Abstract
In fetal females, oogonia proliferate immediately after sex determination. The progress of mitosis in oogonia proceeds so rapidly that the incompletely divided cytoplasm of the sister cells forms cysts. The oogonia will then initiate meiosis and arrest at the diplotene stage of meiosis I, becoming oocytes. Within each germline cyst, oocytes with Balbiani bodies will survive after cyst breakdown (CBD). After CBD, each oocyte is enclosed by pre-granulosa cells to form a primordial follicle (PF). Notably, the PF pool formed perinatally will be the sole lifelong oocyte source of a female. Thus, elucidating the mechanisms of CBD and PF formation is not only meaningful for solving mysteries related to ovarian development but also contributes to the preservation of reproduction. However, the mechanisms that regulate these phenomena are largely unknown. This review summarizes the progress of cellular and molecular research on these processes in mice and humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Preface
In mammals, a fixed population of primordial follicles (PFs) is generally established in the ovaries during early life that serves as the sole source of developing follicles and oocytes throughout reproductive life [1, 2]. Despite substantial prolongation of female life expectancy during the past century, the female reproductive lifespan remains approximately 51 years. The physiological reason for this limit is the gradual and irreversible exhaustion of the PF pool and decreased egg quality with increasing age. In general, because of the adverse effects of environmental, genetic or other unknown factors, the PF pool shrinks steadily, thus shortening the reproductive lifespan [3]. Primary ovarian insufficiency (POI), the clinical term for premature ovarian failure (POF), which is characterized by the disappearance of menstrual cycles associated with premature follicular depletion, is a condition affecting at least 1% of women under the age of 40 years worldwide [4, 5]. Most recent reports show that upon mutation, many genes responsible for the formation of PFs are also associated with POI [6, 7]. Therefore, clarifying the mechanisms underlying the PF reserve is one of the most important topics in female reproductive research. Alternatively, because cancer therapy can adversely affect germ cell survival and cause POF and infertility [8], the requirement for fertility preservation in young cancer patients has increased significantly since the beginning of this century, although a better prognosis has been achieved after cancer therapy [9]. Clinically, cryopreservation of ovarian tissue before cancer therapy is becoming one of the most effective ways to preserve fertility [10, 11]. Nearly, 80% of human PFs were shown to survive after ovarian tissue cryopreservation using the slow-freezing method in the 1990s [12, 13], although this method is not currently the most effective.
Excitingly, with the progress of research on the molecular mechanisms of PF activation, in vitro activation of PFs has become feasible. Mammalian target of rapamycin complex 1 (mTORC1) signaling in the granulosa cells of PFs is responsible for secreting the Kit Ligand (KITL) to activate phosphatidyl inositol 3-kinase (PI3K) within oocytes, which in turn activates PF development in neonatal mouse ovaries and human ovarian cortical tissue in vitro [14–17]. This novel in vitro activation approach has been successfully used in human POF patients, and one healthy baby has been delivered in 2013 [18].
Applying pluripotent stem cells to reproduce functional gametogenesis in vitro is currently one of the key goals in developmental and reproductive biology. Physiologically, functional oocytes derive from intact follicles, implying that the communication between germ cells and ovarian somatic cells within follicles is pivotal for full oocyte development. However, the mechanisms underlying this process are not fully elucidated. Thus, the identification of effective ways to preserve PFs to the greatest extent may not only ensure the preservation of fertility for cancer patients in the future, but may also help to postpone ovarian aging in healthy women. Understanding the formation mechanism of PFs will become the basis for better controlling the in vitro reconstitution of oogenesis, cryopreservation, and the activation of PFs within ovarian tissues. This review aims to summarize the recent progress regarding PF formation in mice and humans to provide a better understanding of the mechanisms controlling female fertility reserves.
Asynchronous PF formation
In mice, immediately after the primordial germ cells (PGCs) migrate to the gonad at 10.5 days post coitus (10.5 dpc) during embryonic development, they form into cysts (multi-nucleated syncytial clones) by rapidly dividing until 13.5 dpc. In humans, germ cell mitosis begins at 5 week post conception (5 wpc) and ends at 10–16 wpc [19–22]. The number of germ cell divisions in Drosophila is markedly different from that in mice. In Drosophila, germ cells undergo four rounds of mitosis, whereas the number of divisions is variable in mice [23]. Unlike spermatogonia, which initiate meiosis postnatally, the oogonia (now referred to as oocytes) within cysts initiate meiosis at approximately 13.5 dpc in mice and 13 wpc in humans [20, 24].
In mammals, the initiation of meiosis is strikingly asynchronous both temporally and spatially [6]. First, along with the increasing number of germ cells initiating meiosis, some oogonia still express stem cell markers and continue to proliferate until at least 16 wpc in humans [21, 25]. Second, this asynchronous development reflects the initiation of meiosis via anterior-to-posterior patterns along the axis of the ovary beginning at 12.5 dpc in the mouse [26, 27]. Third, this asynchronous development is also reflected in the depth of maturation of both germ cells and pre-granulosa cells in the cortical and medullar regions of the ovary. The onset of programmed germ cell cyst breakdown (CBD) starts in the medullary (dorsal) region and moves to the cortical (ventral) side; that is, CBD starts in the medullary region prior to the time of birth and expands toward the cortical surface region of the ovaries in humans and rodents [28–30]. Fewer mature germ cells are located at the periphery of the ovary, with progressively more mature germ cells being found in the medulla area of fetal ovarian tissue [31].
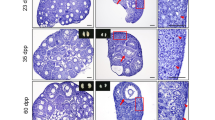
Accordingly, asynchrony also occurs in ovarian somatic cell development. Forkhead box L2 (FOXL2)-positive pre-granulosa cells undergo two waves of differentiation that contribute to two discrete populations of follicles [32]. The source of both populations of pre-granulosa cells is the ovarian surface epithelium. The first wave of differentiation supports bipotential somatic cell differentiation, which is involved in forming the earliest PFs that develop within the medulla region. Consequently, such PFs undergo rapid maturation and die. The second wave of differentiation occurs at the ovarian cortex, where PFs are formed and arrested until puberty and beyond [32]. Additional studies support the conclusion that there are two waves of perinatal PF formation in the rodent and human ovary [17, 33–36]. Based on a visual in vivo follicle tracing system, Zheng et al. demonstrated that the fates of the two classes of PFs follow distinct, age-dependent developmental paths, and play different roles throughout the reproductive lifespan [37]. Pre-granulosa cells recruited in fetal mouse ovaries form the initial PFs in the medullary region. Once formed, these PFs are synchronously activated and become the first wave of follicles activated after birth [38, 39], which contributes to the onset of puberty and to fertility until 3 months of age. Pre-granulosa cells recruited in postnatal mouse ovaries form PFs in the cortical region, which are gradually activated as a means of providing mature oocytes over the entire course of the reproductive life of the animal [32, 38, 40, 41]. As a result, PFs from the second wave gradually replace those from the first until they become the sole source of follicles from 3 months onward [37]. The spatiotemporal patterns of major cellular events that occur during CBD and PF formation in the mouse ovary are shown in Fig. 1.
Schematic representation showing the spatiotemporal patterns of major cellular events during cyst breakdown (CBD) and primordial follicle (PF) formation in the mouse ovary. a After cyst formation, the oocyte with an established Balbiani body in its cytoplasm will survive, whereas the nurse cells surrounding the oocyte may subsequently undergo apoptosis. b Adherens junctions (AJs) between oocytes are sustained by the Wnt4/β-Catenin (β-Cat) signaling pathway before CBD and are disassembled via c-Jun amino-terminal kinase (JNK) signaling after CBD. c Before PF assembly, oocyte-derived factors, such as Jagged 1, induce Notch signaling activation in pre-granulosa cells to promote their differentiation and proliferation. After Jagged1 binds to the extracellular domain of Notch, the proteolytic cleavage of Notch2 is mediated by a disintegrin and metalloproteinase domain 10 (ADAM10). After cleavage, the Notch intercellular domain (NICD) is released and translocates to the nucleus to interact with the transcriptional complex to activate target genes. d Maternal hormones, including estrogen (E2), progesterone (P4), and follicle-stimulating hormone (FSH), exert their inhibitory actions on oocyte apoptosis, CBD and PF formation through classical (nuclear receptor) or non-classical (membrane receptor) pathways. Forkhead box L2 (FOXL2) is one of the most important agents driving granulosa cell differentiation and ovary maintenance. Only FOXL2-positive pre-granulosa cells start to invade cysts and separate germ cells to form PF structures. The differentiation of FOXL2-positive pre-granulosa cells derives from G protein-coupled receptor (LGR5)-positive cells. LGR5-positive cells are restricted to the cortical region of the ovaries from 12.5 dpc to perinatal stages. FOXL2-positive cells first emerge in the medulla and then gradually emerge in the cortical region of the ovary from 16.5 dpc to 4 dpp in the mice
Prerequisite cellular events for the establishment of PFs
In mammals, PF formation consists of a series of cellular events, including the formation of germline cysts through oogonia mitosis, meiosis initiation, CBD, and PF assembly when germ cells are arrested at the dictyate stage of meiosis I. The entire process involves at least two cell types: the germ cells within the cyst and the surrounding pre-granulosa cells [42]. Studies have demonstrated that timely, synchronized development of ovarian somatic cells and oocytes as well as their mutual communication determine PF formation and oocyte survival in mice [43, 44]. Communication occurs not only between ovarian somatic cells and oocytes but also between the somatic cells themselves [45, 46]. Accordingly, the basic criteria for the formation of PFs in mouse include two prerequisites: sufficient numbers of squamous pre-granulosa cells that express the FOXL2 protein and a readily available oocyte arrested at the diplotene stage [32, 47–53].
Pre-granulosa cell recruitment and differentiation
Ovarian differentiation is a coordinate event, possibly driven by secreted factors, including canonical wingless-type MMTV integration site family member 4 (WNT4) and Rspondin 1 [RSPO1, an LGR5 (Leucine Rich Repeat Containing G Protein-Coupled Receptor 5) receptor ligand], transforming growth factor (TGF) beta superfamily-binding proteins, such as Follistatin (Fst), and transcriptional regulators, such as β-Catenin and FOXL2 [54]. FOXL2 is a winged helix/forkhead domain transcription factor that is extensively expressed within somatic cells from as early as 12.5 dpc until PF formation in the mouse. It participates in various molecular events in fetal ovary development [55, 56]. FOXL2 is considered as one of the most important agents during granulosa cell differentiation and ovary maintenance [52, 55–57]. The first hint that FOXL2 was involved in ovarian development came from an analysis in 2001 of human patients suffering from eyelid malformation and POF, which was found to be due to a mutation of the Foxl2 gene [58]. In the mouse, Foxl2 deficiency impairs CBD by affecting granulosa cell differentiation and proper formation of the basal lamina around forming follicles [49].
Physiologically, at 17.5 dpc, only FOXL2-positive pre-granulosa cells start to invade cysts and separate germ cells to form the PF structure [30]. However, the development of pre-granulosa cells is complex. A series of studies have demonstrated that the ovarian surface epithelium, which is LGR5 positive, may be a major source of pre-granulosa cells [32, 59, 60]. Activated WNT4 and RSPO1 are pivotal for ovarian pre-granulosa cell differentiation [61, 62]. LGR5 not only enhances WNT/RSPO1/β-Catenin signaling pathways in various morphogenetic processes, and it is also a marker of stem cells in the ovarian surface epithelium [60, 63]. In general, LGR5-positive cells are restricted to the cortical region of ovaries from 12.5 dpc to perinatal stages. Based on the specific expression patterns of Lgr5 and Foxl2, it has been confirmed that LGR5-positive cells are recruited to differentiate into FOXL2-positive pre-granulosa cells, starting at the initiation of folliculogenesis (approximately 1 dpp) [60, 64–66]. Accordingly, FOXL2-positive cells first emerge in the medulla and then gradually emerge in the cortical region of the ovary (Fig. 1) from 16.5 dpc to 4 dpp in mice [30].
Oocyte meiosis
In mice, meiosis in the germ cells starts at approximately 13.5 dpc and progresses from leptotene to diplotene stages until arrest at 18.5 dpc. Retinoic acid (RA) derived from the somatic environment of either mesonephroi or the fetal ovary is the key signal for the induction of meiosis in mice as well as humans [67–71]. RA is the active derivative of vitamin A, and participates in the regulation of embryonic development, cellular proliferation, and differentiation, as well as reproductive systems [71, 72]. By binding to intracellular RA receptors (RARs) and retinoid X receptors (RXRs), RA stimulates the expression of Stra8 (stimulated by retinoic acid 8), which promotes both the replication of germ cell chromatin and the transition of oogonia to meiotic division [67, 68, 73, 74] (Fig. 2a). As a result, various signals that regulate the homeostasis between RA synthesis by retinaldehyde dehydrogenases (RALDHs) and RA degradation by CYP26 (CYP26A1, CYP26B1, and CYP26C1) control Stra8 expression and the timing of meiosis initiation [75, 76] (Fig. 3a).
Molecular events in developing oocytes and pre-granulosa cells before PF formation. a Retinoic acid (RA) secreted from either ovarian somatic cells or mesonephric tissue is responsible for the initiation of meiosis in oocytes. b Gap junctions (GJs) established between oocytes and pre-granulosa cells may be more important for controlling the development of ovarian somatic cells, instead of oocytes, during CBD and PF assembly. c In the pre-granulosa cells, ADAM10 is responsible for the cleavage of the Notch receptor in both LGR5-positive and FOXL2-positive cells and thus controls the development of pre-granulosa cells in perinatal ovaries. d Small GTPase Rac1 may facilitate the import of STAT3 to the nucleus to activate the expression of oocyte-specific proteins that are secreted from the oocyte
Actions of Act, WNT4, and RSPO1 in pre-granulosa cell development. a Oocyte-secreted Act acts on ovarian somatic cells to prevent kitl expression as well as regulates retinoic acid (RA) synthesis and degradation. b In pre-granulosa cells, WNT4 and RSPO1 signals activate the β-Catenin protein, which is responsible for the expression of many genes during CBD and PF formation, including Kitl, Fst, and Wnt4
In this context, TGF beta superfamily-binding proteins have been demonstrated to participate in the initiation of oogonium meiosis. Activin A (Act A) and RA may cooperate in promoting meiosis in female germ cells [77] (Figs. 2, 3). In vitro, Act A accelerates the progression of oocytes throughout meiotic prophase I stages, which is related to increased expression of premeiotic and meiotic genes (including Dazl, Spo11, Stra8, Scp3, and Rec8) in ovarian tissues [77]. An earlier study also suggested that CYP26B1 is a novel target of Act [78], because Act A-dependent SMAD3 signaling downregulates the expression of Cyp26b1 and upregulates the expression of Rars [67, 68, 78, 79].
In addition to RA and Act, WNT4 and Rspondin signals from ovarian somatic cells have been demonstrated to promote not only the meiotic initiation of female germ cells but also oogonial proliferation and survival [54, 80, 81] (Fig. 3b). Nuclear β-Catenin is involved in ovarian development through either RSPO1-mediated pathways or WNT4 signaling [82, 83], thereby inhibiting Sox9 expression or promoting Wnt4, Bmp2, and Fst expression, respectively [54]. In somatic cells, RSPO1 promotes ovarian differentiation via the activation of WNT/β-Catenin signaling. In Rspo1-deficient mouse ovaries, germ cell proliferation, expression of Stra8, and entry into meiosis are all impaired [81].
Is oocyte entry into meiosis and arrest at the dictyate stage necessary for PF formation? The answer seems to be yes. Meiotic progression is not merely concomitant with CBD but also causative [84]. During meiosis, germ cells experience DNA double-strand breaks (DSBs) to undergo recombination, which potentiates genetic diversity in progeny. The extraordinary DSB tolerance of each oocyte could be one of the characteristics of oocytes in a meiotic state [85]. For instance, mutations in genes involved in the creation and repair of DSBs, such as MutS homolog genes (MSHs) and disrupted meiotic cDNA 1 (DMC1), negatively affect fertility and lead to POI [86, 87]. Premature loss of mouse germ cells is observed by 4 dpp in Msh4-deficient ovaries and by 2 months in Msh5-deficient ovaries [88, 89]. Dmc1 deficiency causes a reduction in mouse follicle numbers and follicles, resulting from the failure of chromosome synapsis [90]. Spo11 (S. cerevisiae homolog)-deficient female mice exhibit a smaller PF pool, showing evident germ cell defects by 15.5 dpc [91]. In the ovaries of Stra8-deficient mice, although some germ cells evidently survived embryonic development despite the meiotic initiation block at E13.5–E14.5, the attrition of the germ cells accelerates from the fetal to postnatal stages, and the ovary is devoid of germ cells by 6–8 weeks of age in this mouse model [92].
In addition, it has been demonstrated that PF formation is closely related to the progress of meiosis. SCP1 (synaptonemal complex protein-1) is expressed specifically in oocytes in females, and its expression declines precipitously within 24 h after birth when CBD begins. Furthermore, Paredes et al. have shown that loss of SCP1 in rat oocytes facilitates follicle assembly [93]. TAF4B, a gonad-enriched subunit of the TFIID complex is critical for oocyte and granulosa cell survival and PF formation in the mouse [94, 95]. TAF4B correlates with ovarian health and oocyte survival in women as well [96, 97]. In addition, TAF4b-deficient female mice are infertile and suffer from POF, which includes persistent estrous, elevated serum FSH levels, and reduced PF numbers [94, 95, 98]. Accordingly, the TAF4B protein has been demonstrated to be responsive to both estrogen and FSH receptors and acts to orchestrate the correct timing of germ cell CBD and PF pool establishment during a critical window of development [95, 99, 100]. Interestingly, TAF4B stimulates the expression of c-Jun in the granulosa cells of rats [101], whereas c-Jun Amino-terminal Kinase (JNK) is important for oocyte survival and CBD, which will be discussed later. Notably, Grive et al. demonstrated that PF assembly may not only follow the arrest of meiosis I progression but also be dependent upon the proper completion of these steps [95]. In accordance with this hypothesis, the most recent study on this topic proposed that proper oocyte meiosis is essential for PF formation under physiological conditions. The meiotic arrest of mouse oocytes at the dictyate stage is indispensable for the formation of PFs [102].
Germ cell cyst formation
The germ line cyst is a structure that is highly conserved among species. The peak number of cysts occurs at 14.5 dpc in mice and 20 wpc in humans, the point at which meiosis begins. At the end of mitosis, each cyst is composed of up to 30 germ cells (oogonia), which are connected via intercellular bridges due to incomplete cytokinesis in mice [103–106]. Contrary to the notion that intercellular bridges are likely restricted to cells of the same genotype [32], some of the growing cysts will break down into smaller cysts by 17.5 dpc and then associate with other unrelated cysts to form aggregated cysts prior to meiosis [33, 107–109]. Although the role of the cysts is still not well understood, it is clear that the structure of the cysts facilitates organelle exchange between germ cells. According to Lei’s and Pepling’s results, mouse oogonia develop into oocytes through both organelle enrichment from sister cyst (nurse-like) germ cells and Balbiani body establishment, whereas the other nurse-like germ cells die [106, 110] (Fig. 1a).
Cyst structure may contribute to the development of perfect oocytes with high quality, which is vital for PF formation [95, 106]. The Balbiani body (named after the nineteenth century Dutch microscopist), or mitochondrial cloud, in the cytoplasmic region near the nucleus is a large, distinctive organelle aggregate that has been found in the developing oocytes of many species, including humans [111, 112]. The advantage of the acquisition of additional organelles may be to ensure that oocytes grow into the largest cells in the mammalian body despite possessing four copies of the DNA complement. Although evidence is lacking, the Balbiani body may also serve a physiological secretory function in oocytes [106]. In addition, cytoplasmic RNA transfer between germ cells may enrich reprogramming factors and protective factors, such as piRNAs that repress transposition in the oocyte [113–115]. Alternatively, through intercellular cytoplasmic bridges, the oocyte nucleus may be relieved of metabolic and biosynthetic duties, which could either contribute to inactivation of the nucleus or reduce its susceptibility to mutagenesis [116].
Although it is difficult to anticipate which germ cell within the cyst will undergo apoptosis, or what kind of germ cells are selected to form into PFs, studies have provided some clues about the possible fate of the living germs cells that are doomed to perinatally form PFs. For example, the size of an oocyte that escapes apoptosis could be relatively larger than the surrounding nurse cells, although this size difference is difficult to identify based on appearance [106]. In relation to this possibility, the Balbiani body within the living oocyte could be a candidate biomarker, according to Pepling’s research; the oocyte within a PF generally contains a Balbiani body [112]. Regarding molecular biomarkers, partitioning-defective protein 6 (PAR6), one of the proteins that is important for cell polarity, could be a potential marker for identifying germ cells that will be selected to form PFs in the mouse ovary [117]. PAR6 plays a role in the asymmetric distribution of cytoplasmic determinants and the regulation of cytoskeleton positioning and asymmetric division [118]. In Drosophila, only the germ cell expressing PARs within a cyst becomes the oocyte, whereas the others undergo degeneration. Mutated PAR disrupts the early polarization of the oocyte and leads to failure to maintain its identity in both C. elegans and Drosophila [119–121]. In the mouse, from 17.5 dpc onward, PAR6 expression shifts from the ovarian somatic cells to the oocytes, and its expression level continues to rise in the nuclei of some oocytes at 19.5 dpc and in of all oocytes at 3 dpp. Importantly, the expression of PAR6 decreases gradually after the PF pool is established. During PF pool establishment, the number of PAR6-positive germ cells remains steady and is consistent with the number of follicles formed at 3 dpp. Wen et al. suggested that the unknown signals inducing oocyte-expressing PAR6 may derive from somatic cells through gap junctions [117]. However, because detailed information is absent, substantial research will be needed to explain the mechanism of PAR6 involvement in PF formation via shifting expression from pre-granulosa cells to oocytes.
Cell–cell adhesion during CBD and PF formation
Cell–cell adhesion is critical for various aspects of multicellular existence, such as morphogenesis, tissue integrity, and differentiation, including in the fetal ovary [105, 122]. Adherens junctions (AJs) are intercellular structures responsible for cell–cell adhesion mediated by Cadherins (Cads) through the calcium-dependent homophilic interaction of their extracellular domains. AJ formation is associated with cytoplasmic Catenin (α, β and p120) proteins, as the cytoplasmic tail of Cad itself exhibits no catalytic activity [123]. Therefore, any possible signaling for the activation of cell–cell adhesion must occur upon the recruitment of signaling molecules to the site of the Cad-Catenin complex [124].
During embryonic development, the dynamic cellular behaviors, such as rearrangement, movement, and shape changes, are regulated by AJs, which constitute a physical bridge between the Cad complex and the cortical actin filaments [125]. In the fetal ovary, elongated pre-granulosa cells extend between germ cells within the same cyst, often making direct contact with intercellular bridges, indicating that they are important for CBD and PF formation [23, 126].
A proper spatiotemporal expression of Cads is required for CBD [127]. Among the Cads, E-Cad was first determined to mediate AJ formation by recruiting PI3K-p85 to the cell membrane, which contributes to activation of the PI3K/AKT pathway and up-regulation of epithelial ovarian cancer growth [128, 129]. Physiologically, E-Cad is intensely expressed during the cyst period, but its expression decreases when CBD occurs in the fetal ovaries of mice [127, 130] and human [131] (Fig. 1b). Along with CBD and PF formation, a V-shaped E-Cad expression pattern is observed in mice. E-Cad is intensely expressed at oocyte–oocyte contact sites inside cysts in 17.5 dpc ovaries. Its expression soon decreases and diffuses at 19.5 dpc but rises again from 2 dpp until 4 dpp. Subsequently, E-Cad is widely expressed in oocytes as well as somatic cells [130]. In hamsters, blocking E-Cad accelerates CBD and PF formation, which is in consistent with the finding that overexpression of E-Cad suppresses CBD in the mouse fetal ovary [127, 130].
Gap junction (GJ) establishment is also essential for murine PF assembly (Fig. 2b). The GJ structure was first observed in fetal ovaries at 17.5 dpc [132]. Earlier studies indicated that GJs formed between pre-granulosa and germ cells and that they at least contained GJ1 (also known as Connexin 43) [132, 133]. According to the most recent report on this topic, GJ structures between the oocyte and surrounding pre-granulosa cells are formed at approximately 19.0 dpc, and communication between them is established from 19.5 dpc to 1 dpp [134]. Among the 20 GJs (Gja through Gjd), 12 elevated genes belong to three distinct patterns that rise and fall from 15.5 dpc to 5 dpp, which imply that GJs are closely related to PF formation and that complex compensatory actions may occur between them [134, 135]. Consistent with this view, global inhibition of GJs not only prevented PF assembly in vitro in perinatal ovaries but also resulted in the failure of antral follicle development in kidney transplant cultures [134]. However, deficiency of either Gja1 or Gja4, the two most essential GJs for follicle growth [136, 137], did not affect PF formation [130, 138, 139]. After systematic inhibition of GJs, ovarian somatic cell-specific genes (such as Notch2, Foxl2, and Irx3) were found to be down-regulated, whereas oocyte-specific genes (such as Ybx2, Nobox, and Sohlh1) and the progress of oocyte meiosis were not, demonstrating that GJ communication may be more important for somatic cell differentiation than for oocyte development [134]. Because of the complexity of GJ genes, future studies should concentrate on the cell-type-dependent functions of each GJ during the transformation of oogonia into oocytes and PF formation [44, 60].
Cross-talk between germ cells and pre-granulosa cells
Mutual communication between oocytes and surrounding pre-granulosa cells is pivotal for CBD and PF formation [140–142]. The contact between germ cells and somatic cells is established around ring canals connecting two oocytes within the cyst as early as 13.5 dpc in the mouse ovary [110]. Disruption of either germ cell- or somatic cell-specific factors (FIG1α and Wnt4, respectively) leads to defects in follicle formation [143, 144]. Studies have shown that many molecules are involved in this process [105]. Unfortunately, it is still unclear how such factors participate in the reorganization of the two distinct cell types into one functional unit through cross-talk.
Notch signaling could be one of the most important pathways participating in folliculogenesis in the perinatal mouse ovary. Notch signaling involves four receptors, Notch1, 2, 3, and 4, and their ligands, Jagged1 and 2 and delta-like 1, 3, and 4 [145]. Jagged1 and Notch2 are expressed in the oocyte and pre-granulosa cells, respectively, from 0 to 6 dpp, suggesting that germ cell CBD is partially coordinated through cellular interactions via Notch signaling [146–150]. Immediately after Jagged1 binds to the extracellular domain of Notch, proteolytic cleavage of the Notch2 receptor begins [145, 150]. Following cleavage, the Notch intercellular domain (NICD) is released and translocates to the nucleus to interact with the transcriptional complex to activate target genes, such as Hes1, Hey2, and Hey1 [151] (Figs. 1c, 2c). Thus, deficiency of Notch, Notch receptor modulator Lunatic fringe (Lfng) or Hes1 results in subfertility and consequent multi-oocyte follicles (MOFs) [147–150, 152].
The regulatory roles of a disintegrin and metalloproteinase domain 10 (ADAM10), the main physiological α-secretase [153], are responsible for the cleavage of the Notch receptor in both LGR5-positive and FOXL2-positive cells, and hence control the development of pre-granulosa cells in perinatal ovaries [66, 154–157] (Fig. 1c). However, it is possible that not all the components of Notch signaling are involved in the regulation of CBD and PF formation, as Notch3, Notch4, and Delta-like 3 mutants are fertile, and Delta-like 1 and Delta-like 4 are not expressed in neonatal ovaries [105]. Although oocyte-specific factors (GDF9 and BMP15) have been demonstrated to regulate the translation of Notch2 via mTORC1 activation in pre-granulosa cells [157], there is a lack of detailed information about the exact action of Notch signaling. Specifically, conditional genetically modified animals will be necessary to evade the problems of embryonic lethality and complex complementary actions between Notch signaling that have occurred in the past studies [105].
Several studies have noted cross-talk between somatic cells and oocytes via the receptor tyrosine kinase c-kit (KIT), which together with the KITL, is important for the control of oocyte reawakening. Activation of KITL in granulosa cells passes signals to oocytes to stimulate PF activation [6, 158]. However, as KIT signaling occurs in the fetal ovary as early as 7.5 dpc and is continuously expressed during ovary development [159, 160], it must exhibit additional functions in fetal ovary development. Indeed, KIT signaling has been demonstrated to play an important role in perinatal oocyte CBD, the determination of oocyte numbers, and PF formation in mice and humans [161, 162]. The regulation of KITL may be related to Act, one of several identified TGFβ family members with major roles in folliculogenesis [163–166]. In humans, oocytes express Act A before CBD, which repress the expression of membrane-bound KITL (KITL2) in neighboring somatic cells, thus preventing the expression of c-Kit in oocytes, because KITL itself can induce the expression of c-kit in germ cells [84]. After CBD, along with the decrease in Act expression in the oocyte, KITL2 expression is increased, which in turn induces the expression of KIT and may subsequently promote PF assembly [161, 167]. Lower KIT levels are then proposed to block either meiotic arrest or PF activation [6, 84, 166].
JNK is involved in the regulation cell proliferation, migration, and apoptosis [167–169]. Studies have demonstrated that the JNK signaling pathway is one of the candidate pathways participating in CBD [170–172], possibly by regulating E-Cad junctions between oocytes in the mouse [130] (Fig. 1b). JNK specifically localizes to oocytes, and its activity is increased as CBD progresses. Interestingly, the action of JNK is inversely correlated with WNT4 expression in the perinatal ovary [130]. In Drosophila epithelial cells, activated JNK relates to E-Cad inactivation and loss of cell polarity [173, 174]. In Xenopus, JNK signaling antagonizes the canonical Wnt3a pathway by regulating β-Catenin transport [175]. JNK was recently found to regulate AJ formation, as the inhibition of JNK kinase activity promoted localization of the E-Cad/β-Catenin complex to cell–cell contact sites [176, 177]. In the mouse, when JNK signaling activity increases, the WNT4 level simultaneously decreases. In vitro, attenuation of JNK signaling leads to WNT4 upregulation, but knockdown of WNT4 does not significantly rescue JNK inhibition-induced CBD failure, as WNT4 regulates E-Cad expression, whereas JNK signaling does not. Therefore, the reciprocal change leads to E-cad junction disassembly and gradual germline CBD. JNK signaling is maintained at high levels until 4 dpp when the oocytes are released from the cysts completely and surrounded by pre-granulosa cells to form PFs [130]. Hence, the homeostasis between JNK and WNT4 activity is fine tuned for cyst structure maintenance and breakdown during the early stage of ovarian development.
The small GTPase family member Rac1 may also play an indispensable role in controlling PF formation in the mouse ovary (Fig. 2d). Rac1 acts as a molecular switch by cycling between an active GTP-bound state and an inactive GDP-bound state. In its active state, Rac1 actively regulates cell shape, adhesion, movement, endocytosis, secretion, and growth [178, 179]. Rac1 is involved in meiotic spindle stability and anchoring in oocytes as well as embryo implantation and embryonic epithelial morphogenesis [180–183]. In the fetal mouse ovary, Rac1 is exclusively expressed in germ cells prior to follicle assembly and in the oocytes of PFs [157]. In vitro, disruption of Rac1 retards CBD, whereas Rac1 overexpression accelerates the formation of PFs. In vivo inhibition of Rac1 results in the formation of MOFs which are similar to those associated with Gdf9 and Bmp mutations in mice [184]. Rac1 may induce the nuclear import of STAT3 through physical binding to activate the expression of oocyte-specific genes, including Jagged1, GDF9, BMP15, and Nobox [157, 185, 186], indicating that signals from the oocyte to the pre-granulosa cells are vital for CBD and PF formation [187]. The action of Rac1 in CBD and PF formation may also be related to E-Cad, as E-Cad can regulate the localization and function of Rho GTPases, including Rac and Cdc42, through a mechanism described as ‘outside-in signaling’ [181]. However, more animal models are needed to clearly elucidate the mechanism of Rac1 in this process.
S100A8, an oocyte-specific chemokine and calcium-binding S100 protein family member, has been demonstrated to direct the migration of ovarian somatic cells during mouse PF assembly [188]. Regarding the initial motivation for PF assembly, apoptosis within cysts is important for yielding isolated oocytes for PF formation, as demonstrated by the BCL2-associated X protein (Bax) mutant mouse model [106, 189]. However, studies of Bax and caspase 2 (Casp2) mutant mice indicate that the formation of PFs may be organized by some stimulus other than apoptosis, such as the migration or invasion of pre-granulosa cells from outside-to-inside the cysts to envelop the oocyte [190, 191]. In contrast to observations made in the 1990s showing that the oocyte itself is motile [192, 193], a recent in vitro study demonstrated that oocytes are functionally immotile during PF formation [188]. S100A8 is significantly expressed in the oocytes of cysts at 19.0 dpc, the point at which PF assembly is about to begin. In addition, S100A8 significantly promotes the number of migrating ovarian somatic cells in vitro, the majority of which are FOXL2-positive cells, implying that pre-granulosa cells are motile. In addition, knockdown of S100A8 not only inhibits follicle reconstruction in a dose-dependent manner but also prevents PF assembly in mice [188]. S100A8 exhibits multiple receptors for performing various functions [194, 195]. Hence, future work should concentrate on defining the ovarian cell-specific receptors of S100A8 to explore its roles in ovarian development.
Hormones regulating CBD and PF formation
In the mouse, circulating hormones and steroid factors, such as diethylstilbestrol [196], bisphenol-A [197] or phytoestrogen genistein [198], as well as estrogen, estradiol, and progesterone [199–202], have negative roles in regulating CBD and PF formation (Fig. 1d). In addition, neonatal mice treated with steroids exhibit a significantly higher incidence of MOFs in adult ovaries, implying that the occurrence of these aberrant follicles results from incomplete CBD [198]. Consequently, under physiological conditions, CBD may be initiated by a dramatic neonatal decrease of estrogen and progesterone, which are derived from the maternal milieu [84, 105, 199] of pregnancy, or from the fetal ovary itself [203]. Estrogen and progesterone may act through classical and non-classical mechanisms to modulate CBD and PF formation [105]. Through binding to estrogen receptors, elevated estrogen, trans-acting transcription factor 1 (SP1), and β-Catenin are involved in regulation of the expression of various genes and cause POF [204, 205]. Estrogen receptor inhibition assures the formation of PF-like structures in vitro [205, 206]. In the hamster and baboon, however, estrogen appears to have positive effects on PF formation [207–211]. Similarly, although estrogen receptors are found in human germ cells at the time of PF formation [212], the situation is quite different in humans compared to mice, as steroid hormones are maintained at high levels during PF formation in the second trimester of embryonic development [213–215]. These differences may be related to the different concentrations before and after CBD or to species specificity [105, 216]. Unfortunately, the exact cause is still unclear.
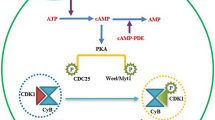
Follicle-stimulating hormone (FSH) may coordinate the action of estrogen in the fetal mouse ovary, while PFs are being established [5, 105, 217, 218]. FSH has been detected in the serum of neonatal mice and hamsters [219, 220]. The FSH receptor, possibly stimulated by FSH or estrogen, is expressed in the fetal ovaries of both mice [221] and hamsters [218]. In the hamster, FSH is involved in inducing PF formation and cAMP production in the ovary [218], the latter of which is important for early oogenesis and folliculogenesis in mice [102]. Neutralization of FSH results in the inhibition of PF formation [222]. These observations are in line with the findings in the mouse, in which PF formation is improved by FSH in vitro [223], and the oocyte-specific genes Fig1α and Nobox are promoted in a low-estrogen environment [217]. Therefore, the coordination of FSH and estrogen may be crucial for the proper timing of CBD and PF formation [5].
The downstream targets of estrogen and progesterone may be the TGFβ family member hormones, such as Inhibin (Inh), Act, and Fst [84]. First, these factors are involved in PF formation. In the human fetal ovary, Act is produced by germ cells in cysts. The level of Act is reduced when CBD begins and is undetectable in oocytes in PFs [215, 224]. Treatment of neonatal mice with Act A results in a greater number of PFs entering the initial follicle pool in vitro and in vivo [77, 225]. Although Act receptors are expressed by both germ and somatic cells in the human fetal ovary [215], the active forms of their downstream transcriptional regulator SMADs are detectable only in the somatic cells, indicating that these cells are the targets of Act signaling [224]. In addition, as the natural antagonist of Act, Fst is specifically expressed in fetal ovary. Conditional knockout of Fst in the granulosa cells of the mouse ovary results in an obvious loss of fertility and reduction of litter numbers [226]. FST288, the strongest Act-neutralizing isoform of Fst, may be involved in germ cell CBD and PF assembly by inhibiting somatic cell proliferation via Notch signaling [227]. Second, an appropriate ratio between Inh-Act-Fst plays a powerful role in regulating germline CBD and PF formation. Acts and Inhs are homo- or hetero-dimeric cysteine knot proteins that share a common β subunit [228]. Exposure of neonatal mice to estrogens suppresses the levels of both Act in the ovary and Inh A in serum [200]. Similarly, in the baboon, estrogen regulates fetal ovarian folliculogenesis by controlling the ratio of Act:Inh within the ovary throughout the second half of gestation [211]. Disruption of the Inh-Act-Fst pathway is observed in TAF4b-deficient mice, indicating that these hormones function downstream of TAF4b in regulating PF formation [229]. Therefore, the effects of Act on germ cell survival and/or proliferation occur indirectly through juxtacrine/paracrine interactions with surrounding somatic cells rather than through autocrine interactions with germ cells [166] (Fig. 3).
Germ cell survival
In many mammalian species, large numbers of oocytes die along with the CBD before individual PFs assemble [25, 110, 230, 231]. During this process, the cytoplasmic bridges between the remaining nuclei are either retracted or cleaved, likely through the protease action of the surrounding somatic cells [84]. In mice, the maximum number of germ cells is observed in the mouse ovary at the time at which oocytes enter meiotic prophase [232], whereas oocyte death is most pronounced at the time of birth, the point at which when PF formation is at its peak. As a result, by 4 dpp in the mouse, approximately 6.4 germ cells on average in each cyst survive from the initial 30 (20%) as primary oocytes [109, 110, 232–234]. In humans, attrition of the ovarian reserve begins at approximately 24 wpc when the number of oocytes starts to decline significantly [235]. Consequently, the ovarian lifespan is perinatally determined through a delicate balance of oocyte survival and apoptosis [87, 161].
An earlier depletion of the ovarian reserve results in earlier loss of estrogen production by the ovary. Estrogen is of great importance for bone, cardiovascular, and cognitive health as well as overall mortality in women [236]. Hence, understanding the mechanism of germ cell loss during this time is pivotal for identifying suitable strategies to preserve the size of the ovarian pool. However, detailed information regarding oocyte death remains limited.
Programmed cell death (PCD) is an important physiological process during embryonic development. Germ cell death can be apoptotic or nonapoptotic, depending on the stimulus or stage of development [237]. There are three types of PCD: type I (apoptosis), type II (autophagy), and type III (non-lysosomal vesiculate degradation) [232, 238]. According to in vivo and in vitro findings, fetal germ cell death occurs via a number of mechanisms during CBD, including Caspase 2-dependent apoptosis [34, 110, 239–243], highly conserved cytoprotective autophagy [241, 244–247], and direct extrusion from the ovaries (ovarian shedding) [245, 246].
Apoptotic and autophagic proteins appear to coexist in fetal oocytes [85] (Fig. 4). Recent reviews showed that autophagy exhibits an unresolved close relationship with apoptosis, as the initiation of either autophagy or apoptosis requires disruption of the same B-cell lymphoma/leukemia-2 (Bcl-2)/Bcl-xL complexes, which bind to either Beclin1 (autophagy pathway) or Bax/Bak (apoptosis pathway) [248, 249]. However, the autophagy protein p62 has been shown to interact with caspase-8, TRAF, and ERK [248]. Autophagy degrades damaged mitochondria and caspases and provides membranes for caspase processing in the regulation of apoptosis [249]. In addition, JNK1 is involved in the regulation of oocyte apoptosis and autophagy via phosphorylation of Bcl-2 [250]. The mutual relationship between autophagy and apoptosis before and after PF formation requires substantial further study.
Schematic representation of autophagy and apoptosis regulation as well as the meiosis initiation mechanism during CBD and PF formation. a Cellular stress induced by starvation, ROS (reactive oxygen species), and other factors causes the displacement of Bcl-2 from either Beclin-1 or Bax, thereby triggering autophagy or apoptosis, respectively. Activated JNK is responsible for the phosphorylation of Bcl-2. The autophagy–apoptosis interactions are not fully understood. b Amino acid deprivation, hypoxia, and an elevated AMP/ATP ratio activate AMPK (5′-AMP-activated protein kinase), which in turn activate TSC2 (tuberous sclerosis complex 2), thus preventing the action of mTORC1 from inhibiting autophagy. c Increase of KIT levels in oocytes induced by KITL or SOHLH2 may activate mTORC1 to inhibit apoptosis through the PI3K/AKT signaling pathway
The regulators of apoptosis consist of two groups of proteins: proapoptotic (Bax, Bak, Bad, Bid, PUMA, Noxa, Nix, Xiap, and Bim) and antiapoptotic (Bcl2, Bcl-xL, and Mcl1) [251]. In most cases, survival or apoptosis results from a balance between the expression of survival (antiapoptotic) and proapoptotic factors [84, 235]. Apoptosis has been recognized as the main cause of the attrition of the ovarian PF pool in both humans and mice [84, 191, 252, 253]. Freshly formed cysts sustain ongoing apoptosis and later fragment into smaller cysts [106]. Apoptosis is triggered by either physiological (developmental cues) or cellular (environmental in origin) stressors [232]. For instance, plummeting levels of estrogen immediately after birth in mice may be responsible for oocyte apoptosis [65, 198, 217, 254]. Most recently, deficiency of Mcl-1 in the mouse oocyte was shown to result in premature exhaustion of the ovarian reserve, characterized by early PF loss due to activation of apoptosis [161, 255]. In humans, Mcl-1 is expressed in germ cells of different sizes at the periphery of the ovary at 14–16 wpc and in the oocytes within newly formed PFs at 21 wpc, indicating a possible role of Mcl-1 in PF formation [256]. Mcl-1 may be essential for the conservation of the postnatal PF pool as well as the survival of growing follicles and effective oocyte mitochondrial function [255]. The roles of other apoptosis-related proteins in PF formation also require further study.
As the mitochondrial load has been positively correlated with the prevention of apoptosis [257], intercellularly transferred proteins, nutrients, or mitochondria may themselves serve as the trigger for apoptosis of the nurse cell, and conversely, for protection of oocytes with a Balbiani body from apoptosis. In addition to the proteins that could be involved in the regulation of oocyte death, recent studies have indicated that miRNAs are involved in PF assembly by regulating either pre-granulosa cell proliferation [217, 258] or oocyte apoptosis [259]. These findings provide insight into new possibilities for systematically evaluating the roles of novel miRNAs in the regulation of target genes during CBD and PF assembly.
The term “autophagy” comes from the Greek words “auto” (self) and “phagein” (to eat). Autophagy is a highly conserved intracellular process that maintains cellular homeostasis through the removal of useless or damaged cytoplasmic organelles and large molecules [260]. Autophagy is both a unique cell-death pathway and an adaptation to stress that promotes cell survival [249]. Recently, autophagy has been demonstrated to be active in the life and death decisions of oocytes, particularly around the time of PF assembly [246, 247, 261]. Compromised autophagy within the perinatal ovary due to deficiency of autophagy-related genes (i.e., Atg7 or Beclin1) results in the premature loss of female germ cells by 1 dpp in mice, which is similar to POI in humans [247, 262]. Therefore, autophagy appears to be a cell protective method for maintaining the endowment of female germ cells prior to establishing PF pools in the ovary [247].
Importantly, if starvation is prolonged before oocytes are enclosed in a PF, the lack of nutrients or growth factors may activate protective autophagy or even result in cell death [85, 262] (Fig. 4a). In addition, the mTOR protein, which senses the availability of nutrients and energy substrates within the intracellular milieu, is the major regulator of autophagy in response to nutritional factors (Fig. 4b). A decrease in the above-mentioned factors results in the inactivation of mTOR and, thus, initiates Beclin1-mediated autophagy [241, 260]. The inducer of autophagy and the prerequisite for activating the process during CBD and PF formation remains a mystery.
KIT is directly involved in and important to the early survival of oocytes (Fig. 4c). As noted above, c-Kit is only expressed in oocytes during CBD, but KITL is expressed in both oocytes and ovarian somatic cells when PFs are formed. The inhibition of KIT results in a reduction in CBD, an increase in oocyte numbers, and a reduction of cell death. Activation of KIT promotes CBD and decreases oocyte numbers [161]. In vitro, KIT inhibitors reduce CBD and the growth of oocytes in both the fetal and neonatal ovary [224, 263]. Previous reports have indicated that naturally mutated KIT ligands in the mouse result in the formation of ovaries with fewer germ cells [264, 265]. When activated by KITL, KIT is autophosphorylated at tyrosine 719, which is also the primary binding site for the p85 of PI3K. Autophagy is regulated through the PI3K/AKT-mTOR pathway as well [260, 266]. Alternatively, spermatogenesis- and oogenesis-specific helix-loop-helix transcription factor 2 (SOHLH2) is effective in upregulating c-kit expression and inhibiting oocyte apoptosis, possibly through activation of the KIT/PI3K/AKT/Foxo3a signaling pathway [267].
In addition, the presence of a particular number of associated granulosa cells is important for sustaining oocyte survival [66, 268]. For example, neurotrophins, a family of growth factors, are involved in cell survival and ovary development. Surviving oocytes are contained in follicles that exhibit sufficient granulosa cells to provide the oocyte with sufficient neurotrophins [66, 269]. Feng et al. (2016) reported that the proper differentiation and proliferation of ovarian supporting cells are essential for the establishment of the PF pool. In particular, the recruited number of somatic cells and speed of recruitment may determine the final size of the female reproductive reserve [66]. Furthermore, water channel aquaporin-8 (AQP8), which is a major plasma membrane water channel protein that is exclusively expressed in granulosa cells and in the neonatal mouse ovary, has been reported to be responsible for the formation of MOFs [270]. Several studies have demonstrated that deficiency of AQP8 may regulate pre-granulosa cell function during folliculogenesis by decreasing apoptosis and impairing cell migration [270–272].
Death receptor signaling factors, such as Tumor Necrosis Factor (TNF)/TNF receptor 1 and Fas ligand/Fas, are expressed in neonatal rodent ovaries and regulate oocyte death [232]. A lack of TNF in ovarian somatic cells results in malfunction of CBD and PF formation in rodents, whereas the deletion of TNFα or Fas in mice increases the number of PFs at birth [273–275].
Finally, Wnt4 and Fst are both specifically expressed in somatic cells of the fetal ovary. Based on Wnt4 acting as an initiator of Fst expression, FOXL2 and Bmp2 cooperate to ensure the correct expression of Fst [276]. Fst may be responsible for preventing the action of Act B, which causes masculinization and female germ cell loss [54]. Germ cells in the ovarian cortex are almost completely lost in both Wnt4- and Fst-deficient gonads before birth, indicating that Wnt4 acts through Fst in the early ovary to regulate vascular boundaries and endothelial cell migration from the mesonephros and to maintain germ cell survival at the pachytene and diplotene stages (approximately 16.5 dpc) in the ovary [276, 277].
Summary
PF formation in mammals is systematically engineered by signals inside and outside the ovary. Previous studies have elucidated many important hormones and factors that play roles in CBD and PF assembly through endocrine, paracrine and autocrine actions. However, additional in vivo assays are needed to accurately elucidate what occur under physiological conditions. Moreover, it is still unclear which organizers are the most important in disassembling germline cysts and regulating PF formation. According to the most recent reports, reconstituted follicles, fertile oocytes, and full-term developed pups have been obtained from in vitro-generated PGCs [205, 206]. However, the low rate of generation of pups produced from the MII oocytes (ranging from 3.5% [205] to 14–40% [206] in vitro vs 61.7% in vivo) implies that the physiological mechanism of oogenesis is far from being well understood. Future work focusing on the mechanism that precisely controls this process is pivotal for potential translation of these findings to produce high-quality PFs in vitro for clinical applications.
Abbreviations
- PF:
-
Primordial follicle
- CBD:
-
Cyst breakdown
- POI:
-
Primary ovarian insufficiency
- POF:
-
Premature ovarian failure
- MOF:
-
Multi-oocyte follicle
- JNK:
-
c-Jun amino-terminal kinase
- mTORC1:
-
Mechanistic target of rapamycin complex 1
- PI3K:
-
Phosphatidyl inositol 3-kinase
- PGCs:
-
Primordial germ cells
- Dpc:
-
Day post coitus
- Wpc:
-
Week post conception
- FOXL2:
-
Forkhead box L2
- WNT4:
-
Canonical wingless-type MMTV integration site family member 4
- RA:
-
Retinoic acid
- RARs:
-
RA receptors
- RXRs:
-
Retinoid X receptors
- RALDHs:
-
Retinaldehyde dehydrogenases
- Act:
-
Activin
- TGF:
-
Transforming growth factor
- DSBs:
-
DNA double-strand breaks
- PAR6:
-
Partitioning-defective Protein 6
- AJs:
-
Adherens junctions
- Cads:
-
Cadherins
- GJs:
-
Gap junctions
- NICD:
-
Notch intercellular domain
- ADAM10:
-
A disintegrin and metalloproteinase domain10
- Bax:
-
Bcl2-associated X protein
- Casp2:
-
Caspase 2
- FSH:
-
Follicle-stimulating hormone
- PCD:
-
Programmed cell death
- SOHLH2:
-
Spermatogenesis- and oogenesis-specific helix-loop-helix transcription factor 2
- AQP8:
-
Water channel aquaporin-8
- Fst:
-
Follistatin
- Inh:
-
Inhibin
- LGR5:
-
Leucine-rich repeat-containing G-protein-coupled receptor 5
- Bcl-2:
-
B-cell lymphoma 2
References
Zuckerman S (1951) The number of oocytes in the mouse ovary. Recent Prog Horm Res 6:63–108
Zhang H, Adhikari D, Zheng WJ, Liu K (2013) Combating ovarian aging depends on the use of existing ovarian follicles, not on putative oogonial stem cells. Reproduction 146:R229–R233
Aaron JWH (2014) Fertility: the role of mTOR signaling and KIT ligand. Curr Biol 24(21):1040–1042
Okeke T, Anyaehie U, Ezenyeaku C (2013) Premature menopause. Ann Med Health Sci Res 3:90–95
Grive KJ, Freiman RN (2015) The developmental origins of the mammalian ovarian reserve. Development 142:2554–2563
Pelosi E, Simonsick E, Forabosco A, Garcia-Ortiz JE, Schlessinger D (2015) Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biol Reprod 92:130
Justine B, Isabelle B, Sara B, Valérie B, Kemal A, Jérôme F, Anne F, Anne LT, Reiner AV, Chérif B, Brigitte D, Catherine D, Jacques Y, Nadine B (2016) Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab 101(12):4541–4550
Jeruss JS, Woodruff TK (2009) Preservation of fertility in patients with cancer. N Engl J Med 360:902–911
Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A, Winer EP (2004) Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 22:4174–4183
Kim SS (2006) Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril 85:1–11
Kim SS (2013) Oocyte biology in fertility preservation. (eBook) Springer New York Heidelberg Dordrecht London. doi:10.1007/978-1-4614-8214-7
Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R (1996) Low temperature storage and grafting of human ovarian tissue. Hum Reprod 11:1487–1491
Gook DA, Edgar DH, Stern C (1999) Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1, 2-propanediol. Hum Reprod 14:2061–2068
Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K (2008) Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319:611–613
John GB, Gallardo TD, Shirley LJ, Castrillon DH (2008) Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 321:197–204
Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ (2010) Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA 107:10280–10284
Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, Bosbach B, Bra¨nnstro¨m M, Liu K (2014) Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol 24:2501–2508
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, Hosoi Y, Yoshioka N, Ishizuka B, Hsueh AJ (2013) Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA 110:17474–17479
Monk M, McLaren A (1981) X-chromosome activity in foetal germ cells of the mouse. J Embryol Exp Morphol 63:75–84
Motta PM, Makabe S, Nottola SA (2000) The ultrastructure of human reproduction. I. The natural history of the female germ cell: origin, migration and differentiation inside the developing ovary. Hum Reprod Update 3:281–295
Kerr CL, Hill CM, Blumenthal PD, Gearhart JD (2008) Expression of pluripotent stem cell markers in the human fetal ovary. Hum Reprod 23:589–599
Cohen PE, Holloway JK (2010) Predicting gene networks in human oocyte meiosis. Biol Reprod 82:469–472
Pepling ME, Spradling AC (1998) Female mouse germ cells form synchronously dividing cysts. Development 125:3323–3328
Jung D, Kee K (2015) Insights into female germ cell biology: from in vivo development to in vitro derivations. Asian J Androl 17(3):415–420
Baker TG (1963) A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond Ser B 158:417–433
Hitomi S, Masami KA, Yoshiakira K (2015) From sex determination to initial folliculogenesis in mammalian ovaries: morphogenetic waves along the anteroposterior and dorsoventral axes. Sex Dev 9:190–204. doi:10.1159/000440689
Menke DB, Koubova J, Page DC (2003) Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 262:303–312
De Felici M, Klinger FG, Farini D, Scaldaferri ML, Iona S, Lobascio M (2005) Establishment of oocyte population in the fetal ovary: primordial germ cell proliferation and oocyte programmed cell death. Reprod Biomed Online 10:182–191
Mazaud S, Guyot R, Guigon CJ, Coudouel N, Le Magueresse-Battistoni B, Magre S (2005) Basal membrane remodeling during follicle histogenesis in the rat ovary: contribution of proteinases of the MMP and PA families. Dev Biol 277:403–416
Pepling ME, Sundman EA, Patterson NL, Gephardt GW, Medico L Jr, Wilson KI (2010) Differences in oocyte development and estradiol sensitivity among mouse strains. Reproduction 139:349–357
Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT (2007) Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol 7:136
Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B (2012) Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod 86:37
Rajah R, Glaser EM, Hirshfield AN (1992) The changing architecture of the neonatal rat ovary during histogenesis. Dev Dyn 194:177–192
De Pol A, Vaccina F, Forabosco A, Cavazzuti E, Marzona L (1997) Apoptosis of germ cells during human prenatal oogenesis. Hum Reprod 12:2235–2241
De Felici M, Di Carlo A, Pesce M, Iona S, Farrace MG, Piacentini M (1999) Bcl-2 and Bax regulation of apoptosis in germ cells during prenatal oogenesis in the mouse embryo. Cell Death. Differentiation 6:908–915
Ghafari F, Gutierrez CG, Hartshorne GM (2007) Apoptosis in mouse fetal and neonatal oocytes during meiotic prophase one. BMC Dev Biol 7:87
Zheng W, Zhang H, Gorre N, Risal S, Shen Y, Liu K (2014) Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet 23:920–928
Hirshfield AN (1992) Heterogeneity of cell populations that contribute to the formation of primordial follicles in rats. Biol Reprod 47:466–472
Eppig JJ, Handel MA (2012) Origins of granulosa cells clarified and complexified by waves. Biol Reprod 86(2):34, 1–2
Peters H (1969) The development of the mouse ovary from birth to maturity. Acta Endocrinol 62:98–116
Hirshfield AN, DeSanti AM (1995) Patterns of ovarian cell proliferation in rats during the embryonic period and the first three weeks postpartum. Biol Reprod 53:1208–1221
Pepling ME (2006) From primordial germ cell to primordial follicle:mammalian female germ cell development. Genesis 44:622–632
Lei L, Zhang H, Jin SY, Wang FC, Fu MY, Wang HB, Xia GL (2006) Stage-specific germ-somatic cell interaction directs the primordial folliculogenesis in mouse Stage-specific ovarian somatic cells in primordial folliculogenesis fetal ovaries. J Cell Physi 208:640–647
Joan SJ (2013) Defining the neighborhoods that escort the oocyte through its early life events and into a functional follicle. Mol Reprod Dev 80(12):960–976
Gilchrist RB, Ritter LJ, Armstrong DT (2004) Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci 82–83:431–446
Chang CL, Wang HS, Soong YK, Huang SY, Pai SY, Hsu SY (2011) Regulation of oocyte and cumulus cell interactions by intermedin/adrenomedullin 2. J Biol Chem 286:43193–43203
Borum K (1961) Oogenesis in the mouse. A study of the origin of the mature ova. Exp Cell Res 45:39–47
Byskov AG, Guoliang X, Andersen CY (1997) The cortex-medulla oocyte growth pattern is organized during fetal life: an in-vitro study of the mouse ovary. Mol Hum Reprod 3:795–800
Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G (2004) Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 13:1171–1181
Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D (2007) Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet 16:2795–2804
Qing T, Liu H, Wei W, Ye X, Shen W, Zhang D, Song Z, Yang W, Ding M, Deng H (2008) Mature oocytes derived from purified mouse fetal germ cells. Hum Reprod 23:54–61
Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schütz G, Cooney AJ, Lovell-Badge R, Treier M (2009) Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139:1130–1142
Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D, Rodgers RJ (2013) A new model of development of the mammalian ovary and follicles. PLoS One 8:e55578
Nicol B, Yao HH (2014) Building an ovary: insights into establishment of somatic cell lineages in the mouse. Sex Dev 8(5):243–251
Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M (2004) The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131(4):933–942
Li G, Zhang H, Wang YJ, Wen J, Teng Z, Mao GP, Wang JW, Guo M, Mu XY, Xia GL (2011) Stage-specific mice ovarian somatic cell is involved in primordial folliculogenesis. Front Biosci (Elite Ed) 3:1025–1033
Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D (2005) Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet 14:2053–2062
Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G (2001) The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166
Ng A, Tan S, Singh G, Rizk P, Swathi Y, Tan TZ, Huang RY, Leushacke M, Barker N (2014) Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat Cell Biol 16(8):745–757
Rastetter RH, Bernard P, Palmer JS, Chassot AA, Chen H, Western PS, Ramsay RG, Chaboissier MC, Wilhelm D (2014) Marker genes identify three somatic cell types in the fetal mouse ovary. Dev Biol 394(2):242–252
Chassot AA, Bradford ST, Auguste A, Gregoire EP, Pailhoux E, de Rooij DG, Schedl A, Chaboissier MC (2012) WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad. Development 139(23):4461–72
Capel B (2014) Ovarian epithelium regeneration by Lgr5(+) cells. Nat Cell Biol 16(8):743–744
Schuijers J, Clevers H (2012) Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J 31(12):2685–2696
Hsueh AJ, Kawamura K, Cheng Y, Fauser BC (2015) Intraovarian control of early folliculogenesis. Endocr Rev 36(1):1–24
Suzuki H, Kanai-Azuma M, Kanai Y (2015) From sex determination to initial folliculogenesis in mammalian ovaries: morphogenetic waves along the anteroposterior and dorsoventral axes. Sex Dev 9(4):190–204
Feng L, Wang Y, Cai H, Sun G, Niu W, Xin Q, Tang X, Zhang J, Wang C, Zhang H, Xia G (2016) ADAM10-Notch signaling governs the recruitment of ovarian pregranulosa cells and controls folliculogenesis in mice. J Cell Sci 129(11):2202–2212
Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P (2006) Retinoid signaling determines germ cell fate in mice. Science 312:596–600
Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC (2006) Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA 103:2474–2479
Le Bouffant R, Guerquin MJ, Duquenne C, Frydman N, Coffigny H, Rouiller-Fabre V, Frydman R, Habert R, Livera G (2010) Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum Reprod 25:2579–2590
Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PT (2011) Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS One 6:e20249
Mu XY, WEN J, Guo M, Wang JW, Li G, Wang ZP, Wang YJ, Teng Z, Cui Y, Xia GL (2013) Retinoic acid derived from the fetal ovary initiates meiosis in mouse germ cells. J Cell Physiol 228(3):627–639
Mammadova A, Zhou H, Carels CE, Von den Hoff JW (2016) Retinoic acid signalling in the development of the epidermis, the limbs and the secondary palate. Differentiation 92(5):326–335
Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ (2005) Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 8:949–961
Griswold MD, Hogarth CA, Bowles J, Koopman P (2012) Initiating meiosis: the case for retinoic acid. Biol Reprod 86(2):35
Niederreither K, McCaffery P, Dräger UC, Chambon P, Dollé P (1997) Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech Dev 62(1):67–78
Tahayato A, Dollé P, Petkovich M (2003) Cyp26C1 encodes a novel retinoic acid-metabolizing enzyme expressed in the hindbrain, inner ear, first branchial arch and tooth buds during murine development. Gene Expr Patterns 3(4):449–454
Liang GJ, Zhang XF, Wang JJ, Sun YC, Sun XF, Cheng SF, Li L, De Felici Massimo, Shen W (2015) Activin A accelerates the progression of fetal oocytes throughout meiosis and early oogenesis in the mouse. Stem Cells Develop 24(20):2455–2465
Kipp JL, Golebiowski A, Rodriguez G, Demczuk M, Kilen SM, Mayo KE (2011) Gene expression profiling reveals Cyp26b1 to be an activin regulated gene involved in ovarian granulosa cell proliferation. Endocrinology 152:303–312
Tedesco M, Desimio MG, Klinger FG, De Felici M, Farini D (2013) Minimal concentrations of retinoic acid induce stimulation by retinoic acid 8 and promote entry into meiosis in isolated pregonadal and gonadal mouse primordia germ cells. Biol Reprod 88:145
Liu CF, Parker K, Yao HH (2010) WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary. PLoS One 5(4):e10382
Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, Adams IR, Chaboissier MC (2011) RSPO1/β-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in themouse fetal ovary. PLoS One 6(10):e25641
Chassot AA, Gregoire EP, Magliano M, Lavery R, Chaboissier MC (2008) Genetics of ovarian differentiation: Rspo1, a major player. Sex Dev 2(4–5):219–227
Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B (2008) Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet 17(19):2949–2955
Tingen CM, Kim A, Woodruff TK (2009) The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod 15(12):795–803
Klinger FG, Rossi V, De Felici M (2015) Multifaceted programmed cell death in the mammalian fetal ovary. Int J Dev Biol 59(1–3):51–54
Mandon-Pépin B, Touraine P, Kuttenn F, Derbois C, Rouxel A, Matsuda F, Nicolas A, Cotinot C, Fellous M (2008) Genetic investigation of four meiotic genes in women with premature ovarian failure. Eur J Endocrinol 158:107–115
Pelosi E, Forabosco A, Schlessinger D (2015) Genetics of the ovarian reserve (OR). Front Genet 6:308. doi:10.3389/fgene.2015.00308
de Vries SS, Baart EB, Dekker M, Siezen A, deRooij DG, deBoer P (1999) Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev 13:523–531
Kneitz B, Cohen PE, Avdievich E, Zhu L, Kane MF, Hou HJ (2000) MutS homolog4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev 14:1085–1097
Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E (1998) Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific Rec A homolog. Mol Cell 1:697–705
Romanienko PJ, Camerini-Otero RD (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 6:975–987
Dokshin GA, Baltus AE, Eppig JJ, Page DC (2013) Oocyte differentiation is genetically dissociable from meiosis in mice. Nat Genet 45:877–883
Paredes A, Garcia-Rudaz C, Kerr B, Tapia V, Dissen GA, Costa M, Cornea A, Ojeda S (2005) Loss of synaptonemal complex protein-1, asynaptonemal complex protein, contributes to the initiation of follicular assembly in the developing rat ovary. Endocrinology 146:5267–5277
Voronina E, Lovasco LA, Gyuris A, Baumgartner RA, Parlow AF, Freiman RN (2007) Ovarian granulosa cell survival and proliferation requires the gonad-selective TFIID subunit TAF4b. Dev Biol 303(2):715–726
Grive KJ, Seymour KA, Mehta R, Freiman RN (2014) TAF4b promotes mouse primordial follicle assembly and oocyte survival. Dev Biol 392:42–51
Di Pietro C, Vento M, Ragusa M, Barbagallo D, Guglielmino MR, Maniscalchi T, Duro LR (2008) Expression analysis of TFIID in single human oocytes: new potential molecular markers of oocyte quality. Reprod Biomed Online 17:338–349
Knauff EA, Franke L, van Es MA, van den Berg LH, van der Schouw YT, Laven JS, Lambalk CB, Hoek A, Goverde AJ, Christin-Maitre S, Hsueh AJ, Wijmenga C, Fauser BC; Dutch POF Consortium (2009) Genome-wide association study in premature ovarian failure patients suggests ADAMTS19 as a possible candidate gene. Hum Reprod 24(9):2372–2378
Lovasco LA, Seymour KA, Zafra K, O’Brien CW, Schorl C, Freiman RN (2010) Accelerated ovarian aging in the absence of the transcription regulator TAF4B in mice. Biol Reprod 82(1):23–34
Ongeri EM, Verderame MF, Hammond JM (2007) The TATA binding protein associated factor 4b (TAF4b) mediates FSH stimulation of the IGFBP-3 promoter in cultured porcine ovarian granulosa. Cells Mol Cell Endocrinol 278(1–2):29–35
Wardell JR, Hodgkinson KM, Binder AK, Seymour KA, Korach KS, Vanderhyden BC, Freiman RN (2013) Estrogen responsiveness of the TFIID subunit TAF4B in the normal mouse ovary and in ovarian tumors. Biol Reprod 89(5):116
Geles KG, Freiman RN, Liu WL, Zheng S, Voronina E, Tjian R (2006) Cell-type-selective induction of C-jun by TAF4b directs ovarian-specific transcription networks. Proc Natl Acad Sci USA 103(8):2594–2599
Wang Y, Teng Z, Li G, Mu X, Wang Z, Feng L, Niu W, Huang K, Xiang X, Wang C, Zhang H, Xia G (2015) Cyclic AMP in oocytes controls meiotic prophase I and primordial folliculogenesis in the perinatal mouse ovary. Development 142(2):343–351
Ginsburg M, Snow MH, McLaren A (1990) Primordial germ cells in the mouse embryo during gastrulation. Development 110:521–528
Edson MA, Nagaraja AK, Matzuk MM (2009) The mammalian ovary from genesis to revelation. Endocr Rev 30:624–712
Pepling ME (2012) Follicular assembly: mechanisms of action. Reprod 143:139–149
Lei L, Spradling AC (2016) Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science 352(6281):95–99
Gomperts M, Garcia-Castro M, Wylie C, Heasman J (1994) Interactions between primordial germ cells play a role in their migration in mouse embryos. Development 120:135–141
Bendel-Stenzel MR, Gomperts M, Anderson R, Heasman J, Wylie C (2000) The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech Dev 91:143–152
Lei L, Spradling AC (2013) Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development 140:2075–2081
Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234:339–351
Carlson JL, Bakst MR, Ottinger MA (1996) Developmental stages of primary oocytes in turkeys. Poult Sci 75:1569–1578
Pepling ME, Wilhelm JE, O’Hara AL, Gephardt GW, Spradling AC (2007) Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci USA 104:187–192
Aravin AA, van der Heijden GW, Castañeda J, Vagin VV, Hannon GJ, Bortvin A (2009) Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLOS Genet 5:e1000764
Lim K, Lorthongpanich C, Chew TG, Tan CW, Shue YT, Balu S, Gounko N, Kuramochi-Miyagawa S, Matzuk MM, Chuma S, Messerschmidt DM, Solter D, Knowles BB (2013) The nuage mediates retrotransposon silencing in mouse primordial ovarian follicles. Development 140:3819–3825
Malki S, van der Heijden GW, O’Donnell KA, Martin SL, Bortvin A (2014) A role for retrotransposon LINE-1 in fetal oocyte attrition in mice. Dev Cell 29:521–533
de Cuevas M, Lilly MA, Spradling AC (1997) Germline cyst formation in Drosophila. Ann Rev Genet 31:405–428
Wen J, Zhang H, Li G, Mao G, Chen X, Wang J, Guo M, Mu X, Ouyang H, Zhang M, Xia GL (2009) PAR6, a potential marker for the germ cells selected to form primordial follicles in mouse ovary. PLoS One 4(10):e7372
Macara IG (2004) Par proteins: partners in polarization. Curr Biol 14:160–162
Cox DN, Seyfried SA, Jan LY, Jan YN (2001) Bazooka and atypical protein kinase C are required to regulate oocyte differentiation in the Drosophila ovary. Proc Natl Acad Sci USA 98:14475–14480
Benton R, Palacios IM, St Johnston D (2002) Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev Cell 3:659–671
Hurov JB, Watkins JL, Piwnica-Worms H (2004) Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol 14:736–741
Gumbiner BM (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84(3):345–357
Takeichi M (2014) Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 15:397–410
Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grübel G, Legrand JF, Shimasaki S, Moore RK, Otsuka F, Erickson GF (2004) The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 25:72–101
Roggiani F, Mezzanzanica D, Rea K, Tomassetti A (2016) Guidance of signaling activations by cadherins and integrins in epithelial ovarian cancer cells. Int J Mol Sci 17(9):E1387
Lechowska A, Bilinksi S, Choi Y, Shin Y, Kloc M, Rajkovic A (2011) Premature ovarian failure in nobox-deficient mice is caused by defects in somatic cell invasion and germ cell cyst break down. J Assist Reprod Genet 28(7):583–589
Wang C, Roy SK (2010) Expression of E-cadherin and N-cadherin in perinatal hamster ovary: possible involvement in primordial follicle formation and regulation by follicle-stimulating hormone. Endocrinology 151:2319–2330
Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, Lundin E, Ottander U, Rytinki M, Liu K (2005) Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol Endocrinol 19:2564–2578
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558
Niu WB, Wang YJ, Wang ZP, Xin QL, Wang Y, Feng LZ, Zhao LH, Wen J, Zhang H, Wang C, Xia G (2016) JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice. Development 143(10):1778–1787
Smith SR, Fulton N, Collins CS, Welsh M, Bayne RA, Coutts SM, Childs AJ, Anderson RA (2010) N- and E-cadherin expression in human ovarian and urogenital duc development. Fertil Steril 93:2348–2353
Mitchell PA, Burghardt RC (1986) The ontogeny of nexuses (gap junctions) in the ovary of the fetal mouse. Anat Rec 214(3):283–288
Pérez-Armendariz EM, Sáez JC, Bravo-Moreno JF, López-Olmos V, Enders GC, Villalpando I (2003) Connexin43 is expressed in mouse fetal ovary. Anat Rec A Discov Mol Cell Evol Biol 271(2):360–367
Teng Z, Wang C, Wang Y, Huang K, Xiang X, Niu W, Feng L, Zhao L, Yan H, Zhang H (2016) Gap junctions are essential for murine primordial follicle assembly immediately before birth. Reproduction 151(2):105–115
Juneja SC (2003) mRNA expression pattern of multiple members of connexin gene family in normal and abnormal fetal gonads in mouse. Indian J Physiology. Pharmacology 47:147–156
Fagbohun C, Downs S (1991) Metabolic coupling and ligand stimulated meiotic maturation in the mouse oocyte–cumulus cell complex. Biol Reprod 45:851–859
Downs SM (1995) The influence of glucose, cumulus cells, and metabolic coupling on ATP levels and meiotic control in the isolated mouse oocyte. Dev Biol 167:502–512
Simon AM, Goodenough DA, Li E, Paul DL (1997) Female infertility in mice lacking connexin 37. Nature 385:525–529
Juneja SC, Barr KJ, Enders GC, Kidder GM (1999) Defects in the germ line and gonads of mice lacking Connexin43. Biol Reprod 60:1263–1270
Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ (2001) Cellular basis for paracrine regulation of ovarian follicle development. Reproduction 121:647–653
Combelles CM, Carabatsos MJ, Kumar TR, Matzuk MM, Albertini DF (2004) Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Mol Reprod Dev 69:347–355
Liu K (2006) Stem cell factor (SCF)-kit mediated phosphatidylinositol 3 (PI3) kinase signaling during mammalian oocyte growth and early follicular development. Front Biosci 11:126–135
Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409
Soyal SM, Amleh A, Dean J (2000) FIGα, a germ cell-specific transcription factor required for ovarian follicle formation. Development 127:4645–4654
Hori K, Sen A, Artavanis-Tsakonas S (2013) Notch signaling at a glance. J Cell Sci 126:2135–2140
Johnson J, Espinoza T, McGaughey RW, Rawls A, Wilson-Rawls J (2001) Notch pathway genes are expressed in mammalian ovarian follicles. Mech Dev 109:355–361
Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J (2005) Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development 132:817–828
Xu J, Gridley T (2013) Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles. BMC Biol 11:13
Manosalva I, Gonzalez A, Kageyama R (2013) Hes1 in the somatic cells of the murine ovary is necessary for oocyte survival and maturation. Dev Biol 375:140–151
Trombly DJ, Woodruff TK, Mayo KE (2009) Suppression of notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 150:1014–1024
Borggrefe T, Oswald F (2009) The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci 66:1631–1646
Vanorny DA, Prasasya RD, Chalpe AJ, Kilen SM, Mayo KE (2014) Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol 28:499–511
Vincent B (2016) Regulation of the α-secretase ADAM10 at transcriptional, translational and post-translational levels. Brain Res Bull 126(Pt 2):154–169
Hayashida K, Bartlett AH, Chen Y, Park PW (2010) Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken) 293:925–937
Reiss K, Saftig P (2009) The “a disintegrin and metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol 20:126–137
Weber S, Saftig P (2012) Ectodomain shedding and ADAMs in development. Development 139: 3693–3709
Zhao L, Du X, Huang K, Zhang T, Teng Z, Niu W, Wang C, Xia GL (2016) Rac1 modulates the formation of primordial follicles by facilitating STAT3-directed Jagged1, GDF9 and BMP15 transcription in mice. Sci Rep 6:23972
Gu Y, Runyan C, Shoemaker A, Surani A, Wylie C (2009) Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development 136:1295–1303
Richardson BE, Lehmann R (2010) Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol 11:37–49
Coutts SM, Childs AJ, Fulton N, Collins C, Bayne RAL, McNeilly AS, Anderson RA (2008) Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev Biol 314:189–199
Jones RL, Pepling ME (2013) KIT signaling regulates primordial follicle formation in the neonatal mouse ovary. Dev Biol 382:186–197
Findlay JK, Drummond AE, Dyson ML, Baillie AJ, Robertson DM, Ethier JF (2002) Recruitment and development of the follicle; the roles of the transforming growth factor-beta superfamily. Mol Cell Endocrinol 191:35–43
Knight PG, Glister C (2003) Local roles of TGF-beta superfamily members in the control of ovarian follicle development. Anim Reprod Sci 78:165–183
Knight PG, Glister C (2006) TGF-beta superfamily members and ovarian follicle development. Reproduction 132(2):191–206
Shimasaki S, Moore RK, Otsuka F, Erickson GF (2004) The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 25:72–101
Childs AJ, Anderson RA (2009) Activin A selectively represses expression of the membrane bound isoform of Kit ligand in human fetal ovary. Fertil Steril 92(4):1416–1419
Deng Y, Ren X, Yang L, Lin Y Wu X (2003) A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell 115:61–70
Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K (2003) JNK phosphorylates paxillin and regulates cell migration. Nature 424:219–223
Pallavi SK, Ho DM, Hicks C, Miele L, Artavanis-Tsakonas S (2012) Notch and Mef2 synergize to promote proliferation and metastasis through JNK signal activation in Drosophila. EMBO J 31:2895–2907
Bagowski CP, Xiong W, Ferrell JEJr (2001) c-Jun N-terminal kinase activation in Xenopus laevis eggs and embryos. A possible non-genomic role for the JNK signaling pathway. J Biol Chem 276:1459–1465
Oktem O, Buyuk E, Oktay K (2011) Preantral follicle growth is regulated by c-Jun-N-terminal kinase (JNK) pathway. Reprod Sci 18:269–276
Etchegaray JI, Timmons AK, Klein AP, Pritchett TL, Welch E, Meehan TL, Li C, McCall K (2012) Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells. Development 139:4029–4039
Igaki T, Pagliarini RA, Xu T (2006) Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol 16:1139–1146
Flora Llense, Enrique, Martı´n-Blanco (2008) JNK Signaling Controls Border Cell Cluster Integrity and Collective Cell Migration. Curr Biol 18:538–544
Liao G, Tao Q, Kofron M, Chen JS, Schloemer A, Davis RJ, Hsieh JC, Wylie C, Heasman J, Kuan CY (2006) Jun NH2-terminal kinase (JNK) prevents nuclear beta-catenin accumulation and regulates axis formation in Xenopus embryos. Proc Natl Acad Sci U S A 103:16313–16318
Lee MH, Koria P, Qu J, Andreadis ST (2009) JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J 23:3874–3883
Lee MH, Padmashali R, Koria P, Andreadis ST (2011) JNK regulates binding of alpha-catenin to adherens junctions and cell-cell adhesion. FASEB J 25:613–623
Aktories K (1997) Bacterial toxins that target Rho proteins. J Clin Invest 99:827
Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279:509
Halet G, Carroll J (2007) Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell 12:309–317
Nicola C, Lala PK, Chakraborty C (2008) Prostaglandin E2-mediated migration of human trophoblast requires RAC1 and CDC42. Biol Reprod 78:976
Ma HL, Gong F, Tang Y, Li X, Li X, Yang X, Lu G (2015) Inhibition of endometrial Tiam1/Rac1 signals induced by miR-22 up-regulation leads to the failure of embryo implantation during the implantation window in pregnant mice. Biol Reprod 92:152
Tu Z, Wang Q, Cui T, Wang J, Ran H, Bao H, Lu J, Wang B, Lydon JP, DeMayo F, Zhang S, Kong S, Wu X, Wang H (2016) Uterine RAC1 via Pak1-ERM signaling directs normal luminal epithelial integrity conducive to on-time embryo implantation in mice. Cell Death Differ 23:169–181
Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL (2001) Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol (Baltimore, Md.) 15:854–866
Suzumori N, Yan C, Matzuk MM, Rajkovic A (2002) Nobox is a homeoboxencoding gene preferentially expressed in primordial and growing oocytes. Mech Dev 111:137–141
Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM (2004) NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 305:1157–1159
Elvin JA, Yan C, Matzuk MM (2000) Oocyte-expressed TGF-beta superfamily members in female fertility. Mol Cell Endo 159:1–5
Teng Z, Wang C, Wang Y, Huang K, Xiang X, Niu W, Feng L, Zhao L, Yan H, Zhang H, Xia G (2015) S100A8, an oocyte-specific chemokine, directs the migration of ovarian somatic cells during mouse primordial follicle assembly. J Cell Physiol 230(12):2998–3008
Greenfeld CR, Roby KF, Pepling ME, Babus JK, Terranova PF, Flaws JA (2007) Tumor necrosis factor (TNF) receptor type 2 is an important mediator of TNF alpha function in the mouse ovary. Biol Reprod 76:224–231
Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, Hara H, Moskowitz MA, Li E, Greenberg A, Tilly JL, Yuan J (1998) Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev 12:1304–1314
Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL (1999) Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 21:200–203
Wordinger R, Sutton J, Brun-Zinkernagel AM (1990) Ultrastructure of oocyte migration through the mouse ovarian surface epithelium during neonatal development. Anat Rec 227:187–198
Mackay S, Smith RA, Haig T (1992) An investigation of the migratory potential of mouse oocytes in vitro. J Anatom 181:437–446
Ruma IM, Putranto EW, Kondo E, Murata H, Watanabe M, Huang P, Kinoshita R, Futami J, Inoue Y, Yamauchi A, Sumardika IW, Youyi C, Yamamoto K, Nasu Y, Nishibori M, Hibino T, Sakaguchi M (2016) MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin Exp Metastasis 33(6):609–627
He Z, Riva M, Björk P, Swärd K, Mörgelin M, Leanderson T, Ivars F (2016) CD14 is a co-receptor for TLR4 in the S100A9-induced pro-inflammatory response in monocytes. PLoS One 11(5):e0156377
Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N (1990) Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod 43:478–484
Zhang HQ, Zhang XF, Zhang LJ, Chao HH, Pan B, Feng YM, Li L, Sun XF, Shen W (2012) Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep 39(5):5651–5657
Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR (2002) Neonatal exposure to genistein induces estrogen receptor (ER)α expression and multioocyte follicles in the maturing mouse ovary: evidence for ERβ-mediated and nonestrogenic actions. Biol Reprod 67:1285–1296
Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME (2007) Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 148:3580–3590
Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE (2007) Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology 148:1968–1976
Guo M, Zhang H, Bian F, Li G, Mu X, Wen J, Mao G, Teng Z, Xia G, Zhang M (2012) P4 down-regulates Jagged2 and Notch1 expression during primordial folliculogenesis. Front Biosci (Elite Ed) 4:2731–2744
Guo M, Zhang C, Wang Y, Feng LZ, Wang ZP, Niu WB, Du XY, Tang W, Li Y, Wang C, Chen ZW (2016) Progesterone receptor membrane component 1 mediates progesterone-induced suppression of oocyte meiotic prophase I and primordial folliculogenesis. Sci Rep 6:36869
Dutta S, Mark-Kappeler CJ, Hoyer PB, Pepling ME (2014) The steroid hormone environment during primordial follicle formation in perinatal mouse ovaries. Biol Reprod 91:68
Levin ER (2005) Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19(8):1951–1959
Morohaku K, Tanimoto R, Sasaki K, Kawahara-Miki R, Kono T, Hayashi K, Hirao Y, Obata Y (2016) Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc Natl Acad Sci USA 113(32):9021–9026
Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M, Hayashi K (2016) Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. doi:10.1038/nature20104
Nicholas CZ, Reinhart BB, Eugene DA, Gerald JP (2002) Developmental regulation of baboon fetal ovarian maturation by estrogen. Biol Reprod 67(4):1148–1156
Wang C, Roy SK (2007) Development of primordial follicles in the hamster: role of estradiol-17beta. Endocrinology 148:1707–1716
Wang C, Prossnitz ER, Roy SK (2008) G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology 149:4452–4461
Zachos NC, Billiar RB, Albrecht ED, Pepe GJ (2004) Regulation of oocyte microvilli development in the baboon fetal ovary by estrogen. Endocrinology 145:959–966
Pepe GJ, Billiar RB, Albrecht ED (2006) Regulation of baboon fetal ovarian folliculogenesis by estrogen. Mol Cell Endocrinol 247(1–2):41–46
Fowler PA, Anderson RA, Saunders PT, Kinnell H, Mason JI, Evans DB, Bhattacharya S, Flannigan S, Franks S, Monteiro A, O’Shaughnessy PJ (2011) Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J Clin Endocrinol Metab 96:1754–1762
Nagamani M, McDonough PG, Ellegood JO, Mahesh VB (1979) Maternal and amniotic fluid steroids throughout human pregnancy. Am J Obstet Gynecol 134:674–680
Wu CH, Mennuti MT, Mikhail G (1979) Free and protein-bound steroids in amniotic fluid of midpregnancy. Am J Obstet Gynecol 133:666–672
Martins da Silva SJ, Bayne RAL, Cambray N, Hartley PS, McNeilly AS, Anderson RA (2004) Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation before primordial follicle formation. Dev Biol 266:334–345
Nilsson E, Dole G, Skinner MK (2009) Neurotrophin NT3 promotes ovarian primordial to primary follicle transition. Reproduction 138:697–707
Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK (2010) The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 315(1–2):63–73
Chakraborty P, Roy SK (2014) Expression of FSH receptor in the hamster ovary during perinatal development. Mol Cell Endocrinol 400:41–47
Vomachka AJ, Greenwald GS (1979) The development of gonadotropin and steroid hormone patterns in male and female hamsters from birth to puberty. Endocrinology 105:960–966
Halpin DM, Jones A, Fink G, Charlton HM (1986) Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic–pituitary ovarian axis. J Reprod Fert 77:287–296
O’Shaughnessy PJ, Marsh P, Dudley K (1994) Follicle-stimulating hormone receptor mRNA in the mouse ovary during post-natal development in the normal mouse and in the adult hypogonadal (hpg) mouse: structure of alternate transcripts. Mol Cell Endocrino 101:197–201
Roy SK, Albee L (2000) Requirement for follicle-stimulating hormone action in the formation of primordial follicles during perinatal ovarian development in the hamster. Endocrinology 141:4449–4456
Wang J, Roy SK (2004) Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod 70:577–585
Saatcioglu HD, Cuevas I, Castrillon DH (2016) Control of oocyte reawakening by kit. PLoS Genet 12(8):e1006215
Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK (2006) Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol 298:132–148
Kimura F, Bonomi LM, Schneyer AL (2011) Follistatin regulates germ cell nest breakdown and primordial follicle formation. Endocrinology 152:697–706
Wang Z, Niu W, Wang Y, Teng Z, Wen J, Xia G, Wang C (2015) Follistatin288 regulates germ cell cyst breakdown and primordial follicle assembly in the mouse ovary. PLoS One 10(6):e0129643
Kelly LW, Yogeshwar M, Craig A (2012) Harrison New insights into the mechanisms of activin action and inhibition. Mol Cell Endo 359:2–12
Freiman RN, Albright SR, Zheng S, Sha WC, Hammer RE, Tjian R (2001) Requirement of tissue-selective TBP-associated factor TAFII105 in ovarian development. Science 293:2084–2087
Brambell FWR (1927) The Development and Morphology of the Gonads of the Mouse—Part I. The morphogenesis of the indifferent gonad and of the ovary. Proc R Soc B 101:391–409
Hirshfield AN (1991) Development of follicles in the mammalian ovary. Int Rev Cytol 124:43–99
Findlay JK, Hutt KJ, Hickey M, Anderson RA (2015) How is the number of primordial follicles in the ovarian reserve established? Biol Reprod 93(5):111
Motta PM, Nottola SA, Makabe S, Heyn R (2000) Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod 2:129–147
Konishi I, Fujii S, Okamura H, Parmley T, Mori T (1986) Development of interstitial cells and ovigerous cords in the human fetal ovary: an ultrastructural study. J Anat 148:121–135
Leung P, Adashi E (2003) The ovary. Elsevier Academic Press, San Diego
Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA (2010) Premature menopause or early menopause: long-term health consequences. Maturitas 65:161–166
Peterson JS, Timmons AK, Mondragon AA, McCall K (2015) The end of the beginning: cell death in the germline. Curr Top Dev Biol 114:93–119
Gozuacik D, Kimchi A (2004) Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23(16):2891–2906
Coucouvanis EC, Sherwood SW, Carswell-Crumpton C, Spack EG, Jones PP (1993) Evidence that the mechanism of prenatal germ cell death in the mouse is apoptosis. Exp Cell Res 209:238–247
Bergeron JM, Gahr M, Horan K, Wibbels T, Crews D (1998) Cloning and in situ hybridization analysis of estrogen receptor in the developing gonad of the red-eared slider turtle, a species with temperaturedependent sex determination. Dev Growth Diff 40:243–254
De Felici M, Lobascio AM, Klinger FG (2008) Cell death in fetal oocytes: many players for multiple pathways. Autophagy 4(2):240–242
Takai Y, Matikainen T, Jurisicova A, Kim MR, Trbovich AM, Fujita E, Nakagawa T (2007) Caspase-12 compensates for lack of caspase-2 and caspase-3 in female germ cells. Apoptosis 12:791–800
Xu B, Hua J, Zhang Y, Jiang X, Zhang H, Ma T, Zheng W, Sun R, Shen W, Sha J (2011) Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS ONE 6:e16046
Morita Y, Maravei DV, Bergeron L, Wang S, Perez GI, Tsutsumi O, Taketani Y, Asano M, Horai R, Korsmeyer SJ (2001) Caspase-2 deficiency prevents programmed germ cell death resulting from cytokine insufficiency but not meiotic defects caused by loss of ataxia telangiectasia mutated (Atm) gene function. Cell Death Differ 8:614–620
Lobascio AM, Klinger FG, Scaldaferri ML, Farini D, De Felici M (2007) Analysis of programmed cell death in mouse fetal oocytes. Reproduction 134(2):241–252
Rodrigues P, Limback D, McGinnis LK, Plancha CE Albertini DF (2009) Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction 137:709–720
Gawriluk TR, Hale AN, Flaws JA, Dillon CP, Green DR, Rucker EB III (2011) Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction 141:759–765
Gump JM, Thorburn A (2011) Autophagy and apoptosis: what is the connection? Trends Cell Biol 21(7):387–392
Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK (2014) Autophagy and apoptosis: where do they meet? Apoptosis 19(4):555–566
Wei Y, Sinha S, Levine B (2008) Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy 4:949–951
Danial NN (2007) BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res 13:7254–7263
Alton M, Taketo T (2007) Switch from BAX-dependent to BAX-independent germ cell loss during the development of fetal mouse ovaries. J Cell Sci 120:417–424
Greenfeld CR, Pepling ME, Babus JK, Furth PA, Flaws JA (2007) BAX regulates follicular endowment in mice. Reproduction, vol 133, Cambridge, England, pp 865–876
Chen Y, Breen K, Pepling ME (2009) Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol 202:407–417
Omari S, Waters M, Naranian T, Kim K, Perumalsamy AL, Chi M, Greenblatt E, Moley KH, Opferman JT, Jurisicova A (2015) Mcl-1 is a key regulator of the ovarian reserve. Cell Death and Disease 6:e1755
Hartley PS, Bayne RAL, Robinson LLL, Fulton N, Anderson RA (2002) Developmental changes in expression of myeloid cell leukaemia-1 in human germ cells during oogenesis and early folliculogenesis. J Clin Endocrinol Metab 87:3417–3427
Perez GI, Trbovich AM, Gosden RG, Tilly JL (2000) Mitochondria and the death of oocytes. Nature 403:500–501
Zhang J, Ji X, Zhou D, Li Y, Lin J, Liu J, Luo H, Cui S (2013) miR-143 is critical for the formation of primordial follicles in mice. Front Biosci (Landmark) 18:588–597
Zhang H, Jiang XH, Zhang YW, Xu B, Hua J, Ma TL, Zheng W, Sun R, Shen W, Cooke HJ, Hao QM, Qiao J, Shi QH (2014) microRNA 376a regulates follicle assembly by targeting Pcna in fetal and neonatal mouse ovaries. Reproduction 148:43–54
Kanninen TT, de Andrade Ramos BR, Tomi WSS (2013) The role of autophagy in reproduction from gametogenesis to parturition. Euro obestetrics genecology. Reprod Biol 171:3–8
Hulas-Stasiak M, Gawron A (2011) Follicular atresia in the prepubertal spiny mouse (Acomys cahirinus) ovary. Apoptosis 16:967–975
Song ZH, Yu HY, Wang P, Mao GK, Liu WX, Li MN, Wang HN, Shang YL, Liu C, Xu ZL, Sun QY, Li W (2015) Germ cell-specific Atg7 knockout results in primary ovarian insufficiency in female mice. Cell Death Dis 6:e1589
Packer AI, Hsu YC, Besmer P, Bachvarova RF (1994) The ligand of the c-kit receptor promotes oocyte growth. Dev Biol 161:194–205
Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P (1993) The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol 157:100–109
Bedell MA, Brannan CI, Evans EP, Copeland NG, Jenkins NA, Donovan PJ (1995) DNA rearrangements located over 100 kb 50 of the Steel (Sl)-coding region in Steel-panda and Steel-contrasted mice deregulate Sl expression and cause female sterility by disrupting ovarian follicle development. Genes Dev 9:455–470
Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley L (2002) Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell 10(1):151–162
Zhang X, Zhang H, Gao Q, Ji S, Bing L, Hao J (2015) Sohlh2 inhibits the apoptosis of mouse primordial follicle oocytes via C-kit/PI3K/Akt/Foxo3a signalling pathway. Reprod Biomed Online 30(5):514–521
Sawyer H, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP (2002) Formation of ovarian follicles during fetal development in sheep. Biol Reprod 66:1134–1150
Norah S, Michael DM, Lynne LLR, Norma F, Helen C, Kohji S, Evelyn ET, Richard AA, David JP (2003) The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development 130:5481–5491
Su WH, Guan XG, Di Z, Sun MY, Yang LF, Yi F, Feng H, Feng XC, Ma TH (2013) Occurrence of multi-oocyte follicles in aquaporin8-deficient mice. Reprod Biol Endocrinol 11:88
Jablonski EM, Webb AN, McConnell NA, Riley MC, Hughes FM Jr (2004) Plasma membrane aquaporin activity can affect the rate of apoptosis but is inhibited after apoptotic volume decrease. Am J Physiol Cell Physiol 286:975–985
Su W, Qiao Y, Yi F, Guan X, Zhang D, Zhang S, Hao F, Xiao Y, Zhang H, Guo L, Yang L, Feng X, Ma T (2010) Increased female fertility in aquaporin 8-deficient mice. IUBMB Life 62:852–857
Sakata S, Sakamaki K, Watanabe K, Nakamura N, Toyokuni S, Nishimune Y, Mori C, Yonehara S (2003) Involvement of death receptor Fas in germ cell degeneration in gonads of Kit-deficient Wv/Wv mutant mice. Cell Death Differ 10:676–686
Moniruzzaman M, Sakamaki K, Akazawa Y, Miyano T (2007) Oocyte growth and follicular development in KIT-deficient Fas-knockout mice. Reproduction 133:117–125
Cui LL, Yang G, Pan J, Zhang C (2011) Tumor necrosis factor alpha knockout increases fertility of mice. Theriogenology 75:867–876
Kashimada K, Pelosi E, Chen H, Schlessinger D, Willhelm D, Koopman P (2011) FOXL2 and BMP2 act cooperatively to regulate Follistatin gene expression during ovarian development. Endocrinology 152(1):272–280
Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B (2004) Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn 230:210–215
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00018-017-2499-8.
Rights and permissions
About this article
Cite this article
Wang, C., Zhou, B. & Xia, G. Mechanisms controlling germline cyst breakdown and primordial follicle formation. Cell. Mol. Life Sci. 74, 2547–2566 (2017). https://doi.org/10.1007/s00018-017-2480-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2480-6