Abstract
Deficiency of decidual NK (dNK) cell number and function has been widely regarded as an important cause of spontaneous abortion. However, the metabolic mechanism underlying the crosstalk between dNK cells and embryonic trophoblasts during early pregnancy remains largely unknown. Here, we observed that enriched glutamine and activated glutaminolysis in dNK cells contribute to trophoblast invasion and embryo growth by insulin-like growth factor-1 (IGF-1) and growth differentiation factor-15 (GDF-15) secretion. Mechanistically, these processes are dependent on the downregulation of EGLN1-HIF-1α mediated by α-ketoglutarate (α-KG). Blocking glutaminolysis with the GLS inhibitor BPTES or the glutamate dehydrogenase inhibitor EGCG leads to early embryo implantation failure, spontaneous abortion and/or fetal growth restriction in pregnant mice with impaired trophoblast invasion. Additionally, α-KG supplementation significantly alleviated pregnancy loss mediated by defective glutaminolysis in vivo, suggesting that inactivated glutamine/α-ketoglutarate metabolism in dNK cells impaired trophoblast invasion and induced pregnancy loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous abortion occurs in 10% of clinically recognized pregnancies in humans [1]. A variety of studies have identified various causes (e.g., chromosomal abnormalities, endocrine abnormalities, uterine anomalies, infectious etiologies, thrombophilia and immune factors) of spontaneous abortion [2]; however, the cases of a large proportion of spontaneous abortions remain unexplained.
Decidualization denotes the transformation that the stromal compartment of the endometrium must undergo to accommodate a successful pregnancy [3]. Additionally, there is a large number of peripheral CD56dimCD16+CXCR4+ NK cells with high cytotoxicity, which are recruited into the decidua and further differentiate into decidual CD56brightCD16− NK cells during early pregnancy [4, 5]. More importantly, these decidual NK (dNK) cells with a high capacity to secrete various cytokines and chemokines constitute approximately 70% of the lymphocytes in the decidua and play important roles in maintaining immune tolerance at the maternal–fetal interface, promoting the decidualization, remodeling of decidual vessels, and the development of placenta and embryo growth, contributing to a successful pregnancy [6,7,8,9,10]. Deficiencies in NK-cell number and function are associated with reproductive diseases and pregnancy complications, including female infertility, spontaneous abortion, intrauterine growth restriction (IUGR) and preeclampsia [8,9,10,11,12]. Unlike dNK cells, there were very limited numbers of ILCs in decidua during early pregnancy, and no significant change of ILCs in decidua between normal and spontaneous abortions [9]. With the development of single cell sequencing technology at the maternal–fetal interface, the characteristics of dNK cells have been explored more deeply. dNK cells were divided into different subgroups based on gene expression, including resting NK (MKI67− TOP2A−), proliferating NK (MKI67+ TOP2A+), peripheral NK (CD16+PLAC8+) and dNK1 (CD39+CYP26A1+B4GALNT1+), dNK2 (ANXA1+ITGB2+) and dNK3 (CD160+KLRB1+CD103+CD127−) [13,14,15,16]. Recently, emerging evidences show that metabolic reprogramming plays a crucial role in the differentiation and functional fate of NK cells, especially in the tumor microenvironment [17,18,19,20]. A heightened capacity for glucose metabolism through glycolysis and oxidative phosphorylation (OXPHOS) supports NK cells with robust cytotoxic capacity. dNK1 cells, which contain more cytoplasmic granules and express higher levels of PRF1, GNLY, GZMA and GZMB, also show higher levels of enzymes involved in glycolysis [14]. Despite this, the role of metabolism in regulating the function and differentiation of decidual NK cells is still very limited. The specific mechanism by which metabolic factors exert their remains to be further explored.
As the most abundant and versatile amino acid in the body, the rate of glutamine consumption by immune cells is similar to or greater than that of glucose [21, 22]. Glutamine is an essential immunonutrient for lymphocyte proliferation and cytokine production, and the phagocytic and secretory activities of macrophages [23,24,25]. It has been reported that glutamine maintains the cell growth and cell response of mouse NK cells in cMyc-dependent and glutaminolysis-independent manners [26]. However, the role of glutamine in NK cell phenotype and function is rarely reported, especially in human tissues.
Therefore, the purpose of this study was to investigate the metabolic characteristics and regulatory mechanism of dNK cell differentiation and function in early pregnancy, and the glutamine metabolism disorders of dNK cells in the pathogenesis of unexplained recurrent spontaneous abortion, and to explore potential intervention strategies.
Materials and methods
Tissue collection
All of the tissues, including decidua, endometria and serum, were collected from patients in the Obstetrics and Gynecology Hospital of Fudan University from February 2018 to June 2021 and were approved by the Ethical Committee (No. 2018-25). Informed consent was obtained from the patients. Decidual tissues of normal early pregnant women (n = 108, age 20–43 years, gestational age of 6–10 weeks) were obtained from patients admitted to the hospital to terminate pregnancy, with no history of spontaneous abortion, stillbirth or other adverse pregnancies. The decidua of unexplained recurrent spontaneous abortion patients (n = 10, age 25–37 years, gestational age of 6–9 weeks) were collected from those who experienced two or more consecutive spontaneous abortions, without distributing factors such as fetal chromosome abnormality, reproductive tract infection or cervical incompetence. Endometrium was collected from patients (age 34–51 years, n = 15) who were diagnosed with curettage or undergoing hysterectomy for benign reasons unrelated to endometrial dysfunction as healthy controls. All of the tissues were stored in ice-cold DMEM/F-12 (HyClone, USA) under sterile conditions and transported to the laboratory in 1 h to further isolate endometrial stromal cells (ESCs), decidual stromal cells (DSCs), and dNK cells as previously described [27]. Peripheral blood samples were obtained from healthy volunteers (age 26–39 years n = 36) and transported to the laboratory immediately under sterile conditions to further isolate peripheral NK (pNK) cells as previously described [27, 28]. Decidual tissue was digested, filtered and density gradient centrifuged to obtain decidual immune cell (DIC). Peripheral blood was density gradient centrifugated to obtain peripheral blood mononuclear cells (PBMCs). Then, pNK and dNK cells were isolated from PBMCs or DIC according to the protocol of the NK-cell isolation kit (MiltenyiBiotec, Bergisch Gladbach, Germany). In brief, immune cells were labeled with NK-cell biotin-antibody cocktail and NK-Cell microbead cocktail separately, incubated in the refrigerator (2–8 °C), and separated through the magnetic field of the MACS separator. Then NK cells were enriched in the unlabeled cells. The purity of isolated NK cells was detected by flow cytometry. NK cells were used in further studies only if the purity was above 90%.
Cell culture
pNK and dNK cells were cultured with or without ESCs, DSCs, or a human trophoblast cell line (HTR-8/SVneo, purchased from the Chinese Academy of Sciences Cell Bank, Shanghai, China) with DMEM/F12 (HyClone Laboratories, Logan, UT, USA) containing 10% fetal bovine serum (Gibco Cell Culture, Carlsbad, CA, USA) and 1% antibiotic–antimycotic (Gibco Cell Culture, Carlsbad, CA, USA). NK92 cells (a cell line of NK cells derived from peripheral NK cells of a non-Hodgkin lymphoma patient) transfected with IL-2 were provided by Professor Qiang Fu at the Department of Immunology, Binzhou Medical College and were cultured with L500 Serum-Free Medium for Lymphocytes (Dakewe Biotech Co., Ltd., Shanghai, China) containing 20% fetal bovine serum and 1% antibiotic–antimycotic. Cells were passaged every two or three days depending on their densities. The temperature of the incubator was set as 37 °C, and the CO2 concentration was 5%. The O2 concentration under normoxic conditions was 20%, and under hypoxic conditions, it was 1%.
LC–MS
The pNK and dNK cells treated in different groups were collected. After washing with PBS, the cells were centrifuged at 1500 rpm at 4 °C for 10 min, and the deposit was fully extracted with 80% methanol precooled at − 80 °C overnight. The supernatant was collected and transferred to another EP tube and dried in a rotary volatilizer. The dried powder was collected for mass spectrometry detection (TSQ-Vantage, Thermo) by the Institute of Biomedical Sciences, Fudan University. Every sample was tested three times, and the results were analyzed in Analyst Software.
Glutamine detection
The glutamine concentration was detected with a glutamine/glutamate determination kit (#GLN1, Sigma-Aldrich Co. LLC, USA) according to a standard procedure. In brief, cell lysate or tissue grinding fluid was first deaminated, and then dehydrogenated, and the absorbance of the reaction products was measured. The glutamine concentration was calculated according to the curve of glutamine standards.
Flow cytometry
In animal experiments, mouse uteruses were mechanically cut, digested with collagenase, and filtrated by sieve to prepare monoplast suspensions. Cells in different groups were collected by centrifugation, washed with PBS and then incubated with different panels of antibodies. The intracellular molecules were stained after fixation and permeation by FOXP3 Fix/Perm Buffer (BioLegend).
Antibodies used in the research are as follows: APC-conjugated anti-human CD56 (#318310), BV421-conjugated anti-human CD45 (#304032), APCCY7-conjugated anti-human CD3 (#344818), FITC-conjugated anti-human CD16 (#360716), PE-conjugated anti-human NKP30 (#325208), PECY7-conjugated anti-human NKG2D (#320806), BV510-conjugated anti-human IFN-γ (#502532), BV421-conjugated anti-human perforin (#308122) and PE-conjugated anti-human Ki-67(#350504, all from Biolegend); rabbit anti-glutaminase antibody (#ab93434) and goat secondary antibody to rabbit IgG Alexa Fluor 647(#ab150079, both from abcam); carboxyfluorescein diacetate succinimidyl ester (CFSE, eBioscience, San Diego, CA, USA), and Annexin V/7-AAD apoptosis kit (BD Biosciences, San Jose, CA, USA); and APCCY7-conjugated anti-mouse CD3 (#100222), Percp-conjugated anti-mouse CD45 (#103132), PE-conjugated anti-mouse NK1.1 (#108707, from Biolegend), Alexa Fluor 488-conjugated anti-mouse IGF-1 (#NBP2-34679AF488, Novus Biologicals, CO, USA) and APC-conjugated anti-mouse GDF-15 (#32572–05161, Assaypro LLC, MO, USA).
Data were collected in a FACS Calibur flow cytometer (Beckman Coulter CyAn ADP or Beckman Coulter Cytoflex, North Carolina, USA) and analyzed with FlowJo 7.6 (BD Biosciences, San Jose, CA, USA). Each experiment was performed three times independently. Statistical analysis was performed by using isotype matched controls as references. Typically, less than 1% positive cells were permitted beyond the statistical marker in the appropriate controls.
Immunofluorescence
The paraffin sections were dehydrated in graded ethanol, retrieved by citric acid buffer or Tris–EDTA buffer, and disposed with 3% hydrogen peroxide. Then, the section was incubated with 5% BSA at room temperature for 1 h, and incubated with anti-NCAM1 (CD56, 1: 200; abcam ab9018), anti-GLS (1:100; abcam ab93434), anti-cytokeratin 7 (1:4000; abcam ab181598), anti-F4/80 (1:500; abcam ab60343), anti-CD86 (1:300; abcam ab 119,857) or rabbit IgG isotypes at 4 °C overnight. After washing with PBS three times, the sections were incubated with fluorescence conjugated secondary antibody at room temperature for 1 h, and stained with DAPI for 10 min. The sections were observed, and pictures were taken under a fluorescence microscope (Leica, Munich, Germany).
RT-PCR
The total RNA of NK cells was extracted by RNAiso Plus reagent (TaKaRa Biotechnology) according to the manufacturer's instructions. Five hundred nanograms of RNA from every sample was reverse transcribed into cDNA using a reverse transcription kit (TaKaRa Biotechnology). Then, the transcription levels of genes were verified by RT‒PCR (ABI QuantStudio 6 Flex, USA). The fold change in gene expression was calculated using the change in cycle threshold value method (ΔΔCt). All values obtained were normalized to the values obtained for β-actin (ACTB). The primer sequences were synthesized by Sangon Biotechnology Co., Ltd. (Shanghai, China) and are listed in Supplementary Table 2.
Western blot
Cells were washed with cold PBS, lysed with lysis buffer (Beyotime Biotechnology, Shanghai, China), diluted with loading buffer (Beyotime Biotechnology) and heated to 95 °C for 10 min. Protein concentrations were detected by a BCA protein assay kit (Beyotime Biotechnology). For western blotting, equal amounts of protein were electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gels (Epizyme Scientific, Shanghai, China), transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), blocked with 5% non-fat milk for 2 h at room temperature, and incubated with corresponding primary SLC1A5 (1:1000, Cell Signaling Technology, D7C12), GLS (1:1000, abcam, ab93434), Actin (1:3000, abcam, ab8226), IGF-1 (1:500, BioVision, 5119–100), GDF-15 (1:500, GeneTex, 10A3) and HIF-1α (1:1000, abcam, ab179483) antibody overnight at 4 °C. The membrane was washed three times and incubated with HRP-linked Anti-rabbit IgG (1:3000, Cell Signaling Technology, 7074) for 1 h at room temperature. After washing for three times, the protein bands were wetted with Immobilon Western Chemiluminescent HRP Substrate (Millipore, Darmstadt, Germany) and detected by Luminescent Image Analyzer LAS 4000 (FUJIFILM, Japan).
Matrigel transwell assay
Matrigel (BD Bioscience) was diluted with DMEM/F12 at a ratio of 1:8, and 35 μL of diluted Matrigel was added to the transwell upper chamber (8 μm, Corning) and placed in a 24-well plate for 1 h at 37 °C. Two hundred microliters of HTR-8 suspension (2 × 104 [4] cells/well) without FBS was added to the upper chamber, and 600 μL medium containing 10% FBS or dNK cells (1 × 105 5 cells/well) in different groups or not was added to the lower chamber. The cells were treated as described in the article. After 48 h, the upper chamber medium and non-penetrating cells were gently wiped off. Cells were fixed with 4% paraformaldehyde for 30 min and stained with crystal violet for 20 min. Random photographs were taken under an inverted microscope (Leica, Munich, Germany), and 5 visual fields were counted in each chamber. The number of invaded cells was counted using the Qupath (Centre for Cancer Research & Cell Biology at Queen’s University Belfast).
ELISA
The concentrations of IGF-1, GDF-15 and FGF-19 were detected by ELISA (R&D Systems, No. DG100, DGD150 and DF1900). Supernatants of dNK cells in different groups were collected and detected according to the manufacturer's instructions.
Cytokine array
dNK cells treated with BPTES (10 μM, MedChemExpress) or 1‰ DMSO were centrifuged and the supernatant was collected. A cytokine array was performed by H-Wayen Biotechnology, Shanghai, China using the Human Cytokine Array AAH-BLG-507(RayBiotech, Norcross, GA, USA) as previously described [29].
Bioinformatics analysis
The intracellular sensing protein of α-KG was predicted based on the molecular structure (http://www.swisstargetprediction.ch). The protein interaction network was constructed using an online database (https://version11.string-db.org/). Transcription factor and target gene prediction was based on the databases TransFac (http://gene-regulation.com/pub/databases.html), TRED (http://rulai.cshl.edu/cgi-bin/TRED/tred.cgi?process=home) and TRRUST (https://www.grnpedia.org/trrust/).
The mRNA of pNK and dNK cells (n = 3) was collected, and transcriptome sequencing was performed by Shanghai Ritz Biotechnology Co., Ltd., as previously described [29].
Animal model and treatment
All of the mice used in this research were of the C57BL/6J strain (from Shanghai Jieijie Experimental Animal Co., Ltd.) and were raised in an SPF experimental animal facility. The Animal Care and Use Committee of Fudan University approved all animal protocols. Eight-week-old female mice and 8-week-old male mice were caged at a ratio of 2:1, and whether the female mice were pregnant was confirmed on the morning of the morning based on vaginal plugs (marked as 0.5 days of gestation). Pregnant mice were randomly divided into different groups, intraperitoneally injected with BPTES (#HY-12683, 12.5 mg/kg, MedChemExpress), EGCG (HY-13653, 10 mg/kg, MedChemExpress), DMOG (HY-15893, 40 mg/kg, MedChemExpress) or PBS at 0.5 days, 4.5 days, 7.5 days and 10.5 days of gestation, and fed a control diet or 5% calcium 2-oxoglutarate (FC40259, Sigma) supplementary diet (MolDiets, Shanghai, China). At 7.5 days of gestation, mice were sacrificed, and the numbers for embryo implantation, embryo absorption and IGF-1 and GDF-15 expression in uterine NK cells were recorded. Similarly, at 13.5 days of gestation, mice were sacrificed, and the number of embryos implanted, embryo absorption, placenta weight and crown-rump length were observed. In addition, the invasion depth of trophoblasts in the uterus was measured by immunofluorescence.
NK depletion and adoptive transfer
The depletion of NK cells in pregnant mice was performed by tail vein injection of anti-NK1.1 or isotype antibody (#108759 and #401508, from Biolegend, 50 μg in 200 μl PBS per mouse) at day 0.5 and day 4.5 of gestation, and adoptive transfer was conducted at Day 5.5 with spleen NK cells (106 [6] cells per mouse in 100 μl PBS) isolated from non-pregnant female mice that were consecutively intraperitoneally injected with Ctrl, BPTES or EGCG for 7 days. At 13.5 days of gestation, mice were sacrificed, and the number of embryos implanted, embryo absorption, placental weight and crown-rump length were observed.
Statistical analysis
All of the data are shown as the mean ± SEM or median and min to mix. Comparisons between two groups were analyzed by Student’s t test or the Mann‒Whitney U test. Comparisons among three or more groups were analyzed by one-way ANOVA. When the data were non-normally distributed, the Mann‒Whitney rank sum test, the Wilcoxon paired test or the Kruskal–Wallis test were generally used. Comparisons of rates or proportions among different groups were analyzed by the chi-square test. All of the analyses were performed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA) for Windows. Differences were considered to be significant at P < 0.05.
Results
Local unique microenvironment induces activated glutamine metabolism in dNK cells at the maternal–fetal interface
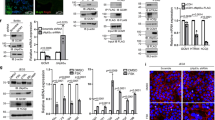
To evaluate the metabolite profiles and differences between peripheral blood NK (pNK) and dNK cells, NK cells from 10 normal early pregnant women were recruited. As shown, metabonomic results showed that there were more than 40 differential metabolites between pNK and dNK cells from normal early pregnant women (Fig. 1a), especially activated arginine/proline metabolism and alanine-aspartate/glutamate metabolism (Fig. 1b). In our data of single-cell RNA sequence (data not shown), we observed dNK cells rarely express ARG1 and P5CS (key factors for arginine/proline metabolism), but a high level of glutaminase (GLS). In both metabolic pathways, glutamine was the key metabolite, which was markedly increased in dNK cells (Fig. 1a, b). To explore the reasons for glutamine enrichment in dNK cells, we further compared the surrounding glutamine concentration, glutamine transport and endogenous synthesis of pNK and dNK cells. As shown, there was no significant difference in the normalized glutamine concentration between serum and decidual tissues from normal early pregnant women (Fig. 1c). As endogenous glutamine synthesis in NK cells was quite low (Supplementary Fig. 1a, b), the main glutamine transporters (SLC1A4, SLC1A5, SLC38A1, SLC38A2, SLC38A5, SLC38A7) of NK cells were detected [26]. We found that SLC1A5, but not SLC38A2, was increased in dNK cells (Fig. 1d), which may contribute to glutamine enrichment. More importantly, we observed that GLS expression was significantly increased in dNK cells compared with pNK and endometrial NK (eNK) cells (Fig. 1e, f). Additionally, the results of RT‒PCR showed that enzymes involved in glutamine metabolism, including glutaminolysis, glycolysis, transamination, and γ-aminobutyric acid (GABA) synthesis, were highly expressed in dNK cells (Fig. 1g), suggesting that dNK cells display an activated and unobstructed glutamate metabolism.
A unique local microenvironment induces activated glutamine metabolism in dNK cells at the maternal–fetal interface. a, b The metabolites in dNK and pNK cells from women with normal pregnancy (n = 10) were detected by LC‒MS, and the KEGG metabolic pathway of differential metabolites was analyzed. c Glutamine in serum and decidua from women (n = 6) with normal pregnancy was detected by the glutamine determination kit. d The expression of the glutamine transporters SLC1A5 and SLC38A2 in dNK (n = 5) and pNK (n = 8) cells from women with normal pregnancy was measured by RT‒PCR. e, f GLS expression in pNK and dNK cells from women (n = 6) with normal pregnancy, and endometrial NK (eNK) cells from control non-pregnant women (n = 6) was tested by flow cytometry and immunofluorescence (green: CD56; red: GLS). g The key enzymes related to glutamine metabolism in dNK and pNK cells from women (n = 3) with normal pregnancy were detected by RT‒PCR, and the relative expression is shown in the heatmap. h–j The effect of the coculture system, hypoxia and sex hormones (estrogen and progesterone) on SLC1A5 and GLS expression in NK92 cells was measured by western blot. ESC: coculture of ESC and NK92 cells; E2: estrogen; P4: progesterone. Data are presented as the mean ± SEM and were analyzed by t test, Mann‒Whitney U test or one-way ANOVA. *P < 0.05, ***P < 0.001, NS no significance
To explore the potential mechanism for the activation of glutamate metabolism in dNK cells during early pregnancy, a coculture system of NK92 cells and decidual stromal cells (DSCs) or human trophoblast HTR-8/SVneo cells was constructed in vitro. As shown, DSCs and embryo-derived trophoblast cells upregulated GLS expression in NK92 cells. Interestingly, estrogen upregulated GLS expression in NK92 cells in a dose-dependent manner, whereas progestogen downregulated GLS expression. To our surprise, SLC1A5 expression seemed to be not affected significantly by coculture, hypoxia or hormones, suggesting that the increased SLC1A5 expression in dNK cells may be induced by other factors (Fig. 1h–j). These findings demonstrate that glutamine metabolism is activated in dNK cells and is induced by the local environment at the maternal–fetal interface.
IGF-1+GDF-1+dNK cells induced by activating glutamine metabolism enhance trophoblast invasion
To investigate the role of glutamine, dNK cells were treated with or without the GLS inhibitor BPTES or cultured with glutamine-free medium. As shown, BPTES obviously decreased the expression of Ki67 and the cell size of dNK cells (Fig. 2a, b). In contrast, glutamine supplementation increased Ki67 expression and cell size (Fig. 2c, d). Additionally, both GLS inhibition and glutamine withdrawal significantly promoted the apoptosis of NK92 cells (Fig. 2e). Interestingly, dNK cells notably promoted the invasion of HTR-8/SVneo cells in vitro, which was significantly impaired when dNK cells were pretreated with BPTES (Fig. 2f). In vivo, trophoblast invasion was impaired in the BPTES-treated mice at D13.5 of pregnancy (Fig. 2g), suggesting that activated glutamine metabolism contributes to dNK cell survival and further enhances trophoblast invasion.
Glutamine metabolism promotes proliferation, inhibits apoptosis of NK cells, and contributes to the invasion of trophoblast cells. a–d Ki67 and cell size were detected by flow cytometry in dNK cells (n = 6) treated with control medium, BPTES (10 μM, 48 h), glutamine-free medium or glutamine (2 mM, 48 h). e Apoptosis was detected by flow cytometry in NK92 cells treated with control medium, BPTES, glutamine-free medium or glutamine. f The invasion of HTR-8 cells was detected by Matrigel invasion assay after coculture with or without control dNK cells or BPTES-pretreated dNK cells (n = 6) for 48 h. g The trophoblast infiltration depth in the placenta of pregnant mice treated with or without BPTES (12.5 mg/kg) was measured by immunofluorescence on D13.5 (on the 13.5th day of pregnancy, n = 6). The ratio of trophoblast infiltration (yellow line) to the depth of the entire placenta was analyzed. Data are presented as the mean ± SEM and were analyzed by t test, Mann‒Whitney U test or one-way ANOVA. *P < 0.05, **P < 0. 01, ****P < 0.0001
Further analysis of the cytokine array showed that there were 89 differential cytokines in Ctrl and BPTES-treated dNK cells. Among these cytokines, 21 were growth factors (Fig. 3a). Subsequently, 5 abundant cytokines with significant differences (IGF-1, GDF-15, VEGF-C, FGF-23, FGF-19) were selected, and the ELISA results showed that the secretion levels of insulin-like growth factor-1 (IGF-1), growth differentiation factor-15 (GDF-15) and fibroblast growth factor-19 (FGF-19) in dNK cells were decreased after BPTES treatment (Fig. 3b). In vivo, intraperitoneal injection of BPTES also decreased the expression of IGF-1 and GDF-15 in mouse uterine NK cells (Fig. 3c, d). Recombinant human IGF-1 protein and GDF-15 protein were found to promote the proliferation of HTR-8 cells within the range of certain concentrations (Supplementary Fig. 2a–c). Notably, treatment with IGF-1 or GDF-15 significantly enhanced the invasiveness of HTR-8/SVneo cells (Fig. 3e, f). The neutralization of IGF-1 and GDF-15 significantly restricted the stimulatory effect of dNK cells on trophoblast invasion (Fig. 3g, h). To confirm the effect of GDF-15, and avoid the contamination with TGF-β [30], we have added the experiment, and found that the pro-invasion effect of GDF-15 could be abrogated by anti-GDF-15 neutralizing antibody. Therefore, the effect of recombinant protein in our study was caused by GDF-15 (Supplementary Fig. 2d). These data suggest that dNK cells with activated glutamine metabolism promote the invasion of trophoblasts by secreting IGF-1 and GDF-15.
Glutamine metabolism in dNK cells promotes trophoblast invasion through IGF-1 and GDF-15. a, b A cytokine array was performed in dNK cells (n = 6) treated with BPTES (10 μM, 48 h) or not. The secretion levels of IGF-1, GDF-15, FGF19, FGF23 and VEGF-C were validated by ELISA. c, d After intraperitoneal injection with or without BPTES, IGF-1 and GDF-15 expression in uterine NK cells of pregnant mice (n = 6) on D7.5 was analyzed by flow cytometry. e, f The invasion of HTR-8 cells treated with or without IGF-1 (20 ng/ml, 48 h) or GDF-15 (50 ng/ml, 48 h) was observed by Matrigel invasion assay. g, h HTR-8 cell invasion in the presence of dNK cells treated with anti-IGF-1 (2 μg/ml, 48 h), anti-GDF-15 (5 μg/ml, 48 h) neutralizing antibody or isotype antibody (n = 6) was observed by Matrigel invasion assay. Data are presented as the mean ± SEM and were analyzed by t test, Mann‒Whitney U test or one-way ANOVA. *P < 0.05, **P < 0. 01, ***P < 0. 001, ****P < 0.0001
Glutamine/α-ketoglutarate metabolism promotes IGF-1+GDF-15+ dNK differentiation
To investigate whether glutamine can be utilized in the tricarboxylic acid cycle, we measured the oxygen consumption and acid production rate of NK cells after supplementation with different substrates. As shown, glutamine supplementation increased the oxygen consumption of NK92 cells immediately, and BPTES markedly inhibited this effect (Fig. 4a). Additionally, the availability of glucose influenced the utilization of glutamine. In the presence of glucose, NK cells were less dependent on glutamine (Fig. 4a). Glucose dramatically increased the glycolysis of NK cells, while glutamine was mainly involved in aerobic respiration (Fig. 4a). To further explore the mechanism of glutamine metabolism on the differentiation of IGF-1+GDF-15+dNK cells, the downstream metabolites of dNK cells treated with or without BPTES were analyzed. As shown, BPTES caused the accumulation of glutamine, a reduction in glutamate and α-ketoglutarate and a compensatory increase in acetyl coenzyme A and citrate (Fig. 4b). Further analysis showed that α-KG increased the secretion of IGF-1 and GDF-15 in a time- and/or dosage-dependent manner (Supplementary Fig. 3a–d), and supplementation with dimethyl alpha-ketoglutarate (DMKG) reversed the inhibitory effect of BPTES (Fig. 4c). Similarly, epigallocatechin gallate sulfate (EGCG, an inhibitor of glutamate dehydrogenase that converts glutamate into α-KG) significantly decreased IGF-1 and GDF-15 production, and DMKG also partly abolished this effect (Fig. 4d). Uterine NK cells from pregnant mice produced more IGF-1 and GDF-15 than those from non-pregnant mice (Fig. 4e, f). Intraperitoneal injection of EGCG impaired IGF-1 and GDF-15 expression in uterine NK cells, and α-KG supplementation in the diet partly rescued it in pregnant mice (Fig. 4e, f). Treatment with EGCG weakened the invasion of HTR-8/SVneo cells stimulated by dNK cells in vitro (Fig. 4g, h) and decreased the infiltration depth of trophoblasts in pregnant mice in vivo (Fig. 4i). These results indicate that α-KG derived from glutamine promotes IGF-1 and GDF-15 production in dNK cells and further enhances trophoblast invasion.
Glutamine/α-ketoglutarate metabolism promotes IGF-1+GDF-15+ dNK differentiation. a The oxygen consumption and extracellular acidification rate of NK92 cells after providing the substance shown in the picture were measured by the Seahorse XFe96 Analyzer. b The metabolites of dNK cells (n = 11) treated with or without BPTES were extracted and detected by LC‒MS. c, d The concentrations of IGF-1 and GDF-15 in the supernatant of dNK cells (n = 6) treated with BPTES or EGCG (50 μM, 48 h) with or without dimethyl-α-ketoglutarate (2 mM, 48 h) were tested by ELISA. e, f IGF-1 and GDF-15 expression in uterine NK cells of pregnant mice (n = 6) on D7.5 intraperitoneally injected with PBS or EGCG (30 mg/kg) with or without an α-KG diet (2%, w/w) was analyzed by flow cytometry. g, h HTR-8 cell invasion was observed in the coculture system with control or EGCG-pretreated dNK cells (n = 6). i The trophoblast infiltration depth in the placentas of pregnant mice treated with or without EGCG was measured by immunofluorescence on D13.5. The ratio of trophoblast infiltration (yellow line) to the depth of the entire placenta was analyzed. Data are presented as the mean ± SEM or median and min to mix and were analyzed by t test, Mann‒Whitney U test, one-way ANOVA or Wilcoxon test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS no significance
α-KG-triggered IGF-1 and GDF-15 production is dependent on HIF-1α degradation in dNK cells
To further explore the mechanism of α-KG on IGF-1 and GDF-15 expression, we predicted the intracellular sensory proteins of α-KG by Swiss Target Prediction (http://swisstargetprediction.ch/) (Supplementary Table 1), analyzed the interaction network of these sensing proteins with IGF-1 and GDF-15, and found that α-KG might regulate the expression of IGF-1 and GDF-15 through Egl Nine Homolog 1 (EGLN1)-hypoxia inducible factor-1α (HIF-1α) (Fig. 5a). Based on three databases, we predicted the potential transcription factors IGF-1 and GDF-15, and observed that most of these transcription factors were significantly upregulated in dNK cells (Fig. 5b). Further analysis showed that five of the transcription factors (TWIST1, TWIST2, EP300, ESR1, TP53) of IGF-1 and GDF-15 could be regulated by HIF-1α (Fig. 5c). It has been reported that α-KG promotes the hydroxylation and degradation of HIF-1α [31]. As an inhibitor of HIF prolyl hydroxylase, dimethyloxallyl glycine (DMOG) significantly increased the accumulation of HIF-1α and decreased the expression and secretion of IGF-1 and GDF-15 in dNK cells (Fig. 5d, e). In vivo, intraperitoneal injection of DMOG inhibited the expression of IGF-1 in uterine NK cells, while the expression of GDF-15 was not significantly changed (Fig. 5f, g). Additionally, DMOG suppressed the stimulatory effects of α-KG on IGF-1 and GDF-15 expression in dNK cells (Fig. 5h), suggesting that the positive regulation of IGF-1 and GDF-15 production by α-KG is dependent on HIF-1α degradation.
α-KG-triggered IGF-1 and GDF-15 production is dependent on HIF-1α degradation in dNK cells. a The protein interaction network between intracellular sensory proteins of α-KG and IGF-1 and GDF-15 was analyzed by Swiss Target Prediction (http://swisstargetprediction.ch/). b The expression of transcription factors regulating IGF-1 or GDF-15 was analyzed based on the transcriptome sequencing of dNK and pNK cells (n = 3). c The upstream transcription factors IGF-1 and GDF-15, which can be regulated by HIF-1α, were analyzed according to online databases. d The expression of HIF-1α, GDF-15, and IGF-1 was measured by western blot in dNK cells (n = 6) treated with or without DMOG (1 mM, 48 h). e The concentrations of IGF-1 and GDF-15 in the supernatant of dNK cells (n = 6) treated with or without DMOG were tested by ELISA. f, g After intraperitoneal injection with PBS or DMOG (40 mg/kg, n = 6), IGF-1 and GDF-15 expression in uterine NK cells of pregnant mice on Day 7.5 was analyzed by flow cytometry (n = 6). h The concentrations of IGF-1 and GDF-15 in the supernatant of dNK cells treated with DMOG, α-KG, or DMOG + α-KG or not were tested by ELISA (n = 6). Data are presented as the mean ± SEM analyzed by t test, Mann‒Whitney U test, or one-way ANOVA. *P < 0.05, **P < 0. 01, ***P < 0. 001, ****P < 0.0001, NS no significance
Defective glutamine/α-KG metabolism in dNK cells leads to pregnancy loss
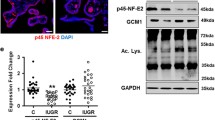
To evaluate the possible differences in glutamine metabolism in dNK cells between normal pregnancy and unexplained recurrent spontaneous abortion, we detected the glutamine concentration and key enzymes in dNK cells from women with normal pregnancy and unexplained recurrent spontaneous abortion. As shown, the level of glutamine and the expression of SLC1A5 and glutamate dehydrogenase (GLUD) were decreased in dNK cells from women with unexplained recurrent spontaneous abortion (Fig. 6a, b), along with a decrease in IGF-1 expression (Fig. 6c). In the pregnant mouse model, intraperitoneal injection of BPTES did not influence the number of embryos implanted, while the rate of embryo absorption was significantly increased in the BPTES-treated group on the 13.5th day of pregnancy (D13.5) (Fig. 6d–f). Additionally, BPTES significantly decreased the crown-rump length and placenta weight (Fig. 6e, g). Of note, EGCG injection led to a decrease in mouse embryo implantation number on D7.5, comparable embryo absorption on D13.5 (Fig. 6h, i), and significant decreases in crown-rump length and placental weight on D13.5 (Fig. 6j, k). These results suggest that α-KG deficiency due to defective glutamine metabolism impairs placental development and embryo growth and contributes to pregnancy loss.
Defective glutamine/α-KG metabolism leads to pregnancy loss. a–c The glutamine concentration, relative expression of glutamine metabolism enzymes, and expression of IGF-1, GDF-15 and HIF-1a were detected in dNK cells from women (n = 5) with normal pregnancy and recurrent spontaneous abortion. d–g After intraperitoneal injection with or without BPTES, the number of embryos implanted, embryo absorption rate, placenta weight and crown-rump length were observed in mice (n = 8) on D13.5. h–k After intraperitoneal injection with or without EGCG, the number of embryos implanted was counted on D7.5, and the embryo absorption rate, placenta weight and crown-rump length were observed in mice (n = 6) on D13.5. Data are presented as the mean ± SEM and were analyzed by t test, Mann‒Whitney U test or Chi-square test. *P < 0.05, **P < 0. 01, ***P < 0. 001, ****P < 0.0001, NS no significance
To further validate the glutamine metabolism of uterine NK cells in successful pregnancy, we depleted NK cells by tail vein injection of anti-NK1.1 antibody at D0.5 and D4.5 (Fig. 7a) and observed that depletion of NK cells significantly increased the embryo absorption rate and decreased the placental weight (Fig. 7b–e). Adoptive transfer of NK cells from BPTES- or EGCG-treated mice led to more embryo absorption and/or decreased placental weight compared with transfer of NK cells from control mice, especially NK cells from EGCG-treated mice (Fig. 7f–i). These results demonstrate that defective glutamine/α-KG metabolism in uterine NK cells increases the risk of pregnancy loss.
Impairment of glutamine/α-KG metabolism in uterine NK cells leads to pregnancy loss. a The procedure of NK depletion and adoptive transfer in mice. The donor mice accepted intraperitoneal injection for seven consecutive days with Ctrl, BPTES or EGCG (n = 5), and spleen NK cells were isolated at day 5.5 of gestation (D5.5). Then, these NK cells were transferred to recipient pregnant mice (n = 10) whose NK cells were depleted by tail vein injection of anti-NK1.1 or isotype antibody at D 0.5 and D4.5. b–e The embryo implantation number, embryo absorption rate, placenta weight and crown-rump length were observed in mice (n = 10) with or without NK depletion at D13.5. f–i The embryo implantation number, embryo absorption rate, placenta weight and crown-rump length were observed in mice (n = 10) with NK depletion on D13.5. Data are presented as the mean ± SEM and were analyzed by t test, Mann‒Whitney U test, Chi-square test or one-way ANOVA. *P < 0.05, **P < 0. 01, NS no significance
α-KG supplementation rescues pregnancy loss caused by the defective glutaminolysis in dNK cells
To explore the possible value of α-KG in the treatment of spontaneous abortion caused by uterine NK cells with defective glutaminolysis during pregnancy, BPETS- or EGCG-injected mice were fed an α-KG supplementary diet. As shown, the α-KG supplementary diet decreased the risk of embryo absorption in BPTES-treated pregnant mice on D13.5 (Fig. 8a, b). More importantly, α-KG resulted in an increase in the embryo implantation number in the EGCG-treated mice on D7.5, as well as the placental weight on D13.5 (Fig. 8c, d). In addition, the infiltration depth of trophoblasts in the placenta was reversed in the α-KG supplementary diet group (Fig. 8e–h). To explore the immunoregulatory effect of dNK cells, we detected the infiltration of macrophages in the uterus by immunofluorescence. As shown, the proportion of macrophages was decreased in the BPTES and EGCG-treated groups, and α-KG partly restored the number of macrophages, while the expression of CD86 seemed to be not significantly changed (Supplementary Fig. 4). These results suggest that the deficiency of α-KG from defective glutamine/glutamate metabolism severely disrupts embryo trophoblast invasion and contributes to impaired embryo implantation and placental development, and the occurrence of pregnancy loss. α-KG supplementation rescues the trophoblast invasion and pregnancy loss caused by inactivated glutaminolysis.
α-KG supplementation rescues the pregnancy loss caused by BPTES and EGCG. (a, b After intraperitoneal injection with PBS or BPTES, supplemented with α-KG diet or not (2%, w/w), the embryo absorption rate and crown-rump length were observed in mice (n = 6) on D13.5. c, d After intraperitoneal injection with PBS or EGCG, supplied with α-KG diet or not (2%, w/w), the number of embryos and placental weight were observed in mice (n = 6) on D7.5 and D13.5. e–h The trophoblast infiltration depth in the placenta of pregnant mice treated with PBS, BPTES or EGCG, supplied with α-KG diet or not (2%, w/w), was measured by immunofluorescence on D13.5. The ratio of trophoblast infiltration (yellow line) to the depth of the entire placenta was analyzed. Data are presented as the mean ± SEM analyzed by one-way ANOVA or the Chi-square test. *P < 0.05, NS no significance
Discussion
The importance of glutamine in the development and differentiation of cells has been gradually emphasized, especially cells with rapid growth and huge demand for energy and nutrients, such as tumor cells and activated immune cells. In tumor cells, glutamine was even more preferentially absorbed and utilized than glucose [22]. In T-cell development, glutamine metabolism, and its effects on chromatin, were proven to promote Th17 but constrain Th1 and CTL effector cell differentiation [32]. Despite the fact that the application of single cell sequencing technology at the maternal–fetal interface has greatly accelerated our understanding of dNK cells in recent years, the role of glutamine metabolism in NK-cell function is still very limited [13,14,15]. Loftus et al. reported that fueling the tricarboxylic acid (TCA) cycle to generate ATP may not be the most important role of glutamine metabolism, rather the large number of metabolites such as succinic acid, fumaric acid and malic acid derived from glutamine through the TCA cycle may be more pivotal in regulating the phenotype and function of NK cells [26]. In the presence of glucose, notably, we observed that NK cells were less dependent on glutamine. More importantly, a low oxygen concentration (hypoxia) is part of normal embryonic implantation and development. BPTES led to significant decreases in aerobic respiration in NK92 cells, suggesting that glutamine metabolism should be an important resource of aerobic respiration.
dNK cells showed an activated glutamine metabolism in our study, represented as increased expression of glutamine transporter SLC1A5 and upregulated key enzymes of glutaminolysis. GLS expression was induced by estrogen and the co-culture system, while SLC1A5 was not significantly changed in our study. Common regulatory factors in NK cells include mTOR, c-MYC, IL-2, IL-15 and the process of ubiquitination-mediated protein degradation [33]. HIF-2α was also shown to induce the expression of SLC1A5 under hypoxic conditions, while this variant of SLC1A5 exists only in mitochondria [34]. Additionally, the relationship between glutamine and glucose metabolism has become increasingly evident in recent years. Lactate was shown to stimulate SLC1A5 expression in cervical cancer cells [35], and inhibition of glucose metabolism was reported to reduce the glycosylation of SLC1A5, suggesting the existence of coordinated regulation of glucose and glutamine metabolism [36].
Our study showed that activated glutamine metabolism enhanced the invasiveness of trophoblasts in vitro and in vivo, depending on the secretion of IGF-1 and GDF-1 in dNK cells. In contrast, blocking glutaminolysis with EGCG or BPTES led to early implantation failure or spontaneous abortion with impaired trophoblast infiltration in the mouse uterus. In addition, we noted that the effect of BPTES and EGCG differed in embryo implantation and fetal abortion. EGCG showed a decrease in implantation number and placental weight, but no change in embryo abortion. While BPTES increased the risk of embryo abortion and placental growth inhibition, it showed no change in implantation number. We attributed these differences to the timing and extent of the glutamine-α-KG metabolism block and trophoblast function. Severe impairment of trophoblast function leads to implantation failure, while moderate and persistent impairment of trophoblast function may result in growth restriction.
α-KG is the key point that links glucose metabolism and amino acid metabolism. Metabolites are recognized as important epigenetic modulators. Some metabolites including α-KG can serve as substrates for enzymes that modify chromatin and nucleic acids, and others such as 2-hydroxyglutarate can modify enzyme activity often by competitively inhibiting substrate utilization [37, 38]. αKG-dependent dioxygenases consume the metabolite α-KG as an obligate cosubstrate and are emerging as key mediators of metabolic control of cell fate [38]. Dioxygenases can catalyze the demethylation or hydroxylation reactions of the target gene or enzyme. DNA methylation and transcription mediated by ten-eleven translocation(TET) methylcytosine dioxygenase is an important way for α-KG to participate in epigenetic regulation of genes [39]. In this study, however, BPTES and EGCG had no influence on IGF-1 and GDF-15 methylation, and the methylation levels of IGF-1 and GDF-15 were not obviously different in dNK cells between normal pregnant women and RSA patients (data not shown). Based on the bioinformatics analysis, we predicted that HIF-1α was controlled by EGLN1 (an α-KG-dependent dioxygenase) [40], possibly regulating the expression of IGF-1 through ESR1, and GDF-15 through TWIST. Further studies verified that DMOG significantly increased the accumulation of HIF-1α and decreased the expression and secretion of IGF-1 and / or GDF-15 in dNK cells in vitro and in vivo. However, the mechanism remains to be further studied.
Decidual NK cells were divided into different subgroups in the latest single cell sequencing studies. dNK4 (CD16+PLAC8+) cells are regarded as cytotoxic decidual NK cells [13]. Granzyme and perforin are the main functional molecules for NK cells to play immune defense and cell killing and play a role in resisting bacterial and viral infection during pregnancy. The latest research showed that decidual NK cells could transport granzymin to trophoblasts through nanotubes to resist bacterial infection [41]. Here we observed that blocking glutamine metabolism with BPTES slightly downregulated the expression of cytotoxicity-related molecules (NKP30, NKP46, perforin, CD107a and Granzyme B), and decreased cell viability in vitro (Supplementary Fig. 5a, b). Regulatory dNK cells have a powerful capacity of cytokine secretion. Through IFN-γ, decidual NK cells are involved in the regulation of the invasion of extravillous trophoblasts [42], the remodeling of placental vessels, the inhibition of Th17-cell differentiation [43, 44], and the maintenance of monocyte and neutrophil immune tolerance [44]. Growth factors are another kind of functional molecule in dNK cells, including VEGF and GM-CSF. With the deepening of research, more growth factors secreted by dNK cells have been discovered, among which pleiotrophin and osteolysin were found to play important roles in promoting fetal growth and bone and joint development [10]. Interestingly, we found that activated glutamine metabolism and α-KG induced the secretion of IGF-1 and GDF-1 in dNK cells and further enhanced the invasiveness of trophoblasts.
IGF-1 is an anabolic hormone that is mainly synthesized by the liver under the stimulation of growth hormone during the non-gestational stage [45]. In acute effects, IGF-1 can promote the synthesis of proteins and carbohydrates, promote the uptake of amino acids in skeletal muscle, and promote the absorption and utilization of glucose in tissues. It also promotes the growth and development of various tissues, such as the heart, bone, muscle and embryo [45,46,47,48]. Additionally, IGF-1 can regulate the immune response through autocrine and paracrine pathways. For example, IGF-1 exerts anti-inflammatory, antioxidant and antiatherogenic effects by suppressing macrophage accumulation [49]. Recently, we reported that WISP2/IGF1 produced by DSCs impaired the cytotoxicity of dNK cells [50]. In pregnancy, IGF-1 was significantly positively correlated with birthweight in both healthy controls and patients with type 1 diabetes [51]. IGF-1 can activate mTOR signaling in the placenta, promoting protein synthesis, mitochondrial function and nutrient transport, which may increase fetal nutrient supply and contribute to fetal growth [52]. Knockdown IGF-1 was found to suppress trophoblast cell migration and invasion [53, 54]. As a member of the TGF-β superfamily, GDF-15 is highly expressed in the placenta under physiological conditions and weakly expressed in most other tissues [55]. Similarly, we observed that GDF15 was mainly expressed by epithelial cells at the maternal–fetal interface, especially extravillus trophoblast (EVT) in our data of single-cell RNA sequence (data not shown). These findings suggest that GDF15 secreted by trophoblasts plays a more important role in the regulation of the biological behavior of trophoblasts. GDF-15 was also associated with the development of NK-cell dysfunction during systemic inflammation [56]. GDF-15 was thought to induce immune tolerance at maternal interface and tumor microenvironment [55, 57], and promotes prostate cancer cell migration and invasion [57, 58]. Here, we identified a subset of dNK cells, IGF-1+GDF-15+ dNK cells, that contribute to trophoblast invasion, embryo implantation and growth under the positive regulation of activated glutamine metabolism and α-KG.
Collectively, as described in Supplementary Fig. 6, the high levels of glutamine transporters and metabolic enzymes involved in glutaminolysis result in activated glutamine metabolism in dNK cells under the influence of local environmental factors. Activated glutamine metabolism promotes proliferation and maturation, inhibits apoptosis, and promotes aerobic respiration in NK cells. More importantly, α-KG derived from glutamine promotes the differentiation of IGF-1+GDF-15+dNK cells through EGLN1/HIF-1α, enhances the invasion of trophoblasts, and further supports embryo growth. However, impaired glutaminolysis leads to a lack of glutamine-derived α-KG, contributing to embryo implantation failure, spontaneous abortion or restricted fetal growth with impaired trophoblast invasion and placental development to different degrees. Patients with unexplained recurrent spontaneous abortion had a decrease in glutamine and defective glutamine metabolism. Interestingly, the α-KG supplementary diet rescued the trophoblast invasion and mouse pregnancy loss caused by inactivated glutaminolysis. Therefore, the potential therapeutic value of α-KG in unexplained biochemical pregnancy and spontaneous abortion should be emphasized due to the activation of IGF-1+GDF-15+dNK cell-mediated trophoblast invasion during early pregnancy. Despite all this, there were some limitations in this study. The detailed mechanism of IGF-1 and GDF-15 in glutamine-α-KG-induced trophoblast invasion and embryo development in vivo still needs to be studied.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Abbreviations
- α-KG:
-
α-Ketoglutarate
- ACTB:
-
β-Actin
- BPTES:
-
Inhibitor of glutaminase
- dNK:
-
Decidual NK cells
- DIC:
-
Decidual immune cells
- DMKG:
-
Dimethyl alpha-ketoglutarate
- DMOG:
-
Dimethyloxallyl glycine, inhibitor of HIF prolyl-hydroxylase
- DSC:
-
Decidual stromal cells
- eNK:
-
Endometrial NK cells
- EGCG:
-
Epigallocatechin gallate sulfate, inhibitor of glutamate dehydrogenase
- EGLN1:
-
Egl nine homolog 1
- EP300:
-
E1A binding protein p300
- ESC:
-
Endometrial stromal cells
- ESR1:
-
Estrogen receptor 1
- FGF-19:
-
Fibroblast growth factor-19
- GABA:
-
γ-Aminobutyric acid
- GDF15:
-
Growth differentiation factor-15
- GLS:
-
Glutaminase
- GLUD:
-
Glutamate dehydrogenase
- HIF-1α:
-
Hypoxia inducible factor-1α
- IGF1:
-
Insulin-like growth factor-1
- IUGR:
-
Intrauterine growth restriction
- OXPHOS:
-
Oxidative phosphorylation
- PBMC:
-
Peripheral blood mononuclear cells
- pNK:
-
Peripheral NK cells
- TCA:
-
Tricarboxylic acid
- TET:
-
Ten-eleven translocation methylcytosine dioxygenase
References
Musallam R, Salem N, Al Halol R, Al Deeb H, Bottcher B, AlHamaida H (2018) Management of pregnancy loss in the first trimester: a retrospective audit. Lancet (London, England) 391(Suppl 2):S34. https://doi.org/10.1016/S0140-6736(18)30400-8
Garrido-Gimenez C, Alijotas-Reig J (2015) Recurrent miscarriage: causes, evaluation and management. Postgrad Med J 91:151–162. https://doi.org/10.1136/postgradmedj-2014-132672
Gellersen B, Brosens JJ (2014) Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 35:851–905. https://doi.org/10.1210/er.2014-1045
Tao Y, Li Y-H, Piao H-L, Zhou W-J, Zhang D, Fu Q et al (2015) CD56(bright)CD25+ NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. Cell Mol Immunol 12:77–86. https://doi.org/10.1038/cmi.2014.26
Keskin DB, Allan DSJ, Rybalov B, Andzelm MM, Stern JNH, Kopcow HD et al (2007) TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16− NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA 104:3378–3383
Jabrane-Ferrat N (2019) Features of human decidual NK cells in healthy pregnancy and during viral infection. Front Immunol 10:1397. https://doi.org/10.3389/fimmu.2019.01397
Erlebacher A (2013) Immunology of the maternal–fetal interface. Annu Rev Immunol 31:387–411. https://doi.org/10.1146/annurev-immunol-032712-100003
Zhou Y, Fu B, Xu X, Zhang J, Tong X, Wang Y et al (2020) PBX1 expression in uterine natural killer cells drives fetal growth. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aax1798
Lu H, Yang H-L, Zhou W-J, Lai Z-Z, Qiu X-M, Fu Q et al (2020) Rapamycin prevents spontaneous abortion by triggering decidual stromal cell autophagy-mediated NK cell residence. Autophagy. https://doi.org/10.1080/15548627.2020.1833515
Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z et al (2017) Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity. https://doi.org/10.1016/j.immuni.2017.11.018
Cartwright JE, James-Allan L, Buckley RJ, Wallace AE (2017) The role of decidual NK cells in pregnancies with impaired vascular remodelling. J Reprod Immunol 119:81–84. https://doi.org/10.1016/j.jri.2016.09.002
Seshadri S, Sunkara SK (2014) Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update 20:429–438. https://doi.org/10.1093/humupd/dmt056
Wang F, Jia W, Fan M, Shao X, Li Z, Liu Y et al (2021) Single-cell Immune landscape of human recurrent miscarriage. Genom Proteom Bioinform 19:208–222. https://doi.org/10.1016/j.gpb.2020.11.002
Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB et al (2018) Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563:347–353. https://doi.org/10.1038/s41586-018-0698-6
Suryawanshi H, Morozov P, Straus A, Sahasrabudhe N, Max KEA, Garzia A et al (2018) A single-cell survey of the human first-trimester placenta and decidua. Sci Adv 4:eaau4788. https://doi.org/10.1126/sciadv.aau4788
Du L, Deng W, Zeng S, Xu P, Huang L, Liang Y et al (2021) Single-cell transcriptome analysis reveals defective decidua stromal niche attributes to recurrent spontaneous abortion. Cell Prolif 54:e13125. https://doi.org/10.1111/cpr.13125
Assmann N, O’Brien KL, Donnelly RP, Dyck L, Zaiatz-Bittencourt V, Loftus RM et al (2017) Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat Immunol 18:1197–1206. https://doi.org/10.1038/ni.3838
Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K et al (2018) Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 19:1330–1340. https://doi.org/10.1038/s41590-018-0251-7
Cong J, Wang X, Zheng X, Wang D, Fu B, Sun R et al (2018) Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. https://doi.org/10.1016/j.cmet.2018.06.021
Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL et al (2021) Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. https://doi.org/10.1016/j.cmet.2021.03.023
Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P (2018) Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. https://doi.org/10.3390/nu10111564
Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR et al (2021) Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593:282–288. https://doi.org/10.1038/s41586-021-03442-1
Leone RD, Zhao L, Englert JM, Sun I-M, Oh M-H, Sun I-H et al (2019) Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science (New York, NY) 366:1013–1021. https://doi.org/10.1126/science.aav2588
Arts RJW, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E et al (2016) Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 24:807–819. https://doi.org/10.1016/j.cmet.2016.10.008
Oh M-H, Sun I-H, Zhao L, Leone RD, Sun I-M, Xu W et al (2020) Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Investig 130:3865–3884. https://doi.org/10.1172/JCI131859
Loftus RM, Assmann N, Kedia-Mehta N, O’Brien KL, Garcia A, Gillespie C et al (2018) Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat Commun 9:2341. https://doi.org/10.1038/s41467-018-04719-2
Lu H, Jin L-P, Huang H-L, Ha S-Y, Yang H-L, Chang R-Q et al (2020) Trophoblast-derived CXCL12 promotes CD56 CD82 CD29 NK cell enrichment in the decidua. Am J Reprod Immunol. https://doi.org/10.1111/aji.13203
Yang S-L, Tan H-X, Niu T-T, Li D-J, Wang H-Y, Li M-Q (2021) Kynurenine promotes the cytotoxicity of NK cells through aryl hydrocarbon receptor in early pregnancy. J Reprod Immunol 143:103270. https://doi.org/10.1016/j.jri.2020.103270
Mei J, Zhou W-J, Zhu X-Y, Lu H, Wu K, Yang H-L et al (2018) Suppression of autophagy and HCK signaling promotes PTGS2 FCGR3 NK cell differentiation triggered by ectopic endometrial stromal cells. Autophagy 14:1376–1397. https://doi.org/10.1080/15548627.2018.1476809
Olsen OE, Skjærvik A, Størdal BF, Sundan A, Holien T (2017) TGF-β contamination of purified recombinant GDF15. PLoS ONE 12:e0187349. https://doi.org/10.1371/journal.pone.0187349
Xiong G, Stewart RL, Chen J, Gao T, Scott TL, Samayoa LM et al (2018) Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat Commun 9:4456. https://doi.org/10.1038/s41467-018-06893-9
Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC et al (2018) Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell. https://doi.org/10.1016/j.cell.2018.10.001
Nachef M, Ali AK, Almutairi SM, Lee S-H (2021) Targeting SLC1A5 and SLC3A2/SLC7A5 as a potential strategy to strengthen anti-tumor immunity in the tumor microenvironment. Front Immunol 12:624324. https://doi.org/10.3389/fimmu.2021.624324
Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH et al (2020) A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. https://doi.org/10.1016/j.cmet.2019.11.020
Pérez-Escuredo J, Dadhich RK, Dhup S, Cacace A, Van Hée VF, De Saedeleer CJ et al (2016) Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell cycle (Georgetown, Tex) 15:72–83. https://doi.org/10.1080/15384101.2015.1120930
Scalise M, Pochini L, Console L, Losso MA, Indiveri C (2018) The human SLC1A5 (ASCT2) amino acid transporter: from function to structure and role in cell biology. Front Cell Dev Biol 6:96. https://doi.org/10.3389/fcell.2018.00096
Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S et al (2012) Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483:484–488. https://doi.org/10.1038/nature10898
Baksh SC, Finley LWS (2021) Metabolic coordination of cell fate by α-ketoglutarate-dependent dioxygenases. Trends Cell Biol 31:24–36. https://doi.org/10.1016/j.tcb.2020.09.010
Rasmussen KD, Helin K (2016) Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev 30:733–750. https://doi.org/10.1101/gad.276568.115
Ivan M, Kaelin WG (2017) The EGLN-HIF O-sensing system: multiple inputs and feedbacks. Mol Cell 66:772–779. https://doi.org/10.1016/j.molcel.2017.06.002
Crespo ÂC, Mulik S, Dotiwala F, Ansara JA, Sen Santara S, Ingersoll K et al (2020) Decidual NK cells transfer granulysin to selectively kill bacteria in trophoblasts. Cell. https://doi.org/10.1016/j.cell.2020.07.019
Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, Stein N, Yamin R et al (2018) Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity. https://doi.org/10.1016/j.immuni.2018.03.030
Chong WP, van Panhuys N, Chen J, Silver PB, Jittayasothorn Y, Mattapallil MJ et al (2015) NK-DC crosstalk controls the autopathogenic Th17 response through an innate IFN-γ-IL-27 axis. J Exp Med 212:1739–1752. https://doi.org/10.1084/jem.20141678
Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D et al (2010) Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci USA 107:11918–11923. https://doi.org/10.1073/pnas.1001749107
Waters MJ, Brooks AJ (2012) Growth hormone and cell growth. Endocr Dev 23:86–95. https://doi.org/10.1159/000341761
Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL et al (2020) Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab 2:278–289. https://doi.org/10.1038/s42255-020-0184-y
Xu J, Wang X, Chen J, Chen S, Li Z, Liu H et al (2020) Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics 10:12204–12222. https://doi.org/10.7150/thno.47683
Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS et al (2016) Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA 113:E7554–E7563
Higashi Y, Sukhanov S, Shai S-Y, Danchuk S, Tang R, Snarski P et al (2016) Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation 133:2263–2278. https://doi.org/10.1161/CIRCULATIONAHA.116.021805
Shi J-W, Yang H-L, Lai Z-Z, Shen H-H, Qin X-Y, Qiu X-M et al (2021) WISP2/IGF1 promotes the survival of DSCs and impairs the cytotoxicity of decidual NK cells. Reproduction 161:425–436. https://doi.org/10.1530/REP-20-0658
Lindsay RS, Hamilton BA, Calder AA, Johnstone FD, Walker JD (2004) The relation of insulin, leptin and IGF-1 to birthweight in offspring of women with type 1 diabetes. Clin Endocrinol (Oxf) 61:353–359
Kelly AC, Powell TL, Jansson T (2020) Placental function in maternal obesity. Clin Sci (Lond, Engl) 134:961–984. https://doi.org/10.1042/CS20190266
Niu Z-R, Han T, Sun X-L, Luan L-X, Gou W-L, Zhu X-M (2018) MicroRNA-30a-3p is overexpressed in the placentas of patients with preeclampsia and affects trophoblast invasion and apoptosis by its effects on IGF-1. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2017.11.568
Wu H-Y, Wang X-H, Liu K, Zhang J-L (2020) LncRNA MALAT1 regulates trophoblast cells migration and invasion via miR-206/IGF-1 axis. Cell cycle (Georgetown, Tex) 19:39–52. https://doi.org/10.1080/15384101.2019.1691787
Wischhusen J, Melero I, Fridman WH (2020) Growth/differentiation factor-15 (GDF-15): from biomarker to novel targetable immune checkpoint. Front Immunol 11:951. https://doi.org/10.3389/fimmu.2020.00951
Kleinertz H, Hepner-Schefczyk M, Ehnert S, Claus M, Halbgebauer R, Boller L et al (2019) Circulating growth/differentiation factor 15 is associated with human CD56 natural killer cell dysfunction and nosocomial infection in severe systemic inflammation. EBioMedicine 43:380–391. https://doi.org/10.1016/j.ebiom.2019.04.018
Roth P, Junker M, Tritschler I, Mittelbronn M, Dombrowski Y, Breit SN et al (2010) GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin Cancer Res 16:3851–3859. https://doi.org/10.1158/1078-0432.CCR-10-0705
Wang W, Yang X, Dai J, Lu Y, Zhang J, Keller ET (2019) Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene 38:4540–4559. https://doi.org/10.1038/s41388-019-0736-3
Acknowledgements
We are grateful to Prof. Shi-Min Zhao from Institute of Metabolism and Integrative Biology (IMIB), School of Life Sciences, Fudan University, for guidance and help. This study supported by the National Natural Science Foundation of China (NSFC) (No. 92057119, 31970798, 32070915), the National Key Research and Development Program of China (2017YFC1001404), the Program for Zhuoxue of Fudan University (JIF157602), the Support Project for Original Personalized Research of Fudan University, and the Yantai Science and Technology Innovation Plan (2021XDHZ082).
Funding
Funding was provided by National Natural Science Foundation of China (92057119, 31970798, 32070915), National Key Research and Development Program of China (2017YFC1001404), Program for Zhuoxue of Fudan University (JIF157602), Support Project for Original Personalized Research of Fudan University, Yantai Science and Technology Innovation Plan (2021XDHZ082).
Author information
Authors and Affiliations
Contributions
SLY and HXT conducted all experiments and prepared the figures and the manuscript. ZZL, HYP and HLY assisted with cell sorting, in vivo experiments, prepared the figures and the manuscript. QF edited the manuscript. MQL, DJL and HYW initiated and supervised the project and edited the manuscript. All the authors were involved in writing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki and all experiments were approved by The Animal Care and Use Committee of Fudan University.
Consent for publication
All authors consent to the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, SL., Tan, HX., Lai, ZZ. et al. An active glutamine/α-ketoglutarate/HIF-1α axis prevents pregnancy loss by triggering decidual IGF1+GDF15+NK cell differentiation. Cell. Mol. Life Sci. 79, 611 (2022). https://doi.org/10.1007/s00018-022-04639-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04639-x