Abstract
Fifty streams, located in southern Ontario, Canada, were visited in September 2008 to investigate the effect of varying land use, land cover, and associated resource inputs on water column bacterial abundance (BACT), production (BP), and extracellular enzyme activity and stoichiometry. Principle components analysis was used to summarize landscape data, producing three components (PCs), which explained 79.2% of the variability in the data. The PCs grouped into the following gradients: (PC1) urban land use and continuous annual cropping to wetland-like cover, (PC2) rotational cropping to forest-like cover, and (PC3) increasing rural and agricultural land uses with increasing watershed size. These landscape gradients created imbalanced resource availability. Nutrient resources were more abundant in streams with more intensive anthropogenic land uses, but carbon availability was primarily controlled by the abundance of natural land covers (wetland and wooded areas). BACT, BP, and enzyme activities were positively related primarily to nutrient availability and/or anthropogenic land use (Stepwise R 2 range: 0.33–0.73). The ratio of β-glucosidase to alkaline phosphatase activity approached a 1:1 balance with increasing anthropogenic land use, decreased wetland and forest cover, and increased total dissolved nitrogen. The ratio of leucine-aminopeptidase to alkaline phosphatase activity approached 1:1 with both increased dissolved organic carbon and nitrogen. Moreover, enzyme C:N:P ratios moved closer to 1:1:1 with faster water column bacterial turnover times. These results suggest that water column microbial communities are better able to balance resource availability with growth in streams receiving nutrient subsidies from anthropogenic sources and under these conditions when carbon resources increase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The landscape through which a stream flows strongly influences the carbon (C), nitrogen (N), and phosphorus (P) concentrations in its waters. Anthropogenic land uses contribute resources to stream ecosystems at rates and in proportions that can differ significantly from natural land covers. This human induced alteration of resource delivery rates can lead to imbalances between resource supply and stream microbial community’s elemental demands. Dissolved organic carbon (DOC) concentrations tend to be higher in streams with a greater proportion of wetland or poorly drained soil in the watershed and lower in streams with higher slope and groundwater contribution (Frost et al. 2006; Wilson and Xenopoulos 2008; Mattsson et al. 2009). In general, DOC concentration in streams does not relate to agricultural and human land uses (Wilson and Xenopoulos 2008), although some studies have shown a decline in DOC concentration or export with increased human land use (Cronan et al. 1999; Mattsson et al. 2009; Wilson and Xenopoulos 2009). In contrast to DOC, stream water column N and P inputs tend to be higher in streams with increased fractions of human land use in their catchment (Boyer et al. 2002; Mattsson et al. 2009; Wilson and Xenopoulos 2009). These contrasting patterns of resource delivery might be expected to differentially impact microbial standing stocks and activity. Moreover, as resource availability is altered the balance of microbial activity devoted to the acquisition of required elements will also change to allow microbial communities to maximize resource use and growth.
Extracellular enzymes are one mechanism by which bacteria and microbes respond to changes in C, N, and P availability in the environment. These enzymes are attached to the outer cell membrane of microorganisms, within the periplasmic space of gram negative bacteria, and can be freely dissolved in the water column. Extracellular enzyme hydrolysis is often the rate limiting step in microbial uptake of carbon and nutrient resources in aquatic systems, as these enzymes target substrates that are generally not able to be taken up directly without extracellular decomposition (Hoppe 1983; Williams and Jochem 2006; Sinsabaugh et al. 2010). As such, extracellular enzyme hydrolysis rates should be sensitive to the effects of differing watershed landscape on microbial function and provide a mechanistic indicator of how land use and cover influence organic matter processing and microbial resource acquisition in streams.
Recently, the balance between C, N, and P acquiring extracellular enzyme activities (e.g., β-glucosidase, aminopeptidase, and phosphatase, respectively) in sediment and soil microbial communities was found to be fairly constrained to stoichiometric C:N:P ratios near 1:1:1. This enzymatic equilibrium is likely maintained through changes in the microbial community in response to altered resource availability (Sinsabaugh et al. 2009). Planktonic microbial communities tend to have slightly higher C:P enzyme activity ratios than sediment/soil community C:P enzyme ratios because planktonic biomass C:P elemental ratios tend to be higher (Sinsabaugh et al. 2010). Enzyme activity N:P ratios are similar between benthic and pelagic communities but decrease below the 1:1 equilibrium when microbial growth rates are high due to disproportionate increases in P demand (Sinsabaugh et al. 2010). In addition, enzymatic N:P ratios have been suggested to be a better indicator of nutrient limitation in streams than ambient nutrient concentrations (Hill et al. 2010b). Given the large C:N:P resource imbalances that can be imposed by differing landscape characteristics, it may be expected that extracellular enzyme ratios in lotic ecosystems will deviate predictably from a 1:1:1 equilibrium in response to altered resource availability and associated microbial growth rates.
In the current study, we investigated the impacts of land use and cover on water column bacteria abundance, production, extracellular enzyme activity, and the stoichiometric ratios of extracellular enzyme activity in streams of mixed land use in southern Ontario, Canada. We hypothesized that agricultural and urban land uses stimulate bacterial communities through nutrient enrichment. This stimulation should be reflected in increased bacterial standing stocks and more rapid processing of stream organic matter (i.e., higher extracellular enzyme activities) in streams with higher nutrient loads. In addition, we predicted that streams with increased anthropogenic land use will have enzyme activity N:P ratios near or below 1:1, indicating that these microorganisms are enzymatically balanced and/or experiencing a growth stimulated elevated P demand. Extracellular enzyme C:P activity ratios are predicted to increase from that found in wetland and forest dominated streams because of increased N and P availability driving the microbial response toward increased C acquisition. Moreover, the current study determined how water column processes across a broad spatial scale vary with respect to resources and landscape and if water column enzymatic activities and their ratios provide evidence of anthropogenically induced imbalances in resource supply.
Materials and methods
Study sites and sampling
Fifty streams were sampled once during September 2008. Streams were located in southern Ontario, Canada, south of the Canadian Shield bedrock formation (Fig. 1). The sampling network included streams with different proportions of natural land covers and human land uses in their catchments. All sampling points, except two tributaries, comprised independent stream drainages and were not nested longitudinally within a stream network. The two sites nested within larger watersheds made up <1% of the total watershed area of the catchment and are considered independent for the current study. This sampling strategy provided a temporally limited but spatially broad snapshot of stream environmental and microbial water column conditions across independent streams with diverse land covers and uses. At each stream, temperature, specific conductivity, and discharge were measured and water was collected from ~10 cm below the surface in acid-washed, sample rinsed polyethylene bottles. For bacterial activity analyses, whole water samples were collected in triplicate from each stream. Water samples were stored cool and in the dark for transport back to the laboratory for processing.
Environmental and microbial variable analyses
Stream discharge (m3 s−1) measurements were taken using a SonTek FlowTracker handheld ADV. Water temperature (°C) and specific conductivity (μS cm−1) were measured with an YSI 30/10 FT handheld probe. Total suspended solids (TSS; mg l−1) were measured by filtering 0.25–1 l of stream water through a pre-weighed 0.7 μm GF/F filter, drying collected particles at 60°C until constant weight, and then subtracting the mass of the filter from that of the filter and collected particles. For DOC (mg C l−1) and total dissolved nitrogen (TDN; mg N l−1) analysis, whole water was filtered serially through 0.7 μm GF/F and 0.2 μm GE polycarbonate filters and the filtrate measured using O. I. Analytical Aurora Model 1030 total organic carbon analyzer with an external total bound nitrogen unit in combustion mode. Total dissolved phosphorus (TDP; mg P l−1) was measured using the above filtrate following persulfate digestion in an autoclave using the molybdate blue colorimetric assay.
Microbial extracellular enzyme potential activities (V max) were determined from unfiltered, whole water samples in the evening (within 12 h of collection) of each sampling day using fluorogenic model substrate analogs (Hoppe 1983; Williams and Jochem 2006). Model substrate analogs and target enzymes were l-Leucine-7-amido-4-methylcoumarin for aminopeptidase (AMA), 4-Methylumbelliferyl (MUF) phosphate for alkaline phosphatase (APA), and MUF β-d-glucopyranoside for β-glucosidase (β-glu). Kinetic enzyme assays were incubated in 96-microwelled plates, in the dark, at 20°C, and in quadruplet, where one replicate was sacrificed at the start of enzyme incubation. Model substrates were added at four concentrations (0.9–181.8 μmol l−1) and incubated for 4–6 h, depending on enzyme. At the start of each enzyme incubation, one replicate was sacrificed by adjusting the pH to ~10.2 with glycine-NaOH buffer and fluorescence was measured immediately using a BioTek FLx800TB computer-controlled plate reader. At the end of each incubation time, glycine-NaOH buffer was added to the triplicate sample sets incubated at ambient pH and then model substrate fluorescence was measured. The fluorescence generated by enzyme hydrolysis was converted to molar concentration using pH adjusted fluorescence end-product standard curves at the appropriate instrument settings and then subtracting initial/background fluorescence determined in the sacrificed replicate from that at the end of the triplicate ambient pH incubations. Apparent enzyme activities (nmol l−1 h−1) were determined by fitting hydrolysis rate and substrate concentration data to the Michaelis–Menten kinetic model, which yielded the potential maximum enzyme hydrolysis velocity (V max) for each enzyme system. The enzyme C:P and C:N activity ratios were calculated using natural log normalized carbon (β-glu), nitrogen (AMA), and phosphorus (APA) acquisition enzymes activities (Sinsabaugh et al. 2009, 2010). In addition, cell specific potential enzyme activities were calculated as V max divided by bacterial count (amol cell−1 h−1) for AMA and β-glu. These cell specific rates should be consider as rough estimates of the true bacterial specific cell activities in the water column because its likely that many of the bacterial cells in the water column were inactive, and enzymes can be transported into flowing waters through nearby soils, produced from benthic microbial biofilms, and/or occur fully dissolved (dissociated from cell membranes) in the water column (Findlay et al. 2001; Freese et al. 2006).
Water column bacterial abundance (BACT; ×109 cells l−1) was determined using a FACSAria flow cytometer. Upon returning to the lab, whole water was fixed with 1% formaldehyde solution (final concentration), flash frozen in liquid nitrogen, and stored at −20°C until analysis. Prior to enumeration, water samples were thawed at 4°C and in the dark for 24 h, passed through a 20 μm screen to remove large particles, and stained with SYBR Green I in presence of 30 mmol l−1 potassium citrate (Marie et al. 1997). Bacterial counts were converted to concentration using weight calibrated instrument flow rates.
Water column bacterial production (BP; μg C l−1 d−1) was measured using 3H-leucine incorporation following the standard centrifuge method (Kirchman 2001). Thirty μl of 3H-leucine working solution (specific activity = 28.25 nmol μCi−1; final leucine concentration = 50 nmol l−1) was added to 1.5 ml of whole water and incubated for 1 h in the dark at 20°C. Incubations were conducted in triplicate with one formaldehyde-fixed control. Proteins were harvested through precipitation with trichloroacetic acid (TCA; 5% final concentration) and centrifugation. Unincorporated 3H-leucine was removed from protein precipitates by repeated washing with 5% TCA and deionized water. Washed precipitates were then allowed to dry, suspended in scintillation cocktail, and incorporated radioactivity measured by liquid scintillation counting. For each stream, water column bacterial turnover time (d) was calculated by converting BACT to carbon units assuming 20 fg C cell−1 and dividing by BP.
Watershed and riparian landscape features
Watershed area, watershed slope, soil drainage characteristics, land cover and land use in stream catchments and riparian zones were determined from Geographic Information Systems (GIS) data for southern Ontario using spatial analyst and hydrology toolboxes in ArcMap 9.0 software. Digital elevation models (DEMs) and stream network data were obtained from the Ontario Ministry of Natural Resources (2002). Land use characteristics were obtained from the Southern Ontario Interim Landcover geospatial data set (SIL 2007). Soil drainage characteristics were obtained from the Ontario Ministry of Agriculture and Food (2004). Watershed area (km2) was calculated using DEMs as the total area upstream of the sampling point that could drain into the sampling point. Watershed slope was calculated as the average percent rise between neighboring DEM cells for the entire watershed using the SLOPE tool in ArcGIS. Riparian land cover/use and soil drainage characteristics were calculated as percent of total land cover/use and drainage within a 100 m buffer of each stream for the complete watershed upstream of the sampling point. In southern Ontario, riparian and total catchment land cover and use positively correlate to each other, but land cover and use determined in the 100 m riparian buffer acts as a slightly better predictor of stream water chemistry (Wilson and Xenopoulos 2008, 2009). Thus, only riparian land cover and use were reported in the current study.
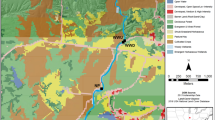
Riparian land cover and use was characterized by SIL (2007) as percent continuous annual cropland, rotational cropland, forage crop and idle land, wooded, wetland, and built-up impervious area among others (Fig. 2). Continuous annual row cropping (classified in SIL as monoculture cropland) is the most intensive from of agriculture in southern Ontario and is defined as continuously farmed cropland with bare soils in spring. Rotational croplands (classified in SIL as mixed croplands) are areas where annual crops are rotated with forage crops as indicated by land cover change analysis of three sequential dates over a 10–15 year period. Forage crop and idle lands are areas that appeared as foliated ground in spring and are generally dominated by grasses and/or legumes. Impervious surfaces include built-up areas such as pavements and buildings. Wooded and wetland areas represent less anthropogenically altered, natural land covers in southern Ontario. In Ontario, surface soils have been classified into seven drainage classes based on the speed at which water permeates through the soil layer. For the current study, only poorly drained soils as the sum of very poorly and poorly drained area are presented because this soil classification was shown to relate well to visible and cryptic wetlands in Southern Ontario (Creed et al. 2003; Wilson and Xenopoulos 2008).
Total watershed area (km2), slope (% rise), percent poorly drained soils, and riparian land use and cover (%) for the complete catchment. Box-Whisker plots display mean (dotted line), median (solid line), and points outside the 10th and 90th percentile (dots). See methods for descriptions of land use categories
Statistical analysis
Prior to statistical analysis, data were natural log, square root, or fourth root transformed to meet the assumptions of normality. Principle components analysis (PCA) was used to reduce watershed variables (i.e., watershed area, slope, poorly drained soils, annual cropland, rotational cropland, forage crop and idle lands, wetlands, wooded area, and impervious surfaces) into statistically distinct components. PCA components with Eigenvalues over one were extracted. Varimax rotation with Kaiser Normalization was applied to the result solution. Pearson’s bivariate correlation (r) and univariate and stepwise multiple linear regression analyses (R 2) were used to determine significant (p < 0.05) relationships between principle component (PC) scores, water chemistry, and microbial variables. Variables were excluded from multiple regression models due to collinearity when the condition index (CI) was >30 and/or variance influence factor (VIF) was >10. Simple and partial Mantel tests were used to check for spatial autocorrelations within the data set (Pearson’s r; King et al. 2005).
Results
Watershed and riparian zone characteristics
The 50 southern Ontario streams under study included headwater through 5th order streams with watershed areas that ranged from 2.9 to 992.6 km2 (Fig. 2). Watershed slopes ranged from fairly flat, 1.2% rise, to moderately steep sloped catchments, 8.8% rise. On average, 14% of the soils in the watershed had poorly drained soils, suggesting that much of the study area comprised rather permeable soils. Riparian land use consisted of composites of built-up urban areas and agricultural lands, which included a wide range of activities from intensive continuously farmed row crops to idle grasslands (Fig. 2). Overall, the current study captured a broad distribution of human associated land uses in stream riparian zones, which ranged from 4 to 93%. Similarly, natural land cover (i.e., wetland and wooded area) ranged from nearly absent (4%) to the dominant land cover (85%) in stream riparian zones (Fig. 2).
Principle components analysis of the above watershed and riparian landscape characteristics (Fig. 2) produced three principle components (PCs) that explained 79.2% of the total variance in the landscape dataset (Table 1). PC1 explained 26.8% of the variability in the data and included poorly drained soils and wetland area in the positive direction and annual cropland and impervious built-up area in the negative direction. PC2 explained 23.7% of the variability in the data and included watershed slope and woodland area in the positive direction and rotational cropland in the negative direction. PC3 explained 18.7% of the variability in the data and included watershed area, forage crops and idle lands, and annual croplands in the positive direction. Overall, the PCA reduced the dataset into the following gradients: (PC1) urban land use and continuous annual cropping to wetland-like cover, (PC2) rotational cropping to forest-like cover, and (PC3) increasing rural and agricultural land uses with increasing watershed size. Simple Mantel tests revealed that PC3 was spatially autocorrelated (r = 0.36, p < 0.001) between sites, but PC1 and PC2 were not influenced significantly by stream location. These PCA results will be used from here forward to determine the influence of the watershed and riparian zone characteristics on stream environmental and microbial parameters.
Environmental variables and water column resources
Across streams, environmental variables did not spatially autocorrelate, and correlations between PCs and environmental variables (Table 2) were not influenced by spatial patterns (Partial Mantel test, p > 0.05). Stream discharge, water column temperature, specific conductivity, and TSS varied widely between streams (Fig. 3a–d). Discharge generally increased with increased watershed slope and wooded area (PC2), but water temperature correlated poorly with PC loadings. Specific conductivity correlated negatively with PC2 but positively with PC3, while TSS increased strongly with PC1.
Stream resource levels varied widely across streams and were influenced differently by watershed and riparian zone characteristics (Table 2; Fig. 3e–g). Stream resource concentrations, other than TDN, were not influenced by spatial autocorrelation. DOC increased with PC1 but decreased with increasing PC2. TDN correlated positively with PC3, though some of this relationship was due to the spatial distance between streams. TDP correlated negatively with PC1 and PC2. DOC:TDN:TDP molar ratios ranged widely across streams (Fig. 3h–j) and tended to suggest P-limiting conditions relative to C across all streams but a mixture of N and P potentially limiting conditions as inferred through TDN:TDP ratios. With respect to landscape influences, DOC:TDP ratios correlated positively with PC1, DOC:TDN ratios correlated negatively with PC3, and TDN:TDP ratios correlated positively with PC3 (Table 2). Overall, N and P resources tended to be higher in streams with increased human land use, while C was higher in streams with increasing wetland and poorly drained soil area and/or decreased wooded area.
Influence of resource and landscape characteristics on microbial parameters
BACT, BP, bacterial turnover time and microbial extracellular enzyme potential activities varied widely across the study streams (Fig. 4a–f). Bacterial specific enzyme activity potentials were also variable and ranged 66–12,638 and 17–576 amol cell−1 h−1 for AMA and β-glu, respectively. These microbial variables were influenced by resource concentrations and landscape characteristics (Tables 2, 3). In general, indicators of microbial activity were lower in streams with a greater fraction of natural land cover and higher in streams with a greater percentage of human land uses and increased water column nutrients. β-glu:APA and AMA:APA ratios ranged from 0.30 to 0.84 and from 0.8 to 2.0, respectively (Fig. 4g, h). Across southern Ontario streams, β-glu:APA ratios increased toward 1:1 (i.e., stoichiometric equilibrium, sensu Sinsabaugh et al. 2009, 2010) with TDN, TDP, anthropogenic land use, and faster bacterial turn over times (Table 2; Fig. 5). AMA:APA ratios decreased toward 1:1 with increased TDN and DOC, loss of natural land cover, and faster water column bacterial turnover times. β-glu:APA ratios declined with increasing DOC:TDP and DOC:TDN resource ratios (r = −0.48 and −0.41, p = 0.002 and 0.012, respectively), but AMA:APA ratios did not relate to resource stoichiometric ratios (data not shown).
Extracellular enzymatic β-glucosidase : alkaline phosphatase (β-glu:APA; C:P, squares) and aminopeptidase : alkaline phosphatase (AMA:APA; N:P, circles) ratios and their relationships to resource levels, water column bacterial turnover times, and the first two landscape principle components. C:P and N:P relationships reflect enzymatic stoichiometric ratios and are not resource or biomass based molar ratios
Stepwise multiple linear regression analysis was used to determine the major ecosystems drivers (PCs and resource concentrations) of water column microbial dynamics (Table 3). BACT and BP were influenced by resource levels and watershed and riparian zone characteristics. BACT was higher in streams with less forest land cover, increased P levels, and increased agricultural and rural riparian land use. Higher BP was associated with higher N concentration, less riparian wetland influence, and higher DOC concentrations. Bacterial turnover time was less predicable than abundance and production. Only 25% of the variation of bacterial turnover time could be explained by TDN concentration and PC1. Thus, bacterial populations tended to be turned over faster with increased water column N concentrations and increased intensive human land use relative to wetland-like areas.
Extracellular enzyme potential activities for the various C, N, and P acquiring systems under study responded similarly to ecosystem drivers (Table 3). Water column AMA was best predicted by including DOC and TDP in a stepwise model, indicating that peptide potential hydrolysis rates increased with decreasing C and increasing P concentrations. In this sense, AMA appears to be important for microbial acquisition of both C and N. APA was best predicted by the PC1 and PC2 axes, where APA was higher in streams with increased human land use and decreased natural land covers. The best stepwise multiple regression for β-glu potential activity was similar to that of APA, but in addition to PC1 and PC2, TDN was also a significant predictor of residual variation. The ratio of β-glu:APA was best predicted by negative relationships with PC2 and PC1 and a positive relationship with TDN. The majority of the variation in the AMA:APA ratio was explained by negative relationships with DOC and TDN. However, when bacterial turnover time was included as stepwise independent variable, the explanatory power was increased from 33 to 55%. This model suggested that microbial demand for nitrogen and phosphorus is more balanced in stream with faster bacterial turn over times and increased carbon availability (Table 3; Fig. 5).
Discussion
The 50 streams under study cover a gradient from 93% human land use to 85% natural land cover. This landscape imposed variability produced contrasts in nutrient supply and created a situation in southern Ontario streams where C resources were less abundant in streams that had increased levels of N and P resources. We found an increase in enzyme activity in the water column of streams with more intensive human land use. This observation supported our hypothesis, which links anthropogenic activity with enhanced organic matter processing rates, but the pattern was not specifically related to one particular category of land use, rather there was a general pattern of higher extracellular enzyme activity and BP in watersheds with less natural land covers (Tables 2, 3). Coupled with these increased nutrient levels, β-glu:APA ratios increased toward 1:1, while DOC:TDP ratios decreased, which supported our hypothesis that C demand would increase in anthropogenically impacted streams. Patterns for AMA:APA ratios only partly supported our hypothesis. Streams with intensive forms of human land use (e.g., annual row cropping and impervious surface areas) did reach N:P enzyme ratios of 1:1, but five streams with ratios <1:1 occurred under less intensive forms of human land use. These low enzyme N:P ratios suggest rapid microbial growth but the mechanism behind these low values was unclear during our study. Together, these results suggest that water column microbial communities have greater access to enzyme degradable resources with human land use and that these resource gains allow for faster population turnover times. These more rapid turnover times and increased availability of enzyme substrates then helped drive extracellular enzymatic activity toward equilibrium rates.
In general, as was found here for southern Ontario streams, agricultural practices and human land uses are associated with increased nutrient loads to streams, while wetlands, forests, water flow path, and soil drainage more strongly impact carbon concentrations (Tables 1, 2; Boyer et al. 2002; Wilson and Xenopoulos 2008; Mattsson et al. 2009). This divergence in landscape associated resource inputs was illustrated in the current study by decreasing DOC:TDP and DOC:TDN molar ratios with increased anthropogenic land use (Table 2). In addition to higher nutrient concentrations relative to C with more intensive land use, the structure of the dissolved organic matter pool has also been observed to become less humic and potentially more labile (e.g., increased protein-like and microbial sourced material; Wilson and Xenopoulos 2009; Williams et al. 2010). Although resource levels in southern Ontario streams correlated with landscape drivers in typical ways (Table 2), individual microbial response variables were not explained consistently by the same combination of environmental and resource factors (Table 3; Fig. 5). Both nutrient concentration and broader scale landscape characteristics were identified as significant predictors of microbial activity indicating that both local resource supply and longer term history of supply are likely important in shaping water column microbial activity.
Resource levels, BACT, BP, and potential enzyme activities were variable between streams and indicated oligotrophic to eutrophic conditions across stream landscapes. Overall, water column BACT and BP were within the range of other pelagic lotic habitats (Chappell and Goulder 1995; Sinsabaugh et al. 1997; Ainsworth and Goulder 2000; Castillo et al. 2004), similar to or slightly lower than that measured for epilithic microbial communities (Hudson and Roff 1992; Carr et al. 2005), and few orders of magnitude lower than that measured in aquatic sediments (Fischer et al. 2002, 2005; Wilczek et al. 2005). Extracellular enzyme activities tend to be variable across habitats and streams. As such, enzyme activity potentials determined in the water column in southern Ontario streams ranged from greater than, to similar to, to lower than activities estimated for soil, sediment, and biofilms (Artigas et al. 2009; Sinsabaugh et al. 2009; Smart and Jackson 2009). When standardized to cell abundance or productivity, extracellular enzyme activities are comparable across soil, sediment, and water column zones (Fischer et al. 2002; Sinsabaugh et al. 2010; Suberkropp et al. 2010; this study). However, average cell specific activities in the current study tended to be high for lotic ecosystems, which might indicate that some enzymes were produced outside the water column and originated from a benthic or terrestrial habitat.
Across ecosystems, extracellular enzyme activities typically follow a common hydrolysis gradient: AMA > APA > β-glu (Fig. 4; Chappell and Goulder 1995; Taylor et al. 2003; Wilczek et al. 2005; Williams and Jochem 2006). In the current study, water column extracellular enzyme activity potentials followed this trend and were within the range of activities reported for lotic ecosystems (Fig. 4; Chappell and Goulder 1995; Sinsabaugh et al. 1997; Ainsworth and Goulder 2000). Extracellular enzyme activities tend to be higher in eutrophic ecosystems than in oligotrophic ones, but enzyme activities can vary by orders of magnitude within a catchment as well as between rivers of similar trophic status due to changes in inorganic and organic matter availability and temperature (Sinsabaugh et al. 1997; Ainsworth and Goulder 2000; Cunha et al. 2000; Wilczek et al. 2005). In the present study, the extracellular enzyme patterns across streams seemed controlled by changes in land use within a catchment and associated impacts to the organic matter pool, and temperature differences between streams did not appear as a significant driver of potential stream enzyme activity. The lack of a temperature effect was likely because samples were collected within the same season.
Extracellular enzyme activities in most aquatic systems, including southern Ontario streams, tend to increase with nutrient availability and organic matter quality (Sinsabaugh et al. 1997; Fischer et al. 2005; Hill et al. 2010b; Williams et al. 2010). For example, β-glu activity can increase with increased anthropogenic land use, indicating greater demand for a more limited supply of organic matter (Wilczek et al. 2005). Hence, more of the bulk organic matter pool was processed in human impacted streams than those with more natural land covers. In contrast, AMA correlated most strongly with decreased DOC:TDP ratios that occurred within anthropogenically impacted streams. An increased bacterial N demand appears to occur in streams where P limitation may be relaxed. Still, correlations between AMA and human land use may be in part a function of the altered composition of the organic matter pool that has been observed in urban and agricultural streams in southern Ontario. In agricultural and urban impacted aquatic system, dissolved organic matter has an increased contribution of labile and protein-like material, which tends to be smaller sized, more accessible to the microbial community, and more N-rich than the organic pool in less impacted systems (Kelton et al. 2007; Williams et al. 2010). In support of this, DOC was a tertiary predictor of BP, indicating that the availability and potentially the composition of the DOC pool (e.g., Williams et al. 2010) regulated water column production to a small degree (Table 3).
Enzyme C:N:P ratios are suggested to provide a better indication of nutrient limitation to the microbial community than resource concentrations (Hill et al. 2010b). When the microbial communities growth is in balance with the available resources, C:N:P ratios based on natural log transformed extracellular enzyme activities tend to be rather invariant, occurring around a 1:1:1 homeostasis (Sinsabaugh et al. 2009). Hence, deviations from this enzymatic homeostasis across landscapes should provide evidence that the microbial community has not approached a steady state with the resource available in the ecosystem. Across southern Ontario streams, mean β-glu:APA (0.59; Fig. 4) was more similar to ratios produced in benthic biofilms (mean = 0.65) than freshwater pelagic microbes (mean = 1.05; Sinsabaugh et al. 2010). Mean AMA:APA (1.20; Fig. 4) were higher on average than both benthic and pelagic enzyme N:P ratios, which tended to be just below 1.0 across aquatic systems (Sinsabaugh et al. 2010). These comparisons indicate that on average the water column microbial community of southern Ontario streams had a high N demand relative to P and a higher P demand relative to C, suggesting that resource supply does not match the water column microbial community demand for growth.
Across landscapes, enzyme C:N:P ratios were variable, but increased nutrients and/or anthropogenic land use allowed water column β-glu:AMA:APA ratios to approach 1:1:1 (Fig. 5). These relationships suggest that elemental demand supplemented through microbial extracellular enzyme activity was better balanced with the substrate supply in streams impacted by anthropogenic land use. In addition, streams with AMA:APA ratios closer to and below 1:1 exhibited faster water column bacterial turnover times than those streams with higher AMA:APA ratios. Overall, pelagic extracellular enzyme activities likely provide a good indicator of organic resource availability and levels of microbial resource limitation in streams, though information regarding inorganic nutrient supply and demand was not collected during our study. Similar conclusions were drawn in a study examining extracellular enzyme activities in both benthic and pelagic habitats (Hill et al. 2010a, b).
Extracellular enzyme activities for C sequestration and BP also increased with anthropogenic land use in our streams. This pattern is predicted to occur when bacterial growth limitations imposed by nutrients are relaxed (Sinsabaugh et al. 1997; Taylor et al. 2003), conditions which occur in our highly anthropogenically impacted stream. Coupled with these increased nutrient levels, β-glu:APA ratios increased toward 1:1, while DOC:TDP ratios decreased. Similarly, across a gradient of agricultural intensity in Pennsylvania, USA, stream biofilm enzyme C:P ratios correlated positively with P levels, indicating that C acquisition increased relative to P with increasing stream nutrient resources (Rier et al. 2011). In urban streams of Melbourne, Australia, β-glu activity decreased but AMA increased with increasing impervious surface area, suggesting that bacterial metabolic demand for substrates shifted and/or substrate availability changed with increased urban influence on streams (Harbott and Grace 2005). However, in the current study, AMA and β-glu potentials both increased with impervious surface area (i.e., PC1), suggesting that the overall demand for C increased in these nutrient rich, human impacted streams rather than a broad-based shift from one C substrate toward another. In addition, bacteria cell specific AMA and β-glu potentials were higher with decreasing DOC concentrations (relationships not shown) further indicating their important role for carbon acquisition.
For the most part, β-glu:APA and AMA:APA ratios deviated greatly from 1:1:1 (Fig. 4), indicating that water column microbial growth rates were not balanced with the available enzyme accessible resources. Only with anthropogenic land use associated resource increases did stream enzyme C:N:P ratios more closely reflect proposed enzymatic homeostasis. Enzyme activities and their ratios were predicted well by water column resource levels and watershed variables, indicating that despite the variability of supply imposed by the dynamics of flowing water these enzyme activities are a broad integrator of conditions within a streams watershed. In this sense, water column activities might broadly provide a good indication of anthropogenic impacts on organic resource availability in stream ecosystems. With increased anthropogenic activity enzyme C:N:P approached 1:1:1 suggesting that with anthropogenic activity and increased resource levels microbial communities where better able to achieve enzymatic homeostasis and balance available resources with their growth demands.
References
Ainsworth AM, Goulder R (2000) Epilithic and planktonic leucine aminopeptidase activity and leucine assimilation along the River Tweed, Scottish Borders. Sci Total Environ 251(252):83–93
Artigas J, Romaní AM, Gaudes A, Muũnoz I, Sabater S (2009) Organic matter availability structures microbial biomass and activity in a Mediterranean stream. Freshw Biol 54:2025–2036
Boyer EW, Goodale CL, Jaworski NA, Howarth RW (2002) Anthropogenic nitrogen sources and relationships to riverine nitrogen export in the northeastern U.S.A. Biogeochemistry 57(58):137–169
Carr GM, Morin A, Chambers PA (2005) Bacteria and algae stream periphyton along a nutrient gradient. Freshw Bio 50:1337–1350. doi:10.1111/j.1365-2427.2005.01401.x
Castillo MM, Allan JD, Sinsabaugh RL, Kling GW (2004) Seasonal and interannual variations of bacterial production in lowland rivers of the Orinoco basin. Freshw Bio 49:1400–1414
Chappell KR, Goulder R (1995) A between-river comparison of extracellular-enzyme activity. Microb Ecol 29:1–17
Creed IF, Sanford SE, Beall FD, Molot LA, Dillion PJ (2003) Cryptic wetlands: integrating hidden wetlands in regression of the export of dissolved organic carbon from forested landscapes. Hydrol Process 17:3629–3648
Cronan CS, Piampiano JT, Patterson HH (1999) Influence of land use and hydrology on exports of carbon and nitrogen in a Maine river basin. J Environ Qual 28:953–961
Cunha MA, Almeida MA, Alcântara F (2000) Patterns of ectoenzymatic and heterotrophic bacterial activities along a salinity gradient in a shallow tidal estuary. Mar Ecol Prog Ser 204:1–12
Findlay S, Quinn JM, Hickey CW, Burrell G, Downes M (2001) Effects of land use and riparian flowpath on delivery of dissolved organic carbon to streams. Limnol Oceanogr 46:345–355
Fischer H, Sachse A, Steinberg CEW, Pusch M (2002) Differential retention and utilization of dissolved organic carbon by bacteria in river sediments. Limnol Oceanogr 47:1702–1711
Fischer H, Kloep F, Wilczek S, Pusch MT (2005) A river’s liver–microbial processes within the hyporheic zone of a large lowland river. Biogeochemistry 76:249–371
Freese HM, Karsten U, Schumann R (2006) Bacterial abundance, activity, and viability in the eutrophic River Warnow, northeast Germany. Microb Ecol 51:117–127
Frost PC, Larson JH, Johnston CA, Young KC, Maurice PA, Lamberti GA, Bridgham SD (2006) Landscape predictors of stream dissolved organic matter concentration and physiochemistry in a Lake Superior river watershed. Aquat Sci 68:40–51
Harbott EL, Grace MR (2005) Extracellular enzyme response to bioavailability of dissolved organic C in streams of varying catchment urbanization. J North Am Benthol Soc 24:588–601
Hill BH, Elonen CM, Jicha TM, Bolgrien DW, Moffett MF (2010a) Sediment microbial enzyme activity as an indicator of nutrient limitation in the great rivers of the Upper Mississippi River basin. Biogeochemistry 97:195–209
Hill BH, McCormick FH, Harvey BC, Johnson SL, Warren ML, Elonen CM (2010b) Microbial enzyme activity, nutrient uptake and nutrient limitation in forested streams. Freshw Bio 55:1005–1019
Hoppe H-G (1983) Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates. Mar Ecol Prog Ser 11:299–308
Hudson JJ, Roff JC (1992) Bacterial production in forested and open streams in southern Ontario. Can J Fish Aquat Sci 49:2412–2422
Kelton N, Molot LA, Dillon PJ (2007) Spectrofluorometric properties of dissolved organic matter from Central and Southern Ontario streams and the influence of iron and irradiation. Water Res 41:638–646
King RS, Baker ME, Whigham DF, Weller DE, Jordan TE, Kazyak PF, Hurd MK (2005) Spatial considerations for linking watershed land cover to ecological indicators in streams. Ecol App 15:137–153
Kirchman DL (2001) Measuring bacterial biomass production and growth rates from leucine incorporation in natural aquatic environments. In: Paul JH (ed) Methods in microbiology, vol 30. Academic, London, pp 227–237
Marie D, Partensky F, Jacquet S, Vaulot D (1997) Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl Environ Microbiol 63:186–193
Mattsson T, Kortelainen P, Laubel A, Evans D, Pujo-Pay M, Räike A, Conan P (2009) Export of dissolved organic matter in relation to land use along a European climatic gradient. Sci Total Environ 407:1967–1976
Ontario Ministry of Agriculture and Food (2004) Soils Ontario packaged product (soils ON). Peterborough, Ontario
Ontario Ministry of Natural Resources (2002) Water resources information project. Peterborough, Ontario
Ontario Ministry of Natural Resources (2007) Southern Ontario Interim Landcover (SIL) geospatial data. Peterborough, Ontario
Rier ST, Nawrocki KS, Whitley JC (2011) Response of biofilm extracellular enzymes along a stream nutrient enrichment gradient in an agricultural region of north central Pennsylvania, USA. Hydrobiologia 669:119–131
Sinsabaugh RL, Findlay S, Franchini P, Fischer D (1997) Enzymatic analysis of riverine bacterioplankton production. Limnol Oceanogr 42:29–38
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ectoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798. doi:10.1038/nature08632
Sinsabaugh RL, Van Horn SJ, Follstad Shah JJ, Findlay S (2010) Ecoenzymatic stoichiometry in relation to productivity for freshwater biofilm and plankton communities. Microb Ecol 60:885–893
Smart KA, Jackson CR (2009) Fine scale patterns in microbial extracellular enzyme activity during leaf litter decomposition in a stream and its floodplain. Microb Ecol 58:591–598
Suberkropp K, Gulis V, Rosemond AD, Benstead JP (2010) Ecosystem and physiological scales of microbial responses to nutrients in a detritus-based stream: results of a 5-year continuous enrichment. Limnol Oceanogr 55:149–160
Taylor GT, Way J, Yu Y, Scranton MI (2003) Ectohydrolase activity in surface waters of the Hudson River and western Long Island Sound estuaries. Mar Ecol Progr Ser 263:1–15
Wilczek S, Fischer H, Pusch MT (2005) Regulation and seasonal dynamics of extracellular enzyme activities in the sediments of a large lowland river. Microb Ecol 50:253–267
Williams CJ, Jochem FJ (2006) Ectoenzymes kinetics in Florida Bay: implications for bacterial carbon source and nutrient status. Hydrobiologia 569:113–127
Williams CJ, Yamashita Y, Wilson HF, Jaffé R, Xenopoulos MA (2010) Unraveling the role of land use and microbial activity in shaping dissolved organic matter characteristics in stream ecosystems. Limnol Oceanogr 55:1159–1171
Wilson HF, Xenopoulos MA (2008) Ecosystem and seasonal control of stream dissolved organic carbon along a gradient of land use. Ecosystems 11:555–568
Wilson HF, Xenopoulos MA (2009) Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat Geosci 2:37–41
Acknowledgments
This research was supported through Canada’s Natural Sciences and Engineering Research Council (NSERC) Discovery and Strategic Grants to M.A.X., an NSERC University Faculty Award to M.A.X., NSERC postgraduate scholarships to H.F.W. and an Early Researcher Award from the Ontario Ministry of Research and Innovation to M.A.X. Geospatial and land use GIS data was supplied under license by Members of the Ontario Geospatial Data Exchange (Ontario Ministry of Natural Resources and Natural Resources Canada). We thank the anonymous reviewers whose comments improved greatly early versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, C.J., Scott, A.B., Wilson, H.F. et al. Effects of land use on water column bacterial activity and enzyme stoichiometry in stream ecosystems. Aquat Sci 74, 483–494 (2012). https://doi.org/10.1007/s00027-011-0242-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-011-0242-3