized water and placed in vials containing 0.8 % KOH-methanol. Ergosterol was extracted according to Gessner and Newell [51]. Briefly, lipids were (i) extracted from sets of six leaf disks by heating for 80 °C during 30 min in 0.8 % KOH-methanol; (ii) purified by solid-phase extraction (Sep-Pak, Waters, Milford, MA, USA); and (iii) quantified at 262 nm by high-performance liquid chromatography (HPLC; Beckman Gold System, Brea, CA, USA) on a C18 column (15 × 0.40 cm, Merck KGaA, Darmstadt, Germany) with methanol as the mobile phase. The ergosterol fractions of two HPLC injections (100 μL sample loop) from each replicate were pooled into a vial containing 10 mL of scintillation fluid (Optiphase Hisafe 2, Perkin-Elmer, MA, USA) and stored overnight before measuring the radioactivity on ergosterol fractions (Packard Tri-Carb 2200, CA, USA).
Bacterial production was determined from incorporation rates of L-[4,5-3H]leucine into protein [52]. Three sets of four leaf disks per replicate were put into screw-top tubes containing 4 mL of filter-sterilized stream water for 1 h at each respective temperature before the assay. In one set, microorganisms were killed by the addition of 500 μL of 40 % trichloroacetic acid (TCA) to determine the background level of radioactivity. In another set, the potential eukaryotic incorporation of leucine was controlled by the addition of 10 μL of 8 % cycloheximide and 5 μL of 8 % colchicine 1 h before the addition of radiolabeled leucine. The third set was used to estimate bacterial production in the samples. Reactions started with the addition of L-[4,5-3H]leucine (final concentration of 400 nM; specific activity, 142 GBq mmol−1, Amersham) and incubated at each temperature for 30 min with gentle mixing every 10 min. The reaction was stopped by adding 500 μL of 40 % TCA. Bacterial cells were dislodged from leaf disks by sonication in a bath (model 2510, Branson, Danbury, CT, USA) for 5 min, and bacterial protein was extracted by heating in a water bath at 95 °C for 30 min. The contents of each tube were filtered through polycarbonate filters (0.2 μm, GTTP, Millipore Corporation, MA, USA), and leaf disks were washed twice with 4 mL of cold deionized water and placed into scintillation vials, and radioactivity counted as described above. Bacterial production (BP) was calculated by the method of Kirchman [52] as BP = (L × F × C) / (P × D), where L is the incorporation rate of L-[4,5-3H]leucine, F is the formula weight of leucine, C is the ratio of cellular carbon to protein (0.86), P is the fraction of leucine in protein (0.073), and D is the mass of leaf disks.
Data Treatment
For each year (ambient and warmed), two-way analyses of variance (ANOVA) were used to test if season and stream half significantly affected stream water variables. The relationships between environmental variables were examined using Pearson correlation coefficients [53].
DGGE gels were aligned and normalized. A DGGE band was considered an OTU, ignoring the fact that DNA from more than one species can co-migrate to the same position in the gel. The relative intensities of bands in the fingerprints were analyzed in BioNumerics 7.1 (Applied Maths, Sint-Martens-Latem, Belgium).
For each year, differences in fungal (based on conidia or DGGE OTUs) and bacterial communities (based on DGGE OTUs) between stream halves and seasons were assessed by non-metric multidimensional scaling (NMDS) based on the Bray-Curtis index [54]. Data were square root transformed prior to construction of similarity matrices. Two-way PERMANOVAs were used to assess if season and stream half affected fungal and bacterial community structures [55].
Repeated measures ANOVAs, with time as repeated factor, were used to test if season and stream half significantly affected bacterial and fungal production in the ambient and warmed year [53]. Tukey’s post-tests were used to determine where significant differences occurred [53].
Kolmogorov-Smirnov’s tests for normality and Levene’s tests for homogeneity of variances were applied to physical and chemical variables of stream water and to fungal and bacterial productivity data [53].
Leaf mass remaining was fit to the negative exponential model W t = W 0 × e − kt, where W t is leaf mass remaining at time t, W 0 is the initial mass, and k is the rate of leaf decomposition. Leaf decomposition rates were compared by ANCOVA with time as continuous variable and season and stream half as categorical variables, followed by Tukey’s post-tests [53]. NMDS and PERMANOVA analyses were done with PRIMER v6 software package for windows (Primer-E Ltd, Plymouth, UK). ANOVAs and ANCOVAs were done with Statistica 8.0 (StatSoft, Inc., Tulsa, OK, USA).
Results
Physical and Chemical Stream Water Characterization
Physical and chemical variables of the stream water during the study are shown in Table 1. In the ambient year, conductivity (26.2–28.6 μS cm−1), discharge (2.3–3.0 L s−1), alkalinity (4.0–4.9 mg CaCO3 L−1), and nitrogen concentration (31.2–40.3 μg L−1) did not differ between stream halves and seasons (two-way ANOVA, F ≤ 2.0, P ≥ 0.2). On the other hand, dissolved oxygen concentration (8.1–12.7 mg L−1) and water temperature (9.8–11.1 °C) were higher in spring than in autumn (two-way ANOVA, F ≤ 85.7, P < 0.0001; Tukey post-tests, P < 0.001), while pH (6.7–7.8) was higher in autumn than in spring (two-way ANOVA, F = 106.7, P < 0.0001; Tukey post-test, P = 0.0001) and no differences were found between stream halves for all parameters (two-way ANOVA, F ≤ 2.7, P ≥ 0.1) (Table S1).
In the warmed year, pH (7.2–7.3) and dissolved oxygen (9.0–9.7 mg L−1) were similar between stream halves and seasons (two-way ANOVA, F ≤ 0.8, P ≥ 0.4). In contrast, conductivity (25.2–27.4 μS cm−1), temperature (9.7–14.8 °C), and SRP concentration (2.7–22.7 μg L−1) decreased, whereas discharge (1.7–2.6 L s−1), alkalinity (4.6–6.1 mg CaCO3 L−1), and nitrogen concentration (16.8–89.3 μg L−1) increased from spring to autumn (two-way ANOVA, F ≥ 7.7, P ≤ 0.009; Tukey post-tests, P < 0.009). In addition, temperature differed between stream halves (two-way ANOVA, F ≥ 12.9, P ≤ 0.001), increasing 2.3 (autumn) and 3.0 °C (spring) from the control to the experimental half (Tukey’s post-test, P < 0.001). Conductivity was also higher in the experimental than the control half in spring (Tukey’s post-test, P = 0.005).
Stream water temperature was positively correlated with conductivity both before and during warming (Pearson’s, r = 0.91 and r = 0.68, respectively, P < 0.05). Positive correlations were also found between temperature and nitrogen concentrations in the ambient year (Pearson’s, r = 0.60, P < 0.05). On the other hand, negative correlations were found between temperature and discharge in the ambient year (Pearson’s, r = − 0.88, P < 0.05) and between temperature and oxygen, temperature and nitrogen, and temperature and alkalinity in the warmed year (Pearson’s, r = −0.56, r = −0.58, and r = −0.69, respectively, P < 0.05).
Microbial Diversity
DNA fingerprints of fungal communities on decomposing oak leaves decreased from 39 to 33 OTUs, from the ambient to the warmed year (Fig. 1). In both years, the maximum number of OTUs increased from spring to autumn: 19 (control half) to 23 fungal OTUs (experimental half) in the ambient year (Fig. 1a) and 20 (control half) to 22 fungal OTUs (experimental half) in the warmed year (Fig. 1c). On the other hand, an increase from a total of 17 to 20 fungal taxa sporulating from leaves was found from the ambient to the warmed year (Fig. 2). In the ambient year, a slight increase from a maximum of 11 (control half) to 13 fungal taxa (control half) was found from spring to autumn (Fig. 2a), while in the warmed year a slight decrease from a maximum of 12 (control half) to 10 species (experimental half) was found from spring to autumn (Fig. 2c). The two dominant contributors to total conidium production regardless of stream half, season, or year, were Tetrachaetum elegans (up to 93 %) and Tricladium chaetocladium (up to 79 %) (Fig. 2a, c).
Denaturing gradient gel electrophoresis (DGGE) fingerprints of fungal communities (a, c) and NMDS ordination diagrams based on fungal communities assessed from DGGE OTUs (b, d), during 60 days of immersion in the control and the experimental half of the Candal stream in spring and autumn at the ambient and the warmed year. M mixture of DNA of four fungal species, AT Articulospora tetracladia, TC Tricladium chaetocladium, TE Tetrachaetum elegans, TS Tricladium splendens
Percentage contribution of aquatic hyphomycete species to total conidial production (a, c) and NMDS ordination diagrams based on fungal communities assessed from conidia released from oak leaves (b, d), during 60 days of immersion in the control and experimental half of the Candal stream in spring and autumn of the ambient and the warmed year. ALAC Alatospora acuminata Ingold, ANFI Anguillospora filiformis Greath., ANFU Anguillospora furtiva Webster & Descals, ARTE Articulospora tetracladia, CASP Casaresia sphagnorum Gonz., CLAQ Clavariopsis aquatica De Wild., CLLO Clavatospora longibrachiata (Ingold) Marvanová & Sv. Nilsson, CUAQ Culicidospora aquatica R. H. Petersen, FLCU Flagellospora curvula Ingold, GOMO Goniopila monticola (Dyko) Marvanová & Descals, HELU Heliscus lugdunensis Sacc. & Thérry, LAUN Lateriramulosa uni-infata Matsush., LEAL Lemonniera alabamensis R.C. Sinclair & Morgan-Jones, LUCU Lunulospora curvula Ingold, MYCA Mycofalcella calcarata Marvanová, STNE Stenocladiella neglecta (Marvanová & Descals) Marvanová & Descals, TEEL Tetrachaetum elegans, TRCH Tricladium chaetocladium, TRSP Tricladium splendens, TRAC Triscelophorus acuminatus Nawawi, TRMO T. monosporus Ingold
DNA fingerprints of bacterial communities on decomposing oak leaves increased from a total of 38 to 47 OTUs from the ambient to the warmed year (Fig. 3). In the ambient year, an increase from a maximum of 19 (experimental half) to 25 bacterial OTUs (both halves) was found from spring to autumn (Fig. 3a), while in the warmed year the opposite was found: a decrease from a maximum of 31 (experimental half) to 27 OTUs (control half) from spring to autumn (Fig. 3c).
Denaturing gradient gel electrophoresis (DGGE) fingerprints of bacterial communities (a, c) and NMDS ordination diagrams of bacterial communities based on DGGE OTUs on oak leaves (b, d) during 60 days of immersion in the control and the experimental half of the Candal stream in spring and autumn of the ambient and the warmed year. M mixture of five bacterial species, BC Bacillus cereus, PV Proteus vulgaris, SA Staphylococcus aureus, PP Pseudomonas putida, EC Escherichia coli
The NMDS ordination diagrams of fungal communities based on OTUs (Fig. 1b, d) or conidia (Fig. 2b, d) and of bacterial communities based on DGGE OTUs (Fig. 3b, d) indicated that season significantly affected community structure (PERMANOVA, P ≤ 0.03) while no differences were found between stream halves (PERMANOVA, P ≥ 0.7), in both years.
Microbial Productivity
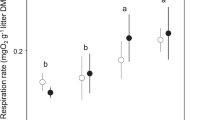
Fungal production attained similar maxima between seasons and stream halves in both years: 2.2 mg C g−1 day−1 in spring (experimental half) and 2.1 mg C g−1 day−1 in autumn (experimental half) in the ambient year (Fig. 4a, b), and 2.8 mg C g−1 day−1 in spring (control half) and 2.5 mg C g−1 day−1 in autumn (control half) in the warmed year (Fig. 4d, e). Bacterial production was low (Fig. 5) and decreased from spring to autumn in both years: 0.056 mg C g−1 day−1 (experimental half) to 0.045 mg C g−1 day−1 (control half), in the ambient year (Fig. 5a, b) and 0.027 mg C g−1 day−1 (experimental half) to 0.016 mg C g−1 day−1 (experimental half), in the warmed year (Fig. 5d, e).
Bacterial production associated with oak leaves during 60 days of immersion in the control and the experimental half of the Candal stream in spring (a, d) and autumn (b, e) and average values for each treatment (c, f) at the ambient and the warmed year. Mean ± SEM, n = 3 (a, b, d, e) and n = 15 (c, f)
No significant differences were found between stream halves and seasons for fungal (two-way ANOVA, F ≤ 2.4, P ≥ 0.2; Fig. 4c) and bacterial production (two-way ANOVA, F ≤ 0.9, P ≥ 0.4; Fig. 5c), in the ambient year. During warming, productivity was higher in spring than in autumn for fungi in both stream halves (Tukey post-test, P = 0.02) and for bacteria in the experimental half (Tukey’s post-test, P = 0.03). No significant differences in production were found between stream halves for fungi (two-way ANOVA, F = 0.4, P = 0.5; Fig. 4f) and bacteria (two-way ANOVA, F = 0.02, P = 0.9; Fig. 5f).
Leaf Decomposition
In the ambient year, leaf mass loss was highest in the experimental half in autumn (40 %) (Fig. 6), but decomposition rates did not significantly differ between treatments (Fig. 6c; ANCOVA, F = 0.8, P = 0.5). In the warmed year, in spring, loss of initial mass was 1.4 times higher in the experimental (50 %) than in the control stream half (32 %) (Fig. 6d), which translated into significant differences in leaf decomposition rates between stream halves (Fig. 6f; ANCOVA, F = 6.2, P = 0.02). In autumn, leaves lost 40 % of their initial mass at both control and experimental halves (Fig. 6e) and leaf decomposition rates did not differ between stream halves (Fig. 6f; ANCOVA, F = 1.2, P = 0.3). In addition, leaf decomposition rate in the experimental half in spring was significantly higher (1.4 times) than in the control half in autumn (Fig. 6f; ANCOVA, F = 7.9, P = 0.009).
Dry mass remaining of oak leaves during 60 days of immersion in the control and the experimental half of the Candal stream in spring (a, d) and autumn (b, e) and decomposition rates for each treatment, in the ambient and the warmed year (c, f). Mean ± SEM, n = 3 (a, b, d, e) and n = 15 (c, f). Dry mass remaining was fitted to the exponential decay model
Discussion
In a stream manipulation experiment, seasonal temperature increases had a greater impact on microbial decomposers than experimental warming of similar magnitude (ca. 3 °C). The approach adopted here allows more realistic predictions of the effects of warming on stream microbial community structure and functioning, when compared with microcosms [e.g., 15, 17–19, 56] or correlative field studies along natural thermal gradients [24, 28, 30]. Microbial decomposers of plant litter are widely recognized as key mediators of carbon (C) and energy flow in freshwaters [4, 9]. Thus, understanding how global change will affect microbial diversity and activity in a more realistic scenario is crucial for freshwater ecologists attempting to forecast changes in biodiversity and key ecosystem processes.
Do Differences in Temperature due to Season and Warming Induce Shifts in the Structure of Decomposer Communities?
Decomposing oak leaves harbored diverse aquatic fungal and bacterial communities regardless of year, seasons, and stream half, as indicated by the numbers of OTUs from DNA fingerprints and taxon richness. Both were similar to those found in previous studies using molecular approaches [57–60]. Fungal species diversity assessed from conidia followed the same pattern before or during warming and was lower than that based on DGGE OTUs. This was expected since DGGE also detects non-sporulating fungal species [57, 58, 60] or non-cultivable bacteria [58–61], giving more accurate results about the true diversity of environmental samples than the traditional approaches.
Fungal and bacterial richness was similar between seasons, but community structure differed between spring and autumn both before and during warming. In temperate regions, seasonal changes in water temperature have been suggested as a main driver of the structure of fungal communities on decomposing plant litter [57, 62, 63]. On the other hand, the experimental warming did not produce any effects on microbial community structure both in spring and autumn. This agrees with a previous study where no effects on fungal sporulating community structure were found with a 3 °C temperature increase, suggesting that warming stimulated most fungal species to a similar degree [64]. However, marked changes in fungal sporulating community have been found with warming of 5 to 10 °C [15, 17, 18, 34]. Greater temperature increases (>3 °C) may favor warm-water species, such as Lunulospora curvula [15, 16, 64] (present in the current study in spring), and inhibit cold-water species, such as Flagellospora curvula or Anguillospora filiformis (present in the current study in autumn in the warmed year). Differences in fungal communities/assemblages and their metabolic rates can alter leaf quality at warmer temperatures [15–17, 21, 65] and therefore affect the detritivore performance and global C cycle of the coupled stream detritus foodwebs [66, 67].
In our study, the lack of an effect of experimental warming on microbial community structure suggests that seasonal changes in water temperature in combination with other environmental factors, in particular the phosphorus levels, may have contributed more to structure microbial communities on leaves than the experimental warming. The trophic status of a stream, defined by nitrogen or phosphorus concentrations, can determine the structure of microbial communities on plant litter [e.g., 60]. Temperature affects most life processes, e.g., the growth rates and life cycles of aquatic biota, and the productivity of the entire system [2]. Therefore, nutrient alterations in stream water may also have structured communities of microbial decomposers of plant litter, as suggested earlier [18].
To our knowledge, the current study is the first attempt to compare the effects of season and warming on leaf-associated bacterial diversity in temperate streams. Data from other biomes, such as arctic lakes [68] or littoral freshwater marshes [69], suggest that increases in temperature can lead to pronounced shifts in the composition of bacterial communities.
Do Warmer Temperatures Increase Microbial Activity and Leaf Litter Decomposition?
In the ambient year, no differences were found in leaf mass loss or microbial productivities between stream halves, which was expected since both halves were at the same temperature. Microbial communities differed between spring and autumn, but these differences were not accompanied by significant alterations in microbial activity. The small variation in temperature between spring and autumn (ca. 1 °C higher in spring) most likely explained the lack of differences in microbial activity in the ambient year between seasons, as previously reported [70, 71].
Although we would expect an increase in the contribution of bacteria to litter decomposition with temperature increase [72], fungal C production was greater than that of bacteria in both years, seasons, and stream halves and slightly increased with temperature. Fungal production exceeded bacterial production 132-fold in spring and 84-fold in autumn in the ambient year and 201-fold in spring and 232-fold in autumn in the warmed year, which agrees with previous reports [9, 50, but see 72], suggesting that even under warming conditions, fungi are the main contributors to litter decomposition. Accordingly, most studies reported a stimulation of fungal biomass and activity with increasing temperature [14–20].
On the other hand, in the warmed year, leaf mass loss and microbial productivity were on average higher in spring than in autumn, particularly in the experimental half in spring. An overall increase in microbial metabolic rates generally accelerates microbial-mediated leaf decomposition [50, 65, 73, 74]. Our results supported this trend since during warming the seasonal increase in temperature stimulated microbial activity and led to faster leaf decomposition in the experimental half (2.8 °C higher in spring than in autumn), but not in the control half (2.1 °C higher in spring than in autumn). In addition, the increase in temperature, simulating global warming in the experimental half, stimulated leaf litter decomposition in spring (3 °C higher in the experimental than in the control half), but not in autumn (2.3 °C higher in the experimental half). The low levels of phosphorus found in the stream water in autumn in the warmed year might help explain the limited response of litter decomposition to temperature increase in this season. Litter decomposition is usually lower under oligotrophic conditions [75, 76], and the effect of warming might have been limited by the low nutrient availability [18]. For instance, fungal biomass and sporulation rates increased with temperature (up to 15 °C) at low (up to 0.01 mg L−1 PO4-P and 1.39 mg NO3-N L−1) and high nutrient levels (up to 0.10 mg L−1 PO4-P and 13.86 mg NO3-N L−1), but leaf decomposition was stimulated with increased temperature only at high nutrient levels [18]. Thus, if nutrient levels had been high in both seasons in the warmed year, we would have expected a stimulation of microbial activity and leaf decomposition in spring due to increased temperature.
An opposite trend was found by Ferreira and Canhoto [36, 37]: a stimulation of litter decomposition was found only in the coolest (ambient temperature of 6.4 °C in winter), but not in the warmest months (ambient temperature of 11.8 °C in spring and 9.7 °C in autumn). However, as in our study, the effects of temperature on litter decomposition, within each season, were not directly explained by the response patterns of microbial variables (fungal biomass accrual and sporulation rates) [37].
A 3 °C experimental increase led to higher prokaryote and chlorophyll density and alterations in bacterial biofilm functional composition [38]. But the use of organic compounds was affected differentially: under warming conditions, biofilms had a higher capacity to use cellulose, hemicellulose, lignin, and peptidic compounds, but a lower capacity to degrade lipids [38]. The effects of temperature on bacterial communities were also complex in arctic lakes and streams in northern Alaska, but strongly influenced bacterial productivity and community composition [68].
In our study, increased temperature in the experimental half during the warmed year could have promoted leaf litter leaching [e.g., 30, 77] or stimulated enzymatic activities [78] and accelerated decomposition without enhancing microbial productivities in spring, as initially expected. Phosphorus concentrations, which are reported to accelerate microbial decomposition [8, 9, 60], were higher in spring than in autumn, in particular in the control half of the stream, which could have dampened differences in microbial activity between treatments [66]. Temperature and dissolved nutrients, which are predicted to increase concomitantly with global change [79], may act synergistically on litter decomposition and associated fungi [18, 80], and the results found in the warmed year lend support to this. Differences in temperature between seasons were small in both years (1.1–1.2 times higher in spring than in autumn, for both stream halves), but phosphorus concentrations were higher in the warmed (8.4 and 2.9 times higher at the control and experimental half, respectively) than in the ambient year in spring (2.6 and 1.8 times higher at control and experimental half, respectively), where an overall stimulation of microbial activity and leaf decomposition was found. Although the slight differences in nutrient concentrations between seasons in the warmed year may have contributed to differences in microbial activity and leaf decomposition between seasons, these concentrations are still low when compared with nutrient-enriched streams [9, 60, 75, 76]. Therefore, the effects of warming on litter decomposition might be exacerbated under eutrophication scenarios [18, 29, 56] leading to a faster disappearance of litter from the stream bed and to a mismatch between consumers and their resources [81]. In addition, if faster leaching occurs in a warmer environment [77], litter conditioning might be stimulated, increasing the availability of high-quality substrate for detritivores but further compromising the functioning of detritus foodwebs if substrates are consumed faster. We must keep in mind, however, that the warming period in our study was restricted to 1 year. Stronger effects of small increases in temperature may be found in streams experiencing longer warming periods and supplied with higher quality litter (e.g., Alnus glutinosa (L.) Gaertn.), where the faster decomposition may lead to food depletion for aquatic invertebrates during part of the year, until the following litter fall [64].
Take-Home Message
A ca. 3 °C temperature increase stimulated decomposition of oak leaves in spring, but this was not accompanied by alterations in the structure of microbial communities or by increased microbial activity. The increase in water temperature due to global warming may stimulate litter decomposition resulting in reduced quantities of benthic organic matter available for stream-dwelling organisms. We should be aware that temperature differences such as those found between the two seasons (up to 2.8 °C) can readily occur due to riparian vegetation alterations, as a result from agricultural or forestry activities (e.g., tree removal from the stream margins) [2, 82]. In fact, the effects of alterations in riparian vegetation on litter decomposition in streams are expected to be more pronounced than the effects of a temperature increase of up to 3 °C [64]. Nevertheless, we speculate that microbial diversity and activity will be affected when global change pushes temperature beyond the natural seasonal differences of 3 °C. Additional effects on ecosystem processes may arise due to concomitant changes in other environmental factors, such as nutrient availability in the stream water.
References
IPCC (2014) Summary for policymakers. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (eds) Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Allan JD, Castillo MM (2007) Stream ecology: structure and function of running waters. Springer, Dordrecht
Stefan HG, Sinokrot BA (1993) Projected global climate change impact on water temperatures in five North Central U.S. streams. Clim Change 24:353–381
Suberkropp K (1998) Microorganisms and organic matter decomposition. In: Naiman R, Bilby RE (eds) River ecology and management: lessons from the pacific coastal ecoregion. Springer, New York, pp 120–143
Bärlocher F (2005) Freshwater fungal communities. Taylor and Francis, CRC Press, Boca Raton, Florida
Chamier A-C, Dixon PA (1982) Pectinases in leaf degradation by aquatic hyphomycetes: the enzymes and leaf maceration. J Gen Microbiol 128:2469–2483
Gessner MO, Gulis V, Kuehn KA, Chauvet E, Suberkropp K (2007) Fungal decomposers of plant litter in aquatic ecosystems. In: Kubicek CP, Druzhinina IS (eds) The mycota: environmental and microbial relationships, vol IV, 2nd edn. Springer, Berlin, pp 301–321
Gulis V, Suberkropp K (2003) Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshw Biol 48:123–134
Pascoal C, Cássio F (2004) Contribution of fungi and bacteria to leaf litter decomposition in a polluted river. Appl Environ Microbiol 70:5266–5273
Bärlocher F, Kendrick B (1975) Leaf-conditioning by microorganisms. Oecologia 20:359–362
Graça MAS, Cressa C (2010) Leaf quality of some tropical and temperate tree species as food resource for stream shredders. Int Rev Hydrobiol 95:27–41
Chauvet E, Suberkropp K (1998) Temperature and sporulation of aquatic hyphomycetes. Appl Environ Microbiol 64:1522–1525
Duarte S, Fernandes I, Nogueira M-J, Cássio F, Pascoal C (2013) Temperature alters interspecific relationships among aquatic fungi. Fungal Ecol 6:187–191
Dang CK, Schindler M, Chauvet E, Gessner MO (2009) Temperature oscillation coupled with fungal community shifts can modulate warming effects on litter decomposition. Ecology 90:122–131
Fernandes I, Uzun B, Pascoal C, Cássio F (2009) Responses of aquatic fungal communities on leaf litter to temperature-change events. Int Rev Hydrobiol 94:410–418
Fernandes I, Pascoal C, Guimarães H, Pinto R, Sousa I, Cássio F (2012) Higher temperature reduces the effects of litter quality on decomposition by aquatic fungi. Freshw Biol 57:2306–2317
Ferreira V, Chauvet E (2011) Future increase in temperature more than decrease in litter quality can affect microbial litter decomposition in streams. Oecologia 167:279–291
Ferreira V, Chauvet E (2011) Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Glob Change Biol 17:551–564
Batista D, Pascoal C, Cássio F (2012) Impacts of warming on aquatic decomposers along a gradient of cadmium stress. Environ Pollut 169:35–41
Geraldes P, Pascoal C, Cássio F (2012) Effects of increased temperature and aquatic fungal diversity loss on litter decomposition. Fungal Ecol 5:734–740
Gonçalves AL, Graça MAS, Canhoto C (2013) The effect of temperature on leaf decomposition and diversity of associated aquatic hyphomycetes depends on the substrate. Fungal Ecol 6:546–553
Drake JA, Huxel GR, Hewitt CL (1996) Microcosms as models for generating and testing community theory. Ecology 77:670–677
Fraser LH (1999) The use of microcosms as an experimental approach to understanding terrestrial ecosystem functioning. Adv Sp Res 24:297–302
Fabre E, Chauvet E (1998) Leaf breakdown along an altitudinal stream gradient. Arch Hydrobiol 141:167–179
Taylor BR, Chauvet E (2014) Relative influence of shredders and fungi on leaf litter decomposition along a river altitudinal gradient. Hydrobiologia 721:239–250
Irons JG, Oswood MW, Stout RJ, Pringle CM (1994) Latitudinal patterns in leaf litter breakdown: is temperature really important? Freshw Biol 32:401–411
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Boyero L, Pearson RG, Gessner MO, Barmuta LA, Ferreira V, Graça MAS et al (2011) A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecol Lett 14:289–294
Friberg N, Christensen JB, Olafsson JS, Gislason GM, Larsen SE, Lauridsen TL (2009) Relationship between structure and function in streams contrasting in temperature: possible impacts of climate change on running water ecosystems. Freshw Biol 54:2051–2068
Chergui H, Pattee E (1990) The influence of season on the breakdown of submerged leaves. Arch Hydrobiol 120:1–12
Swan CM, Palmer MA (2004) Leaf diversity alters litter breakdown in a Piedmont stream. J N Am Benthol Soc 23:15–28
Woodward G, Dybkjaer JB, Ólafsson JS, Gíslason GM, Hannesdóttir ER, Friberg N (2010) Sentinel systems on the razor’s edge: effects of warming on Arctic geothermal stream ecosystems. Glob Change Biol 16:1979–1991
Hogg ID, Williams DD (1996) Response of stream invertebrates to a global-warming thermal regime: an ecosystem-level manipulation. Ecology 77:395–407
Bärlocher F, Seena S, Wilson KP, Williams DD (2008) Raised water temperature lowers diversity of hyporheic aquatic hyphomycetes. Freshw Biol 53:368–379
Canhoto C, de Lima J, Traça de Almeida A (2013) Warming up a stream reach: design of a hydraulic and heating system. Limnol Oceanogr Methods 11:410–417
Ferreira V, Canhoto C (2014) Effect of experimental and seasonal warming on litter decomposition in a temperate stream. Aquat Sci 76:155–163
Ferreira V, Canhoto C (2015) Future increase in temperature may stimulate litter decomposition in temperate mountain streams: evidence from a stream manipulation experiment. Freshw Biol 60:881–892
Ylla I, Canhoto C, Romaní AM (2014) Effects of warming on stream biofilm organic matter use capabilities. Microbiol Ecol 68:132–145
Miranda P, Coelho FES, Tomé AR, Valente MA (2002) 20th century Portuguese climate and climate scenarios. In: Santos FD, Forbes K, Moita R (eds) Climate change in Portugal. Scenarios, impacts and adaptation measures. SIAM project, Gradiva Publications, Lda, Lisbon, pp 23–83
Ferreira V, Encalada AC, Graça MAS (2012) Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshw Sci 31:945–962
Pozo J, González E, Díez JR, Molinero J, Elósegui A (1997) Inputs of particulate organic matter to streams with different riparian vegetation. J North Am Benthol Soc 16:602–611
González E, Pozo J (1996) Longitudinal and temporal patterns of benthic coarse particulate organic matter in the Agüera stream (northern Spain). Aquat Sci 58:355–366
Morrill JC, Bales RC, Conklin MH (2005) Estimating stream temperature from air temperature: implications for future water quality. J Environ Eng 131:131–139
Gore JA (1996) Discharge measurements and stream flow analysis. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology. Academic press, New York, pp 53–74
APHA (1995) Standard methods for the examination of water and watershed. American Public Health Association, Washington DC
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press Inc, New York, pp 315–322
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Bärlocher F (2005) Sporulation by aquatic hyphomycetes. In: Graça MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition: a practical guide. Springer, Dordrecht, pp 185–188
Gulis V, Marvanová L, Descals E (2005) An illustrated key to the common temperate species of aquatic hyphomycetes. In: Graça MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition: a practical guide. Springer, Dordrecht, pp 153–168
Suberkropp K, Weyers H (1996) Application of fungal and bacterial production methodologies to decomposing leaves in streams. Appl Environ Microbiol 62:1610–1615
Gessner MO, Newell SY (2002) Biomass, growth rate, and production of filamentous fungi in plant litter. In: Hurst CJ, Crawford RL, Knudsen C, McIerney M, Stetzenbach LD (eds) Manual of environmental microbiology, 2nd edn. ASM Press, Washington DC, pp 390–408
Kirchman DL (1993) Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, pp 509–512
Zar JH (2010) Biostatistical analysis. Pearson Prentice-Hall, Upper Saddle River
Kruskal JB, Wish M (1978) Multidimensional scaling. Sage Publications, Beverley Hills
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Fernandes I, Seena S, Pascoal C, Cássio F (2014) Elevated temperature may intensify the positive effects of nutrients on microbial decomposition in streams. Freshw Biol 59:2390–2399
Nikolcheva LG, Bärlocher F (2005) Seasonal and substrate preferences of fungi colonizing leaves in streams: traditional versus molecular evidence. Environ Microbiol 7:270–280
Duarte S, Pascoal C, Alves A, Correia A, Cássio F (2010) Assessing the dynamic of microbial communities during leaf decomposition in a low-order stream by microscopic and molecular techniques. Microbiol Res 165:351–362
Das M, Royer TV, Leff LG (2007) Diversity of fungi, bacteria, and actinomycetes on leaves decomposing in a stream. Appl Environ Microbiol 73:756–767
Duarte S, Pascoal C, Garabétian F, Cássio F, Charcosset J-Y (2009) Microbial decomposer communities are mainly structured by trophic status in circumneutral and alkaline streams. Appl Environ Microbiol 75:6211–6221
Muyzer G, Brinkoff T, Nübel U, Santegoeds C, Schäfer H, Wawer C (2004) Denaturing gradient gel electrophoresis (DGGE) in microbial ecology. In: Kowalchuk GA, de Bruijn FJ, Head IM, Akkermans ADL, van Elsas JD (eds) Molecular microbial ecology manual. Kluwer Academic Publishers, The Netherlands, pp 743–770
Suberkropp K (1984) The effect of temperature on the seasonal occurrence of aquatic hyphomycetes. Trans Br Mycol Soc 82:53–62
Bärlocher F (2000) Water-borne conidia of aquatic hyphomycetes: seasonal and yearly patterns in Catamaran Brook, New Brunswick, Canada. Can J Bot 78:157–167
Ferreira V, Chauvet E, Canhoto C (2015) Effects of experimental warming, litter species, and presence of macroinvertebrates on litter decomposition and associated decomposers in a temperate mountain stream. Can J Fish Aquat Sci 72:206–216
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Towards a metabolic theory of ecology. Ecology 85:1771–1789
Mas-Martí E, Muñoz I, Oliva F, Canhoto C (2015) Effects of increased water temperature on leaf litter quality and detritivore performance: a whole-reach manipulative experiment. Freshw Biol 60:184–197
Lecerf A, Dobson M, Dang CK, Chauvet E (2005) Riparian plant species loss alters trophic dynamics in detritus-based stream ecosystems. Oecologia 146:432–442
Adams HE, Crump BC, Kling GW (2010) Temperature controls on aquatic bacterial production and community dynamics in arctic lakes and streams. Environ Microbiol 12:1319–1333
Flury S, Gessner MO (2011) Experimentally simulated global warming and nitrogen enrichment effects on microbial litter decomposers in a marsh. Appl Environ Microbiol 77:803–809
Finlay BJ, Maberly SC, Cooper JI (1997) Microbial diversity and ecosystem function. Oikos 80:209–213
Verma B, Robarts RD, Headley JV (2003) Seasonal changes in fungal production and biomass on standing dead Scirpus lacustris litter in a Northern prairie wetland. Appl Environ Microbiol 69:1043–1050
Buesing N, Gessner MO (2006) Benthic bacterial and fungal productivity and carbon turnover in a freshwater marsh. Appl Environ Microbiol 72:596–605
Acuña V, Wolf A, Uehlinger U, Tockner K (2008) Temperature dependence of stream benthic respiration in an Alpine river network under global warming. Freshw Biol 53:2076–2088
Young RG, Matthaei CD, Townsend CR (2008) Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. J N Am Benthol Soc 27:605–625
Gulis V, Ferreira V, Graça MAS (2006) Stimulation of leaf litter decomposition and associated fungi and invertebrates by moderate eutrophication: implications for stream assessment. Freshw Biol 51:1655–1669
Woodward G, Gessner MO, Giller PS, Gulis V, Hladyz S, Lecerf A et al (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336:1438–1440
Park S, Cho KH (2003) Nutrient leaching from leaf litter of emergent macrophyte (Zizania latifolia) and the effects of water temperature on the leaching process. Korean J Biol Sci 7:289–294
Baldrian P, Šnajdr J, Merhautová V, Dobiášová P, Cajthaml T, Valášková V (2012) Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol Biochem 56:60–68
Murdoch PS, Baron JS, Miller TL (2000) Potential effects of climate change on surface-water quality in North America. J Am Water Res Assoc 36:347–366
Martínez A, Larrañaga A, Pérez J, Descals E, Pozo J (2014) Temperature affects leaf litter decomposition in low-order forest streams: field and microcosm approaches. FEMS Microbiol Ecol 87:257–267
Durant JM, Hjermann DØ, Ottersen G, Stenseth NC (2007) Climate and the match or mismatch between predator requirements and resource availability. Clim Res 33:271–283
Lecerf A, Richardson J (2010) Litter decomposition can detect effects of high and moderate levels of forest disturbance on stream condition. Forest Ecol Manag 259:2433–2443
Acknowledgments
We thank Ana Lírio and João Rosa for valuable help in the field, Amado & Amado Company for the efficacy and constant maintenance of the warming system, and the Lousã Town Hall for its collaboration and logistic support. We also want to thank two anonymous reviewers and Felix Bärlocher for the comments and suggestions made on an earlier version of the manuscript. This study was funded by IMAR-CMA, CBMA-UM, the European Fund for Economic and Regional Development (FEDER) through the Program Operational Factors of Competitiveness (COMPETE), and National Funds through the Portuguese Science and Technology Foundation (FCT) under the projects “Predicting the effect of global warming on stream ecosystems” (PTDC/CLI/67180/2006; FCOMP-01-0124-FEDER-007112) and “Development of molecular tools for assessing fungal diversity and activity in freshwaters” (PTDC/AAC-AMB/113746/2009; FCOMP-01-0124-FEDER-013954) and PEst-C/BIA/UI4050/2011. Financial support granted by the FCT to VF (SFRH/BPD/34368/2006 and SFRH/BPD/76482/2011, program POPH/FSE; IF/00129/2014) and SD (SFRH/BPD/47574/2008 and SFRH/BPD/109842/2015) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Duarte, S., Cássio, F., Ferreira, V. et al. Seasonal Variability May Affect Microbial Decomposers and Leaf Decomposition More Than Warming in Streams. Microb Ecol 72, 263–276 (2016). https://doi.org/10.1007/s00248-016-0780-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0780-2