Abstract
The PTEN gene is one of the most frequently inactivated tumor suppressor genes in sporadic cancers. Inactivating mutations and deletions of the PTEN gene are found in many types of cancers, including melanoma. However, the exact frequency of PTEN alteration in melanoma is unknown. In this study, we comprehensively reviewed 16 studies on PTEN genetic changes in melanoma cell lines and tumor biopsies. To date, 76 PTEN alterations have been reported in melanoma cell lines and 38 PTEN alterations in melanoma biopsies. The rate of PTEN alterations in melanoma cell lines, primary melanoma, and metastatic melanoma is 27.6, 7.3, and 15.2%, respectively. Three mutations were found in both melanoma cell lines and biopsies. These mutations are scattered throughout the gene, with the exception of exon 9. A mutational hot spot is found in exon 5, which encodes the phosphatase activity domain. Evidence is also presented to suggest that numerous homozygous deletions and missense variants exist in the PTEN transcript. Studying PTEN functions and implications of its mutations and other genes could provide insights into the precise nature of PTEN function in melanoma and additional targets for new therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphatase and tensin homolog (PTEN) is 47-kDa protein and was first identified as a candidate tumor suppressor gene in 1997 after its positional cloning from a region of chromosome 10q23 known to exhibit loss in a wide spectrum of tumor types [1–3]. Since then, mutations of PTEN have been detected in a variety of human cancers including breast, thyroid, glioblastomas, endometrial, prostate, and melanoma [4–14]. Inherited mutations in this gene also predispose carriers to develop Cowden’s disease, a heritable cancer risk syndrome, and several related conditions [15–17]. PTEN is classified as a tumor suppressor because its activity is lost by deletion, mutation, or through epigenetic changes [18–21]. Molecular mechanistic studies of PTEN have provided a great deal of insight into the basis for its involvement in tumor suppression. The PTEN protein has both protein phosphatase and lipid phosphatase activity [22, 23]. Although the tumor suppressive function of PTEN has mainly been attributed to its lipid phosphatase activity, a role for PTEN protein phosphatase activity in cell-cycle regulation and inhibition of cell invasion in vitro has been suggested as well [24–28]. Loss of PTEN function seems to be responsible for many of the phenotypic features of melanoma, thus PTEN may serve as a potential target for drug development. However, most types of tumors with PTEN alteration also carry other genetic changes, making the role of PTEN more ambiguous. As discussed below, PTEN homozygous deletions and missense mutations alone is sufficient to cause tumorigenesis in certain tissues but not in others. However, even when mutation of PTEN alone has minimal effects, it frequently contributes to tumorigenesis in the context of other genetic alterations. In this review, signaling pathways mediated by the lipid and protein phosphatase activities of PTEN, and the implication of PTEN loss in melanoma tumorigenesis, will be discussed.
PTEN, a tumor suppressor gene
The most convincing initial insight into the potential involvement of chromosome 10 in melanoma was reported by Fountain et al. [29]. Many Studies on the relative frequency of chromosomal aberrations revealed that several chromosomes were more commonly altered than chromosome 10; however, chromosomes 9 and 10 were unique in their early alteration and dysplastic lesions. The presence of a tumor suppressor gene(s) on chromosome 10q had long been suspected, since loss of heterozygosity (LOH) on regions of chromosome 10q was observed frequently in a number of cancer types [30–34]. In melanoma, loss of chromosome 10 was first reported by Parmiter et al. [35]. Since then, LOH of chromosome 10q has been studied extensively and a frequency of 30–50% has been found in melanoma, suggesting that the presence of tumor suppressor gene(s) on chromosome 10q is critical for inhibiting melanoma tumorigenesis [32, 33]. However, LOH studies in melanoma did not eventually yield the identification of a tumor suppressor gene on chromosome 10q. In 1997, by homozygous deletion mapping in gliomas and breast tumors, PTEN was finally identified as a candidate tumor suppressor gene on chromosome 10q. That year, three research groups independently reported the cloning of PTEN, MMAC1 and TEP1, which turned out to be the same tumor suppressor gene. In 1997, Li et al. [1] first isolated PTEN by mapping of homozygous deletions on chromosome 10q23 in breast tumors. The predicted PTEN protein contained the phosphatase consensus motif and had ~40% homology with the focal adhesion protein tensin. It was named PTEN (phosphatase and tensin homolog deleted in from chromosome ten). Similarly, MMAC1 was cloned based on homozygous deletion studies in glioma tumor cells by Steck and colleagues [2]. Coding region mutations of this gene were observed in numerous cancer types including glioblastomas, prostate, kidney and breast cancers, thus it was named MMAC1 (mutated in multiple advanced cancers). TEP1, on the other hand, was identified as a protein tyrosine phosphatase by searching Genebank sequences containing phosphatase consensus motifs. The expression level of this gene was found to be altered in a number of tumor cells and it was rapidly downregulated by transforming growth factor-β (TGF-β). Therefore, it was called TEP1 (TGF-β-regulated and epithelial cell-enriched phosphatase) [3]. Sequence identity between PTEN, MMAC1, and TEP1 confirmed that they were of the same gene. Subsequently, a high frequency of PTEN mutations have been reported in malignant melanoma, squamous cell carcinoma, endometrial, and thyroid tumors in addition to glioma, prostate, and breast tumors [4, 5, 7, 36]. These findings placed PTEN among the most mutated tumor suppressor genes in human cancers.
PTEN signaling
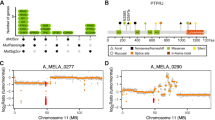
The PTEN gene spans 105 kb and includes nine exons (Fig. 1a). PTEN was first predicted to be a protein phosphatase since it contained (I/V)-H-C-X-A-G-X-X-R-(S/T)-G, the critical motif found in protein tyrosine phosphatases (PTPs) and dual-specificity phosphatases (DSPs) [1, 37, 38]. However, the recombinant PTEN protein exhibited higher catalytic activity towards negatively charged phosphorylated polypeptides than phosphoproteins [39]. Phosphatidylinositol (3,4,5)-tris-phosphate [PtdIns(3,4,5)P3] was then identified as a substrate of PTEN [40]. In 1999, the crystal structure of PTEN was unraveled, showing an overall phosphatase domain structure similar to that of the DSP Vaccinia Hi-related phosphatase (VHR). However, the active site pocket of PTEN appeared deeper and wider, and two basic residues (Lys125 and Lys128) were localized within the active site loop, which were absent in PTP and VHR [41]. It was suggested that PTEN, as a phosphatase, might have preference towards PtdIns(3,4,5)P3 and highly acidic residues present in polypeptides, although it could use both protein and lipid as substrates.
Schematic representation of PTEN gene and genetic alterations identified in melanoma. a Structural representation of PTEN with 9 exons represented by boxes. The exon 5 represents the hot spot of mutations and the different numbers show the mutated codons. The vertical lines correspond to the localization of PTEN mutations in the exons and the height of the lines indicates the mutation frequencies. The horizontal lines refer to different extent of deletions. b Schematic representation of PTEN protein and its biological functions. PTEN contains two key domains: the phosphatase domain (in red; amino acids 14–185), which possesses lipid and protein phosphatase activities; and the C2 domain (in grey; amino acids 190–350), which is responsible for lipid binding and membrane localization. There are two other important domains; the carboxy-terminal region (amino acids 351–400), which is involved in cell migration and regulation of protein stability; and the PDZ-binding domain (in yellow; amino acids 401–403), which is important for protein–protein interactions
As a phosphatase, PTEN acts to remove phosphates from lipids. The best described substrate of PTEN is PtdIns(3,4,5)P3. PTEN removes the phosphate in PtdIns(3,4,5)P3 to generate PtdIns(4,5)P2. PTEN serves to counter-balance the effects of phosphoinositide 3′ kinase (PI3K), which normally adds a phosphate to PtdIns(4,5)P2 to generate PtdIns(3,4,5)P3. PtdIns(3,4,5)P3 recruits kinases such as phosphoinositide-dependent kinase 1 (PDK1), which in turn phosphorylates Akt that phosphorylates other downstream proteins involved in regulation of apoptosis and cell-cycle progression. PTEN removal of the phosphate from PtdIns(3,4,5)P3 inhibits this pathway by preventing localization of proteins with pleckstrin homology domains to the cell membrane. In addition to this activity, other functions could be affected following alterations (deletion/mutation) of the PTEN gene.
The PTEN tumor suppressor function requires both the phosphatase and the lipid membrane-binding domains (Fig. 1b), and the lipid phosphatase activity of PTEN dephosphorylates the 3-phosphoinositide products of PI3K. 3-phosphoinositides can activate important survival kinases, such as PDK1 and Akt, as well as other proteins that are not kinases. PTEN therefore negatively regulates the Akt pathway, leading to decreased phosphorylation of Akt substrates such as tuberous sclerosis 2 (TSC2) and PRAS40 (encoded by AKT1S1) that control mTOR activity, p27 (encoded by CDKN1B), p21 (encoded by CDKN1A), glycogen synthase kinase 3 (GSK3α and GSK3β), BCL-2-associated agonist of cell death (BAD), apoptosis signal regulating kinase 1 (ASK1, also known as MAP3K5), WT1 regulator PAWR (also known as PAR4) and CHK1, as well as members of the forkhead transcription factor family (for example, FOXO1, FOXO3, and FOXO4) [42]. Changes in phosphorylation alter the activity and/or localization of these proteins, which in turn affects processes such as cell cycle progression, metabolism, migration, apoptosis, transcription, and translation.
Mechanism of PTEN regulation
There are multiple mechanisms for the regulation of PTEN, including transcription, mRNA stability, microRNA (miRNA) targeting, translation, and protein stability. PTEN is transcriptionally silenced by promoter methylation in endometrial, gastric, lung, thyroid, breast and ovarian tumors, as well as glioblastoma [43–49]. In glioma, lung, and prostate cancer, PTEN expression is decreased by overexpression of miRNA-26a (miR-26a), miR-106b-25 cluster, or the miR-21 [50–52]. PTEN can also be post-translationally regulated by phosphorylation, ubiquitylation, oxidation, acetylation, proteosomal degradation, and subcellular localization [53, 54]. Although many of these post-translational changes in PTEN have been shown to alter various cellular phenotypes in vitro, most have not been validated as key regulators of PTEN in human cancers or mouse models. PTEN amino acids Lys13 and Lys289 are monoubiquitinated, which leads to nuclear import in vitro, and mutations at Lys289 have been observed in Cowden syndrome and associated with nuclear exclusion [55]. Recently, a regulatory role has been reported by Poliseno et al., [56] showing that mRNA molecules from PTENP1 (PTEN pseudogene) can act as competitive endogenous RNA sequestering miRNA molecules. In a given tissue, there would be a balance in the level of expression from both PTEN and PTENP1; if PTENP1 transcription decreases, more miRNAs are able to target PTEN. On the other hand, an increase in PTENP1 transcription implies that less miRNA will target PTEN. Therefore, PTENP1 indirectly regulates PTEN by competing for binding to the miRNA. In conclusion, PTENP1 transcripts can act as indirect post-transcriptional regulators decoding miRNAs that target the PTEN gene. However, the role of PTENP1 in disease (tumorigenesis, in particular) has not been proven.
Other criteria for PTEN regulation is the importance of its gene dosage in tumorigenesis events. Indeed, a generated complete or partial loss of PTEN knockout mice allowed to understand its tumor suppressive activity in specific cells and/or tissues in vivo, [57]. Using mouse genetic engineering, several studies have contributed to show the impact of partial PTEN level reduction in cancer. A number of cancers, including mammary, prostate, and uterine were found in PTEN heterozygous mice [58–60]. Also, studies on knockout mice showed that complete PTEN loss results in exhaustion of the hematopoietic stem cell compartment prior to leukemia development [61, 62] or a novel cellular senescence program [63]. As BRAF mutation induces senescence in melanocytes, a process that is important for melanomagenesis, the cooperation between PTEN and BRAF on cellular senescence warrants further investigation.
PTEN, a mutated gene in human cancers
Numerous mutations have been reported throughout this gene (Fig. 1a). Mutations resulting in the loss of function or reduced levels of PTEN, as well as PTEN deletions or alteration are found in many sporadic tumors [64]. PTEN mutations are found throughout most of the PTEN coding region, with the exception of exon 9, which encodes the carboxy-terminal 63 amino acids [65]; more than 40% occur within exon 5, which encodes the phosphatase domain [65]. Allelic or total deletion of PTEN is a frequent occurrence in cancers such as breast, prostate cancer, and melanoma. A subset of patients with melanoma carries mutations in the PTEN promoter or in potential splice donor and acceptor sites [5, 66–69]. Splicing alterations can lead to exon skipping that alters PTEN functions. In mice, decreasing PTEN level correlates with increasing tumor susceptibility [70, 71]. This suggests that reduced levels of normal PTEN are insufficient for its tumor suppressor function and raises the possibility that reduction of PTEN activity could be an important driving mechanism for cancer.
PTEN mutations have been extensively characterized and found in three linked autosomal dominant cancer predisposition syndromes: Cowden’s disease (CD), Lhermitte–Duclos disease, and Bannayan-Zonana syndrome. These cancer syndromes share similar phenotypic characteristics including mental retardation, gastrointestinal hamartomas, thyroid adenomas, breast fibroadenomas, macrocephaly, and mucocutaneous lesions [72, 73]. Over 80% of patients with CD harbored germline PTEN mutations. LOH studies in 20 hamartomas using markers flanking and within PTEN showed that wild-type PTEN locus was indeed lost in two breast fibroadenomas, one thyroid adenoma, and one pulmonary hamartoma, confirming that PTEN functions as a tumor suppressor gene in CD [74]. Somatic PTEN alteration is common in many sporadic tumor types [75]. Various tissue-specific and/or inducible homozygous deletions of PTEN have been generated in mice to model sporadic PTEN loss in tumorigenesis. In the endometrium [76], mammary gland [77], prostate [78], and in T cells [79], homozygous deletion of PTEN led to rapid tumor formation in the targeted tissue. Similarly, PTEN-deficient mice developed tumors in the liver [80], bladder [81], and lung [82]. By contrast, when PTEN was deleted the intestine [83], no malignant tumors developed, although intestinal polyps were common, as observed in Cowden syndrome. Loss of other tumor suppressors or the activation of oncogenes can nonetheless combine with PTEN loss to cause cancer in these organs.
PTEN mutations in melanoma
PTEN mutations in melanoma were reported shortly after its cloning. Initial studies demonstrated a mutation rate of ~30–40% in melanoma cell lines and ~10% in primary melanomas [5, 66]. Functional studies supported the hypothesis that PTEN played an important role in melanoma. Indeed, in PTEN-deficient melanoma cells, ectopic expression of PTEN was able to reduce melanoma tumorigenicity and metastasis [3, 84], implicating PTEN as a critical tumor suppressor in melanoma tumorigenesis. To elucidate the role of PTEN loss in melanoma tumorigenesis, a thorough understanding of the functions of PTEN on the structural/molecular level and PTEN-mediated signaling events is necessary.
The PTEN mutations are scattered along the length of the gene, with the exception of exon 9 (no mutation reported). A mutational hot spot is found in exon 5 (33%), which encodes the phosphatase catalytic core motif (Fig. 1), and recurrent mutations are also found at CpG dinucleotides suggesting deamination-induced mutations. The genetic alterations included point missense mutations, insertions, splice site mutations, small and gross deletions of the gene (Tables 1, 2). The majority of these alterations lead to premature termination with small transcripts or functional inactivation of the protein in some cases.
A similar mutational profile has been found for PTEN mutations in human cancers. The highest frequency of PTEN mutations is found in endometrial carcinomas and glioblastomas. PTEN mutations are also found in lymphoma, thyroid, breast, prostate carcinomas, and melanomas [85]. In melanoma, PTEN mutation rates of 27.6% in melanoma cell lines (Table 3) and 12.0% in melanoma biopsies have been reported (Table 4). In 1997, Guldberg et al. [5] first reported that 42.9% (15/35) of examined melanoma cell lines harbored PTEN mutations. Nine of these cell lines showed homozygous deletion of PTEN gene, and six lines had mutations in one allele in combination with the loss of the second. Tsao et al. [66] examined 45 melanoma cell lines and found PTEN mutations in 28.9% of the melanoma cell lines, including 20.0% (9/45) homozygous deletions and 8.9% (4/45) frameshift, nonsense, and intronic splice mutations. Teng et al. [86] examined seven melanoma cell lines and found that four cell lines contained homozygous deletions in the PTEN gene. Pollock et al. [87] reported that 22.8% (13/57) of melanoma cell lines carried mutations in PTEN. Eight of these cell lines showed mutations in one allele and five had homozygous deletion of PTEN. In total, 76 different alterations were found in all the cell lines studied (Tables 1, 3). Among these alterations, there are 20 deletions for which the exact regions were not determined. All information on PTEN mutational status in melanoma biopsies was obtained from a single tumor of the patients. To expand our knowledge on PTEN functions, it would be important to study its status in multiple tumors from the same patient.
PTEN mutations are uncommon in uncultured melanoma biopsies. Tsao et al. [66] examined 17 uncultured metastatic melanoma samples; only one case of homozygous deletion and another case of premature stop mutation were identified (Tables 2, 4). When Boni et al. [88] tried to identify mutations within the exons 5, 6, 7, and 8 of the PTEN gene, no mutations were found. Teng et al. [86] found 10% (1/10) missense mutation of PTEN in primary melanoma tumors. Birck et al. screened a panel of 77 melanoma biopsies including 16 primary and 61 metastatic tumors. PTEN mutations were identified in 6.6% (4/61) of the metastatic tumors, while no mutation was found in primary melanoma [13]. In this study, they have detected a nonsense mutation (L139X) that had already been reported by Guldberg et al. [5]. By examining two intragenic biallelic polymorphisms, 53.8% (21 out of 39) informative specimens showed loss of one PTEN allele [13]. Reifenberger et al. [89] examined 40 melanomas and found 20% (3/15) of primary melanomas and 12% (3/25) of metastatic melanomas contained PTEN mutations. Two different mutations were found in both primary and metastatic melanomas. Celebi et al. [14] also detected PTEN sequence alterations in four of 21 (19%) metastatic melanoma samples. Two other mutations were found in the putative splice site of PTEN, making the total alterations at 28.6%. Similar results were reported by Poetsch et al. [90] and Abdel-Rahman et al. [91] for metastatic melanomas (Tables 2, 4). Taken together, these data supported the notion that PTEN alterations occur in melanomas and loss of PTEN may contribute to melanoma development.
Functional studies support a role for PTEN in melanoma tumor suppression. An in vitro LOH study by Robertson et al. [92] showed that PTEN was indeed targeted for LOH in melanoma. A melanoma cell line UACC903 with duplicated mutant chromosome 10 was used to build the in vitro LOH model. A wild-type chromosome 10 was transferred into the cells and underwent spontaneous breakage and deletions over time in culture. During this process, the introduced wild-type copy of PTEN was lost. In parallel, another melanoma cell line with wild-type PTEN gene maintained a transferred 10q23–24 region that contained the exogenous PTEN gene. Ectopic expression of PTEN into UACC903 cells was also demonstrated to suppress tumor cell growth [92]. Similar findings have been reported by Tsao et al. [67] as well. In a number of melanoma cell lines, overexpression of PTEN uniformly inhibited colony formation, implicating a tumor-suppressive function of PTEN in melanoma [67].
In contrast, three groups have failed to detect significant PTEN mutation rates in melanoma. Boni et al. [88] used two microsatellite markers flanking PTEN gene to search for LOH surrounding the PTEN locus, and found no LOH for either of the markers in 40 (23 primary and 17 metastatic) melanoma tissue. Further SSCP analysis for exons of PTEN gene did not yield any abnormal bands [88]. Herbst et al. [93] analyzed LOH at loci closely linked or intragenic to PTEN in 65 melanomas. A rate of LOH of lower than 16% with eight different polymorphism markers led to the conclusion that it rather represented random genetic events than indicating that PTEN was the target in melanoma [93]. Poetsch et al. [90] screened 25 primary and 25 metastatic melanomas for PTEN mutation, and found two missense and eight silent mutations (Table 2).
These findings are in contrast with the abundant evidence implicating PTEN as an important tumor suppressor in melanoma and other cancers. However, it must be taken into consideration that although an overall mutational rate of PTEN in cultured melanoma cell lines is around 27.6%, only seven cases with PTEN mutations has been detected in over 96 primary melanomas. There are several possible explanations for this observation: (1) although PTEN loss is important in melanoma, it occurs late in melanoma tumorigenesis since mutation is rarely detected in primary melanomas; (2) PTEN loss may in fact be relatively rare in melanoma, and the establishment of cell lines selects for melanomas with PTEN alterations; (3) the biology of PTEN alteration in early melanomas makes detection of alteration difficult (e.g., from dosage reduction, epigenetic downregulation of expression or homozygous deletion); (4) the number of primary melanoma samples examined is small and the subtypes of tumors have not been examined; or (5) PTEN loss following the epigenetic mechanisms, such as DNA methylation.
The negative cases in PTEN alteration studies could be explained by the homozygous deletion rate observed by several laboratories. As homozygous deletion makes LOH of chromosome 9p21 (at CDKN2A) difficult to detect in tumor samples [94], it is possible that chromosome 10q23 PTEN deletions in melanomas may have been underdetected too. Moreover, epigenetic studies recently suggested that the involvement of PTEN function loss in melanoma might have been in fact underestimated. Zhou et al. [95] analyzed PTEN protein expression, instead of analyzing PTEN gene mutations in melanomas. Using immunohistochemistry, they found no PTEN protein expression in 15% (5/34) and low expression in 50% (17/34) of melanoma samples (four primary and 30 metastatic). Surprisingly, among the five melanomas with no PTEN protein expression, four showed no deletion or mutation of PTEN gene, indicating the presence of an epigenetic mechanism of biallelic functional inactivation of PTEN [95].
The timing of PTEN alterations in melanoma development is also not understood. Cytogenetic studies, cited above, suggested an early involvement of PTEN. However, Birck et al. [13] examined primary melanoma samples and detected no PTEN mutations. However, they also found allelic loss of PTEN gene in 37.5% (3/8) primary melanomas, indicating a decreased PTEN dosage possibly occurring early in melanoma development [13]. Thus, different types of genetic changes may lead to higher frequency of PTEN alterations in melanoma cell lines, providing some explanation for the discrepancies in the literature. Additional work will be needed to accumulate data to allow for reconciliation of divergent mutation rates from different studies.
Sporadic melanomas frequently have a loss of PTEN through LOH, deletion, and mutation [5]. PTEN can also be epigenetically silenced in melanoma, as decreased PTEN transcript levels were associated with PTEN promoter methylation [96]. PTEN methylation also correlated with decreased survival [97]. In another study, low PTEN expression was associated with melanoma ulceration, which is characteristic of aggressive tumors, but did not significantly correlate with overall survival [98]. A link between DNA damage and PTEN mutation in melanoma has been suggested by Wang et al. [99] who showed that more than 50% of the melanomas from patients with xeroderma pigmentosum have PTEN mutations that are typically associated with ultraviolet radiation exposure.
In mice, PTEN deletion in pigmented mouse cells does not lead to the development of spontaneous melanoma, despite an increase in the number of dermal melanocytes. However, in this model, topical carcinogen treatment led to melanoma formation in nearly 50% of the mice within 20 weeks [100]. In conjunction with CDKN2A (encoding p14ARF) deletion, nearly 10% of PTEN +/− mice developed spontaneous melanoma [101].
In all the melanoma biopsies analyzed, 12.0% (41/342) contained PTEN alterations. Three mutations were found twice in different tumors, giving a total of 38 different alterations (Tables 2, 4). The huge proportion of these alterations was found in metastatic melanoma biopsies (34/224, 15.2%). Only 7.3% (7/96) of primary melanoma specimens were found to carry PTEN alterations.
PTEN mutations and other genes
In addition to PTEN alterations, mutations of CDKN2A, as well as NRAS mutations have been frequently observed in melanoma [102, 103]. CDKN2A is the most often mutated tumor suppressor gene in melanoma, with 60% homozygous deletions and additional 15–20% point mutations [104–106]. The two proteins encoded by CDKN2A, p16, and p14ARF, function in the pRB and p53 pathway, respectively. p16 is a cyclin-dependent kinase inhibitor. It binds to and inhibits cyclin D/CDK4, which in turn blocks pRB phosphorylation, leading to G1 cell-cycle arrest. p14, on the other hand, binds mdm2 and relieves p53 from mdm2-mediated p53 degradation. p53 is known to block cell proliferation by inducing cell-cycle arrest or apoptosis.
As RAS is a prominent oncogene involved in melanoma tumorigenesis, like PTEN, it has several biological functions. RAS gene family members include HRAS, NRAS, and KRAS. They encode 21-kDa proteins with GTPase activity. RAS is involved in regulating receptor tyrosine kinase-induced MAPK activation. RAS activates MEK and MAPK through RAF. RAS also binds and activates lipid kinase PI3K, and therefore activates Akt pathway. Finally, RAS interacts with p53 and p16. In primary mouse embryonic fibroblasts, for example, HRAS was shown to induce premature cell senescence, which was associated with the accumulation of p16 and p53 [107]. Pathways controlled by these three elements, RAS, p53, and p16, therefore appear to be central to control of the malignant phenotype.
PTEN functions as a lipid and protein phosphatase that downregulates Akt and MAPK, potentially suggesting that RAS and PTEN have opposite functions in both protein and lipid kinase signaling pathways. Is it possible that PTEN loss and RAS oncogenic activation are redundant in tumor development? Tsao et al. [67] reported a reciprocal mutational status for PTEN and NRAS in human melanoma cells. Among 53 cutaneous melanoma cell lines, 16 cell lines (30%) harbored PTEN mutations and 11 lines (20.7%) had oncogenic NRAS mutations. Only one cell line showed mutations in both genes, so a total of 50% cell lines had mutations in either PTEN or NRAS (Table 2; [67]). Similar reciprocal findings have been reported in endometrial cancer [108]. Furthermore, Davies et al. [109] showed that loss of PTEN expression was not detected in melanoma cases harboring NRAS mutations. However, Nogueira et al. [110] recently found that PTEN loss cooperates with RAS activation to drive melanoma cell invasion and promote metastasis.
In mouse melanoma models, RAS and CDKN2A loss cooperate to lead to melanoma development [111]. It has been shown that CDKN2A loss coupled with PTEN loss lead to melanoma; however, it is not clear that PTEN loss confers greater susceptibility to melanoma development than CDKN2A loss alone [101]. Recently, Dankort et al. [112] showed that a simultaneous activation of BRAF and deletion of PTEN in melanocytes leads to early onset spontaneous melanomas, with metastasis to the lymph nodes and lung. Notably, the mTOR inhibitor rapamycin increased survival in these mice by more than twofold [112]. These mouse studies indicate that PTEN is probably not a driving mutation in melanoma, but can contribute to a malignant phenotype in the presence of other genetic alterations. Further studies are needed to elucidate the details of PTEN, RAS, and CDKN2A interaction in murine models.
A direct downstream target of RAS, BRAF, has been shown to exhibit a higher mutation frequency in melanoma [113]. In all, 806 of 2,346 (34.3%) uncultured melanomas, 568 of 1,336 (42.4%) cutaneous melanomas, and 251 of 389 (64.5%) melanoma cell lines harbored mutated BRAF gene. In that study, NRAS mutations were also detected in 21.2% (379/1,790) uncultured melanomas, 26.4% (282/1,067) cutaneous melanomas, and 13.6% (58/426) of the melanoma cell lines. Furthermore, cell lines were detected with oncogenic-activated RAS–RAF–MAPK pathway. Thus, BRAF is a second gene whose mutations are reciprocally distributed with regard to RAS. Like RAS, RAF can activate PI3K, and PI3K and Akt can directly alter RAF kinase activity [114, 115]. Thus, understanding the relation of PTEN, RAS, and RAF, in the context of PI3K-Akt and RAS–MAPK pathways will be crucial to understanding melanoma tumorigenesis.
The PTEN expression in pre-malignant lesions was conducted by two different groups. Tsao et al. [116] found uniformly strong PTEN expression in the cytoplasm of almost all benign nevi, while Singh et al. [117] showed strong cytoplasmic staining of PTEN for eleven of 17 (64%) benign nevi. In accordance of these findings, PTEN is present in pre-malignant melanoma as opposed to its notable absence in a significant proportion of primary melanomas. These findings support the role of PTEN loss in the pathogenesis of melanoma.
PTEN genetic alterations in melanoma result in the activation of critical signaling pathways promoting growth and survival of tumors cells. Alterations in the RAS–RAF–MAP kinase and PI3-kinase signaling pathways are commonly altered in melanoma. Mutations in BRAF and NRAS occur in a mutually exclusive pattern and lead to MAP-kinase activation. The most common known genetic alteration in the PI3-kinase cascade is the loss of PTEN function, and was commonly associated with BRAF mutations [67, 68, 118].
Alterations in membrane receptors or mutations in downstream effectors such as RAS or RAF can initiate aberrant MAPK signaling in melanoma [119]. About 20% of melanoma patients harbor NRAS mutations, but it is BRAF (the RAS substrate) that harbors the most frequent mutations in melanoma (~40%) [113]. About 80% of these mutations display a valine to glutamic acid substitution (V600E), causing constitutive kinase activation, and about 16% harbor a valine to lysine substitution (V600K) [120, 121]. MAPK signaling is required for proliferation of both RAS and RAF-transformed melanocytes, as it was shown that RAF and MEK inhibitors decreased ERK activity and blocked their cell cycle progression. Provided the large number of melanomas that harbor activating mutations in the BRAF oncogene and their reliance on BRAF activity, targeted inhibition of this protein became of high interest.
The treatment of human melanoma at advanced stage using biotherapeutics or chemotherapeutics has rarely provided response rates higher than 20% [122]. This clinical aspect is changing with the advent small molecule inhibitors to treat metastatic melanoma [123, 124]. The BRAF mutation in melanoma provided an opportunity to target a cancer-specific oncogene and develop compounds to curb its aberrant activity. Recently, through structure-guided approaches, the specific BRAF(V600E) inhibitor PLX4032 was developed, which provided increasing proof that targeting BRAF in melanoma is a real therapeutic approach [123, 124]. PLX4032 is a well-tolerated small-molecule inhibitor inducing ~80% partial or complete tumor regression for melanomas containing BRAF(V600E) mutations and has received FDA approval for the treatment of late-stage human melanoma.
In melanoma, ERK mutations have not been identified and MEK mutations are not frequent. However, as the MAPK pathway is constantly active in the tumor cells, these effectors can also be targeted. From preclinical models, MEK inhibitors induce significant reduction in melanoma growth [125, 126]. However, these inhibitors have not shown significant clinical efficacy in melanoma clinical trials [127]. Interestingly, inhibitors of BRAF and MEK were reported to have similar transcriptional targets; therefore, MEK inhibitors could be useful in patients with acquired BRAF inhibitor resistance if toxicities can be controlled [128].
Interestingly, BRAF V600E mutations are also observed in benign nevi; suggesting that BRAF mutations alone are insufficient for tumorigenesis and that additional factors are needed for cancer progression [129]. In fact, a mouse genetic model of BRAFV600E/PTEN−/− that mimics melanoma progression indicates that the PI3K pathway also plays an important role in the development of aggressive tumors [112]. PI3K pathway activity was shown to be increased in melanoma through loss of activity of the tumor suppressor PTEN. This loss occurs through PTEN mutation, deletion, or methylation, which can also coincide with BRAF mutations but not NRAS [68]. PTEN loss is found in 5–20% of noninherited melanomas, and similar to other neoplasia, may regulate inhibition of the MAPK pathway, cell-cycle arrest, and survival via effects on Bcl-2 and caspases [130]. Thus, as melanomas favor the deregulation of both the MAPK and PI3K pathways, their combined targeting has therapeutic merit [131]. Although multiple other signaling pathways may be involved in melanoma oncogenesis, finding which ones are essential for melanoma survival and progression will determine their therapeutic value.
Recently, several studies have identified PTEN loss in multiple mechanisms of BRAF inhibitor resistance [132–135], suggesting that PTEN inactivation can affect sensitivity to BRAF inhibition. These findings are useful in developing a new generation of BRAF inhibitors. In fact, the growth advantage conveyed by the constitutive activation of these pathways leads to positive selection of cells that have acquired the mutations and in many instances leads to critical dependency of the cancer cells on their activation. This creates opportunities for therapeutic interventions targeted at signaling components within these pathways that are amenable for pharmacological inhibition.
Relationship between PTEN alterations and functions
To date, 111 different alterations of the PTEN gene have been reported (Tables 1, 2). Three mutations have been found in both melanoma cell lines and tissues. Some alterations have been reported twice or more. These mutations, scattering along the whole gene, include point-stop mutations, point missense mutations, insertions, duplications, splice site mutations, and small and gross deletions of the gene. A great number of the mutations are found in exon 5 coding for the phosphatase domain, and likely alter the phosphatase activity of PTEN. The majority of mutations occurring in PTEN result either in abnormal RNA splicing, truncation, or gross deletion of the gene, thus predicting inactivation of the protein and supporting loss of various functions assigned to PTEN.
A review of the literature (considering only the mutations found in tumoral specimens and not those found in cell lines) provides 38 PTEN mutations occurring in various types of primary tumors or metastases (Table 2). These mutations affecting PTEN in melanoma patients have been found predominantly in metastatic tissues. It is mainly nonsense, frameshift, or splicing mutations resulting in a truncation of the protein. In addition, bi-allelic inactivation of PTEN has been evidenced with both point mutations in one allele and deletions of the other allele resulting in loss of heterozygosity (LOH). Similarly to cell lines, a great number of missense mutations occur in the exon 5 (hot spots of PTEN mutations). Interestingly, two of the three mutations found in both cell lines and biopsies were located in the exon 5.
PTEN alterations in different functional domains will lead to the loss of expression, thereby affecting its tumor suppressor functions. In fact, PTEN contains two key domains required for its tumor suppressor function: the lipid membrane-binding (C2) domain (amino acids 190–350), and the catalytic (phosphatase) domain (amino acids 14–185) with an active site constituted by the residues 123–130 (Fig. 1b). Many mutations affect these domains leading to a loss of function (Tables 1, 2). There are other domains such as the PDZ-binding domain (amino acids 401–403), which binds to proteins containing PDZ domains (an acronym of three proteins: Psd95, Dlg1, and Zo-1), and the carboxy-terminal region (amino acids 351–400), which contains PEST (rich in amino-acids P, E, S, T) sequences and may contribute to protein stability and activity. Only deletions were reported for these two domains and their importance in the tumor suppressor function of PTEN is less well defined.
PTEN alterations occur in both the N-terminal catalytic core motif and the C-terminal non-catalytic regulatory domain. Five phosphorylation sites (S370, S380, T382, T383, and S385) were reported for the latter [136]. When phosphorylated at these residues, PTEN is targeted for degradation through the ubiquitin/proteasome system [137]. In addition, phosphorylation may protect the carboxyl terminus from caspase 3-mediated cleavage during apoptosis [138]. Interestingly, it has been reported that phosphorylation at T383 requires the protein phosphatase activity, but not the lipid phosphatase activity [136]. Consequently, each alteration affecting these areas could disturb the functional activity of PTEN.
As reported PTEN has many roles, including: (i) lipid phosphatase activity removing the phosphate on either PtdIns(3,4,5)P 3 or (PtdIns(3,4)P 2 [40, 136, 139]; (ii) protein phosphatase activity [3, 40, 136, 139]; and (iii) as a substrate for phosphorylation by kinases [1, 3, 39]. These different roles result in reduced activation of the (PI3K)/Akt and other pathways leading to an anti-invasive, anti-proliferative and tumor suppressive effects. PTEN is also involved in mediating growth arrest and other cellular functions of the MAPK pathway [140–143]. Each of reported alterations could affect one or several functions. The biological significance of the protein phosphatase effects of PTEN is less well characterized than the lipid phosphatase effects. The relationships between genetic alterations and PTEN functions are outlined in Fig. 1b.
Although the lipid phosphatase activity of PTEN is important for its tumor suppressor functions, other functions of PTEN may also prove to be important. Indeed, several studies have demonstrated that PTEN protein phosphatase activity is important for its functions in cell cycle arrest and inhibition of cell invasion in vitro [24–28]. The lipid phosphatase activity of PTEN is thought to mostly occur at the cell membrane, but PTEN has also been demonstrated to exert nuclear functions. The binding of PTEN to centromere protein C1 (CENP-C1) is required for centrosome stability, and its nuclear localization is required for DNA double-strand break (DSB) repair that is mediated by DNA repair protein RAD51 [144]. PTEN also regulates the tumor suppressor function of anaphase-promoting complex (APC) and its regulator E-cadherin (encoded by CDH1) in the nucleus, independently of its lipid phosphatase activity [145]. Altered APC–CDH1 activity has been implicated in multiple tumor types [146].
Conclusions and perspectives
The analysis of PTEN alteration in cell lines and tissues provides evidence that the development of many melanoma cases seems to be driven by the loss of PTEN expression and function. In this work, we have discussed function and signaling of PTEN as a lipid phosphatase as well as a protein phosphatase. The consequences of PTEN loss are alterations in the control of cell-cycle progression, apoptosis, cell contact, and migration. Together, these aberrations contribute to the malignant cell phenotype (Fig. 1b).
We have discussed several lines of evidence implicating PTEN in the development of melanoma. However, one key question that remains to be answered is whether tumors that develop as a consequence of PTEN attenuation are attributed to which biological function of this tumor suppressor. The importance of 10q23 loss in melanoma is clear, and studies of PTEN in tumors and cultured melanoma lines suggest strongly that mutated PTEN lead to a loss of function, although much remains to be learned about the precise role of PTEN in melanoma tumorigenesis. The exact frequency of PTEN loss in primary tumors, in metastases and the relation of these observations to the findings in cell lines require further confirmation. The inter-relation of PTEN mutation and other genes important in melanomagenesis needs to be studied. Modeling of these genetic discoveries in mouse models could be used to show whether re-expression of PTEN in PTEN-deficient melanoma could lead to tumor regression. Finally, the discovery of reagents to stimulate PTEN activity in cells that lack functional PTEN could be an important advance in cancer therapies.
References
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947
Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV (1997) Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15:356–362
Li DM, Sun H (1997) TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 57:2124–2129
Dahia PL, Marsh DJ, Zheng Z, Zedenius J, Komminoth P, Frisk T, Wallin G, Parsons R, Longy M, Larsson C, Eng C (1997) Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res 57:4710–4713
Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J (1997) Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res 57:3660–3663
Risinger JI, Hayes AK, Berchuck A, Barrett JC (1997) PTEN/MMAC1 mutations in endometrial cancers. Cancer Res 57:4736–4738
Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH (1997) Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res 57:3935–3940
Halachmi N, Halachmi S, Evron E, Cairns P, Okami K, Saji M, Westra WH, Zeiger MA, Jen J, Sidransky D (1998) Somatic mutations of the PTEN tumor suppressor gene in sporadic follicular thyroid tumors. Genes Chromosomes Cancer 23:239–243
Maxwell GL, Risinger JI, Gumbs C, Shaw H, Bentley RC, Barrett JC, Berchuck A, Futreal PA (1998) Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res 58:2500–2503
Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, Parsons R (1997) Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res 57:4183–4186
Yang J, Ren Y, Wang L, Li B, Chen Y, Zhao W, Xu W, Li T, Dai F (2010) PTEN mutation spectrum in breast cancers and breast hyperplasia. J Cancer Res Clin Oncol 136:1303–1311
Barbosa M, Henrique M, Pinto-Basto J, Claes K, Soares G (2011) Prostate cancer in Cowden syndrome: somatic loss and germline mutation of the PTEN gene. Cancer Genet 204:224–225
Birck A, Ahrenkiel V, Zeuthen J, Hou-Jensen K, Guldberg P (2000) Mutation and allelic loss of the PTEN/MMAC1 gene in primary and metastatic melanoma biopsies. J Invest Dermatol 114:277–280
Celebi JT, Shendrik I, Silvers DN, Peacocke M (2000) Identification of PTEN mutations in metastatic melanoma specimens. J Med Genet 37:653–657
Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R (1997) Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16:64–67
Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z, Liaw D, Caron S, Duboue B, Lin AY, Richardson AL, Bonnetblanc JM, Bressieux JM, Cabarrot-Moreau A, Chompret A, Demange L, Eeles RA, Yahanda AM, Fearon ER, Fricker JP, Gorlin RJ, Hodgson SV, Huson S, Lacombe D, Eng C et al (1998) Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet 7:507–515
Eng C, Hobert JA (2009) PTEN hamartoma tumor syndrome: an overview. Genet Med 11:687–694
Eng C (2003) PTEN: one gene, many syndromes. Hum Mutat 22:183–198
Waite KA, Eng C (2002) Protean PTEN: form and function. Am J Hum Genet 70:829–844
Di Cristofano A, Pandolfi PP (2000) The multiple roles of PTEN in tumor suppression. Cell 100:387–390
Mutter GL (2001) Pten, a protean tumor suppressor. Am J Pathol 158:1895–1898
Furnari FB, Huang HJ, Cavenee WK (1998) The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res 58:5002–5008
Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H (2000) Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res 60:7033–7038
Hlobilkova A, Guldberg P, Thullberg M, Zeuthen J, Lukas J, Bartek J (2000) Cell cycle arrest by the PTEN tumor suppressor is target cell specific and may require protein phosphatase activity. Exp Cell Res 256:571–577
Weng LP, Brown JL, Eng C (2001) PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet 10:599–604
Dey N, Crosswell HE, De P, Parsons R, Peng Q, Su JD, Durden DL (2008) The protein phosphatase activity of PTEN regulates SRC family kinases and controls glioma migration. Cancer Res 68:1862–1871
Davidson L, Maccario H, Perera NM, Yang X, Spinelli L, Tibarewal P, Glancy B, Gray A, Weijer CJ, Downes CP, Leslie NR (2010) Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene 29:687–697
Poon JS, Eves R, Mak AS (2010) Both lipid- and protein-phosphatase activities of PTEN contribute to the p53-PTEN anti-invasion pathway. Cell Cycle 9:4450–4454
Fountain JW, Bale SJ, Housman DE, Dracopoli NC (1990) Genetics of melanoma. Cancer Surv 9:645–671
Fults D, Pedone C (1993) Deletion mapping of the long arm of chromosome 10 in glioblastoma multiforme. Genes Chromosomes Cancer 7:173–177
Isshiki K, Elder DE, Guerry D, Linnenbach AJ (1993) Chromosome 10 allelic loss in malignant melanoma. Genes Chromosomes Cancer 8:178–184
Herbst RA, Weiss J, Ehnis A, Cavenee WK, Arden KC (1994) Loss of heterozygosity for 10q22–10qter in malignant melanoma progression. Cancer Res 54:3111–3114
Healy E, Rehman I, Angus B, Rees JL (1995) Loss of heterozygosity in sporadic primary cutaneous melanoma. Genes Chromosomes Cancer 12:152–156
Ittmann M (1996) Allelic loss on chromosome 10 in prostate adenocarcinoma. Cancer Res 56:2143–2147
Parmiter AH, Balaban G, Clark WH Jr, Nowell PC (1988) Possible involvement of the chromosome region 10q24–q26 in early stages of melanocytic neoplasia. Cancer Genet Cytogenet 30:313–317
Poetsch M, Lorenz G, Kleist B (2002) Detection of new PTEN/MMAC1 mutations in head and neck squamous cell carcinomas with loss of chromosome 10. Cancer Genet Cytogenet 132:20–24
Tonks NK, Neel BG (1996) From form to function: signaling by protein tyrosine phosphatases. Cell 87:365–368
Yuvaniyama J, Denu JM, Dixon JE, Saper MA (1996) Crystal structure of the dual specificity protein phosphatase VHR. Science 272:1328–1331
Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK (1997) P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA 94:9052–9057
Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3, 4, 5-trisphosphate. J Biol Chem 273:13375–13378
Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP (1999) Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99:323–334
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274
Alvarez-Nunez F, Bussaglia E, Mauricio D, Ybarra J, Vilar M, Lerma E, de Leiva A, Matias-Guiu X (2006) PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid 16:17–23
Garcia JM, Silva J, Pena C, Garcia V, Rodriguez R, Cruz MA, Cantos B, Provencio M, Espana P, Bonilla F (2004) Promoter methylation of the PTEN gene is a common molecular change in breast cancer. Genes Chromosomes Cancer 41:117–124
Ho CM, Lin MC, Huang SH, Huang CJ, Lai HC, Chien TY, Chang SF (2009) PTEN promoter methylation and LOH of 10q22–23 locus in PTEN expression of ovarian clear cell adenocarcinomas. Gynecol Oncol 112:307–313
Kang YH, Lee HS, Kim WH (2002) Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest 82:285–291
Koul D (2008) PTEN signaling pathways in glioblastoma. Cancer Biol Ther 7:1321–1325
Salvesen HB, MacDonald N, Ryan A, Jacobs IJ, Lynch ED, Akslen LA, Das S (2001) PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int J Cancer 91:22–26
Soria JC, Lee HY, Lee JI, Wang L, Issa JP, Kemp BL, Liu DD, Kurie JM, Mao L, Khuri FR (2002) Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res 8:1178–1184
Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T, Holland EC (2009) The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev 23:1327–1337
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH (2010) MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta 411:846–852
Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele G, Rameh L, Loda M, Pandolfi PP (2010) Identification of the miR-106b ~ 25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal 3:ra29
Tamguney T, Stokoe D (2007) New insights into PTEN. J Cell Sci 120:4071–4079
Wang X, Jiang X (2008) Post-translational regulation of PTEN. Oncogene 27:5454–5463
Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP (2007) Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128:141–156
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038
Nardella C, Carracedo A, Salmena L, Pandolfi PP (2010) Faithfull modeling of PTEN loss driven diseases in the mouse. Curr Top Microbiol Immunol 347:135–168
Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS (1998) Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res 58:204–209
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R (1999) Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 96:1563–1568
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP (1998) Pten is essential for embryonic development and tumour suppression. Nat Genet 19:348–355
Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ (2006) Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441:475–482
Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, Wu H, Li L (2006) PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441:518–522
Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436:725–730
Chalhoub N, Baker SJ (2009) PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 4:127–150
Orloff MS, Eng C (2008) Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene 27:5387–5397
Tsao H, Zhang X, Benoit E, Haluska FG (1998) Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene 16:3397–3402
Tsao H, Zhang X, Fowlkes K, Haluska FG (2000) Relative reciprocity of NRAS and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res 60:1800–1804
Tsao H, Goel V, Wu H, Yang G, Haluska FG (2004) Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol 122:337–341
Jonsson G, Dahl C, Staaf J, Sandberg T, Bendahl PO, Ringner M, Guldberg P, Borg A (2007) Genomic profiling of malignant melanoma using tiling-resolution arrayCGH. Oncogene 26:4738–4748
Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, Salmena L, Sampieri K, Haveman WJ, Brogi E, Richardson AL, Zhang J, Pandolfi PP (2010) Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 42:454–458
Shen-Li H, Koujak S, Szablocs M, Parsons R (2010) Reduction of Pten dose leads to neoplastic development in multiple organs of Pten (shRNA) mice. Cancer Biol Ther 10:1194–1200
Kim DK, Myung SJ, Yang SK, Hong SS, Kim KJ, Byeon JS, Lee GH, Kim JH, Min YI, Lee SM, Jeong JY, Song K, Jung SA (2005) Analysis of PTEN gene mutations in Korean patients with Cowden syndrome and polyposis syndrome. Dis Colon Rectum 48:1714–1722
Sato K, Tamura G, Tsuchiya T, Endoh Y, Sakata K, Motoyama T, Usuba O, Kimura W, Terashima M, Nishizuka S, Zou T, Meltzer SJ (2002) Analysis of genetic and epigenetic alterations of the PTEN gene in gastric cancer. Virchows Arch 440:160–165
Nelen MR, van Staveren WC, Peeters EA, Hassel MB, Gorlin RJ, Hamm H, Lindboe CF, Fryns JP, Sijmons RH, Woods DG, Mariman EC, Padberg GW, Kremer H (1997) Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet 6:1383–1387
Keniry M, Parsons R (2008) The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene 27:5477–5485
Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK (2008) Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res 68:5619–5627
Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, Hennighausen L, Wu H (2002) Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development 129:4159–4170
Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H (2003) Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4:209–221
Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW (2001) T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14:523–534
Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, Watanabe S, Mak TW, Nakano T (2004) Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest 113:1774–1783
Tsuruta H, Kishimoto H, Sasaki T, Horie Y, Natsui M, Shibata Y, Hamada K, Yajima N, Kawahara K, Sasaki M, Tsuchiya N, Enomoto K, Mak TW, Nakano T, Habuchi T, Suzuki A (2006) Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer Res 66:8389–8396
Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, Whitsett JA, Mak TW, Nakano T, Nakazato M, Suzuki A (2007) Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest 117:2929–2940
Marsh V, Winton DJ, Williams GT, Dubois N, Trumpp A, Sansom OJ, Clarke AR (2008) Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat Genet 40:1436–1444
Hwang PH, Yi HK, Kim DS, Nam SY, Kim JS, Lee DY (2001) Suppression of tumorigenicity and metastasis in B16F10 cells by PTEN/MMAC1/TEP1 gene. Cancer Lett 172:83–91
Bonneau D, Longy M (2000) Mutations of the human PTEN gene. Hum Mutat 16:109–122
Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen KL, Vinson VL, Gumpper KL, Ellis L, El-Naggar A, Frazier M, Jasser S, Langford LA, Lee J, Mills GB, Pershouse MA, Pollack RE, Tornos C, Troncoso P, Yung WK, Fujii G, Berson A, Steck PA et al (1997) MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res 57:5221–5225
Pollock PM, Walker GJ, Glendening JM, Que Noy T, Bloch NC, Fountain JW, Hayward NK (2002) PTEN inactivation is rare in melanoma tumours but occurs frequently in melanoma cell lines. Melanoma Res 12:565–575
Boni R, Vortmeyer AO, Burg G, Hofbauer G, Zhuang Z (1998) The PTEN tumour suppressor gene and malignant melanoma. Melanoma Res 8:300–302
Reifenberger J, Wolter M, Bostrom J, Buschges R, Schulte KW, Megahed M, Ruzicka T, Reifenberger G (2000) Allelic losses on chromosome arm 10q and mutation of the PTEN (MMAC1) tumour suppressor gene in primary and metastatic malignant melanomas. Virchows Arch 436:487–493
Poetsch M, Dittberner T, Woenckhaus C (2001) PTEN/MMAC1 in malignant melanoma and its importance for tumor progression. Cancer Genet Cytogenet 125:21–26
Abdel-Rahman MH, Yang Y, Zhou XP, Craig EL, Davidorf FH, Eng C (2006) High frequency of submicroscopic hemizygous deletion is a major mechanism of loss of expression of PTEN in uveal melanoma. J Clin Oncol 24:288–295
Robertson GP, Furnari FB, Miele ME, Glendening MJ, Welch DR, Fountain JW, Lugo TG, Huang HJ, Cavenee WK (1998) In vitro loss of heterozygosity targets the PTEN/MMAC1 gene in melanoma. Proc Natl Acad Sci USA 95:9418–9423
Herbst RA, Podewski EK, Mommert S, Kapp A, Weiss J (1999) PTEN and MXI1 allelic loss on chromosome 10q is rare in melanoma in vivo. Arch Dermatol Res 291:567–569
Cairns P, Polascik TJ, Eby Y, Tokino K, Califano J, Merlo A, Mao L, Herath J, Jenkins R, Westra W et al (1995) Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet 11:210–212
Zhou XP, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C (2000) Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol 157:1123–1128
Mirmohammadsadegh A, Marini A, Nambiar S, Hassan M, Tannapfel A, Ruzicka T, Hengge UR (2006) Epigenetic silencing of the PTEN gene in melanoma. Cancer Res 66:6546–6552
Lahtz C, Stranzenbach R, Fiedler E, Helmbold P, Dammann RH (2010) Methylation of PTEN as a prognostic factor in malignant melanoma of the skin. J Invest Dermatol 130:620–622
Mikhail M, Velazquez E, Shapiro R, Berman R, Pavlick A, Sorhaindo L, Spira J, Mir C, Panageas KS, Polsky D, Osman I (2005) PTEN expression in melanoma: relationship with patient survival, Bcl-2 expression, and proliferation. Clin Cancer Res 11:5153–5157
Wang Y, Digiovanna JJ, Stern JB, Hornyak TJ, Raffeld M, Khan SG, Oh KS, Hollander MC, Dennis PA, Kraemer KH (2009) Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci USA 106:6279–6284
Inoue-Narita T, Hamada K, Sasaki T, Hatakeyama S, Fujita S, Kawahara K, Sasaki M, Kishimoto H, Eguchi S, Kojima I, Beermann F, Kimura T, Osawa M, Itami S, Mak TW, Nakano T, Manabe M, Suzuki A (2008) Pten deficiency in melanocytes results in resistance to hair graying and susceptibility to carcinogen-induced melanomagenesis. Cancer Res 68:5760–5768
You MJ, Castrillon DH, Bastian BC, O’Hagan RC, Bosenberg MW, Parsons R, Chin L, DePinho RA (2002) Genetic analysis of Pten and Ink4a/Arf interactions in the suppression of tumorigenesis in mice. Proc Natl Acad Sci USA 99:1455–1460
Nikolaou V, Kang X, Stratigos A, Gogas H, Latorre MC, Gabree M, Plaka M, Njauw C, Kypreou K, Mirmigi I, Stefanaki I, Tsao H (2011) Comprehensive mutational analysis of CDKN2A and CDK4 in Greek patients with cutaneous melanoma. Br J Dermatol. doi:10.1111/j.1365-2133.2011.10551.x
Si L, Kong Y, Xu X, Flaherty KT, Sheng X, Cui C, Chi Z, Li S, Mao L, Guo J (2011) Prevalence of BRAF V600E mutation in Chinese melanoma patients: Large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. doi:10.1016/j.ejca.2011.06.056
Liu L, Lassam NJ, Slingerland JM, Bailey D, Cole D, Jenkins R, Hogg D (1995) Germline p16INK4A mutation and protein dysfunction in a family with inherited melanoma. Oncogene 11:405–412
Flores JF, Walker GJ, Glendening JM, Haluska FG, Castresana JS, Rubio MP, Pastorfide GC, Boyer LA, Kao WH, Bulyk ML, Barnhill RL, Hayward NK, Housman DE, Fountain JW (1996) Loss of the p16INK4a and p15INK4b genes, as well as neighboring 9p21 markers, in sporadic melanoma. Cancer Res 56:5023–5032
Haluska FG, Hodi FS (1998) Molecular genetics of familial cutaneous melanoma. J Clin Oncol 16:670–682
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602
Ikeda T, Yoshinaga K, Suzuki A, Sakurada A, Ohmori H, Horii A (2000) Anticorresponding mutations of the KRAS and PTEN genes in human endometrial cancer. Oncol Rep 7:567–570
Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, Woodman SE, Calderone TC, Ju Z, Lazar AJ, Prieto VG, Aldape K, Mills GB, Gershenwald JE (2009) Integrated molecular and clinical analysis of AKT activation in metastatic melanoma. Clin Cancer Res 15:7538–7546
Nogueira C, Kim KH, Sung H, Paraiso KH, Dannenberg JH, Bosenberg M, Chin L, Kim M (2010) Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene 29:6222–6232
Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW 2nd, DePinho RA (1997) Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev 11:2822–2834
Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr, You MJ, DePinho RA, McMahon M, Bosenberg M (2009) Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 41:544–552
Hocker T, Tsao H (2007) Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat 28:578–588
Mograbi B, Bocciardi R, Bourget I, Busca R, Rochet N, Farahi-Far D, Juhel T, Rossi B (2001) Glial cell line-derived neurotrophic factor-stimulated phosphatidylinositol 3-kinase and Akt activities exert opposing effects on the ERK pathway: importance for the rescue of neuroectodermic cells. J Biol Chem 276:45307–45319
Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M (2002) Regulation of Raf-Akt Cross-talk. J Biol Chem 277:31099–31106
Tsao H, Mihm MC Jr, Sheehan C (2003) PTEN expression in normal skin, acquired melanocytic nevi, and cutaneous melanoma. J Am Acad Dermatol 49:865–872
Singh RS, Diwan AH, Zhang PS, Prieto VG (2007) Phosphoinositide 3-kinase is not overexpressed in melanocytic lesions. J Cutan Pathol 34:220–225
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC (2005) Distinct sets of genetic alterations in melanoma. N Engl J Med 353:2135–2147
Fecher LA, Cummings SD, Keefe MJ, Alani RM (2007) Toward a molecular classification of melanoma. J Clin Oncol 25:1606–1620
Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R (2004) Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116:855–867
Rubinstein JC, Sznol M, Pavlick AC, Ariyan S, Cheng E, Bacchiocchi A, Kluger HM, Narayan D, Halaban R (2010) Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med 8:67
Sun W, Schuchter LM (2001) Metastatic melanoma. Curr Treat Options Oncol 2:193–202
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516
Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N (2006) BRAF mutation predicts sensitivity to MEK inhibition. Nature 439:358–362
Smalley KS, Contractor R, Haass NK, Lee JT, Nathanson KL, Medina CA, Flaherty KT, Herlyn M (2007) Ki67 expression levels are a better marker of reduced melanoma growth following MEK inhibitor treatment than phospho-ERK levels. Br J Cancer 96:445–449
Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, Hanson LJ, Gore L, Chow L, Leong S, Maloney L, Gordon G, Simmons H, Marlow A, Litwiler K, Brown S, Poch G, Kane K, Haney J, Eckhardt SG (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 26:2139–2146
Packer LM, East P, Reis-Filho JS, Marais R (2009) Identification of direct transcriptional targets of (V600E)BRAF/MEK signalling in melanoma. Pigment Cell Melanoma Res 22:785–798
Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS (2003) High frequency of BRAF mutations in nevi. Nat Genet 33:19–20
Wu H, Goel V, Haluska FG (2003) PTEN signaling pathways in melanoma. Oncogene 22:3113–3122
Smalley KS, Flaherty KT (2009) Integrating BRAF/MEK inhibitors into combination therapy for melanoma. Br J Cancer 100:431–435
Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, Santiago-Walker AE, Letrero R, D’Andrea K, Pushparajan A, Hayden JE, Brown KD, Laquerre S, McArthur GA, Sosman JA, Nathanson KL, Herlyn M (2010) Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 18:683–695
Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR, Ribas A, Palma MD, Nathanson KL, Koomen JM, Messina JL, Smalley KS (2011) PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 71:2750–2760
Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, Davies MA (2010) Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res 70:8736–8747
Xing F, Persaud Y, Pratilas CA, Taylor BS, Janakiraman M, She QB, Gallardo H, Liu C, Merghoub T, Hefter B, Dolgalev I, Viale A, Heguy A, De Stanchina E, Cobrinik D, Bollag G, Wolchok J, Houghton A, Solit DB (2011) Concurrent loss of the PTEN and RB1 tumor suppressors attenuates RAF dependence in melanomas harboring (V600E)BRAF. Oncogene. doi:10.1038/onc.2011.250
Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A (2004) Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303:1179–1181
Torres J, Pulido R (2001) The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem 276:993–998
Torres J, Rodriguez J, Myers MP, Valiente M, Graves JD, Tonks NK, Pulido R (2003) Phosphorylation-regulated cleavage of the tumor suppressor PTEN by caspase-3: implications for the control of protein stability and PTEN-protein interactions. J Biol Chem 278:30652–30660
Myers MP, Tonks NK (1997) PTEN: sometimes taking it off can be better than putting it on. Am J Hum Genet 61:1234–1238
Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J (2000) A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol 20:1448–1459
Mahimainathan L, Choudhury GG (2004) Inactivation of platelet-derived growth factor receptor by the tumor suppressor PTEN provides a novel mechanism of action of the phosphatase. J Biol Chem 279:15258–15268
Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK (1998) The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci USA 95:13513–13518
Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA (2004) JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia 18:189–218
Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y (2007) Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128:157–170
Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP (2011) Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144:187–199
Qiao X, Zhang L, Gamper AM, Fujita T, Wan Y (2010) APC/C-Cdh1: from cell cycle to cellular differentiation and genomic integrity. Cell Cycle 9:3904–3912
Gast A, Scherer D, Chen B, Bloethner S, Melchert S, Sucker A, Hemminki K, Schadendorf D, Kumar R (2010) Somatic alterations in the melanoma genome: a high-resolution array-based comparative genomic hybridization study. Genes Chromosomes Cancer 49:733–745
Daniotti M, Oggionni M, Ranzani T, Vallacchi V, Campi V, Di Stasi D, Torre GD, Perrone F, Luoni C, Suardi S, Frattini M, Pilotti S, Anichini A, Tragni G, Parmiani G, Pierotti MA, Rodolfo M (2004) BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene 23:5968–5977
Packer L, Pavey S, Parker A, Stark M, Johansson P, Clarke B, Pollock P, Ringner M, Hayward N (2006) Osteopontin is a downstream effector of the PI3-kinase pathway in melanomas that is inversely correlated with functional PTEN. Carcinogenesis 27:1778–1786
Acknowledgments
This work is funded by research Grants from the Canadian Institutes of Health Research (MOP-84559, MOP-93810 and MOP-110974), Canadian Cancer Society Research Institute (2011-700714) and Canadian Dermatology Foundation to G.L. A.A.T. receives postdoctoral fellowship from Canadian Institutes of Health Research Skin Research Training Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguissa-Touré, AH., Li, G. Genetic alterations of PTEN in human melanoma. Cell. Mol. Life Sci. 69, 1475–1491 (2012). https://doi.org/10.1007/s00018-011-0878-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0878-0