Abstract
Wettability and retention capacity of leaf surfaces are parameters that contribute to interception of rain, fog or dew by forest canopies. Contrary to common expectation, hydrophobicity or wettability of a leaf do not dictate the stickiness of drops to leaves. Crucial for the adhesion of drops is the contact angle hysteresis, the difference between leading edge contact angle and trailing edge contact angle for a running drop. Other parameters that are dependent on the static contact angle are the maximum volume of drops that can stick to the surface and the persistence of an adhering drop with respect to evaporation. Adaption of contact angle and contact angle hysteresis allow one to pursue different strategies of drop control, for example efficient water shedding or maximum retention of adhering water. Efficient water shedding is achieved if contact angle hysteresis is low. Retention of (isolated) large drops requires a high contact angle hysteresis and a static contact angle of 65.5°, while maximum retention by optimum spacing of drops necessitates a high contact angle hysteresis and a static contact angle of 111.6°. Maximum persistence with respect to evaporation is obtained if the static contact angle amounts to 77.5°, together with a high contact angle hysteresis. It is to be expected that knowledge of these parameters can contribute to the capacity of a forest to intercept water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A significant amount of water that precipitates within canopies as rain, fog or dew is intercepted by the forest canopy. Interception comprises various processes that occur after the water has come into contact with plant surfaces: evaporation of water retained inside the canopy (interception loss), down drip off the canopy (drip) and down flow along the stems (stemflow). Canopy interception is a process of considerable importance for the hydrological cycle since annual interception losses in forests can amount to more than a quarter of total rainfall (Hörman et al. 1996; Dingman 2002). Determination of hydrological input by fog is partially very difficult due to the exact determination of interception. The actual rate of interception loss is dependent on various factors, such as forest structure, fog or rain intensity and meteorological parameters. Precise knowledge on interception losses are of substantial importance for predicting and modeling hydrological processes, such as effects of woodland, climate or land cover on water resources (Gash 1979; Calder 1990; Aboal et al. 1999; Muzylo et al. 2009).
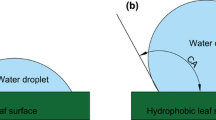
The maximum amount of water that can be temporarily held by a canopy is mainly distributed between bark and leaves (Herwitz 1985). For the leaves, the amount of stored water is a function of leaf area index (LAI), but with substantial interspecific differences (Aston 1979). Besides leaf area, wettability of the leaves is expected to be important. Wettability describes the behaviour of water after coming in contact with a surface. Water repellent surfaces are hydrophobic, and droplets upon these surfaces develop spherical forms with contact angles of >90° (Calies and Quéré 2005). On hydrophilic surfaces, droplets attain contact angles of <90°. Complete wetting leads to the spreading of drops into films. Water repellency differs substantially between upper and lower sides of leaves, between species and between forest types (Holder 2007).
Wettability and the resulting contact angle also influences the gliding angle, that is, the angle of inclination of an object that leads to the rolling off of drops lying upon an object or being attached to the underside of the object. Wettability of a leaf depends on the chemical nature of the leaf waxes, as well as on structures of the leaf surface, including papillae and trichomes (Barthlott and Neinhuis 1997; Shirtcliffe et al. 2009). It is intuitively expected that the more water repellent a surface is, the lower is the gliding angle. This would mean that a low inclination would be sufficient to remove drops from the object, and species with water repellent leaves would, therefore, show a lower storage capacity for rain interception than a species with more hydrophilic leaves.
However, the gliding angle is not only dependent on wettability. Rather, contact angle hysteresis governs the gliding behavior of droplets on a surface (Quéré 2008), that is, the difference between the developing contact angle when a droplet moves forwards (advancing contact angle) or backwards (receding contact angle). For example, petals can be very sticky with respect to droplet behavior despite their low wettability (Feng et al. 2008). In this contribution we consider the interrelationship between wettability and contact angle hysteresis and gliding behaviour of drops attached to surfaces. In particular, we will address the following questions: which contact angle leads to (1) a maximum storage capacity of a surface with respect to sitting or hanging drops, and (2) a maximum persistence of drops with respect to evaporation (under a given humidity and temperature.)
2 Basic Properties of Droplets Attached to a Plane
In this section we derive volumes and areas of droplets attached to a horizontal or inclined plane and the surface and gravitational forces acting on them.
2.1 Droplet Hanging Down from a Horizontal Plane
We consider a droplet hanging down from a horizontal plane under its own weight (Fig. 1). Assuming that the droplet is shaped as a segment of a sphere of radius R and that the contact angle formed between droplet and substrate is θ the volume of the spherical segment amounts to
a Droplet hanging down from a horizontal plane. b Forces acting on the droplet. F g denotes the force of gravity, F σ the force due to the surface tension of the water/air interface at the contact line. Notice that the vectors \(\mathbf{d F_{\sigma}}\) represent infinitesimal forces contributed by two infinitesimal elements of the contact line. Because these are diametrically located, the horizontal component of F σ vanishes if integrated around the circular contact line. The vertical component of F σ may surmount F g , in which case the droplet remains attached to the horizontal plane. For F σ < F g the droplet falls down
The second version is a result of the substitution \(s=R \sin\theta,\) where s denotes the radius of the circle which forms the contact line where water, air and the solid of the plane meet. The surface area of the droplet is the sum M + S, where
S denotes the attachment area between drop and plane and M denotes that part of the droplet surface which is in contact with air.
As long as the droplet is pending the force due to its weight is compensated by the force which originates from the surface tension of the water/air interface at the contact line (see Fig. 1). The infinitesimal force arising from an infinitesimal element of this circle can be decomposed into a horizontally and a vertically oriented component. Integrating along the contact line (i.e. the circle) the integral of the horizontal force component vanishes because of the axial symmetry of the situation while the vertical component of the tension force adds up to
where σ denotes the surface tension between water and air. For a droplet with maximum volume F σ should be balanced by the gravitational force
caused by the droplet’s weight. Exploiting expression (1) and then solving F g = F σ for the radius s m of the contact circle corresponding to a maximum volume droplet we arrive at
The quantity
depends merely on the natural constants surface tension (between air and water) σ ≈ 72 × 10−3 N/m, the gravitational acceleration g≈ 10 m/s2 and the density of water ρ≈ 103 kg/m. Thus, it represents a characteristic length of the problem. (In the literature the expression \(\sqrt{{\sigma}/{(\rho g)}}\) is known as the capillary constant.) From \(s=R \sin\theta\) and (6) we find
and (upon insertion of (6) into expression (1)) we obtain the maximum volume V m of the droplet:
Notice that although s m(θ), R m(θ) and V m(θ) represent a droplet which is—due to its weight—on the verge of falling down these functions depend still on the contact angle θ (see Fig. 2). Hence, we may calculate the contact angle(s) where they attain their maxima. Interestingly, the maxima of s m(θ) and V m(θ) do not coincide. We, rather, find:
a Contact circle radius s m(θ) related to the maximum volume of a droplet as a function of contact angle θ. The maximum of s m(θ) is located at \( \theta_{s_{\rm m}}\) = 0° (see (10)). b Volume V m(θ) related to the maximum volume of a droplet as a function of contact angle θ. The maximum of V m(θ) is located at \( \theta_{V_{\rm m}}\) ≈ 65.5° (see (12))
2.2 Droplet and Inclined Plane
In conjunction with horizontally oriented planes, only hanging droplets are interesting, because the vertically directed force of gravitation has no horizontal component which could push around droplets attached to the upper side of the plane. If the plane is inclined with respect to the horizontal the situation changes. Hence, we consider now both hanging and sitting droplets.
2.2.1 Forces Acting on a Hanging Droplet, Conditions for Detachment and Downslide
Consider a droplet of given volume V hanging down from a plane which is inclined against the horizontal by an angle α (Fig. 3). If α is increased, the shape of the droplet deviates—at first slightly, then increasingly—from being a segment of a sphere. The droplet as a whole, however, does not move. Eventually—when a critical angle of inclination has been reached—the droplet either starts to slide down or it detaches from the plane and falls down. Similarly, as in Sect. 2.2.1 (Eq. 6), we would like to find a relation between the “system defining” variables like s, θ and α which characterise the onset of slide or detachment.
a Droplet hanging down from a plane which is inclined against the horizontal by an angle α. θr and θa denote the receding and advancing contact angle, respectively. b Forces acting on a droplet hanging down from a plane which is inclined against the horizontal by an angle α. θr and θa denote the receding and advancing contact angle, respectively. \(\mathbf{F_{g}}\) denotes the force of gravity, \(\mathbf{F_{\sigma}}\) the force due to the surface tension of the water/air interface at the contact line. Subscripts n and t denote normal and tangential components with respect to the plane. Because the infinitesimal force vectors \(\mathbf{d F_{\sigma}}\) are oriented tangentially to the water/air interface, the inequality θr ≠ θa implies that the magnitude of their (infinitesimal) components parallel and normal to the plane vary along the contact line
Experimentally, it has been found that at the critical inclination the contact angle θ assumes along the “upstream” segment of the contact circle the receding value θr. At the “downstream” segment of the contact circle, however, the advancing contact angle θa is realised.
Decomposition of F g and F σ into components parallel and normal to the inclined plane (Fig. 3) leads to two conditions:
-
(a)
Downslide of the droplet sets in when the tangential component of gravity surmounts the tangential component of the surface tension
$$ {F_{g,t}} \equiv \rho gV \sin\alpha \ge k \sigma w (\cos\theta_{\rm r}-\cos\theta_{\rm a}) \equiv {F_{\sigma,t}} $$(13)w denotes denotes the maximum halfwidth of the droplet. The detailed theory of a sliding droplet is rather involved (see Extrand and Kumagai 1995; Petrissans and Cscapo 2003; Podgorski et al. 2001; Dimitrakopoulos and Higdon 1998, 1999; Glasner 2007). Most authors agree on the structure of Eq. 13 but disagree on the value of the numerical constant k to which various values in the range k = π/4…2 have been assigned. Moreover, k depends also on the shape of the droplet.
-
(b)
Detachment of the droplet from the plane occurs if the normal gravity component is greater than the normal component of the surface tension
$$ {F_{g,n}} \equiv \rho gV \cos\alpha \ge \frac{\pi}{2} k \sigma w (\sin\theta_{\rm r}+\sin\theta_{\rm a}) \equiv {F_{\sigma,n}} $$(14)
As they stand, Eqs. 13 and 14 are of limited use because they contain the droplet volume V which is difficult to obtain experimentally. If, however, the shape of the droplet deviates not too much from a segment of a sphere, the contact line is a circle with radius s and we can use Eq. 1 to express V in terms of s and the contact angle θ which is in this context defined as the arithmetic mean of the receding and the advancing contact angle:
Moreover, if the droplet is quasi-spherically shaped, we may conclude w = s and k = 2 (see Petrissans and Cscapo 2003), which we shall assume in the sequel. Then, insertion of (1) into (13) and (14) allows us to solve these expressions for the contact circle radii s at which the equals signs in (13) and (14) are realised. Employing the definition
and the relations
conditions (a) and (b) can be stated as follows:
-
(a)
Downslide sets in if s > s σ, where
$$ s_{\sigma} := \frac{l (1+\cos\theta)}{ \sqrt{2+\cos\theta}} \sqrt{\frac{2}{\pi} \frac{\sin\chi}{\sin\alpha}} $$(18) -
(b)
Detachment occurs if s > s g , where
$$ s_g := \frac{l (1+\cos\theta)}{ \sqrt{2+\cos\theta}} \sqrt{\frac{\cos\chi}{\cos\alpha}} $$(19)
Meaningful ranges of θ and χ are: 0 ≤ θ ≤ π and 0 ≤ χ ≤ π/2. The latter is equivalent to 0 ≤ θa − θr ≤ π.
Whether a droplet of contact radius s detaches itself from or slides down along the inclined plane depends on the relation between s, s σ and s g : for s > s g > s σ, sliding sets in, for s > s σ > s g , however, detachment occurs.
The relation between s σ and s g reduces via (18) and (19) to a relation between α and χ:
2.2.2 Critical Volume of a Hanging Droplet, Contact Angle and Gliding Angle
Combination of (18) and (19) with (1) results in an expression for the critical droplet volume:
The condition that a pending droplet starts to move (either by downslide or by detachment) can be stated as V > V c. Critical droplet volumes V c(α) for a few combinations of θ and χ are depicted in Fig. 4. This figure illustrates also that the curves V c(α) exhibit maxima at \(\alpha_{\rm m} = \arctan\left(\frac{2}{\pi} \tan\chi\right).\)
Critical volume V c of a hanging droplet (solid lines) and of a sitting droplet (broken lines) as a function of the inclination angle α. Droplets characterised by (V, α)-pairs lying below a curve (V denotes the volume of the droplet) remain immobile, whereas droplets characterised by a (V, α)-pair above this curve either detach from the leaf and fall down (curve segments to the left of the cusps) or slide down while keeping contact with the inclined leaf (curve segments to the right of the cusps). a The different curves are generated by insertion of the values (θ, χ) = (60°, 20°), (90°, 20°), (30°, 20°) and (120°, 20°) (from top to bottom) into expression (22). b The different curves are generated by insertion of the values (θ, χ) = (60°, 30°), (60°, 20°) and (60°, 10°) (from top to bottom) into expression (22)
Notice, that the expression in braces equals the volume V m (defined in (9)) which emerged in the context of droplets hanging down from a horizontal plane. In order to clarify the role of χ with respect to droplet motion we divide the equation V = V c, which separates immobile (V < V c) and falling or sliding (V > V c) droplets, by V m (see (9)). It then becomes feasible to reformulate the conditions for droplet (im-)mobility in terms of relations between the inclination angle α and the half-difference of advancing and receding contact angle χ: depending on the value of V/V m, the pair of curves
divides the (χ, α)-plane into either (a) three sections, if V is in the interval \( 0 < V \le (2/\pi )^{{3/2}} V_{{\text{m}}} \approx 0.5V_{{\text{m}}} \) (Fig. 5a), or (b) two sections, if \( (2/\pi )^{{3/2}} V_{{\text{m}}} < V < V_{{\text{m}}} \) applies (Fig. 5b). (χ, α)-pairs lying between the two curves indicate that a droplet of volume V remains immobile. Droplets (of this same volume) characterised by a (χ, α)-pair above/left of the upper curve slide down, droplets below/right of the lower curve detach from the plane and fall down.
Areas of moving (α > γ and α < δ) and immobile (γ < α < δ) droplets hanging from a plane which is inclined against the horizontal by an angle α. χ denotes the half-difference of advancing and receding contact angle. γ(α, χ, V) and δ(α, χ, V) denote gliding angle and “detachment angle” defined in Eqs. 24 and 25. Immobile droplets are related to (χ, α)-pairs lying within the tetragon. Droplets characterised by (χ, α)-pairs outside the tetragon either slide down (area denoted α > γ) or detach from the plane and fall down (area denoted α < δ). a For \( V_{1} = {\text{0}}{\text{.14}}V_{{\text{m}}} < (2/\pi )^{{3/2}} V_{{\text{m}}} \) the curves γ(α, χ, V 1) and δ(α, χ, V 1) form a “tetragon of immobility” which covers the complete interval 0 < α < π/2. Hence, by adjusting χ appropriately, a droplet of this volume can be made to adhere to an arbitrarily inclined plane. b For \( V_{2} = {\text{0}}{\text{.7}}V_{{\text{m}}} > (2/\pi )^{{3/2}} V_{{\text{m}}} \) the curves γ(α, χ, V 2) and δ(α, χ, V 2) intersect and form a “triangle of immobility” which covers only part of the interval 0 < α < π/2. Hence, droplets of this volume cannot be kept immobile for every inclination 0 < α < π/2, even if χ can be arbitrarily chosen. The broken curve indicates whether a droplet slides down (α > γ) or detaches (α < δ) from the plane
The important conclusion is, that droplets of a given volume that fall into category (a) can be made to adhere to an arbitrarily inclined plane if χ can be adjusted appropriately, whereas droplets belonging to category (b) cannot be kept immobile for every inclination 0 < α < π/2, even if χ can be arbitrarily chosen.
It is thus justified to identify the upper expression of (23) with the gliding angle γ (the angle of inclination of an object that leads to the rolling off of drops lying upon the object) and its lower counterpart with a “detachment angle” δ (the angle of inclination of an object that leads to the detachment of drops from the object). Upon insertion of (9) expression (23) transforms to
where it is implicit that the condition V ≤ V m should be fulfilled, if (24) and (25) are applied to hanging droplets. If so, the condition that a droplets starts to slide down can be expressed by the statement α > γ, and the condition that a droplets starts to detach from the plane is equivalent to α < δ. Droplet immobility is realised where γ < α < δ applies (cf. Fig. 5). If δ > γ is valid (as in the upper, right part of Fig. 5b) immobile droplets cannot exist.
2.2.3 Sitting Droplet, Conditions for Downslide
A droplet sitting on the upper surface of an inclined leaf cannot choose between “downslide” and “free fall” (although free fall may eventually become an option, if the droplet reaches the leaf margin). Thus, the lower line of (22) and “detachment condition” (25) become void. The condition that a droplet starts to move can be expressed either by the statement V > V c, where
or by the condition γ > α (see (24)) which is in this case valid without the restriction V ≤ V m.
Similarly, as in the case of the hanging droplet, an increase of droplet volume brings about a drastic decrease of the (χ, α)-area wherein droplets remain sessile (Eq. 24; Fig. 6).
Areas of moving and immobile droplets sitting on a plane which is inclined against the horizontal by an angle α. χ denotes the half-difference of advancing and receding contact angle. The curves γ(α, χ, V k ) (k = 1, …, 4) represent the gliding angle defined in Eq. 24 for the droplet volume V k . Immobile droplets are related to (χ, α)-pairs lying above/to the left of a curve related to a given V k , droplets characterised by (χ, α)-pairs below/to the right of the same curve slide along the plane. For \( V_{1} = {\text{0}}{\text{.14}}V_{{\text{m}}} < (2/\pi )^{{3/2}} V_{{\text{m}}} \) the curve covers the complete interval 0 < α < π/2. Hence, by adjusting χ appropriately, a droplet of this volume can be made to adhere to an arbitrarily inclined plane. The curves γ(α, χ, V k ) related to \( V_{2} = {\text{0}}{\text{.7}}V_{{\text{m}}} > (2/\pi )^{{3/2}} V_{{\text{m}}} \), V 3 = V m and V 4 = 8 V m, however, cover only part of the interval 0 < α < π/2. Hence, droplets of these volumes cannot be kept immobile for every inclination 0 < α < π/2, even if χ can be arbitrarily chosen
The main result of this section concerns the dependence of the critical volume V c(θ, χ, α) on the contact angle θ, the half-difference of advancing and receding contact angle χ and the inclination α of the plane to which the hanging or sitting droplet is attached (cf. Figs. 4, 5):
-
The critical volume shows a maximum with respect to θ at \(\theta_{V_{\rm m}} =\hbox{arccos}(\sqrt{2}-1) \approx{65.5}\deg\) (for constant values of χ and α, Fig. 2b illustrates—apart from a constant factor—the θ-dependence of V c(θ, χ, α)).
-
For sitting droplets (and for hanging droplets, provided \(\tan\alpha > \frac{2}{\pi} \tan\chi\)) the critical volume increases with increasing χ.
-
For sitting droplets (and for hanging droplets, provided \(\tan\alpha > \frac{2}{\pi} \tan\chi\)) the critical volume decreases with increasing α.
Consequently, if a leaf surface wants to dispose of hanging or sitting water droplets for a given leaf inclination α, it has two options: (1) it may minimise contact angle hysteresis (i.e. aiming for χ → 0), or (2) it can try to produce a contact angle which either much smaller or much larger than the value \(\theta_{V_{\rm m}} \) ≈ 65.5° for which V c attains its maximum. Minimising χ is probably the more promising choice, since it has—according to Fig. 4 or Eq. 22—the effect of shifting the maxima of the V c-curves (which exist for hanging droplets only) to smaller values of α, thereby enhancing the tendency to detach or slip down of a droplet.
Contrariwise, if a leaf wants to store much water it should try to arrange for (1) a large angle χ and (2) a contact angle close to \(\theta_{V_{\rm m}} \) ≈ 65.5°.
3 Maximum Storage Capacity and Optimum Spacing of Droplets
Consider a leaf that wants to store as much water as possible in the form of droplets attached to its lower or upper surface. Two questions arise: (1) which geometric pattern should the droplets form, and, (2) exists an optimum contact angle?
Obviously, the optimum pattern is realised by partitioning the leaf surface into hexagons and “inscribing” into each of them one droplet. If the contact angle is in the range 0 ≤ θ ≤ π/2 the contact circle represents the greatest lateral extension of the droplet, hence the radius of the hexagon’s incircle should equal s. For π/2 ≤ θ ≤ π, however, R > s, thus the incircle radius should equal R. The respective areas amount to
In order to calculate the contact angle related to maximum storage capacity, we form the quantity \(\mu:= [\hbox {maximum water volume stored in one droplet}]/[\hbox {leaf area required for one droplet}].\)
3.1 Horizontal Plane
Recalling that the volume of hanging droplets exhibits a maximum value V m which depends, according to (9), on the contact angle θ, we find from (6), (8), (9) and (27)
It appears that μm features a maximum with respect to θ which is located at
i.e. in the hydrophobic range of contact angles. Insertion into (28) produces
Since droplets sitting on a horizontal plane cannot be detached by gravitation, their volume is—in principle—unlimited; that is, both s and R can be increased (almost) indefinitely. Forming μ = V/A hex from expressions (1) and (27) results in
The absence of θ-dependent upper limits for s and R effects the θ-dependence of μ (compared to μm): other than (28), expression (31) exhibits no maximum with respect to θ.
3.2 Inclined Plane
Assuming that the shape of droplets attached to an inclined plane deviates not much from a segment of a sphere, expression (31) is valid, provided that V < V c (where V and V c are defined in (1) and (22), respectively).
In the limit V = V c, expression (27) assumes upon use of (22) (31) (respectively, (26)), (18) (19) and (27) the form
Notice that the θ-dependence of μc is contained in μm(θ) (as given in (28)). Therefore, it shares with μm(θ) the maximum described in (29). As it stands, expression (32) is valid for hanging droplets of critical (maximum) volume V c. Sitting droplets of volume V c are represented by the upper line of (32), only.
4 Lifetime of a Droplet
In Sect. 3 we explored the maximum storage capacity of a leaf. In this section we evaluate (1) the lifetime of a droplet subjected to evaporation, and (2) whether its lifetime depends on its radius and on the leaf contact angle.
4.1 Horizontal Plane
A droplet which is attached to a leaf loses water by evaporation through the droplet’s water/air interface M (see (3)). This particle loss leads to a decrease of the droplet volume V, according to
For what follows, we assume that the evaporation flux j M is a constant with respect to t and θ. Applying textbook thermodynamics (e.g. Reif 1974; Atkins 1998), j M can—under these assumptions—be expressed as
D wv denotes the diffusional constant of water vapour in air, V wv the molar volume of water vapour, b is the thickness of the boundary layer surrounding the leaf. c wv denotes the atmospheric molar concentration of water vapour, and c sat the saturation value related to it.
Employing the differentiation rule dV(s)/dt = (dV/ds) (ds/dt) and expressions (1)–(3), Eq. 33 becomes, after a few rearrangements, a simple differential equation for s(t)
with the solution
where s 0 denotes the radius of the contact line at time t = 0 when evaporation and/or absorption set in (cf. Fig. 1). The lifetime τ of the droplet is obtained by letting s = 0 in (36) and solving for t,
If the initial radius of the contact line attains the value s m (corresponding to the maximum volume V m(θ) of a hanging droplet), the droplet’s lifetime becomes
It is interesting to calculate the contact angle θτ for which the lifetime τm becomes a maximum. It turns out that it depends neither on l nor on j M. Its value amounts to
which is in the hydrophilic range of contact angles. Upon insertion of θτ into τm we find
An explicit expression for V(t) results from insertion of (36) into (1):
4.2 Inclined Plane
If the plane is inclined, the basic Eq. 33 remains valid both for hanging and sitting droplets. Treating the droplets as quasi-spherical segments, the same reasoning as above applies with the same results regarding the t-dependance of s and V and the droplet lifetime (Eqs. 36, 37 and 41, respectively).
In the case of hanging droplets we have to take into account that the initial value s 0 of the contact circle should—due to the inclination of the leaf—fulfill the conditions s 0 < s σ and s 0 < s g [see (18) and (19)].
If a hanging droplet is initially of maximum volume (i.e. s 0 = s σ or s 0 = s g , depending on the values of the inclination angle α and on the half-difference of advancing and receding contact angle χ), τ simplifies to
where the expression in braces is just τm, the lifetime of a droplet hanging from a horizontal plane.
5 Discussion
The easiness with which water drops roll off a surface is mainly dependent on the contact angle hysteresis of a surface, and not on the static contact angle. The smaller the contact angle hysteresis the easier a drop will roll off the surface. Thus, hydrophobicity does not necessarily lead to a low stickiness of drops to the surface. To determine the stickiness of drops to surfaces it is not sufficient to measure the static contact angle θ. It is necessary to also measure χ. Potential benefits of efficient shedding of drops for a leaf are frequently discussed with respect to self cleaning. Pathogens, such as fungal spores, are easily washed off then, or are prevented from germination on the constantly dry surface (Barthlott and Neinhuis 1997). If a plant species pursues the strategy to efficiently get rid of water drops upon its surface, it should minimize χ. This quantity is usually influenced by the often heterogeneous nature of surfaces, with respect to surface micro/nanostructure and/or local contact angle variations (Quéré 2008). In leaves, these surface effects will be mostly created by cuticle wax structures and/or trichomes.
Another benefit has to do with photosynthesis since the development of a water film above stomata, the gas exchange pores of a leaf, is deleterious for photosynthesis. To keep stomata free from a water film, it is beneficial—besides to get rid of the drops by a low χ—to reduce the contact area between a droplet and the surface. Consequently, high water repellency, together with low stickiness for drops, is expected for species in foggy habitats and especially for stomatous leaf sides (frequently the abaxial side). Holder (2007) found that for different forest species θ tended to be unexpectedly low for many cloud forest species. He discussed this result as being caused by the high erosion of cuticle waxes due to intense precipitation. However, since χ was not determined in Holder’s study, leaves may pursue also other strategies by their surface properties than easy shedding of spherical drops. For example, it may be speculated that fog harvesting species are interested in collecting large drops before they roll off the leaf and fall to the ground. Fog drip can be an important source of water input (Feng et al. 2008). The shedding of large drops is expected to be more efficient in wetting the soil around the plant than the shedding of many small or very small droplets because large drops have a higher potential for throughfall. Furthermore, due to their low surface-to-volume ratio, they will resist evaporation much more strongly than small droplets. Maximum drop size can be achieved with a θ ≈ 65.5°. A good ability for water retention may have also other beneficial aspects. There are various plants that are able to absorb water via the leaves. This was unambiguously demonstrated for Californian redwoods (Burgess and Dawson 2004), but also indicated for other species (Breshears et al. 2008). If water absorption by leaves represents an important water source for a species, then it should be expected that both a good wetting behavior (low θ and a high χ) leading to a high persistence of the water on the leaf is beneficial. This would then prolong the water amount and the time interval available for absorption. The highest leaf storage capacity of a leaf would be obtained with a contact angle of θ ≈ 111.6° and a high χ. For maximum persistence of a drop under given meteorological conditions, the contact angle should amount to θ ≈ 77.5°.
Furthermore, nutrient input via aerosols are important for many ecosystems, and it was shown that hygroscopic mineral salts can be found upon leaf surfaces (Burkhardt 2010). Absorption not only of water but also of minerals dissolved in leaf surface water may, therefore, be of substantial importance for species living on nutrient-poor soils (Burkhardt 2010) and a good water retention should be also beneficial in these cases.
Leaf surface properties do not only affect the hydrological cycle via canopy interception but can also be important for the plant. The ecophysiological interrelationships between leaf surface and drops can be manyfold, and may additionally be changed by external factors, such as dust particles, abrasion or insect-mediated structural changes. Furthermore, the mechanical impacts of wind currents or animal activities upon leaves will frequently be sufficient to lead to detachment of drops that otherwise would be attached quite firmly to the leaf surface. The consequences and impacts of the behaviour of deposited drops upon leaf surfaces are complex for a considered species and depend on its habitat, ecological niche and other ecophysiological traits. Often, an interrelationship to any vital function cannot be provided. The ability of hydrophobic petals, for example, to retain a water droplet firmly, even if upside down (Petal Effect) (Feng et al. 2008), can presently not be explained as being of any adaptive value. Considering the various complex interrelationships of leaves with their environment, the interaction of leaf surfaces with water is a fascinating and important topic that deserves further attention.
References
Aboal, J.R., Jiménez, M.S., Morales, D., Hernández, J.M. (1999), Rainfall interception in laurel forest in the Canary Islands. Agricultural and Forest Meteorology 97, 73–86.
Aston, A.R. (1979), Rainfall interception by eight small trees. Journal of Hydrology 42, 383–396.
Atkins, P., Physical Chemistry (W.H. Freeman, New York 1998).
Barthlott, W., Neinhuis, C. (1997), Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202, 1–8.
Breshears, D., McDowell, N.G., Goddard, K. L., Dayem, K. E., Martens, S. N., Meyer, C. W., Brown, K. M. (2008), Foliar absorption of intercepted rainfall improves woody plant water status most during drought. Ecology 89, 41–47.
Burgess, S.S.O., Dawson, T.E., (2004), The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration. Plant, Cell and Environment 27, 1023–1034.
Burkhardt, J. (2010), Hygroscopic particles on leaves: nutrients or desiccants? Ecological Monographs, 80, 369–399.
Calder, I. (1990), Evaporation in the uplands. John Wiley and sons Inc., Chichester. 149 pp.
Calies, M., Quéré, D. (2005), On water repellency. Soft Matter 1, 55–61.
Dimitrakopoulos, P., and Higdon, J.J.L. (1998), On the displacement of three-dimensional fluid droplets from solid surfaces in low-Reynolds-number shear flows, J. Fluid Mech. 377, 189–222.
Dimitrakopoulos, P., and Higdon, J.J.L. (1999), On the gravitational displacement of three-dimensional fluid droplets from inclined solid surfaces, J. Fluid Mech. 395, 181–209.
Dingman, S., Physical hydrology (Prentice Hall, Upper Saddle River 2002).
Extrand, C.W., and Kumagai, Y., (1995), Liquid drops on a inclined plane: the relation between contact angles, drop shape, and retentive force. Journal of Colloid and Interface Science 170, 515–521.
Feng, L., Zhang, Y.A., Xi, J.M., Zhu, Y., Wang, N., Xia, F., Jiang, L. (2008), Petal effect: Two major examples of the Cassie-Baxter model are the Petal effect and Lotus effect. A superhydrophobic state with high adhesive force. Langmuir 24, 4114–4119.
Gash, J.H.C. (1979), An analytical model of rainfall interception by forests. Journal of Hydrology 105, 43–55.
Glasner, K.B. (2007), The dynamics of pendant droplets on a one-dimensional surface, Physics od Fluids 19, 102–104.
Herwitz, S.R. (1985), Interception storage capacities of tropical rainforest canopy trees. Journal of Hydrology 77, 237–252.
Hörman, G., Branding, A., Clemen, T., Herbst, M., Hinrichs, A. (1996), Calculation and simulation of wind controlled canopy interception of beech forest in Northern Germany. Agricultural and Forest Meteorology 79, 131–148.
Holder, C.D. (2007), Leaf water repellency of species in Guatemala and Colorado (USA) and its significance to forest hydrology studies. Journal of Hydrology 336, 147–154.
Muzylo, A., Llorens, P., Valente, F., Keizer, J.J., Domingo, F., Gash, J.H.C. (2009), A review of rainfall interception modelling. Journal of Hydrology 370, 191–206.
Petrissans, M., and Cscapo, E. (2003), Retention of glycerol sessile drop on MDF wood material, Holz als Roh- und Werkstoff, 61, 12–116.
Podgorski, T., Flesselles, J.-M., and Limat, L. (2001), Corners, Cusps, and Pearls in Running Drops, Phys. Rev. Lett. 87, 036102.
Quréré, D. (2008), Wetting and roughness. Annual Review of Materials Research 38, 71–99.
Reif, F., Fundamentals of statistical and thermal physics (McGraw-Hill, New York 1974).
Shirtcliffe, N.J., McHale, G., Newton, M.I. (2009), Learning from superhydrophobic plants: The use of hydrophilic areas on superhydrophobic surfaces for droplet control. Langmuir 25, 14121–14128.
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research by a grant to A. R. -N., within the project 02WT0906.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konrad, W., Ebner, M., Traiser, C. et al. Leaf Surface Wettability and Implications for Drop Shedding and Evaporation from Forest Canopies. Pure Appl. Geophys. 169, 835–845 (2012). https://doi.org/10.1007/s00024-011-0330-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00024-011-0330-2