Abstract
Aquaporin 5 (AQP5) promotes the progression and invasion of several cancers, but its role in the tumorigenesis of human gastric carcinoma (GC) has not been clearly defined. Here, we investigated the potential functions of AQP5 in the proliferation and migration of human GC. RT-PCR and western blotting were used to detect the expression of AQP5 in human GC cell lines. Immunohistochemistry was applied to evaluate the expression of AQP5 in human GC tissues and corresponding normal tissues. Following ectopic overexpression of AQP5 or inhibition of AQP5 by its inhibitor, acetazolamide (AZA), cell proliferation and migration of AGS cells were analyzed by MTT assay, colony formation assay, and wound healing assay. Heterogeneous expression of AQP5 mRNA and protein was observed in human GC cell lines MKN45, MKN28, AGS, and SGC7901. AQP5 was up-regulated in GC tissues in comparison to corresponding normal tissues. AQP5 protein was mainly localized in the cell membrane. Overexpression of AQP5 was correlated with enhanced lymph node metastasis. In vitro, overexpression of AQP5 notably enhanced, while inhibition of AQP5 by AZA significantly attenuated the proliferation and migration of AGS cells. Our data indicate that AQP5 may play an important role in the tumorigenesis and progression of human GC and suggest that AQP5 is a potential therapeutic target against GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric carcinoma (GC), with an estimated one million new cases every year [1], remains a major cause of mortality and morbidity worldwide [2]. To improve the diagnosis, treatment and prognosis of GC, it is urgent to elucidate the molecular mechanisms of the carcinogenesis of GC.
Aquaporin (AQP) family, a class of integral membrane proteins is responsible for the transportation of water, glycerol, and urea [3]. It has been well documented that AQP is associated with cell migration in a variety of cell types in vitro and in vivo in mice [4–8]. Importantly, earlier studies revealed that AQP facilitates tumor progression and invasion [5, 9]. Therefore, AQP is a novel anti-tumor target for the development of molecular targeted cancer therapeutics.
AQP5, an isoform of AQP family, was first cloned from the salivary gland and known as an exocrine-type water channel with a unique tissue expression such as the salivary gland, eyes, lacrimal gland, lung, trachea, and gastrointestinal glands [10, 11]. Overexpression of AQP5 is seen in a variety of cancers and correlated with the clinicopathological characteristics of patients such as colon cancer [12], lung cancer [13], chronic myelogenous leukaemia [14], breast cancer [15], and biliary tract carcinoma [16]. There is an up-regulation of AQP5 in lung cancer tissues compared to the surrounding normal tissues, and overexpression of AQP5 associates with poor prognosis [17]. Moreover, overexpression of AQP5 associates with liver metastasis of patients with colon cancer [12]. In addition, recent studies reported that AQP5 was highly expressed in human GC [18, 19]. However, the roles of AQP5 in the tumorigenesis of GC remain to be determined. In the present study, we attempted to examine the expression of AQP5 in GC cell lines and GC tissue samples, and studied the effects of AQP5 expression on the proliferation and migration in GC cell line AGS.
Materials and methods
Cell culture and tissue specimens
Human gastric adenocarcinoma cell lines MKN45, MKN28, AGS, and SGC7901 were obtained from the Institute of Digestive Disease, First Affiliated Hospital of Nanchang University, and cultured in DMEM medium supplemented with 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in humidified air at 37 °C with 5 % CO2. When confluent, cells were subcultured by trypsinization. Exponentially grown cells were used throughout the study.
Specimens were obtained from 40 gastric adenocarcinoma patients in the Department of Pathology, First Affiliated Hospital, Nanchang University, Nanchang, China. All the patients had not received chemotherapy or radiotherapy before surgery. Forty specimens of corresponding normal mucosal tissues, which were 5 cm apart from the carcinoma, were collected simultaneously. All patients were diagnosed by pathological analyses based on the American Joint Committee on Cancer criteria. The correlation between AQP5 expression and clinicopathological characteristics of patients was evaluated, and these characteristics included age (≤50 or >50 years), gender (male or female), tumor size (≤3 or >3 cm), cell differentiation (well and moderately differentiated or poorly differentiated), lymph node (LN) metastasis (positive or negative), depth of invasion (T1 and T2 or T3 and T4) and TNM stage (I, II, III, or IV). All patients gave their informed consent. The methods for experiments were approved by the local institutional review boards.
Immunohistochemistry
Standard hematoxylin and eosin-stained slides from the Department of Pathology were examined, and the best sections were chosen for immunohistochemistry. Formalin-fixed and paraffin-embedded GC specimens were prepared for 5-μm-thick sections on the slides. The sections were deparaffinized in xylene and rehydrated through graded series of ethanol. Endogenous peroxidase activity was blocked by 3 % H2O2 solution at room temperature for 8 min. Antigen retrieval was performed using boiling sodium citrate buffer (pH 6.0) in a microwave for 15 min. The sections were incubated with anti-AQP5 antibody (1:600; Abcam, Hong Kong, China) at 4 °C overnight, and then with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:200; Santa Cruz, CA, USA) at 37 °C for 20 min. The immunoreactive product was visualized with 3,3′-diaminobenzidine, and the slides were counterstained with hematoxylin. AQP5 immunolabeling was reviewed by pathologists, and the intensity and proportion were scored. Immunolabeling intensity of AQP5 was scored as negative (score 0), light yellow (score 1), yellow (score 2), and brown yellow (score 3). Proportion is the approximate numbers of AQP5-positive tumor cells among the total tumor cells, scored as negative (score 0), ≤25 % (score 1), 26–50 % (score 2), 51–80 % (score 3), and >80 % (score 4). AQP5 expression in the cancer tissues was defined as positive when the product of intensity score by percentage score was 4 or more. Negative reaction controls (tissue sections that were incubated with PBS) and positive reaction controls (lung tissues that were known to be reactive the AQP5 antibodies) were used.
Plasmid construction and production of stable cell lines
The mammalian cell expression vector, pcDNA3.1/myc-His, was gifted by Dr. Shi-Wen Luo. Human cDNA was amplified by PCR using primers for AQP5 (sense: 5′-ATGAAGAAGGAGGTGTGCTCCGT-3′; antisense: 5′-TCAGCGGGTGGTCAGCTCC-3′) and inserted into BamHI and EcoRI site of pcDNA3.1/myc-His vector. Clones were confirmed by restriction analysis and DNA sequencing (Generay, Shanghai, China). Expression constructs were transfected into AGS cells with Lipofectamine 2000 (Invitrogen, USA) according to manufacturer’s recommendations. All transfectants were selected with 400 μg/mL G418 (Merck, Germany) for 3 weeks, and the selected clones were confirmed by RT-PCR and western blotting.
RT-PCR
Total RNA from human GC cell lines was extracted using TRIzol reagent (Invitrogen, USA), and 4 μg of total RNA was used for reverse transcription using RT-PCR Kit (Invitrogen, USA) in a 40-μL reaction vial according to the manufacturer’s instruction. PCR amplifications were performed with the primers for AQP5 (sense: 5′-ATGAAGAAGGAGGTGCTCCGT-3′; antisense: 5′-TCAGCGGGTGGTCAGCTCC-3′), which yielded a 798-bp product. The constitutive gene β-actin was also amplified as control, using the primers for β-actin (sense: 5′-CGGGAAATCGTGCGTGAC-3′; antisense: 5′-TGGAAGGTGGACAGCGAGG-3′), which yielded a 432-bp product. PCR was carried out according to the following program: 35 cycles at 94 °C for 1 min, 55 °C for 30 s, and 72 °C for 1 min. Amplified PCR products were electrophoresed on 1 % agarose gel containing ethidium bromide and imaged with a gel imaging system (Bio-Rad, USA).
Western blotting
The cell lysates were obtained in RIPA lysis buffer containing 1 mM phenylmethanesulfonyl fluoride. The total protein concentration was measured using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA), and all samples were adjusted with RIPA lysis buffer to reach the identical final protein concentration. Total protein lysates (40 μg) were fractionated by 10 % sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes (Millipore Corporation, Billerica, MA), and blocked for 1 h with 5 % nonfat dry milk in Tris-buffered saline (pH 7.5) with 0.1 % Tween 20, followed by overnight incubation with anti-AQP5 antibody (1:1,000; Abcam, Hong Kong, China) or anti-β-actin antibody (1:500; Santa Cruz, CA, USA). HRP-conjugated secondary antibody (1:5,000; Santa Cruz, CA, USA) was applied for 2 h at room temperature. Immunoreactive bands were detected by enhanced chemiluminescent substrate (Pierce, Rockford, IL, USA). The film was scanned, and band intensity was quantified by Image J. The intensity for each protein was corrected by intensity of β-actin and was normalized to facilitate comparison.
MTT assay
MTT assay was used to analyze the proliferation of AGS cells in both plasmid transfection and acetazolamide (AZA) treatment experiments. For plasmid transfection experiment, AGS cells (800 cells/well), either pcDNA3.1/myc-His vector-transfected cells (mock cells) or AQP5-pcDNA3.1/myc-His-transfected cells (AQP5-transfected cells), were plated in 96-well culture plates for 9 days. Medium was replaced every 48 h. For AZA treatment experiment, AGS cells (4 × 103 cells/well), either vehicle (dimethylsulfoxide, DMSO)-treated cells (control group) or 10 mM AZA-treated cells (AZA group), were plated in 96-well culture plates for 3 days, and the medium was replaced every 24 h with fresh vehicle or AZA. Cell proliferation was measured by the same way at each day. Briefly, 10 μL of the MTT reagent (5 mg/mL) was added to each well for 4 h at 37 °C. After addition of 150 μL DMSO and oscillation, the absorbance (A value) of each well was then measured using a spectrophotometric plate reader (Thermo Labsystem, USA) at a wavelength of 490 nm. The experiments were performed in triplicate.
Colony formation assay
Three hundred cells were plated into 6-cm dishes and incubated in DMEM with 10 % FBS at 37 °C. Two weeks later, the cells were fixed with methanol and stained with 0.1 % crystal violet. The number of colonies, defined as ≥50 cells/colony, was counted. The experiments were performed in triplicate.
Wound healing assay
Cells were cultured to a confluent monolayer, synchronized in 1 % FBS for 24 h, and scratched by removing about 400-μm-wide strips of cells across the well with a standard 200-μL pipette tip. Floating cells were removed by washing with PBS. Five representative images of the scratched area under each condition were photographed. To estimate the relative migration of the cells, the unclosed cell-free area from five prints under each condition were excised. “Average gap” (the percentage of the unclosed cell-free areas according to 0 h of scratch distance) was used to quantify the data by Image J software, and scratch distance at 0 h was considered as 100 % of the averaged gap.
Statistics analysis
Values were indicated as mean ± standard deviation. The differences between the groups were evaluated by Student’s t test or one-way analysis of variance. For the relationship between AQP5 expression and clinicopathological factors, the χ 2 test was used. All analyses were performed using SPSS software (version 17.0). P < 0.05 was considered to be significant. All given P values were two-sided.
Results
AQP5 is ubiquitously expressed in GC cell lines
We first checked the expression of AQP5 in human GC cell lines MKN45, MKN28, AGS, and SGC7901 by RT-PCR and western blotting. RT-PCR analysis showed that the AQP5 products (798 bp) were detected in all four human GC cell lines with relatively higher levels in AGS and SGC7901 (Fig. 1a). In consistence with mRNA levels, AQP5 protein was detected in each of these human GC cell lines but relatively higher in AGS and SGC7901, suggesting that AQP5 expression is mainly controlled at transcriptional levels (Fig. 1b). These results showed that AQP5 is ubiquitously expressed in GC cell lines.
Heterogeneous expression of AQP5 in human GC cell lines. a Total RNA was isolated from human GC cell lines MKN45, MKN28, AGS, and SGC7901, and analyzed by RT-PCR (upper panel). Relative expression of AQP5 mRNA was plotted with β-actin as control (lower panel). b Total protein was extracted from human GC cell lines MKN45, MKN28, AGS, and SGC7901, and probed with an AQP5 antibody (upper panel). Relative expression levels of AQP5 protein were expressed as the relative intensity compared with β-actin (lower panel)
AQP5 is overexpressed and correlated with LN metastasis in GC
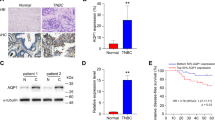
To look into the effects of AQP5 expression on the tumorigenesis of GC, we examined AQP5 expression of GC samples and corresponding normal tissues by immunohistochemistry, and found that there was differential expression of AQP5 between GC and corresponding normal tissues. Weak expression of AQP5 was seen in corresponding normal tissues (Fig. 2a). In contrast, AQP5 was highly expressed in 27 out of 40 carcinomas (Table 1) and mainly localized in the cell membrane (Fig. 2c, d). Interestingly, AQP5 expression was observed in the tissues of intestinal metaplasia (Fig. 2b), suggesting that AQP5 might be involved in the tumorigenesis of GC. For this, we further analyzed the correlation between AQP5 expression and clinicopathological characteristics of patients with GC. As shown in Table 2, there was no statistically significant correlation between AQP5 expression and demographic and pathologic factors such as age, gender, tumor size, cell differentiation, TNM stage, and depth of invasion, whereas AQP5 expression associates with LN metastasis, indicating that AQP5 might be involved in GC metastasis.
Differential expression of AQP5 in human GC and corresponding normal tissues. a Weak staining of AQP5 protein in accompanying normal tissue. b Positive staining in intestinal metaplasia tissue. c Positive staining in moderately differentiated GC tissue. d Positive staining in poorly differentiated GC tissue. Scale bar: 40 μm. Expression of AQP5 in paraffin-embedded sections of specimens was determined by immunohistochemistry
Overexpression of AQP5 promotes the proliferation and migration of AGS cells
The initial results suggest that AQP5 may be involved in the progression and metastasis during the multiple processes of GC tumorigenesis. To test this hypothesis, we first stably transfected AGS cells with AQP5 plasmids to overexpress AQP5. AQP5 mRNA expression in the AQP5-transfected cells significantly increased with 3.6-fold increase in comparison with mock control (Fig. 3a). Similarly, a 2.9-fold increase of AQP5 protein was observed in the AQP5-transfected cells (Fig. 3b).
Effects of AQP5 overexpression in proliferation and migration of AGS cells. a AQP5 mRNA in the AQP5-transfected cells significantly increased when compared to the mock cells (upper panel). Relative mRNA was plotted with β-actin as control (lower panel). b Increased AQP5 protein in the AQP5-transfected cells (upper panel). Relative protein was plotted with β-actin as control (lower panel). c Determination of cell proliferation by MTT assay. The A values in the AQP5 group were significantly higher than that in the mock group. d The ability of cell proliferation was evaluated by colony formation assay. The rate of colony formation in the AQP5-transfected cells was higher than mock control. Right panel represents a set of experiments. e Cell migration was determined by wound healing assay. The average gap in the AQP5-transfected cells was shorter than mock cells. Single asterisk indicates P < 0.05 and double asterisks indicate P < 0.01 versus mock group. Right panel represents a set of the experiments
We next checked the effects of AQP5 overexpression on the proliferation of AGS cells by MTT assays. Overexpression of AQP5 significantly promoted the cell proliferation of AGS cells (Fig. 3c), indicating that overexpression of AQP5 in human GC cells increases the cell proliferation. Moreover, the AQP5-transfected cells formed 1.4-fold more colonies than mock cells (Fig. 3d), which correlates with the increased cell proliferation in human GC cells overexpressing AQP5. In addition, in vitro wound healing assay shows that the average gap in the AQP5-transfected cells (41.73 % of 0 h) was shorter than that in mock cells (60.46 % of 0 h) (Fig. 3e), suggesting that overexpression of AQP5 in human GC cells was associated with increased cell migration.
Inhibition of AQP5 by AZA leads to decreased proliferation and migration AGS cells
AZA is an AQP inhibitor and able to reduce AQP expression and AQP-related functions in various cells [20–22]. We, thus, investigated AQP5 expression in AGS cells treated with AZA. AGS cells were cultured in the presence of 5, 10, and 20 mM of AZA for 24 h. AQP5 mRNA in AGS cells treated with AZA significantly decreased in a concentration-dependent manner, with 0.75-, 0.64-, and 0.44-fold in the experimental group after treatment with 5, 10, and 20 mM AZA for 24 h, respectively (Fig. 4a). In addition, reduced AQP5 protein was observed in the AGS cells exposed to 5, 10, and 20 mM AZA for 24 h (0.72-, 0.62-, and 0.37-fold in the experimental group, respectively) (Fig. 4b). The results show that AZA can downregulate the expression of AQP5 at both mRNA and protein levels in GC cell lines.
Inhibition of AQP5 by AZA leads to decreased proliferation and migration AGS cells. AGS cells were treated with 5, 10, and 20 mM AZA for 24 h. RT-PCR and western blotting were adopted to evaluate the expression of AQP5 at both mRNA and protein levels. a and b Expression of AQP5 mRNA and protein in AGS cells was significantly decreased in a concentration-dependent manner, compared with that in the control group. The lower panels are the quantification of the corresponding upper panels. c AGS cells were cultured with 10 mM AZA treatment for 24, 48, and 72 h, and the A value of 490 nm was measured by MTT assays as described in the “Material and methods” section. The A values in AZA-treated group were significantly lower in a time-dependent manner compared with that in the control group. d Migration of AGS cells with 10 mM AZA treatment was evaluated by wound healing assays, and the average gap of AZA-treated cells was significantly longer than control cells. Right panel represents a set of the experiments. Single asterisk indicates P < 0.05, double asterisks indicate P < 0.01, and triple asterisks indicate P < 0.001 versus control group; the double black triangles indicate P < 0.01 versus AZA group at 24 h, and the number sign indicates P < 0.05 versus AZA group at 48 h
To test the effects of the inhibition of AQP5 expression by AZA on cell proliferation, AGS cells were cultured in the presence of 10 mM AZA for 24, 48, and 72 h, and cell proliferation was monitored by MTT assays. The A values in the AZA-treated group were significantly decreased in a time-dependent manner compared to that of the control group (Fig. 4c). Moreover, 10 mM AZA treatment leads to 1.68-fold increase of the average gap when compared with that of control cells (Fig. 4d). Therefore, inhibition of AQP5 expression reduces the proliferation and migration of human GC cells.
Discussion
AQP is overexpressed and correlated with metastatic potential in a variety of tumor tissues [13, 23]. AQP is an important oncogenic factor for tumor progression [5, 9, 24]. Among the AQP, AQP5 is expressed in the epithelial cells of secretory tissues such as lung, salivary, and gastrointestinal glands [10, 11] and overexpressed in human cancer tissues including GC [18, 19]. However, the roles of AQP5 in human GC progression have not yet been clarified.
In the present study, we found that AQP5 was ubiquitously but heterogeneously expressed in human GC cell lines MKN45, MKN28, AGS, and SGC7901, which is different from the previous study showing that AQP5 was not expressed in MKN45 cells [18]. Differential expression of AQP5 was also found between human GC and corresponding normal tissues. AQP5 is localized in the cell membrane. AQP5 expression was correlated with LN metastasis of GC. Interestingly, expression of AQP5 was observed in the tissues of intestinal metaplasia, which was different from the previous results that AQP5 was not expressed in the tissues of intestinal metaplasia [18]. It is known that intestinal metaplasia is the precursor of GC [25]. Therefore, our data suggest that AQP5 may play an important role in the tumorigenesis and progression of GC.
Tumor metastasis is a complex process involving detaching, migration, gaining access to blood or lymphatic vessels, and disseminating into the body. Previous studies show that AQP facilitates cell migration independent of cell types [4]. It was proposed that the significantly increased water permeability might contribute to the enhanced metastatic potential of AQP5-expressing tumor cells [26]. In addition, upregulation of MUC5AC mucin production [17] and induction of epithelial mesenchymal transition [27] may play a role in the enhanced metastatic tendency associated with AQP5 expression.
The results in this study were consistent with the observation from other tumor types that AQP5 expression was involved in the cell proliferation and migration. In vitro, stable transfection of AQP5 in lung cancer cell lines SPC-A1 and PC-9 resulted in enhanced cell proliferation and migration [17]. In contrast, AQP5 knockdown in SPC-A1 cells by RNAi leads to decreased cell migration and invasion potential, presumably by changing cell shape and volume through the decreased osmotic water permeability of cell membrane [26]. AQP5 was also proposed as a marker for proliferation and migration of human breast cancer cells [15]. In the present study, we found that AQP5 promoted the cell proliferation and migration of GC cell line AGS. Thus, they provide evidence that AQP5 may promote tumor progression of GC by enhancing cell proliferation and migration.
Previous studies suggested that AQP5 might induce cell proliferation and migration through transporting membrane-associated signaling molecules. RAS enhanced cell proliferation in AQP5-overexpressed NIH3T3 cells [28], and AQP5 promoted tumor invasion by interacting with SH3 domains of c-Src in non-small cell lung cancer [27]. In addition, AQP5 promotes cell proliferation and migration via activation of the EGFR/ERK/p38 MAPK signaling pathway in lung cancer [17]. Therefore, it will be intriguing to investigate whether AQP5 increases GC cell proliferation and migration via receptor tyrosine kinase-mediated signaling pathways such as PI3K-AKT and RAS-MAPK axes.
Taken together, the present study suggests that AQP5 might play an important role in the tumorigenesis and progression of GC, and indicates that AQP5 is a potential therapeutic target against GC.
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Nielsen S, Frokiaer J, Marples D, et al. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–44.
Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflügers Arch. 2007;456:693–700.
Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–4.
Cao C, Sun Y, Healey S, et al. EGFR-mediated expression of aquaporin-3 is involved in human skin fibroblast migration. Biochem J. 2006;400:225–34.
Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–92.
Saadoun S, Papadopoulos MC, Watanabe H, et al. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–98.
Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:326–32.
Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–12.
Parvin MN, Tsumura K, Akamatu T, Kanamori N, Hosoi K. Expression and localization of AQP5 in the stomach and duodenum of the rat. Biochim Biophys Acta. 2002;1542:116–24.
Kang SK, Chae YK, Woo J, et al. Role of human Aquaporin 5 in colorectal carcinogenesis. Am J Pathol. 2008;173:518–25.
Najbauer J, Kim M-J, Woo J, et al. Expression of Aquaporin 5 (AQP5) Promotes tumor invasion in human non small cell lung cancer. PLoS One. 2008;3:e2162.
Blagosklonny MV, Chae YK, Kang SK, et al. Human AQP5 plays a role in the progression of chronic myelogenous leukemia (CML). PLoS One. 2008;3:e2594.
Jung HJ, Park J-Y, Jeon H-S, Kwon T-H. Aquaporin-5: a marker protein for proliferation and migration of human breast cancer cells. PLoS One. 2011;6:e28492.
Sekine S, Shimada Y, Nagata T, et al. Prognostic significance of aquaporins in human biliary tract carcinoma. Oncol Rep. 2012;27:1741–47.
Zhang Z, Chen Z, Song Y, et al. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J Pathol. 2010;221:210–20.
Watanabe T, Fujii T, Oya T, et al. Involvement of aquaporin-5 in differentiation of human gastric cancer cells. J Physiol Sci. 2009;59:113–22.
Shen L, Zhu Z, Huang Y, et al. Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed Pharmacother. 2010;64:313–8.
Ameli PA, Madan M, Chigurupati S, et al. Effect of acetazolamide on aquaporin-1 and fluid flow in cultured choroid plexus. Acta Neurochir Suppl. 2012;113:59–64.
Tanimura Y, Hiroaki Y, Fujiyoshi Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J Struct Biol. 2009;166:16–21.
Ran X, Wang H, Chen Y, et al. Aquaporin-1 expression and angiogenesis in rabbit chronic myocardial ischemia is decreased by acetazolamide. Hear Vessel. 2010;25:237–47.
Zhang Z-Q, Zhu Z-X, Bai C-X. Lung fluorescence imaging to evaluate tumor metastasis induced by AQP5 expression in murine model. Med Oncol. 2010;29:205–11.
Moon C, Soria JC, Jang SJ, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–703.
Sakitani K, Hirata Y, Watabe H, et al. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. Gastroenterol Hepatol. 2011;26:1570–5.
Chen Z, Zhang Z, Gu Y, Bai C. Impaired migration and cell volume regulation in aquaporin 5-deficient SPC-A1 cells. Respir Physiol Neurobiol. 2011;176:110–7.
Chae YK, Woo J, Kim MJ, et al. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS One. 2008;3:e2162.
Woo J, Lee J, Kim MS, et al. The effect of aquaporin 5 overexpression on the Ras signaling pathway. Biochem Biophys Res Commun. 2008;367:291–8.
Acknowledgements
We thank Dr. Shi-wen Luo for suggestion. This work was supported by the National Science Foundation of China (no. 81270479) and the Health Foundation of Jiangxi Province (no. 20112011).
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, YH., Zhou, XY., Wang, HM. et al. Aquaporin 5 promotes the proliferation and migration of human gastric carcinoma cells. Tumor Biol. 34, 1743–1751 (2013). https://doi.org/10.1007/s13277-013-0712-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0712-4