Abstract
Insulin is the main glucoregulator that promotes the uptake of glucose by tissues and the subsequent utilization of glucose as an energy source. In this paper, we describe a novel glucoregulator, the alpha-synuclein (SNCA) protein, that has previously been linked to Parkinson’s disease. Treatment with recombinant SNCA promotes glucose uptake in vitro in preadipocytes and in vivo in the adipose tissues and skeletal muscles of mice through the LPAR2/Gab1/PI3K/Akt pathway; these effects occur independently of the insulin receptor. This function of SNCA represents a new mechanistic insight that creates novel avenues of research with respect to the process of glucose regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin is the primary glucoregulator in mammals; in combination with other hormones, insulin maintains glucose uptake and glycogen synthesis in tissues and regulates the utilization of glucose as an energy source. A better understanding of glucose regulation and the molecules that participate in this process will facilitate better targeting of novel therapeutic candidates that can optimize and/or control glucose utilization in insulin target organs, such as skeletal muscle and adipose tissue.
In this study, we identified a novel player that is involved in glucose regulation, alpha-synuclein (SNCA). The SNCA has been well studied because its mutated variants are associated with Parkinson’s disease [1]. Non-mutant SNCA has important functions in the brain, regulating exocytosis through the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex in the presynaptic aspects of neurotransmissions during the process of aging [2] and preventing neurodegeneration through cooperation with the cysteine-string protein-α (CSPα) [3]. In fact, SNCA is a protein that is located not only in the brain but also in various extracerebral locations, including but not limited to serum, bone marrow, red blood cells and platelets; its function and significance in these locations are poorly understood [4–7]. Interestingly, it has recently been reported that SNCA could be associated with glucose metabolism. Kurz et al. [8] demonstrated that glyoxalase I expression and glycation damage were induced by SNCA ablation. This finding suggests that SNCA deficiency directly or indirectly affects the regulation of glucose metabolism in the brain. Seo et al. [9] revealed the ability of SNCA to initiate v-akt murine thymoma viral oncogene homolog 1 (Akt) activation and thereby increase B cell lymphoma protein (Bcl)-dependent cell survival in kidney and prostate cancer cell lines. Similarly, Geng et al. [10] have described a role for SNCA in the insulin secretion of beta cells, suggesting that SNCA may possess an undiscovered role in glucose regulation.
Given these observations, we ask the logical question of whether SNCA has any direct effects on glucose metabolism in insulin target tissues. In this paper, we report the existence of a novel SNCA-dependent transduction signal pathway, the LPAR2/Gab1/PI3K/Akt pathway, that contributes to glucose uptake in adipose tissues.
Materials and methods
Cell culture and extracts
We maintained 3T3-L1, HepG2, C2C12, SK-N-SH and mouse primary culture cells in DMEM (Dulbecco’s Modified Eagle Medium) with 10 % fetal bovine serum supplemented with Penicillin–Streptomycin. Dr. Yoshihiro Ogawa (Tokyo Medical and Dental University) generously provided 3T3-L1 cells that stably expressed CAR (coxsackie-adenovirus receptor). Adipocyte differentiation was induced in these 3T3-L1 cells through the use of the STEMPRO Adipogenesis Differentiation Kit (Invitrogen, USA) for 7 days. All of the cells were grown in a cell incubator at 37 °C in an atmosphere containing 5 % CO2. Additional detailed information is included in the supplemental methods portion of this paper.
Immunoblotting and immunoprecipitation
Samples were obtained using either RIPA 1× (Thermo, USA) (for immunoblotting) or NP-40 lysis buffer (for immunoprecipitation) and then electrophoresed in a multigradient gel (Multigel II Mini, Cosmo Bio). Transference was performed using Polyvinylidene difluoride (PVDF) transfer membranes (Amersham Hybond, GE Health Care). The membranes were blocked with 5 % skim milk (Nacalai Tesque) in PBS-T (containing 0.05 % Tween 20) for 1 h and incubated overnight with the primary antibody. An Image Quant LAS 4000 mini system (GE, USA) was used in accordance with the manufacturer’s instructions to obtain images. Additional detailed information is contained in the supplemental methods portion of this paper.
Phosphatidylinositol 3-OH kinase (PI3K) assay
The PI3K assay was performed using a modified version of thin layer chromatography procedures that were described by the previously published studies [11]. Additional detailed information is contained in the supplemental methods portion of this paper.
Animal procedures
All of the in vivo experiments were performed in accordance with the regulations of Osaka University’s Animal Facility. The C57/BL6 mice were obtained from CLEA (Japan) and SNCAKO mice were acquired from The Jackson Laboratory (USA) [12]. We used mice of the same body weight for group comparisons. Serum insulin (Morinaga, Japan), glucagon (R&D, USA) and cortisol (Cayman Chemical Company, USA) were quantified by ELISA.
Intraperitoneal glucose and insulin tolerance test (ipGTT and ipITT) [13]
WT and SNCAKO mice were fasted overnight (8 h) and water was supplied ad libitum. Subsequently, glucose (2 g/kg) or insulin (0.8 IU/kg) (Humulin R, Lilly) were injected into these mice, and blood glucose levels were measured with a Glucometer AS-R (SKK, Japan) at the time points of 0, 15, 30, 60, 90 and 120 min after the injection. Additional blood samples (20 μl) were extracted at 0, 30, 60 and 120 min after the injection for the evaluation of serum insulin levels by ELISA (Mouse insulin, Morinaga, Japan).
Indirect calorimetry
All of the mice were fed ad libitum during the experiment in separate chambers at 24–25 °C following previous cardiac catheterization and mice habituation. The habituation to a new environment was performed over the course of a minimum of three days. The auto-calibration process was performed in accordance with the manufacturer’s protocol. We collected data beginning on the day after the first calorimeter measurements to avoid possible artifacts. Metabolic parameters were measured in WT (C57/BL6) and SNCAKO mice for 24 h. Data were obtained using an MM202R O2/CO2 analyzer (Muromachi Kikai, Japan).
Cardiac catheterization
Mice weighing 24 g were intraperitoneally anesthetized with medetomidine (0.3 mg/kg), midazolam (0.8 mg/kg) and butorphanol (1 mg/kg). The catheterization procedure was performed in accordance with previously published methods [14]. Briefly, the installation of a silicon cardiac catheter was performed in the right jugular vein of mice. These catheters were fixed and heparinized, and the experiments were performed 1–3 days later. During this time, the mice were habituated to the handling procedures of this study. The intravascular localization of the catheter was confirmed postmortem in all of the mice after the conclusion of the experiment.
Glucose uptake in vivo [15]
After the cardiac catheterization and habituation of the mice, the mice were fasted for 5 h. Under the same glycemic conditions, SNCA (14 μg/kg) or insulin (0.8 IU/kg) was then infused through the cardiac catheter and allowed to incubate for 10 min. Following this incubation, 6.25 μCi of [3H]-2-deoxy-d-glucose and 1.25 μCi of [14C] sucrose in saline were infused. Subsequently, we obtained blood samples at 0, 7, 10, 15 and 20 min to create a tritium-sucrose disappearance curve. Tissue samples were obtained after the last blood sample collection. Measurements of serum insulin, glucagon, cortisol and SNCA were performed in independent experiments, although the original conditions of this experiment were conserved through an extended time course, as indicated in the figures. Additional detailed information is contained in the supplemental methods portion of this paper.
GPCR screening
Briefly, we used the silencer select GPCR siRNA Library V4 from Ambion (USA) in accordance with the manufacturer’s instructions to transfect siRNA (5 nM) into HEK293 and neuroblastoma (SK-N-SH) cells. After 24 h of transfection, the cells were serum starved for 1 day and then stimulated with either SNCA (10 nM) or insulin (100 nM). The phosphorylation of Akt was assessed using the PathScan Phospho-Akt1 (Ser473) ELISA kit from Cell Signaling (USA). We used three different siRNAs for each gene and repeated the experiments three times to obtain consistent data. We transfected mouse siRNA for LPAR2 (s79393, s79394, s79395) and CD97 (s76982, s76983) from Applied Biosystems (USA) into 3T3-L1 cells. The results of these transfections were confirmed through immunoblotting analyses. Additional detailed information is contained in the supplemental methods portion of this paper.
Statistical analysis
We used Stat View, v5.0 to perform the analysis of the in vivo and in vitro data. We presented the data in terms of mean ± SD. We used Student’s t test and ANOVA assessments to determine statistical significance. Bonferroni’s test was used for multiple comparisons as appropriate.
Results
First, we investigated whether SNCA could initiate Akt signaling in preadipocytes (3T3-L1), myocytes (C2C12) or hepatocytes (HepG2), as these cell types are the most important insulin target cells for assessing glucose metabolism in vitro [16, 17]. Interestingly, we detected Akt phosphorylation after recombinant SNCA treatment in both preadipocytes and mature adipocytes (Fig. 1a and supplemental Fig 1); however, this phosphorylation was less evident in myocytes and hepatocytes (supplemental Fig. 2). Importantly, SNCA treatment could induce 2.5-fold greater [3H] glucose incorporation in both preadipocytes and mature adipocytes, suggesting that insulin signaling could be involved in SNCA effects (Fig. 1b and supplemental Fig. 1b). This reaction was neutralized by treatment with an SNCA monoclonal antibody, indicating the specific role of SNCA in the induction of glucose uptake.
The role of SNCA in the glucose regulation of 3T3-L1 cells (preadipocytes). a Upper panel immunoblot indicating the phosphorylation of Akt1 (at Ser473) and Akt2 (at Ser474) by SNCA, which acts downstream of PI3K in preadipocytes. Lower panel the densitometry results of the upper panel. b SNCA and insulin (200 nM) activate [3H]-2-deoxy-d-glucose uptake in preadipocytes. AbSNCA indicates the SNCA antibody neutralization group (1:1,000 dilution). For each group, n = 5. c Upper panel as indicated by immunoblot analyses, SNCA treatment induced Gab1 (Tyr627) phosphorylation from 5 to 30 min but not the phosphorylation of IRS1 (Tyr 895), IR-β (Tyr1146) or Gab2 (Tyr 452). Lower panel the densitometry results of the upper panel. d The immunoprecipitation results demonstrating the tyrosine phosphorylation of Gab1 after 5 min of stimulation with SNCA or insulin (200 nM). PY indicates phosphotyrosine monoclonal antibody. e The immunoprecipitation results revealing the binding of Gab1 to PI3K (p85) after 5 min of stimulation with SNCA. f The PI3K assay indicating phosphatidylinositol triphosphate (PIP-3) production after SNCA treatment. The catalytic subunit of PI3K, p110 alpha, significantly affects PIP-3 production at the plasma membrane, functioning as a second messenger to further translocate and activate Akt. g Left panel the immunoblotting of the concentration-dependent phosphorylation of Gab1 and Akt1 after 5 min of stimulation with SNCA or insulin (100 nM). Right panel the densitometry results for the left panel. AU indicates arbitrary units. h The immunoblots illustrating phosphorylation of AS160 (at Thr642) and GSK3-beta (at Ser9) after 5 min of stimulation with SNCA. In a and c, the cells were treated with 50 nM vascular endothelial growth factor (VEGF) for 10 min and 100 nM insulin for 120 min as controls. In all of the above experiments the SNCA stimulation was performed at a concentration of 10 nM in 3T3-L1 cells. AU indicates arbitrary units. All histogram bars indicate mean ± SD. ** P < 0.01, and *** P < 0.001
Subsequently, based on these findings, we assessed the impact of SNCA treatment on participants in insulin signaling pathways to examine whether these molecules could be potential upstream players for the effects of SNCA [18]. We detected no phosphorylation of either the insulin receptor or the insulin receptor substrate (IRS)-1 (Fig. 1c) after SNCA treatment, and SNCA did not appear to promote the binding of IRS-2 to p85 (supplemental Fig. 3). Because Growth factor receptor bound protein 2-associated binding (Gab) proteins possess similar structures to IRS proteins and play important roles in inducing cellular glucose uptake during insulin and hepatocyte growth factor-dependent signaling [19, 20], we decided to investigate the possibility that SNCA affects Gab1 protein activation. Indeed, we detected the phosphorylation of Gab1 but not Gab2 after treatment with SNCA (Fig. 1c). Gab1 has been reported to initiate and maintain Akt phosphorylation and regulate cell proliferation through the src homology 2 domain-containing tyrosine phosphatase 2 (SHP2)-extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) complex [19, 21, 22]. Because Gab1/SHP2/ERK-dependent proliferation does not actively participate in the glucose metabolism of adipocytes, we focused on investigating the final output of the Gab1/phosphatidylinositol 3-OH kinase (PI3K)/Akt pathway. We found that SNCA increased Gab1 phosphorylation and Gab1-p85 binding in 3T3-L1 cells (Fig. 1d, e). IRS and Gab proteins bind to the p85 (regulatory subunit) and the p110 (catalytic subunit) of PI3K to increase the activation of Akt [21]. In accordance with this type of effect, SNCA-induced PI3K activity was detected in preadipocytes (Fig. 1f). Furthermore, following SNCA treatment, we observed the activation of Akt substrate of 160 kDa (AS160), which is an activator of glucose transporter 4 (GLUT4), a protein that allows glucose uptake by cells [23]. In addition, the Ser-9 phosphorylation (inactivation) of glycogen synthase kinase-3 beta (GSK3-β), which promotes glucose utilization in tissues [24, 25], was also observed after the SNCA treatment (Fig. 1h).
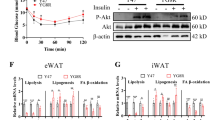
To establish the importance of each participant in this signal transduction process, we pre-treated preadipocytes with the PI3K inhibitors LY294002 and Wortmannin. We observed that this pre-treatment caused the attenuation of SNCA-induced Akt phosphorylation and glucose uptake and the deterioration of the full phosphorylation of Gab1 in 3T3-L1 cells (Fig. 2a, b). It has been reported that the positive feedback effects of PI3 K on Gab1 occur just after Gab1 translocates to the plasma membrane and permit a more prolonged and efficient activation of the Gab1 pathway [26]. In this study, we demonstrate that PI3K inhibition impairs the full phosphorylation of Gab1. In addition, to elucidate the importance of Gab1 as the leading player in this pathway, we depleted Gab1 in 3T3-L1 by siRNA. Under these conditions, we observed the suppression of SNCA-induced Akt phosphorylation and glucose uptake (Fig. 2c, d). Moreover, after we overexpressed Gab1 by adenovirus transfection in preadipocytes, SNCA-induced Akt phosphorylation and glucose uptake were enhanced (Fig. 2e, f). In combination, these results demonstrate that Gab1 activation is crucial for sustaining the downstream activation of PI3K and Akt, which is followed by glucose uptake after SNCA treatment in preadipocytes. Given this result, we investigated the involvement of other insulin receptor-independent glucose uptake pathways, such as the 5′ adenosine monophosphate-activated protein kinase (AMPK) pathway [27, 28], but the activation of this pathway was not detected after SNCA treatment in preadipocytes (Fig. 2g). The AMPK pathway is particularly important for inducing insulin-independent glucose uptake in tissues and thereby promoting the use of glucose as an energy source; biguanides and physical exercise have been proposed as agonists of AMPK and mitochondrial capacity, respectively [29, 30]. In addition, it has been demonstrated that the AMPK pathway provides important contributions to glucose metabolism by mediating metabolic signals through the central nervous system affecting distal tissues such as skeletal muscle [27].
Gab1 is critical for SNCA-induced glucose uptake in vitro. a Pre-incubation with PI3K inhibitors. The immunoblots illustrate the phosphorylation of Akt1 after 5 min of SNCA treatment. PI3K inhibitors were used at a pre-incubation dose of 20 μM (30 min) for the LY294002 group and 50 nM (30 min) for the Wortmannin group. b Pre-incubation with PI3K inhibitors. The immunoblots indicate glucose uptake after SNCA treatment (30 min); n = 5 for each group. c Gab1-depleted 3T3-L1 cells. The immunoblots indicate the phosphorylation of Akt1 after 5 min of SNCA treatment. d Gab1-depleted 3T3-L1 cells. The immunoblots indicate the glucose uptake after SNCA treatment (30 min); n = 5 for each group. e Gab1 overexpression in 3T3-L1 cells. The immunoblots indicate the phosphorylation of Akt1 after 5 min of SNCA treatment. f Gab1 overexpression in 3T3-L1 cells. The immunoblots indicate the glucose uptake after SNCA treatment (30 min); n = 5 for each group. g AMPK in 3T3-L1 cells. The immunoblots show the catalytic (α, Thr 172) and regulatory (β1, Ser 108) subunit of AMPK after SNCA treatment. Insulin was used at a concentration of 100 nM for 120 min and forskolin at a concentration of 40 μM for 24 h. In all of the experiments, the SNCA stimulation was performed at a concentration of 10 nM in 3T3-L1 cells. All of the histogram bars indicate mean ± SD. * P < 0.05, ** P < 0.01 and *** P < 0.001, respectively
Furthermore, to evaluate the in vivo effects of SNCA on adipose tissue, we intravenously administered SNCA to C57/BL6 mice, which are hereafter referred to as wild type ‘WT’ mice. We found that, in mice, SNCA activates the Gab1 pathway in the white adipose tissue (WAT) and soleus muscle (Fig. 3a), but not in the liver (supplemental Fig. 5). In addition, we measured the glucose uptake in an animal model under euglycemic conditions (supplementary Fig. 6). We observed that SNCA activates glucose uptake, as assessed by the 2DG uptake in vivo in not only epididymal and retroperitoneal white adipose tissue but also the soleus and gastrocnemius muscles. We also confirmed that co-treatment with an SNCA monoclonal antibody attenuated SNCA-induced glucose uptake (Fig. 3b). Similarly, in mice, glucose utilization, as assessed by GSK3-β inactivation and calculated glycogen synthesis, was increased after SNCA treatment both in the visceral white adipose tissue and in skeletal muscle (Fig. 3a, c).
SNCA promotes glucose uptake and utilization through the Gab1 pathway in vivo. a Immunoblot indicating the phosphorylation of Gab1, Akt1 and GSK3-beta (Ser9) but not IR-β (Tyr1146) after 5 min of SNCA administration (i.v.) in WT mice. WAT epididymal white adipose tissue. b The glucose uptake assay in mice that were treated with SNCA. White adipose tissue (eWAT, epididymal; retroWAT, retroperitoneal) and skeletal muscle (soleus and gastrocnemius) incorporate glucose as a result of the SNCA treatment. The SNCA antibody was used at a dilution of 1:1,000. c Glycogen synthesis is increased by SNCA or insulin treatment. For the experiments in mice, SNCA (14 μg/kg, i.v.) or insulin (0.8 IU/kg) was injected into the mice and incubated for 10 min. For the saline group, n = 5; for the SNCA group, n = 10; for the Insulin group, n = 5; and for the SNCA–SNCAAb group, n = 5. All of the histogram bars indicate the mean ± SD. * P < 0.05, ** P < 0.01 and *** P < 0.001, respectively
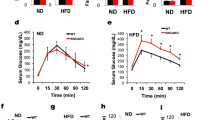
To further investigate the physiological function of SNCA in vivo, we used SNCA-deficient mice (SNCAKO) [12]. SNCAKO mice are viable and fertile, exhibit intact brain architecture and have normal dopaminergic cell bodies, fibers and synapses [31]. Because our previous findings indicate a potential role of SNCA in glucose uptake and utilization in tissues, we decided to assess the glucose metabolism in SNCAKO mice. Interestingly, we observed that these mice exhibit a modest glucose dysmetabolism in response to glucose and insulin (supplemental Fig. 7). Given that the main objective of glucose metabolism and regulation is to ensure that energy substrate supplies are provided to tissues, we then sought to quantitatively assess the whole-body energy metabolism of these mice by indirect calorimetry. The energy source (carbohydrates, lipids or proteins) that is used is determined by the respiratory quotient rate (RQ) in mammals. Normal carbohydrate (particularly glucose) utilization is observed when the RQ is close to 1.0, as measured by indirect calorimetry, and decreased RQ levels represent the utilization of a non-carbohydrate energy source (i.e., lipids and/or proteins) [32]. Using this method, we found that SNCAKO mice displayed an evident impairment in whole-body glucose utilization compared with WT mice; no differences between the two types of mice were observed with respect to oxygen consumption, temperature or locomotor activity (Fig. 4). This result indicates that SNCA is an important player in glucose metabolism in vivo.
SNCA particularly promotes glucose metabolism in vivo. a Measurements of oxygen consumption over 24 h (VO2) in SNCAKO and WT mice; n = 8 for each group. b Measurements of respiratory quotient over 24 h (RQ) in SNCAKO and WT mice; n = 8 for each group. c The area under the curve (AUC) of (b), indicating the evident impairment in RQ per min during the light phase of SNCAKO (red bars) mice compared with WT (black bars); n = 8 for each group. d The rectal temperatures of SNCAKO and WT mice; n = 10 for each group. e The locomotor activity in SNCAKO and WT mice (30 min); n = 10 for each group. We performed all the experiments using mice of similar body weight (25 ± 1 g). All bars indicate mean ± SD. ** P < 0.01 by ANOVA
We then sought to identify the upstream players of Gab1. To address this issue, we tested the signal transduction characteristics of several growth factors that are well-known activators of Gab1 (Fig. 5a). Hepatocyte-growth factor (HGF) has been reported to play an important role in activating the met proto-oncogene (the c-Met receptor, also known as the HGF receptor), and Gab1 can act as an adaptor protein for downstream signal transduction [20]. However, in our experiments in preadipocytes, we could not detect a strong HGF-dependent Gab1 signal of the SNCA-dependent activation of the c-Met receptor at the direct binding site of Gab1 (Fig. 5a, b). Interestingly, epidermal-growth factor (EGF) stimulation in preadipocytes resembled SNCA-dependent Gab1 and Akt activation. In addition, pretreatment with AG1478, an inhibitor of the EGF receptor (EGFR), attenuated the SNCA-induced phosphorylation of Gab1 and Akt1 (Fig. 5a). However, SNCA did not induce the activation of the EGF receptor at the Gab1 binding site (Fig. 5c).
The activation of Gab1 through GPCR and LPAR2 in preadipocytes. a Immunoblot indicating Gab1 and Akt activation after the stimulation of preadipocytes with various growth factors. S indicates SNCA. b Immunoblot assessing c-Met receptor phosphorylation at the Gab1 binding site. c Immunoblot assessing EGF receptor phosphorylation at the Gab1 binding site. d The fold increase in Akt after SNCA treatment in HEK293 cells that have been depleted of the preliminary GPCR candidates. e Immunoblot indicating Gab1 and Akt phosphorylation after SNCA treatment in LPAR2- and CD97-depleted preadipocytes. f Glucose uptake after SNCA treatment in LPAR2- and CD97-depleted preadipocytes. In all of these experiments, SNCA was used at a concentration of 10 nM. In a and b, insulin (100 nM, 10 min), VEGF (50 nM, 10 min), HGF (100 ng/mL, 10 min), EGF (10 ng/mL, 10 min), BAPTA (10 μM, 30 min) and AG1478 (250 nM, 30 min) were used as controls. All bars indicate mean ± SD. * P < 0.05 and ** P < 0.01
Based on recent evidence indicating that SNCA and Gab1 interact with G protein-coupled receptors (GPCR) and the fact that a number of GPCRs have not been fully characterized [33–35], we decided to further investigate the role of GPCRs in the transduction of SNCA signals. We depleted each of the 379 human genes encoding a GPCR in human-embryonic kidney cells (HEK293) and then treated these cells with SNCA. In preliminary experiments, we found that the SNCA-dependent transduction pathway is present in HEK293 cells. We then sought to identify the GPCR-depleted cells that demonstrated the smallest differences in Akt activation between the SNCA-treated groups and the untreated groups. Through this strategy, we identified 27 preliminary candidates (supplementary Fig. 8). Next, we independently analyzed each experiment and searched for consistent matches; we obtained only ten candidates from this examination (Table 1). In particular, we repeated the GPCR depletion experiments for these ten candidates and observed that three out of the ten candidates, lysophosphatidic acid receptor-2 (LPAR2), CD97 and vomeronasal type 1 receptor 4 (VN1R4) displayed the most stable suppression of SNCA-dependent Akt1 phosphorylation (Fig. 5d). In a second screening approach, we again evaluated the effects of SNCA treatment in the Gab1 activation in cells. Moreover, LPAR2 depletion decreased SNCA-induced Gab1 activation in HEK293 cells; this effect was not produced by CD97 depletion, VN1R4 depletion or pre-incubation with pertussis toxin (PTX) (supplementary Fig. 9). We noticed that in HEK293 cells, the basal activity of Gab1 was increased after the depletion of LPAR2. Thus, we decided to confirm these findings in our initial target cell, the preadipocyte. To accomplish this purpose, we chemically antagonized the LPAR family through the pretreatment of preadipocytes with Kil6425 [36]. Under these conditions, we assessed the transduction of the SNCA signal to Gab1 and Akt1. Interestingly, in this experiment, SNCA-dependent Gab1 and Akt1 phosphorylation was suppressed by 40–50 % and by more than 90 %, respectively (supplemental Fig. 10). Notably, LPA-dependent Gab1 and Akt1 phosphorylation through LPAR family members resembled the SNCA transduction signal pattern with respect to the types of cell that were affected. By contrast, CD55 (the ligand of CD97) weakly increased the phosphorylation of Gab1/Akt1 through its receptor CD97. To confirm these results, we repeated the knockdown experiments separately and observed that the depletion of either LPAR2 or CD97 resulted in the significant suppression of SNCA-dependent Akt activation in preadipocytes (Fig. 5e). In this manner, we observed that LPAR2 depletion in preadipocytes could suppress not only SNCA-dependent Gab1 and Akt signaling but also glucose uptake (Figs. 5f, 6). Although CD97 depletion resulted in an attenuation of SNCA-induced Akt signaling and glucose uptake, CD97 depletion failed to inhibit Gab1 activation.
Discussion
In this study, we demonstrate that the acute administration of SNCA activates the LPAR2/Gab1/PI3K/Akt pathway in adipose tissue and skeletal muscle, promoting glucose metabolism in the examined organisms. The absence of SNCA impairs the glucose metabolism in vivo.
We described for the first time the glucoregulatory effect of SNCA, which is dependent on Gab1 in peripheral tissues. To address this issue, we discussed how SNCA-dependent Gab1 activation might occur. SNCA’s bilayer affinity [37] or the activation of a cognate receptor were contemplated as potential upstream factors for this activation. The acute and transient signal patterns induced by SNCA in tissues strongly suggest the existence of a cognate receptor. Therefore, we decided to first investigate the most frequent receptors that could be involved in Gab1 activation, such as EGFR and c-Met; however, these efforts were unsuccessful at identifying the cognate receptor in question. Fortunately, our deeper analysis involving the screening of novel potential receptors that are unstudied or not fully characterized proved more fruitful. In particular, by restricting our search to candidates that could most plausibly be involved in this transduction signal, such as GPCRs, we discovered two receptors, LPAR2 and CD97, that are necessary for conveying SNCA-dependent signals. These receptors can be found either alone or together as a heterodimer, as observed by Ward et al. [38].
LPAR2 is a receptor for lysophosphatidic acid (LPA) that can regulate the efficiency and specificity of signal transduction in various signaling pathways [39]. Interestingly, in previous reports associating Gab1 with GPCR, the potential of LPA to induce Gab1 activation was demonstrated; however, the receptor that was involved in this signaling mechanism was not clearly established [34]. Moreover, it has been demonstrated that the LPAR family is important for blood glucose metabolism; in rodents, LPAR can act in various trophic roles, including functions that oppose obesity and adipogenesis [39]. Consistent with these previous results, in this study, we demonstrate that through its coupling to G11/q and G12/13 proteins, LPAR2 is important for maintaining the integrity of the SNCA signal pathway and allowing this pathway to exert its effects on tissues. However, the physiological and pathological role of LPAR2 in SNCA-induced signal transduction must be investigated through future studies. By contrast, CD97 depletion did not prevent Gab1 activation, although it did impact Akt1. We surmised that CD97 may be more important at the PI3K/Akt level of glucose regulation but not at higher levels in this cascade. CD97 is considered to be one of the EGF-transmembrane 7 family receptors that is expressed as a noncovalently associated heterodimer, and it has been reported that the CD55 molecule [also known as complement decay-accelerating factor (DAF)] and chondroitin sulfate are its ligands [40]. To date, this is the first evidence that links CD97 alone with glucose metabolism. However, we cannot exclude the possibility of heterodimerization of CD97 with the LPAR family; this interaction was proposed by Ward et al. [38] and would allow for the co-participation of CD97 and LPAR2 in the transduction of SNCA-dependent signals in tissues. In our experiments, we found that through the LPAR family, LPA could mimic SNCA-dependent transduction signals in preadipocytes. In addition, albeit in a weaker manner than LPA, CD55 could act through CD97 to increase Gab1 and Akt1 phosphorylation. This result, in combination with the fact that LPAR antagonism or LPAR2 depletion could significantly suppress SNCA-dependent transduction signals, suggests that SNCA exerts its effects primarily through LPAR2, either alone or dimerized with CD97. Thus, the nature of the LPAR family members to heterodimerize with CD97 [38] may certainly be functionally important for the transduction of SNCA signals in tissues.
Moreover, the glucoregulatory effect of SNCA in vivo and in vitro was dependent on the subsequent activation of Gab1 in peripheral tissues. It is known that Gab1 functions in insulin signaling and HGF-dependent signaling as an adaptor protein for cell proliferation, growth and metabolism [20, 21]. In addition, Gab1 is ubiquitously expressed in various tissues [41], enabling these tissues to respond to SNCA. In this study, we demonstrated that SNCA-dependent Gab1 activation is an important regulator of glucose metabolism in skeletal muscle and adipose tissues. It is noteworthy that the activation of the Gab1/PI3K/Akt/GLUT4 pathway has been proposed previously as a therapeutic target for improving glucose diffusion in preadipocytes [42]. In this study, we describe a novel endogenous candidate that may fulfill the desired profile for these therapeutic applications. In addition, as shown by previously published research, the activation of Gab1 may also generate implications for cell growth and proliferation in tissues [20].
In our in vivo results, we did not discover significant differences among various other glucoregulatory hormones (i.e., insulin, glucagon and cortisol) after the administration of SNCA; instead we observed only a modest difference in glucose metabolism after glucose administration in the SNCAKO mice under normal chow conditions. With respect to this result, we suggested the possibility that the interplay between several other homeostatic systems (e.g., hepatic glucose release or autonomous nervous system counter-activation, among other possibilities) [21] may compensate in part for the phenotype of the SNCAKO mice. However, if we used a more sensitive method to assess the intrinsic metabolism of the SNCAKO mice, we observed a clear impairment in the glucose metabolism of these mice. In previously published reports, SNCA has been implicated in protecting against glycation damage in neurons [8] and regulating the insulin release of beta cells [10]. Building upon these previous findings, in this study, we provide the first evidence of the direct involvement of SNCA in both systemic and tissue-specific glucose metabolism.
In summary, in this report we describe a novel role of SNCA, namely, the promotion of glucose metabolism in tissues. This function is performed in at least two different ways: the activation of glucose uptake in white adipose tissues and skeletal muscle, independently of insulin receptor phosphorylation (in particular, through the LPAR2-CD97/Gab1/PI3K/Akt pathway); and the maintenance of glucose metabolism in tissues (through glucose utilization).
This discovery represents a new mechanistic insight that creates a novel area of research with respect to the complex process of glucose regulation.
References
Goedert M (2001) Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2:492–501
Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329:1663–1667
Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123:383–396
Duran R, Barrero FJ, Morales B, Luna JD, Ramirez M, Vives F (2010) Plasma alpha-synuclein in patients with Parkinson’s disease with and without treatment. Mov Disord 25:489–493
Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P et al (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5:55–59
Park SM, Jung HY, Kim HO, Rhim H, Paik SR, Chung KC, Park JH, Kim J (2002) Evidence that alpha-synuclein functions as a negative regulator of Ca(++)-dependent alpha-granule release from human platelets. Blood 100:2506–2514
Hong S, Lee HK, Kim CY, Seong GJ (2008) Identification and localization of alpha-synuclein in human cornea. Korean J Ophthalmol 22:145–146
Kurz A, Rabbani N, Walter M, Bonin M, Thornalley P, Auburger G, Gispert S (2011) Alpha-synuclein deficiency leads to increased glyoxalase I expression and glycation stress. Cell Mol Life Sci 68:721–733
Seo JH, Rah JC, Choi SH, Shin JK, Min K, Kim HS, Park CH, Kim S, Kim EM, Lee SH et al (2002) Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. FASEB J 16:1826–1828
Geng X, Lou H, Wang J, Li L, Swanson AL, Sun M, Beers-Stolz D, Watkins S, Perez RG, Drain P (2011) alpha-Synuclein binds the K(ATP) channel at insulin-secretory granules and inhibits insulin secretion. Am J Physiol Endocrinol Metab 300:E276–E286
Hayashi H, Nakagami H, Takami Y, Koriyama H, Mori M, Tamai K, Sun J, Nagao K, Morishita R, Kaneda Y (2009) FHL-2 suppresses VEGF-induced phosphatidylinositol 3-kinase/Akt activation via interaction with sphingosine kinase-1. Arterioscler Thromb Vasc Biol 29:909–914
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A et al (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252
Begriche K, Massart J, Abbey-Toby A, Igoudjil A, Letteron P, Fromenty B (2008) Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity (Silver Spring) 16:2053–2067
Shiuchi T, Nakagami H, Iwai M, Takeda Y, Cui T, Chen R, Minokoshi Y, Horiuchi M (2001) Involvement of bradykinin and nitric oxide in leptin-mediated glucose uptake in skeletal muscle. Endocrinology 142:608–612
Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB et al (2009) Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab 10:466–480
Heart E, Choi WS, Sung CK (2000) Glucosamine-induced insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 278:E103–E112
Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP (2003) Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA 100:7569–7574
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96
Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ (1996) A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379:560–564
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4:915–925
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
Wohrle FU, Daly RJ, Brummer T (2009) Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun Signal 7:22
Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE (2005) AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J 391:87–93
Wang Y, Feng W, Xue W, Tan Y, Hein DW, Li XK, Cai L (2009) Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes 58:1391–1402
Mariappan MM, Shetty M, Sataranatarajan K, Choudhury GG, Kasinath BS (2008) Glycogen synthase kinase 3beta is a novel regulator of high glucose- and high insulin-induced extracellular matrix protein synthesis in renal proximal tubular epithelial cells. J Biol Chem 283:30566–30575
Eulenfeld R, Schaper F (2009) A new mechanism for the regulation of Gab1 recruitment to the plasma membrane. J Cell Sci 122:55–64
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ et al (2004) AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574
Rose AJ, Richter EA (2005) Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20:260–270
Bertrand L, Ginion A, Beauloye C, Hebert AD, Guigas B, Hue L, Vanoverschelde JL (2006) AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol 291:H239–H250
O’Neill HM, Maarbjerg SJ, Crane JD, Jeppesen J, Jorgensen SB, Schertzer JD, Shyroka O, Kiens B, van Denderen BJ, Tarnopolsky MA et al (2011) AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci USA 108:16092–16097
Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R et al (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci 22:8797–8807
Hogberg H, Engblom L, Ekdahl A, Lidell V, Walum E, Alberts P (2006) Temperature dependence of O2 consumption; opposite effects of leptin and etomoxir on respiratory quotient in mice. Obesity (Silver Spring) 14:673–682
Pronin AN, Morris AJ, Surguchov A, Benovic JL (2000) Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem 275:26515–26522
Bisotto S, Fixman ED (2001) Src-family tyrosine kinases, phosphoinositide 3-kinase and Gab1 regulate extracellular signal-regulated kinase 1 activation induced by the type A endothelin-1 G-protein-coupled receptor. Biochem J 360:77–85
Lin SH, Civelli O (2004) Orphan G protein-coupled receptors: targets for new therapeutic interventions. Ann Med 36:204–214
Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T et al (2003) Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol 64:994–1005
Haque F, Pandey AP, Cambrea LR, Rochet JC, Hovis JS (2010) Adsorption of alpha-synuclein on lipid bilayers: modulating the structure and stability of protein assemblies. J Phys Chem B 114:4070–4081
Ward Y, Lake R, Yin JJ, Heger CD, Raffeld M, Goldsmith PK, Merino M, Kelly K (2011) LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res 71:7301–7311
Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN et al (2010) LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50:157–186
Kwakkenbos MJ, Kop EN, Stacey M, Matmati M, Gordon S, Lin HH, Hamann J (2004) The EGF-TM7 family: a postgenomic view. Immunogenetics 55:655–666
Vaughan TY, Verma S, Bunting KD (2011) Grb2-associated binding (Gab) proteins in hematopoietic and immune cell biology. Am J Blood Res 1:130–134
Bertola A, Bonnafous S, Cormont M, Anty R, Tanti JF, Tran A, Le Marchand-Brustel Y, Gual P (2007) Hepatocyte growth factor induces glucose uptake in 3T3-L1 adipocytes through A Gab1/phosphatidylinositol 3-kinase/Glut4 pathway. J Biol Chem 282:10325–10332
Acknowledgments
This project was supported by the Grant-in-Aid for Scientific Research and the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (Monbukagakusho).
Conflict of interest
The authors declare that they have no competing financial interests with respect to this study.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodriguez-Araujo, G., Nakagami, H., Hayashi, H. et al. Alpha-synuclein elicits glucose uptake and utilization in adipocytes through the Gab1/PI3K/Akt transduction pathway. Cell. Mol. Life Sci. 70, 1123–1133 (2013). https://doi.org/10.1007/s00018-012-1198-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1198-8