Abstract
It has been known for several decades that cyclic AMP (cAMP), a prototypical second messenger, transducing the action of a variety of G-protein-coupled receptor ligands, has potent immunosuppressive and anti-inflammatory actions. These actions have been attributed in part to the ability of cAMP-induced signals to interfere with the function of the proinflammatory transcription factor Nuclear Factor-kappaB (NF-κB). NF-κB plays a crucial role in switching on the gene expression of a plethora of inflammatory and immune mediators, and as such is one of the master regulators of the immune response and a key target for anti-inflammatory drug design. A number of fundamental molecular mechanisms, contributing to the overall inhibitory actions of cAMP on NF-κB function, are well established. Paradoxically, recent reports indicate that cAMP, via its main effector, the protein kinase A (PKA), also promotes NF-κB activity. Indeed, cAMP actions appear to be highly cell type- and context-dependent. Importantly, several novel players in the cAMP/NF-κB connection, which selectively direct cAMP action, have been recently identified. These findings not only open up exciting new research avenues but also reveal novel opportunities for the design of more selective, NF-κB-targeting, anti-inflammatory drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The omnipresent second messenger 3′-5′-cyclic adenosine monophosphate (cAMP) plays a key role in diverse cellular processes, including energy metabolism, spermatogenesis and steroidogenesis [1]. It was proposed a few decades ago that cAMP is also an important modulator of immune cell function [2]. A wealth of literature is indeed available on the effects of cAMP-modulating agents, either physiological or pharmacological, on multiple aspects of immune function. The overall conclusion of these studies is that cAMP mainly has an anti-inflammatory action, interfering with both innate and adaptive immune responses at multiple levels [3–5].
The inflammatory response is a tightly regulated physiological process, involving orchestrated expression of inflammatory mediators. One of the master regulators of inflammatory gene expression is the transcription factor Nuclear Factor-kappa B (NF-κB) [6]. NF-κB binding to the promoters of a plethora of inflammatory mediators is instrumental in successful coordination of the immune response. Nevertheless, excessive NF-κB activation is associated with the onset and propagation of auto-immune diseases and cancer. Therefore, NF-κB is among the “most wanted” targets for therapeutical intervention. Side effects associated with global inhibition of NF-κB have, however, hampered the clinical use of synthetic NF-κB inhibitors, and the search for more selective modulators of NF-κB action is still in progress [7, 8].
NF-κB activation has been extensively studied in cells triggered with typical proinflammatory stimuli. Yet, in physiological settings, cells are exposed to a multitude of environmental signals that interact with the NF-κB cascade and modulate the outcome of inflammation-associated NF-κB activation. The second messenger cAMP is involved in a plethora of cellular responses, making interactions between this pathway and the NF-κB signaling cascade highly relevant.
In this review, we will provide a concise report on the “anatomy”of the cAMP and NF-κB signaling cascades. Next, we will summarize the state of the art on cAMP/NF-κB interactions, focusing on molecular mechanisms of crosstalk. A more elaborate discussion of the most recent reports on cAMP effects will focus on novel mechanisms that might contribute to a better understanding of the cell type specificity and gene selectivity of these cAMP effects.
Intracellular cAMP: synthesis and effectors
Physiological modulation of intracellular cAMP levels, [cAMP]i, occurs in response to activation of G-protein-coupled receptors (GPCRs), a large family of transmembrane receptors that sense a variety of extracellular signals and transduce them to intracellular signals, hence modulating a plethora of cellular responses [9–12]. According to the classical paradigm of GPCR activation, the encounter of GPCRs with their ligands induces a conformational change that confers the receptor with guanine nucleotide exchange factor (GEF) activity. Ligand-bound GPCRs next activate associated heterotrimeric G-proteins by exchanging their bound GDP for GTP, which induces detachment of the Gα subunit from the β and γ subunits. Several subtypes of Gα proteins exist (Gαs, Gαi, Gαq/11, Gα12/13), which exert different effects on intracellular signaling cascades. Briefly, Gαs and Gαi proteins are connected with the cAMP signaling cascade, whereas the Gαq/11 and Gα12/13 subunits activate phospholipase C and small GTPases, respectively. This description of the cascade of GPCR-induced signaling events is tremendously oversimplified and, in recent years, many sophisticated mechanisms fine-tuning GPCR signaling have been reported [13], the discussion of which is, however, beyond the scope of this review.
The Gαs and Gαi-coupled receptors that modulate [cAMP]i can be triggered by very diverse ligands, including hormones, neurotransmitters, chemokines, lipid mediators and even micro-organisms. Active Gαs and Gαi proteins respectively stimulate or inhibit the activity of the adenylyl cyclases (ACs), the enzymes that catalyze the conversion of ATP to cAMP. To date, ten known AC isoforms with differential cell type-specific expression have been identified [14, 15]. Interestingly, the activity of adenylyl cyclases is not only regulated via interaction with G-proteins but is also modulated in response to growth factors, allowing crosstalk between these pathways [16–18]. Intracellular cAMP levels are furthermore regulated by phosphodiesterases (PDEs), enzymes that catalyze the conversion of cAMP to 5′AMP. The PDEs consist of 11 distinct gene families, which, like the ACs, are expressed in a tissue-specific manner [19]. In short, [cAMP]i is balanced by the opposing actions of adenylyl cyclases and phosphodiesterases. Besides the physiological GPCR ligands, multiple pharmacological tools are available that allow modulation of [cAMP]i. For instance, [cAMP]i can be altered directly using cell permeable, often phosphodiesterase-resistant, cAMP analogues or, indirectly, by more or less selective activators or inhibitors of adenylyl cyclase or phosphodiesterase activity.
The most studied transducer of cAMP signals, the cAMP-dependent protein kinase A (PKA), was identified over 40 years ago [20]. Unactivated PKA resides in the cytoplasm as an inactive tetrameric holoenzyme, which dissociates into two free regulatory and two catalytic subunits upon binding of cAMP to the regulatory subunits. For the catalytic PKA subunits, three isoforms, Cα, Cβ and Cγ, have been described. The liberated active catalytic PKA subunits can phosphorylate serine (ser) and threonine residues on substrate proteins, including the transcription factor cAMP-response element binding protein (CREB) [21, 22]. However, PKA-independent actions of cAMP have been described in several cell types, and evidence for the importance of these PKA-independent pathways in the immunomodulatory effects of cAMP is emerging. Although in many cases the alternative cAMP effectors remain to be identified, the guanine exchange proteins directly activated by cAMP (EPAC-1 and EPAC-2) are possible effector candidates [23, 24]. Upon binding of cAMP, the EPACs elicit downstream responses via activation of the small Ras-like GTPase Rap-1 and, moreover, they have recently been implicated as regulators of monocyte and macrophage function [25–27]. Finally, in specialized tissues such as olfactory neurons, cAMP can directly activate cyclic nucleotide-gated ion channels [28]. Importantly, in addition to cell type-specific expression of PKA, AC and PDE isoforms, intracellular compartmentalization of PKA, ACs and PDEs by A kinase anchoring proteins (AKAPs) adds an extra dimension to the regulation of cAMP effect specificity [29, 30]. It is indeed well accepted that AKAPs compartmentalize cAMP signals to discrete subcellular regions, by tethering PKA and other cAMP regulators to particular cellular organelles, creating “microdomains” of cAMP pools within the cell. Figure 1 provides an illustration of the above-described signaling cascades.
The cAMP signaling cascade. Cyclic AMP is generated by activation of adenylyl cyclases, which convert ATP to cAMP. Physiologically, adenylyl cyclase activity is regulated by GPCRs that are either coupled to Gs or Gi proteins, which respectively stimulate and inhibit adenylyl cyclase activity. Several substances in addition allow pharmacological modulation of adenylyl cyclase activity, such as for instance cholera toxin, which stimulates adenylyl cyclase activity via activating Gs, and forskolin which directly activates adenylyl cyclases. [cAMP]i is, furthermore, negatively regulated by phosphodiesterases which degrade cAMP to 5′AMP. At the center of the canonical cAMP signaling pathway is PKA (1). Briefly, cAMP molecules bind the PKA regulatory subunits of the PKA holoenzyme, which results in release of the two catalytic subunits that subsequently translocate to the nucleus. In the nucleus, the catalytic subunits can phosphorylate different substrates, the best known of which is the transcription factor CREB. Phosphorylated CREB induces the transcription of a plethora of genes harbouring CREB-responsive elements. Alternatively, cAMP can bind to exchange proteins directly activated by cAMP (EPACs) (2). This cascade results in the activation of Rap1
The NF-κB signaling cascade
NF-κB is the generic name of a family of transcription factors that functions as dimers and regulates genes involved in immune, inflammatory and anti-apoptotic responses [6, 31, 32]. The NF-κB proteins are sequestered in the cytoplasm through physical interaction with IκB (inhibitor of κB) family proteins. Today, eight IκB family members are known: IκBα, IκBβ, IκBγ, IκBε, IκBζ, Bcl-3, p100 and p105, all characterized by the presence of ankyrin repeats. Following stimulation with proinflammatory cytokines, pathogen-associated molecular patterns (PAMPs) or upon engagement of the antigen receptors of B- and T-cells, the IκB kinase (IKK), a cytoplasmic kinase complex, becomes activated and phosphorylates the IκB molecules, leading to their subsequent degradation through the ubiquitin–proteasome pathway. NF-κB dimers then translocate to the nucleus where they can bind to κB consensus sequences in the promoters of their target genes. The NF-κB family consists of five members: p65 (RelA), RelB, c-Rel, p50 and p52, which can act as homo- or heterodimers. All NF-κB (or Rel) proteins are characterized by the presence of an N-terminal Rel homology domain (RHD), which mediates dimerization, DNA binding and cytoplasmic retention via its interaction with an IκB protein. Only the p65, c-Rel and RelB NF-κB subunits contain the C-terminal activation domain (TAD) necessary to activate target gene expression. The most abundant, ubiquitously expressed, heterodimer is the p50/p65 combination, which is also most commonly associated with the regulation of inflammatory responses. Although it was hypothesized that particular variations in κB consensus sequences could convey selectivity via preferential DNA binding of specific dimers, it appears NF-κB binds to κB sites in a rather promiscuous fashion, indicating there must be additional levels of control to explain its selectivity [33].
The IKK complex encompasses three known subunits: two protein kinases (IKKα and IKKβ) and a structural/regulatory subunit, called NF-κB essential modulator (NEMO or IKKγ). More recently, a so-called non-canonical pathway has been uncovered, which causes NF-κB activation in response to a specific set of stimuli, including B-cell activating factor (BAFF), lymphotoxin β (LTβ) and CD40 ligand (CD40L). In the canonical pathway, IκB degradation is mediated by the IKKβ kinase. The non-canonical pathway instead depends on IKKα and specifically targets the p100 IκB family member, which preferentially interacts with RelB in the cytoplasm. Following stimulation, p100 is partially degraded to generate p52, thereby liberating p52/RelB NF-κB complexes, that activate a subset of NF-κB-dependent target genes, involved in lymphoid organ formation and B-cell maturation [34].
Although activation of the IKK signalosome and consequent modification and degradation of IκBs provides the cytoplasmic switch for NF-κB activation in both the canonical and non-canonical cascade, several additional regulatory mechanisms have evolved to provide selectivity. For instance, Rel subunits are subject to multiple posttranslational modifications (including phosphorylation, acetylation, ubiquitinylation, sumoylation and nitrosylation), which regulate the transactivating ability and stability of NF-κB complexes, as well as their affinity for particular promoters [35]. NF-κB-dependent gene expression is furthermore regulated by context-dependent interactions with other transcription factors and cofactors, and by the accessibility of the κB binding sites in a given promoter [36–39]. The integration of all regulatory mechanisms at promoters containing NF-κB-binding sites ultimately determines the gene expression signature triggered by a specific stimulus in a specific cell type. A scheme of possible events leading to NF-κB-dependent gene expression is presented in Fig. 2.
The NF-κB signaling cascade. The NF-κB signaling cascade is initiated at the cell membrane and depends on the IKK complex, which, in addition to its γ regulatory subunit, contains two catalytic subunits, IKKα and IKKβ. The canonical NF-κB pathway is triggered by binding of pathogen-associated molecular patterns, cytokines or antigen to their cognate receptors. This leads to phosphorylation and consequent activation of IKKβ, which in turn phosphorylates IκB, leading to its ubiquitination and subsequent proteasomal degradation. IκB degradation exposes the nuclear localization signal of the NF-κB p65 subunit, allowing the NF-κB dimer to translocate to the nucleus, where it can switch on the transcription of its target genes, including among others cytokines, chemokines, enzymes and adhesion molecules involved in orchestrating the inflammatory response. The non-canonical pathway is initiated by extracellular stimuli involved in B-cell maturation and lymphoid organogenesis and depends on IKKα, which is activated by the NF-κB-inducing kinase (NIK). Active IKKα preferentially phosphorylates the p100 IκB family protein, which sequesters RelB in the cytoplasm. Once phosphorylated, p100 is partially degraded to p52 in the ubiquitin–proteasome pathway, allowing translocation of the p52-RelB NF-κB dimer to the nucleus. The p52-RelB NF-κB complex induces the transcription of a distinct set of NF-κB target genes, including chemokines, cytokines and other genes involved in lymphocyte function and lymphoid organogenesis
Mechanisms of NF-κB modulation by cAMP
NF-κB was originally identified as a transcription factor regulating the expression of the κ immunoglobulin light chain in B lymphocytes [40]. As a consequence, the two earliest studies undertaken to assess the effect of cAMP on NF-κB function were performed in the 70Z/3 pre-B cell line [41, 42]. Even in these two early studies, using a straightforward mode of [cAMP]i elevation by means of membrane-permeable cAMP analogues, conflicting results were obtained. Whereas one group found that cAMP could induce NF-κB binding to the κ light chain enhancer and activated a κB reporter gene [42], the other group found no effect of cAMP by itself and reported that cAMP instead inhibits early IL-1β-induced NF-κB, but stimulates late phase NF-κB activation [41]. Many other groups later assessed the effect of pharmacological elevation of [cAMP]i by means of permeable cAMP analogues, adenylyl cyclase activators or phosphodiesterase inhibitors on NF-κB activity in different cell types, using different NF-κB activators and different readouts of NF-κB activation (Table 1). In addition, there is an extensive literature on modulation of (putative) NF-κB-dependent responses by GPCR agonists, some of which might work by modulating [cAMP]i (reviewed in [43]). Although most studies report inhibition of NF-κB activity by cAMP-inducing stimuli, as apparent from Table 1, several papers have, in contrast, found that cAMP positively affects NF-κB activity or that it does not interfere with NF-κB activation. Cyclic AMP modulates NF-κB when activated by typical stimuli, such as proinflammatory cytokines, B-and T-cell activators, PAMPs, and oxidative stress, but also upon triggering of NF-κB by less common activators such as amyloidogenic peptides, thrombin and high glucose. Whereas Table 1 indicates that cAMP inhibits NF-κB largely irrespective of the NF-κB triggering stimulus, a few studies reported stimulus-specific effects of cAMP. For instance, in HUVECs, cAMP inhibited TNF-α-activated NF-κB, but had no effect on NF-κB induced by high glucose [44]. In another example, in human pancreatic cancer cells, cAMP inhibited the activation of NF-κB by LPS and IL-1β, but did not affect PMA-triggered NF-κB [45]. Furthermore, cAMP was reported to inhibit CD3-, but not PMA/A23187-induced NF-κB in T cells [46]. Several other studies, however, did find inhibition of NF-κB in PMA/A23187-activated T cells [47–50], exemplifying the contradictory findings in literature.
Only a few studies have reported positive effects of cAMP on NF-κB activity which became apparent only after prolonged treatment with proinflammatory stimuli [41, 51] and these effects were probably indirect. Moreover, most positive effects of cAMP were reported in cells that were not exposed to typical NF-κB triggers [42, 52–62]. Interestingly, in one of these studies, it was shown that the positive effect on NF-κB was not mediated by the prototypical cAMP effector PKA, but instead was dependent on EPAC activation [53]. Although it is tempting to speculate that differential effects of cAMP could be the result of preferential activation of PKA or EPAC, to date there is very little evidence supporting this theory.
Interactions between cAMP and NF-κB cascades have been described in various cell types, including, among others, diverse leukocyte subsets, fibroblasts, epithelial and endothelial cells, smooth muscle cells and brain cells (Table 1). Some studies have reported cell type-specific effects of cAMP. For instance, cAMP inhibited NF-κB in 3T3 fibroblasts, whereas it induced NF-κB in brown adipocytes [63]. Recently, it was also reported that cAMP enhances TNF-α-induced NF-κB activity in breast cancer cells, whereas it inhibited NF-κB in HEK293 cells [64]. However, inspection of Table 1 does not allow the correlation of effects of cAMP only with particular cell types, as positive and negative effects of cAMP have been described in all tissue types. A more systematic analysis investigating cAMP effects in a given cell type subjected to different NF-κB-activating stimuli or in different cell types treated with the same NF-κB stimulus, which is currently lacking, would be required to allow straightforward conclusions regarding the cell type and stimulus specificity of cAMP/NF-κB crosstalk.
Multiple mechanisms have been proposed to explain how cAMP-mediated signals interfere with the NF-κB signaling cascade. Here, we will summarize the reported mechanisms of molecular crosstalk and discuss current controversies in the literature.
Effects of cAMP on IKK activation and cellular IκB levels
As mentioned in “The NF-κB signaling cascade”, IKK activation is the initial “switch” for triggering NF-κB activation. To our knowledge, the literature does not report on cAMP effects on non-canonical NF-κB activation, which is quite remarkable considering the important role of this cascade in B cells, wherein cAMP/NF-κB crosstalk was initially reported. Hence, all effects described below probably concern IKKβ [65]. Neumann et al. [50] were the first to propose that inhibition of NF-κB in activated T cells by the adenylyl cyclase activator forskolin or prostaglandin E2 (PGE2) was the result of elevation of intracellular IκB levels by cAMP. Since then, this mechanism has been reported in a variety of cell types, using different stimuli to activate both NF-κB and cAMP signaling cascades [44, 45, 66–85]. Conversely, dopamine signaling via the Gi-coupled D4 receptor inhibited IκB expression, probably by reducing [cAMP]i [86], indicating that it is a mechanism that can act in two directions. Whereas the augmentation of cellular IκB levels by cAMP inducers appears to be a common mechanism, some groups did not find cAMP-mediated effects at this level of the NF-κB signaling cascade [87–90]. It should be noted, however, that many investigators did not perform a kinetic analysis of IκB expression levels, which might confound some of the conclusions. In fact, only a few studies have reported rapid inhibition of IκB degradation due to blocking of IKK activity by cAMP [44, 67, 71, 85, 91]. In one study, it was shown that the neurotransmitter serotonin, via the cAMP-inducing 5HT1 receptor, could induce PP2A phosphatase activity, which in turn led to IκB dephosphorylation and inhibition of its degradation [92]. This observation indicates that effects on IκB phosphorylation do not necessarily reflect cAMP-mediated targeting of the IKK kinase. Most studies, however, did not find effects of cAMP at the level of early stimulus-induced IκB degradation, but instead reported enhanced levels of resynthesized IκB [45, 66, 83]. In a few studies, both mechanisms were operative [69, 93]. The mechanisms at the basis of the elevated expression of resynthesized IκB remain largely unresolved. There is some evidence that cAMP enhances IκB resynthesis at the transcriptional level [45, 93]. Other studies rather indicated that increased IκB levels are the result of stabilization at the protein level, but where precisely cAMP intersects the IκB degradation cascade (i.e. via interfering with IκB ubiquitinylation, or by decreasing proteasomal activity) was not addressed [66, 69]. Interestingly, in J774 murine macrophages, cAMP activated IKK, resulting in NF-κB activation instead of inhibition [56]. The cAMP effects on IKK activity were inhibited by the PKA inhibitor H89. However, cAMP also induced protein kinase C (PKC) activity in this study, which might explain the discrepant results. Activation of IKK by cAMP was recently also demonstrated in acute lymphoblastic leukemia cells [94]. The fact that the role of cAMP is often supported solely by the use of pharmacological cAMP/PKA activators/inhibitors, the selectivity of which is disputable, is indeed an important obstacle in the interpretation of many of the reported studies [95, 96]. For instance, in the case of H89, the most widely used PKA inhibitor, it is well established that it also targets other kinases, such as the mitogen- and stress-activated kinase-1 (MSK-1), which happens to be an important regulator of NF-κB activity [97].
Posttranslational modification of Rel proteins induced by cAMP
The prototypical cAMP effector kinase is PKA. In the canonical PKA activation pathway cAMP binds to the regulatory units of the cytosolic PKA tetramer (R2C2), which induces an allosteric change that leads to release of the catalytic subunits. Posttranslational modification of NF-κB by PKA was first described by Zhong et al. [98]. Interestingly, in this study, PKA was activated in a non-canonical fashion that did not involve cAMP. Rather, it was proposed that a subpool of PKA-C, independent of its regulatory subunits, resides in the cytoplasm in complex with IκB and NF-κB. Activation of this NF-κB-associated PKA-C did not depend on cAMP, but required LPS-induced activation of IKK and concomitant degradation of IκB to release the PKA catalytic unit. Importantly, LPS-activated PKA phosphorylated the p65 subunit at its ser 276 residue and this phosphorylation was later reported to be important for the recruitment of the CREB-binding protein (CBP) transcriptional co-activator, which is instrumental for turning on NF-κB-dependent transcription [99]. This cAMP-independent mode of PKA activation has been reported since then by many other groups, in several other cell types, subjected to a variety of NF-κκB-activating stimuli [100–105].
However, several recent studies have reported phosphorylation of p65 at its ser 276 residue in different cell types by cAMP agonists, indicating that cAMP-induced PKA might also target the p65 ser 276 residue, thus enhancing NF-κB transactivation ability [60, 64, 90]. We have also investigated cAMP-dependent phosphorylation of the p65 ser 276 residue upon treatment of different cell types using different cAMP inducers, but were unable to demonstrate phosphorylation of this residue using the currently available phosphospecific anti-phospho-p65 ser 276 antibodies [83, 106]. Importantly, as cAMP-mediated activation of PKA is dependent on the PKA regulatory subunits, it is highly likely that cAMP-dependent phosphorylation of p65 does not proceed via the non-canonical mechanism described by Zhong et al. [98], but will require alternative mechanisms to orchestrate the encounter of the PKA catalytic subunit with p65.
Whether it is cAMP-dependent or not, several observations suggest PKA-dependent activation of NF-κB might have physiological relevance. For instance, it was proposed that blocking of PKA-dependent p65 ser 276 phosphorylation is exploited by the human adenovirus E1A-12 as an immune evasion mechanism [107]. A model has also been suggested in which the glucocorticoid receptor (GR) represses NF-κB p65 by sequestering PKA-Cα, hence preventing PKA-induced p65 phosphorylation [108]. The importance of the p65 ser276 phosphorylation event is furthermore supported by a recent study, showing that knock-in mice, expressing NF-κB p65 with a phosphomimetic mutation of ser 276, were characterized by a severe systemic hyperinflammatory phenotype, due to exaggerated TNF-α production [109]. Nevertheless, phosphorylation of ser 276 of p65 was shown to be important for the expression of only a subset of NF-κB target genes [110, 111].
Although most studies have reported an NF-κB-activating function for p65 ser 276 phosphorylation, this phosphorylation instead promotes the formation of p65/relB heterodimers in TNF-stimulated mouse embryonic fibroblasts (MEFs), ultimately leading to inhibition of RelB DNA binding and consequently reduced expression of RelB target genes [112]. Whether cAMP and PKA are involved in this mechanism remains to be investigated. Recently, a small molecule inhibitor of p65 ser 276 phosphorylation was described, which could be a promising lead for development of novel, selective NF-κB inhibitors [113].
Interestingly, one group reported that PKA-Cα does not phosphorylate p65 at ser 276, but instead activates NF-κB by phosphorylating the p65 C-terminal TAD [89]. PKA-Cα has also been shown to be involved in a TGF-β-induced acetylation of p65, which is also associated with enhanced transactivation. This modification would require a preceding phosphorylation at ser 276, thus allowing docking of CBP/p300, that in turn could acetylate NF-κB p65 at lysine 221 [114, 115]. In addition to PKA-Cα, PKA-Cβ has also been shown to stimulate NF-κB transcriptional activity, by phosphorylating the c-Rel subunit [116]. It is, however, undetermined whether cAMP inducers can mimick this effect.
The idea that cAMP and/or PKA would enhance NF-κB transactivation via posttranslational modification of Rel proteins appears to be in conflict with the widely available indications that cAMP mainly inhibits NF-κB activation. Indeed, cAMP has been shown to block the MEKK/JNK cascade [47], the p38 MAPK [117–120] and PI3 K [121], which all stimulate NF-κB transactivation. In line with these findings, PKA also mediates modifications of other NF-κB subunits that are associated with transcriptional repression. For instance, PKA-Cα was reported to phosphorylate p50 at its ser 337 residue (which is homologous to the p65 ser 276 residue) [107, 122], and this phosphorylation was required for DNA binding of the p50 homodimer, which functions as a repressor complex [122]. In a recent study, it was shown that, in Raw264.7 macrophage-like cells, cAMP-activated PKA also phosphorylates the p105 Rel protein at ser 940 which thus inhibited degradation of p105 to p50, concomitantly reducing TNF-α expression. Interestingly, p105 has been shown to interact with the A kinase-anchoring protein AKAP 95 and this interaction was instrumental for mediating the inhibitory effects of cAMP [90].
A possible explanation for the seemingly conflicting effects of cAMP/PKA on NF-κB activation could lie in the existence of different PKA pools, with distinct subcellular localization and different functions, depending on the composition of the multiprotein complexes of which they are a part. It is indeed well known that the specificity of PKA action, like that of many other signaling enzymes, is regulated at the molecular level by scaffolding, anchoring, and adaptor proteins. Clearly, further research will be required to elucidate the mechanisms that determine in which direction PKA, albeit in a cAMP-dependent or -independent manner, modulates NF-κB activity. Interestingly, a tip of the iceberg was recently revealed in a publication by Gao et al., showing that the nuclear PKA scaffolding protein AKIP-1 (A kinase-interacting protein 1) promotes PKA-dependent p65 ser 276 phosphorylation and nuclear retention of p65, by preferentially targeting PKA to p65-containing promoters [123]. Importantly, they subsequently demonstrated that PKA positively affects p65 transactivation in cells expressing high levels of AKIP-1, whereas PKA oppositely inhibited NF-κB activity in cells containing low levels of AKIP-1, suggesting AKIP-1 functions as a molecular switch determining whether PKA positively or negatively affects NF-κB activity [64].
Effects of cAMP on NF-κB dimer composition
The composition of the NF-κB complex that interacts with a given promoter is an important determinant of the outcome of NF-κB promoter binding. For instance, NF-κB dimers that contain only Rel proteins lacking a TAD, such as the p50-p50 homodimer, often repress gene transcription [124]. Remarkably, very few studies have addressed the effect of cAMP on the composition of the NF-κB dimer. It was shown that the complement protein C1q, by activating the cAMP/PKA pathway, induced the formation of p50 homodimers in peripheral blood mononuclear cells (PBMCs), concomitantly reducing NF-κB transactivation [125]. Cyclic AMP also enhanced the formation of p50 homodimers in LPS-treated Raw264.7 macrophages [90]. In accordance with these findings, PKA promoted p50 homodimer binding to DNA by phosphorylating the p50 ser 337 residue [122]. In another study, cAMP induced binding of the p50-RelB heterodimer to an intronic enhancer of the c-myb proto-oncogene, concomitantly enhancing c-myb expression and dedifferentiation [57]. Finally, it has also been postulated that PKA-mediated phosphorylation of p65 at ser 276 preferentially induces p65 homodimer formation, and that these homodimers selectively activate a subset of NF-κB-dependent genes, including TNF-α [99, 109, 126].
Effects of cAMP on NF-κB-containing enhanceosomes
Efficient transcriptional activation by NF-κB requires the formation of so-called enhanceosomes, containing multiple coactivators and corepressors, other transcription factors and components of the basal transcription machinery [127]. The formation of enhanceosomes at NF-κB target promoters is instrumental in allowing combinatorial control and selectivity in NF-κB-induced gene expression patterns [36]. In this context, a competitive mechanism between the cAMP/PKA-activated transcription factor CREB and NF-κB for binding to limiting amounts of the transcriptional co-activator CBP has often been proposed to account for inhibitory effects of cAMP on NF-κB activity [128]. This model was initially reported by Parry et al., who found that, in human monocytes and endothelial cells, cAMP inhibited NF-κB-dependent gene expression without affecting NF-κB nuclear translocation [129]. Since then, other groups have reported this cofactor competition model in different cellular systems. Using a variety of methods (overexpression studies, co-immunoprecipitation, DNA-affinity purification), it was shown that cAMP/PKA stimulation enhanced the formation of CREB/CBP complexes at the expense of NF-κB/CBP complexes [70, 71, 91, 129–134]. Cyclic-AMP-activated PKA not only reduced the association of p65 with CBP but also blocked the interaction of p65 with the TATA-binding protein (TBP), a component of the basal transcriptional machinery [132, 133]. In contrast, in J774 macrophage cells, cAMP treatment enhanced the interaction of NF-κB and CBP [56]. Additionally, we have recently demonstrated, using chromatin immunoprecipitation (ChIP), that cAMP-activated CREB and TNF-α-activated NF-κB, cooperatively recruited CBP to the endogenous Interleukin-6 (IL-6) promoter in human astrocytes, thus promoting synergistic gene expression. Moreover, this effect was gene-selective, as TNF-induced IL-8 expression was inhibited by cAMP. We would therefore like to nuance the generality of the current CREB/NF-κB competition model and, instead, propose a model in which CREB and NF-κB antagonism or cooperativity for cofactor recruitment can occur, depending on the promoter context. A possible explanation for this promoter selectivity could be the presence of and relative distance between CREB and NF-κB sites in a given promoter. When these sites lie in close proximity (e.g., in the IL-6 promoter) cooperative cofactor recruitment would be preferred over antagonism. Further studies, involving transcriptome analysis combined with ChIP-on-chip or ChIP-sequencing will, however, be required to support this hypothesis.
Cyclic AMP not only affects NF-κB-dependent gene expression by modifying NF-κB’s association with cofactors but also affects the recruitment of other transcription factors to κB-containing gene promoters. For instance, in Raw264.7 and THP-1 cells, cAMP induced the exchange of LPS-induced c-jun for CREB at the TNF-α promoter cAMP-response element (CRE) [135], which resulted in reduced TNF-α expression. Another study reported that, in the TNF-α promoter, a composite CRE/κB response element is present, which binds CREB, c-jun and NF-κB (p65-p50) in LPS-stimulated cells. The cAMP inducer calcitonin gene-related peptide (CGRP) induced the binding of the inducible cAMP early repressor (ICER) protein to this composite CRE/κB response element, reducing TNF-α transcription. Interestingly, the CCL4 promoter contained a similar composite element and, like TNF-α gene expression, CCL4 transcription was inhibited by CGRP [136]. Alternatively, at the IL-12p40 promoter Ets-2 site, combined treatment with LPS and IFN-γ induced the binding of a complex, containing Ets-2, c-Rel and IRF-1. Cyclic AMP elevation by either vasoactive intestinal peptide (VIP) or pituitary adenylate cyclase-activating peptide (PACAP) removed c-Rel and IRF-1 from this complex, concomitantly reducing IL-12p40 expression [131].
Finally, it has recently been shown that cAMP induces the expression of the c-Fos transcription factor, which in turn interacts with NF-κB p65, thereby reducing p65 recruitment to the TNF-α promoter. This observation provided a mechanistic basis for the inhibition of LPS-induced TNF-α expression by cAMP in bone marrow-derived dendritic cells (BMDCs) [137]. Interestingly, c-Fos was shown to interact with NF-κB in a selective manner, removing p65 from the TNF-α but not from the IL-6 gene promoter. The authors proposed that this promoter selectivity was the result of preferential association of c-Fos with p65 homodimers, the NF-κB dimer assumed to be responsible for TNF-α expression, whereas it did not interfere with DNA-binding of the p50–p65 heterodimer, which is the predominant NF-κB complex associating with the IL-6 promoter.
Obviously, the repertoire of actions via which cAMP-dependent signals can affect enhanceosome composition at selected promoters is far from clear. All studies performed to date have assessed the composition of the NF-κB-containing enhanceosomes by gel shift analysis, DNA-affinity purification or ChIP, and these techniques are all hampered by researcher expectancy-introduced bias as well as the availability of antibodies. Future experiments, in which DNA affinity purification is combined with sophisticated modern proteomics, might, however, reveal novel cAMP-dependent alterations in NF-κB enhanceosome composition.
In Table 2, we have summarized the reported effects of cAMP on the expression of selected NF-κB target genes. As evident from the Table, the expression of several prototypical NF-κB target genes, such as, for instance, TNF-α and ICAM-1, appears to be consistently inhibited by cAMP, whereas the effects on other target genes, such as IL-6 and iNOS, are less straightforward. The variability in the reported effects probably reflects the different model systems used and further illustrates the importance of cell type and stimulus specific parameters in determining the outcome of cAMP/NF-κB crosstalk.
Concluding remarks
As evident from the summarized literature, the cAMP-PKA pathway interacts at multiple levels with the NF-κB cascade and the outcome of these interactions at the level of NF-κB-dependent gene expression can be either positive (Fig. 3) or negative (Fig. 4). A recurrent theme in cAMP/NF-κB crosstalk studies is the cell type specificity of many mechanisms. Even when the outcome of [cAMP]i elevation is ultimately the same, very different mechanisms might be at play. On the one hand, some studies explain the inhibitory effects of cAMP on NF-κB function merely by cytoplasmic regulatory mechanisms, that lead to a global inhibition of the NF-κB cascade, due to enhanced IκB action preventing NF-κB to migrate to the nucleus. On the other hand, other studies do not find any effects of cAMP on canonical NF-κB activation, but rather report nuclear “fine tuning” interference of cAMP effectors, that is operative only at selected promoters. Currently, there is no theory that allows reconciling both models, but it could be speculated that the PKA scaffolding proteins, which regulate the intracellular compartmentalization of PKA, as well as of AC and PDE isoforms, might play a noteworthy role.
Mechanisms explaining positive effects of cAMP on NF-κB activity. a cAMP activates PKA, which phosphorylates p65 at its ser 276 residue, leading to enhanced NF-κB transactivation. b At promoters, containing CREB and NF-κB responsive elements in close proximity, both transcription factors co-operatively recruit the CBP co-activator, leading to enhanced NF-κB-dependent gene expression. c In cells expressing AKIP-1, cAMP-activated PKA is targeted to NF-κB-dependent promoters, where it phosphorylates p65 at ser 276, leading to recruitment of CBP and enhanced transcriptional activation. In cells that do not express AKIP-1, PKA preferentially phosphorylates CREB, leading to competition between CREB and NF-κB for CBP and consequently reduced NF-κB-dependent gene expression (see also Fig. 4b). For more detailed information and references related to the mechanisms presented here, we refer to the text “Mechanisms of NF-κB modulation by cAMP”
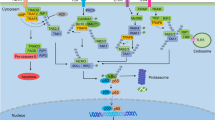
Mechanisms explaining negative effects of cAMP on NF-κB activity. a cAMP inhibits NF-κB activity by elevating cytoplasmic levels of IκB via: 1 inducing CREB-mediated transcription of the IκB gene, 2 blocking IKKβ activity, hence preventing IκB degradation, and 3 enhancing IκB levels by interfering with IκB ubiquitinylation and/or subsequent proteasomal degradation. b cAMP and NF-κB both depend on the limiting cofactor CBP for transcriptional activation of their respective target genes. As elevated [cAMP]i leads to the phosphorylation of CREB, and phosphorylated CREB has a higher affinity for CBP than NF-κB, CBP will preferentially associate with active CREB, enhancing CREB-dependent transcription at the cost of NF-κB-dependent transcription. c cAMP induces the exchange of transactivating NF-κB complexes (i.e. p50–p65) for repressive complexes (i.e. p50–p50). d At certain NF-κB promoters containing CREB responsive elements (i.e. the TNF-α promoter), cAMP induces replacement of transactivating CREB-c-jun complexes at the CRE by repressive CREB-ICER complexes, leading to transcriptional inhibition. e cAMP induces the expression of c-Fos which prevents p65 homodimers from binding to their cognate responsive elements, leading to inhibited transcription of a subset of NF-κB target genes. For more detailed information and references related to the mechanisms presented here, we refer to the text “Mechanisms of NF-κB modulation by cAMP”
Nearly all studies reporting positive effects of cAMP on NF-κB action involve PKA-dependent phosphorylation of the p65 Rel protein at ser 276, leading to the subsequent recruitment of CBP. How this observation can be reconciled with the reported PKA-mediated inhibition of NF-κB activity has puzzled many researchers in the field. Recently, the AKIP1 PKA-interacting protein has been appointed the role of molecular switch, determining whether cAMP positively or negatively affects NF-κB activity. Although this field is still in its infancy, further studies on how PKA-interacting and -anchoring proteins are involved in interconnecting the cAMP and NF-κB signaling cascades will undoubtedly shed light on at least some of the most intriguing questions in this field of study. Importantly, whereas to date all information on the formation of NF-κB/PKA complexes comes from biochemical analyses or microscopical evaluation of fixed cells, major breakthroughs in resolving the spatiotemporal interaction of NF-κB family members with components of the cAMP signaling cascade can be expected from studies using state-of-the art live cell imaging technologies.
Finally, it was formerly assumed that cAMP has a negative effect on the expression of inflammatory mediators. Recent studies, however, indicate cAMP and its main effector PKA instead influence NF-κB-dependent gene expression in a selective manner. Selectivity is probably associated with the particular architecture of certain κB-dependent gene promoters and involves complex combinatorial control depending on enhanceosome composition. Although recent studies have provided some insight into how cAMP accomplishes this selectivity, a systematic analysis is currently lacking and will require more integrated systems biology approaches. For example, combining transcriptome analysis with ChIP-sequencing could reveal how effects of cAMP on the NF-κB-dependent gene expression signature correlate with recruitment of CREB and/or NF-κB to gene promoters, and might identify gene networks and promoter sequences prone to cAMP/NF-κB coregulation.
In summary, although a number of paradoxes concerning cAMP/NF-κB crosstalk remain unsolved to date, and in particular the basis of selectivity of cAMP action warrants further research, we believe cAMP-modulating therapeutical strategies might hold promise for treatment of inflammatory disorders.
References
McKnight GS (1991) Cyclic AMP second messenger systems. Curr Opin Cell Biol 3(2):213–217
Bourne HR, Lichtenstein LM, Melmon KL, Henney CS, Weinstein Y, Shearer GM (1974) Modulation of inflammation and immunity by cyclic AMP. Science 184(132):19–28
Kammer GM (1988) The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today 9(7–8):222–229
Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M (2008) Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 39(2):127–132
Tasken K, Ruppelt A (2006) Negative regulation of T-cell receptor activation by the cAMP-PKA-Csk signalling pathway in T-cell lipid rafts. Front Biosci 11:2929–2939
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27:693–733
Greten FR, Karin M (2004) The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett 206(2):193–199
Karin M (2005) Inflammation-activated protein kinases as targets for drug development. Proc Am Thorac Soc 2(4):386–390 discussion 394–385
Bockaert J, Pin JP (1999) Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J 18(7):1723–1729
Gether U (2000) Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev 21(1):90–113
Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ (1991) Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem 60:653–688
Gilman A (1987) G Proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649
Premont RT, Gainetdinov RR (2007) Physiological roles of G protein–coupled receptor kinases and arrestins. Annu Rev Physiol 69:511–534
Cooper DM (2003) Regulation and organization of adenylyl cyclases and cAMP. Biochem J 375(Pt 3):517–529
Sunahara RK, Taussig R (2002) Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2(3):168–184
Tan CM, Kelvin DJ, Litchfield DW, Ferguson SS, Feldman RD (2001) Tyrosine kinase-mediated ser phosphorylation of adenylyl cyclase. Biochemistry 40(6):1702–1709
Ding Q, Gros R, Gray ID, Taussig R, Ferguson SS, Feldman RD (2004) Raf kinase activation of adenylyl cyclases: isoform-selective regulation. Mol Pharmacol 66(4):921–928
Kawabe J, Iwami G, Ebina T, Ohno S, Katada T, Ueda Y, Homcy CJ, Ishikawa Y (1994) Differential activation of adenylyl cyclase by protein kinase C isoenzymes. J Biol Chem 269(24):16554–16558
Bender AT, Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58(3):488–520
Walsh DA, Perkins JP, Krebs EG (1968) An adenosine 3’, 5’-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem 243(13):3763–3765
Mayr B, Montminy M (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2(8):599–609
Montminy MR, Gonzalez GA, Yamamoto KK (1990) Regulation of cAMP-inducible genes by CREB. Trends Neurosci 13(5):184–188
Bos JL (2003) Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4(9):733–738
de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396(6710):474–477
Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M (2005) Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 174(2):595–599
Bryn T, Mahic M, Enserink JM, Schwede F, Aandahl EM, Tasken K (2006) The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol 176(12):7361–7370
Gerlo S, Verdood P, Kooijman R (2010) Modulation of cytokine production by cyclic adenosine monophosphate analogs in human leukocytes. J Interferon Cytokine Res 30(12):883–891
Kaupp UB, Seifert R (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82(3):769–824
Baillie GS, Scott JD, Houslay MD (2005) Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett 579(15):3264–3270
Dessauer CW (2009) Adenylyl cyclase–A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol 76(5):935–941
Gilmore TD (2006) Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25(51):6680–6684
Gilmore TD, Herscovitch M (2006) Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 25(51):6887–6899
Hoffmann A, Leung TH, Baltimore D (2003) Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO J 22(20):5530–5539
Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M (2001) Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293(5534):1495–1499
Perkins ND (2006) Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 25(51):6717–6730
Vanden Berghe W, Ndlovu MN, Hoya-Arias R, Dijsselbloem N, Gerlo S, Haegeman G (2006) Keeping up NF-kappaB appearances: epigenetic control of immunity or inflammation-triggered epigenetics. Biochem Pharmacol 72(9):1114–1131
Vanden Berghe W, Vermeulen L, Delerive P, De Bosscher K, Staels B, Haegeman G (2003) A paradigm for gene regulation: inflammation, NF-kappaB and PPAR. Adv Exp Med Biol 544:181–196
Natoli G (2006) Tuning up inflammation: how DNA sequence and chromatin organization control the induction of inflammatory genes by NF-kappaB. FEBS Lett 580(12):2843–2849
Natoli G, Saccani S, Bosisio D, Marazzi I (2005) Interactions of NF-kappaB with chromatin: the art of being at the right place at the right time. Nat Immunol 6(5):439–445
Singh H, Sen R, Baltimore D, Sharp PA (1986) A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature 319(6049):154–158
Bomsztyk K, Toivola B, Emery DW, Rooney JW, Dower SK, Rachie NA, Sibley CH (1990) Role of cAMP in interleukin-1-induced kappa light chain gene expression in murine B cell line. J Biol Chem 265(16):9413–9417
Shirakawa F, Chedid M, Suttles J, Pollok BA, Mizel SB (1989) Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol 9(3):959–964
Fraser CC (2008) G protein-coupled receptor connectivity to NF-kappaB in inflammation and cancer. Int Rev Immunol 27(5):320–350
Wu X, Mahadev K, Fuchsel L, Ouedraogo R, Xu SQ, Goldstein BJ (2007) Adiponectin suppresses IkappaB kinase activation induced by tumor necrosis factor-alpha or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab 293(6):E1836–E1844
Kamthong PJ, Wu M (2001) Inhibitor of nuclear factor-kappaB induction by cAMP antagonizes interleukin-1-induced human macrophage-colony-stimulating-factor expression. Biochem J 356(Pt 2):525–530
Watanabe S, Yssel H, Harada Y, Arai K (1994) Effects of prostaglandin E2 on Th0-type human T cell clones: modulation of functions of nuclear proteins involved in cytokine production. Int Immunol 6(4):523–532
Ho HY, Lee HH, Lai MZ (1997) Overexpression of mitogen-activated protein kinase kinase kinase reversed cAMP inhibition of NF-kappaB in T cells. Eur J Immunol 27(1):222–226
Jimenez JL, Punzon C, Navarro J, Munoz-Fernandez MA, Fresno M (2001) Phosphodiesterase 4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-kappaB and nuclear factor of activated T cells activation. J Pharmacol Exp Ther 299(2):753–759
Navarro J, Punzon C, Jimenez JL, Fernandez-Cruz E, Pizarro A, Fresno M, Munoz-Fernandez MA (1998) Inhibition of phosphodiesterase type IV suppresses human immunodeficiency virus type 1 replication and cytokine production in primary T cells: involvement of NF-kappaB and NFAT. J Virol 72(6):4712–4720
Neumann M, Grieshammer T, Chuvpilo S, Kneitz B, Lohoff M, Schimpl A, Franza BR Jr, Serfling E (1995) RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J 14(9):1991–2004
Kikumori T, Kambe F, Nagaya T, Funahashi H, Seo H (2001) Thyrotropin modifies activation of nuclear factor kappaB by tumour necrosis factor alpha in rat thyroid cell line. Biochem J 354(Pt 3):573–579
Chen CC, Chiu KT, Sun YT, Chen WC (1999) Role of the cyclic AMP-protein kinase A pathway in lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages. Involvement of cyclooxygenase-2. J Biol Chem 274(44):31559–31564
Moon EY, Pyo S (2007) Lipopolysaccharide stimulates Epac1-mediated Rap1/NF-kappaB pathway in Raw 264.7 murine macrophages. Immunol Lett 110(2):121–125
Muroi M, Suzuki T (1993) Role of protein kinase A in LPS-induced activation of NF-kappa B proteins of a mouse macrophage-like cell line, J774. Cell Signal 5(3):289–298
Serkkola E, Hurme M (1993) Activation of NF-kappa B by cAMP in human myeloid cells. FEBS Lett 334(3):327–330
Chio CC, Chang YH, Hsu YW, Chi KH, Lin WW (2004) PKA-dependent activation of PKC, p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell Signal 16(5):565–575
Suhasini M, Reddy CD, Reddy EP, DiDonato JA, Pilz RB (1997) cAMP-induced NF-kappaB (p50/relB) binding to a c-myb intronic enhancer correlates with c-myb up-regulation and inhibition of erythroleukemia cell differentiation. Oncogene 15(15):1859–1870
Kleinert H, Euchenhofer C, Ihrig-Biedert I, Forstermann U (1996) In murine 3T3 fibroblasts, different second messenger pathways resulting in the induction of NO synthase II (iNOS) converge in the activation of transcription factor NF-kappaB. J Biol Chem 271(11):6039–6044
Yin F, Wang YY, Du JH, Li C, Lu ZZ, Han C, Zhang YY (2006) Noncanonical cAMP pathway and p38 MAPK mediate beta2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol 40(3):384–393
Yoon C, Korade Z, Carter BD (2008) Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor-kappaB promotes Schwann cell differentiation into a myelinating phenotype. J Neurosci 28(14):3738–3746
Islam KN, Mendelson CR (2002) Potential role of nuclear factor kappaB and reactive oxygen species in cAMP and cytokine regulation of surfactant protein-A gene expression in lung type II cells. Mol Endocrinol 16(6):1428–1440
Wang P, Zhu F, Konstantopoulos K (2010) Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol 298(6):C1445–C1456
Dobashi K, Asayama K, Shirahata A (2003) Differential effects of cyclic AMP on induction of nitric oxide synthase in 3T3–L1 cells and brown adipocytes. Free Radic Biol Med 35(1):94–101
Gao N, Hibi Y, Cueno ME, Asamitsu K, Okamoto T (2010) A-kinase interacting protein 1 (AKIP1) acts as a molecular determinant of the role of PKA in NF-{kappa}B signaling. J Biol Chem. 285(36):28097–28104
DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M (1997) A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388(6642):548–554
Farmer P, Pugin J (2000) beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol 279(4):L675–L682
Manna SK, Aggarwal BB (1998) Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J Immunol 161(6):2873–2880
Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y (2000) Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 102(11):1296–1301
Chong YH, Shin SA, Lee HJ, Kang JH, Suh YH (2002) Molecular mechanisms underlying cyclic AMP inhibition of macrophage dependent TNF-alpha production and neurotoxicity in response to amyloidogenic C-terminal fragment of Alzheimer’s amyloid precursor protein. J Neuroimmunol 133(1–2):160–174
Delgado M, Ganea D (2001) Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol 167(2):966–975
Delgado M, Gonzalez-Rey E, Ganea D (2005) The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol 175(11):7311–7324
Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL (2004) Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J Neuroinflammation 1(1):9
Deree J, Martins JO, Melbostad H, Loomis WH, Coimbra R (2008) Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition. Clinics (Sao Paulo) 63(3):321–328
Li W, Wang T, Ma C, Xiong T, Zhu Y, Wang X (2006) Calcitonin gene-related peptide inhibits interleukin-1beta-induced endogenous monocyte chemoattractant protein-1 secretion in type II alveolar epithelial cells. Am J Physiol Cell Physiol 291(3):C456–C465
Kelschenbach J, Ninkovic J, Wang J, Krishnan A, Charboneau R, Barke RA, Roy S (2008) Morphine withdrawal inhibits IL-12 induction in a macrophage cell line through a mechanism that involves cAMP. J Immunol 180(6):3670–3679
Kim A, Son M, Kim KI, Yang Y, Song EY, Lee HG, Lim JS (2009) Elevation of intracellular cyclic AMP inhibits NF-kappaB-mediated thymosin beta4 expression in melanoma cells. Exp Cell Res 315(19):3325–3335
Kim SY, Seo M, Kim Y, Lee YI, Oh JM, Cho EA, Kang JS, Juhnn YS (2008) Stimulatory heterotrimeric GTP-binding protein inhibits hydrogen peroxide-induced apoptosis by repressing BAK induction in SH-SY5Y human neuroblastoma cells. J Biol Chem 283(3):1350–1361
Chen YJ, Zhang LQ, Wang GP, Zeng H, Lu B, Shen XL, Jiang ZP, Chen FP (2008) Adiponectin inhibits tissue factor expression and enhances tissue factor pathway inhibitor expression in human endothelial cells. Thromb Haemost 100(2):291–300
Garcin G, Le Gallic L, Stoebner PE, Guezennec A, Guesnet J, Lavabre-Bertrand T, Martinez J, Meunier L (2009) Constitutive expression of MC1R in HaCaT keratinocytes inhibits basal and UVB-induced TNF-alpha production. Photochem Photobiol 85(6):1440–1450
Park SY, Lee SW, Shin HK, Chung WT, Lee WS, Rhim BY, Hong KW, Kim CD (2010) Cilostazol enhances apoptosis of synovial cells from rheumatoid arthritis patients with inhibition of cytokine formation via Nrf2-linked heme oxygenase 1 induction. Arthritis Rheum 62(3):732–741
Crisafulli C, Mazzon E, Galuppo M, Paterniti I, Caminiti R, Cuzzocrea S (2010) Olprinone attenuates the development of ischemia/reperfusion injury of the gut. Intensive Care Med 36(7):1235–1247
Hong G, Zhang B, Harbrecht BG (2010) Cyclic AMP inhibits IL-1beta plus IFNgamma-induced NF-kappaB translocation in hepatocytes by a PKA independent mechanism. J Surg Res 159(1):565–571
Spooren A, Kooijman R, Lintermans B, Van Craenenbroeck K, Vermeulen L, Haegeman G, Gerlo S (2010) Cooperation of NFkappaB and CREB to induce synergistic IL-6 expression in astrocytes. Cell Signal 22(5):871–881
Gavrilyuk V, Horvath P, Weinberg G, Feinstein DL (2001) A 27-bp region of the inducible nitric oxide synthase promoter regulates expression in glial cells. J Neurochem 78(1):129–140
Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T (2005) Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur J Immunol 35(1):31–41
Zhen X, Zhang J, Johnson GP, Friedman E (2001) D(4) dopamine receptor differentially regulates Akt/nuclear factor-kappa b and extracellular signal-regulated kinase pathways in D(4)MN9D cells. Mol Pharmacol 60(4):857–864
Hickey FB, Brereton CF, Mills KH (2008) Adenylate cycalse toxin of Bordetella pertussis inhibits TLR-induced IRF-1 and IRF-8 activation and IL-12 production and enhances IL-10 through MAPK activation in dendritic cells. J Leukoc Biol 84(1):234–243
Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N (1996) Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem 271(34):20828–20835
Takahashi N, Tetsuka T, Uranishi H, Okamoto T (2002) Inhibition of the NF-kappaB transcriptional activity by protein kinase A. Eur J Biochem 269(18):4559–4565
Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, Simon MI, Fraser ID (2009) Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95–p105. Sci Signal 2(75):ra28
Delgado M, Ganea D (2001) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit expression of Fas ligand in activated T lymphocytes by regulating c-Myc, NF-kappa B, NF-AT, and early growth factors 2/3. J Immunol 166(2):1028–1040
Hsiung SC, Tin A, Tamir H, Franke TF, Liu KP (2008) Inhibition of 5-HT1A receptor-dependent cell survival by cAMP/protein kinase A: role of protein phosphatase 2A and Bax. J Neurosci Res 86(10):2326–2338
Mustafa SB, Olson MS (1998) Expression of nitric-oxide synthase in rat Kupffer cells is regulated by cAMP. J Biol Chem 273(9):5073–5080
Kloster MM, Naderi EH, Carlsen H, Blomhoff HK, Naderi S (2011) Hyperactivation of NF-kappaB via the MEK signaling is indispensable for the inhibitory effect of cAMP on DNA damage-induced cell death. Mol Cancer 10:45
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351(Pt 1):95–105
Murray AJ (2008) Pharmacological PKA inhibition: all may not be what it seems. Sci Signal 1(22):re4
Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G (2003) Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22(6):1313–1324
Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S (1997) The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89(3):413–424
Zhong H, Voll RE, Ghosh S (1998) Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1(5):661–671
Dulin NO, Niu J, Browning DD, Ye RD, Voyno-Yasenetskaya T (2001) Cyclic AMP-independent activation of protein kinase A by vasoactive peptides. J Biol Chem 276(24):20827–20830
Gambaryan S, Kobsar A, Rukoyatkina N, Herterich S, Geiger J, Smolenski A, Lohmann SM, Walter U (2010) Thrombin and collagen induce a feedback inhibitory signaling pathway in platelets involving dissociation of the catalytic subunit of PKA from an NF-{kappa}B-I{kappa}B complex. J Biol Chem 285(24):18352–18363
Profirovic J, Gorovoy M, Niu J, Pavlovic S, Voyno-Yasenetskaya T (2005) A novel mechanism of G protein-dependent phosphorylation of vasodilator-stimulated phosphoprotein. J Biol Chem 280(38):32866–32876
Sriwai W, Zhou H, Murthy KS (2008) G(q)-dependent signalling by the lysophosphatidic acid receptor LPA(3) in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by Rho kinase and cAMP-independent PKA. Biochem J 411(3):543–551
Vinciguerra M, Hasler U, Mordasini D, Roussel M, Capovilla M, Ogier-Denis E, Vandewalle A, Martin PY, Feraille E (2005) Cytokines and sodium induce protein kinase A-dependent cell-surface Na, K-ATPase recruitment via dissociation of NF-kappaB/IkappaB/protein kinase A catalytic subunit complex in collecting duct principal cells. J Am Soc Nephrol 16(9):2576–2585
Zieger M, Tausch S, Henklein P, Nowak G, Kaufmann R (2001) A novel PAR-1-type thrombin receptor signaling pathway: cyclic AMP-independent activation of PKA in SNB-19 glioblastoma cells. Biochem Biophys Res Commun 282(4):952–957
Spooren A, Kolmus K, Vermeulen L, Van Wesemael K, Haegeman G, Gerlo S (2010) Hunting for ser 276-phosphorylated p65. J Biomed Biotechnol 2010:275892
Guan H, Jiao J, Ricciardi RP (2008) Tumorigenic adenovirus type 12 E1A inhibits phosphorylation of NF-kappaB by PKAc, causing loss of DNA binding and transactivation. J Virol 82(1):40–48
Doucas V, Shi Y, Miyamoto S, West A, Verma I, Evans RM (2000) Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA 97(22):11893–11898
Dong J, Jimi E, Zeiss C, Hayden MS, Ghosh S (2010) Constitutively active NF-kappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory disease. Genes Dev 24(16):1709–1717
Nowak DE, Tian B, Jamaluddin M, Boldogh I, Vergara LA, Choudhary S, Brasier AR (2008) RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol Cell Biol 28(11):3623–3638
Prasad RC, Wang XL, Law BK, Davis B, Green G, Boone B, Sims L, Law M (2009) Identification of genes, including the gene encoding p27Kip1, regulated by ser 276 phosphorylation of the p65 subunit of NF-kappaB. Cancer Lett 275(1):139–149
Jacque E, Tchenio T, Piton G, Romeo PH, Baud V (2005) RelA repression of RelB activity induces selective gene activation downstream of TNF receptors. Proc Natl Acad Sci USA 102(41):14635–14640
Law M, Corsino P, Parker NT, Law BK (2010) Identification of a small molecule inhibitor of ser 276 phosphorylation of the p65 subunit of NF-kappaB using in silico molecular docking. Cancer Lett 291(2):217–224
Ishinaga H, Jono H, Lim JH, Komatsu K, Xu X, Lee J, Woo CH, Xu H, Feng XH, Chen LF, Yan C, Li JD (2009) Synergistic induction of nuclear factor-kappaB by transforming growth factor-beta and tumour necrosis factor-alpha is mediated by protein kinase A-dependent RelA acetylation. Biochem J 417(2):583–591
Ishinaga H, Jono H, Lim JH, Kweon SM, Xu H, Ha UH, Koga T, Yan C, Feng XH, Chen LF, Li JD (2007) TGF-beta induces p65 acetylation to enhance bacteria-induced NF-kappaB activation. EMBO J 26(4):1150–1162
Yu SH, Chiang WC, Shih HM, Wu KJ (2004) Stimulation of c-Rel transcriptional activity by PKA catalytic subunit beta. J Mol Med 82(9):621–628
Li S, Ni Z, Cong B, Gao W, Xu S, Wang C, Yao Y, Ma C, Ling Y (2007) CCK-8 inhibits LPS-induced IL-1beta production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-kappaB pathway. Shock 27(6):678–686
Qi XF, Kim DH, Yoon YS, Li JH, Song SB, Jin D, Huang XZ, Teng YC, Lee KJ (2009) The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF-kappaB in HaCaT keratinocytes. Mol Immunol 46(10):1925–1934
Rahman A, Anwar KN, Minhajuddin M, Bijli KM, Javaid K, True AL, Malik AB (2004) cAMP targeting of p38 MAP kinase inhibits thrombin-induced NF-kappaB activation and ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol 287(5):L1017–L1024
Zhang J, Bui TN, Xiang J, Lin A (2006) Cyclic AMP inhibits p38 activation via CREB-induced dynein light chain. Mol Cell Biol 26(4):1223–1234
Sousa LP, Carmo AF, Rezende BM, Lopes F, Silva DM, Alessandri AL, Bonjardim CA, Rossi AG, Teixeira MM, Pinho V (2009) Cyclic AMP enhances resolution of allergic pleurisy by promoting inflammatory cell apoptosis via inhibition of PI3 K/Akt and NF-kappaB. Biochem Pharmacol 78(4):396–405
Hou S, Guan H, Ricciardi RP (2003) Phosphorylation of ser 337 of NF-kappaB p50 is critical for DNA binding. J Biol Chem 278(46):45994–45998
Gao N, Asamitsu K, Hibi Y, Ueno T, Okamoto T (2008) AKIP1 enhances NF-kappaB-dependent gene expression by promoting the nuclear retention and phosphorylation of p65. J Biol Chem 283(12):7834–7843
Hoffmann A, Natoli G, Ghosh G (2006) Transcriptional regulation via the NF-kappaB signaling module. Oncogene 25(51):6706–6716
Fraser DA, Arora M, Bohlson SS, Lozano E, Tenner AJ (2007) Generation of inhibitory NFkappaB complexes and phosphorylated cAMP response element-binding protein correlates with the anti-inflammatory activity of complement protein C1q in human monocytes. J Biol Chem 282(10):7360–7367
Dong J, Jimi E, Zhong H, Hayden MS, Ghosh S (2008) Repression of gene expression by unphosphorylated NF-kappaB p65 through epigenetic mechanisms. Genes Dev 22(9):1159–1173
Kim TK, Maniatis T (1997) The mechanism of transcriptional synergy of an in vitro assembled interferon-beta enhanceosome. Mol Cell 1(1):119–129
McManus KJ, Hendzel MJ (2001) CBP, a transcriptional coactivator and acetyltransferase. Biochem Cell Biol 79(3):253–266
Parry GC, Mackman N (1997) Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol 159(11):5450–5456
Delgado M (2002) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit CBP-NF-kappaB interaction in activated microglia. Biochem Biophys Res Commun 297(5):1181–1185
Delgado M, Ganea D (1999) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit interleukin-12 transcription by regulating nuclear factor kappaB and Ets activation. J Biol Chem 274(45):31930–31940
Delgado M, Ganea D (2001) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-kappa B-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J Biol Chem 276(1):369–380
Delgado M, Ganea D (2003) Vasoactive intestinal peptide inhibits IL-8 production in human monocytes by downregulating nuclear factor kappaB-dependent transcriptional activity. Biochem Biophys Res Commun 302(2):275–283
Uchiba M, Okajima K, Kaun C, Wojta J, Binder BR (2004) Inhibition of the endothelial cell activation by antithrombin in vitro. Thromb Haemost 92(6):1420–1427
Delgado M, Munoz-Elias EJ, Kan Y, Gozes I, Fridkin M, Brenneman DE, Gomariz RP, Ganea D (1998) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-kB and cAMP response element-binding protein/c-Jun. J Biol Chem 273(47):31427–31436
Harzenetter MD, Novotny AR, Gais P, Molina CA, Altmayr F, Holzmann B (2007) Negative regulation of TLR responses by the neuropeptide CGRP is mediated by the transcriptional repressor ICER. J Immunol 179(1):607–615
Koga K, Takaesu G, Yoshida R, Nakaya M, Kobayashi T, Kinjyo I, Yoshimura A (2009) Cyclic adenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylated c-Fos protein. Immunity 30(3):372–383
Safa M, Zand H, Mousavizadeh K, Kazemi A, Bakhshayesh M, Hayat P (2010) Elevation of cyclic AMP causes an imbalance between NF-kappaB and p53 in NALM-6 cells treated by doxorubicin. FEBS Lett 584(15):3492–3498
Chen D, Rothenberg EV (1994) Interleukin 2 transcription factors as molecular targets of cAMP inhibition: delayed inhibition kinetics and combinatorial transcription roles. J Exp Med 179(3):931–942
Vincenti MP, Burrell TA, Taffet SM (1992) Regulation of NF-kappa B activity in murine macrophages: effect of bacterial lipopolysaccharide and phorbol ester. J Cell Physiol 150(1):204–213
Mandrika I, Muceniece R, Wikberg JE (2001) Effects of melanocortin peptides on lipopolysaccharide/interferon-gamma-induced NF-kappaB DNA binding and nitric oxide production in macrophage-like RAW 264.7 cells: evidence for dual mechanisms of action. Biochem Pharmacol 61(5):613–621
Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C (2006) Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 291(4):G681–G688
Gobejishvili L, Barve S, Joshi-Barve S, McClain C (2008) Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 295(4):G718–G724
Manna SK, Mukhopadhyay A, Aggarwal BB (2000) Human chorionic gonadotropin suppresses activation of nuclear transcription factor-kappa B and activator protein-1 induced by tumor necrosis factor. J Biol Chem 275(18):13307–13314
Shames BD, McIntyre RC Jr, Bensard DD, Pulido EJ, Selzman CH, Reznikov LL, Harken AH, Meng X (2001) Suppression of tumor necrosis factor alpha production by cAMP in human monocytes: dissociation with mRNA level and independent of interleukin-10. J Surg Res 99(2):187–193
Sarkar A, Sreenivasan Y, Manna SK (2003) alpha-Melanocyte-stimulating hormone induces cell death in mast cells: involvement of NF-kappaB. FEBS Lett 549(1–3):87–93
Zhou W, Hashimoto K, Goleniewska K, O’Neal JF, Ji S, Blackwell TS, Fitzgerald GA, Egan KM, Geraci MW, Peebles RS Jr (2007) Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol 178(2):702–710
Sousa LP, Lopes F, Silva DM, Tavares LP, Vieira AT, Rezende BM, Carmo AF, Russo RC, Garcia CC, Bonjardim CA, Alessandri AL, Rossi AG, Pinho V, Teixeira MM (2010) PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3 K/Akt-dependent and NF-{kappa}B-independent manner. J Leukoc Biol 87:895–904
Cavicchi M, Whittle BJ (1999) Potentiation of cytokine induced iNOS expression in the human intestinal epithelial cell line, DLD-1, by cyclic AMP. Gut 45(3):367–374
Hershko DD, Robb BW, Luo G, Hasselgren PO (2002) Multiple transcription factors regulating the IL-6 gene are activated by cAMP in cultured Caco-2 cells. Am J Physiol Regul Integr Comp Physiol 283(5):R1140–R1148
Haddad JJ, Land SC, Tarnow-Mordi WO, Zembala M, Kowalczyk D, Lauterbach R (2002) Immunopharmacological potential of selective phosphodiesterase inhibition. II. Evidence for the involvement of an inhibitory-kappaB/nuclear factor-kappaB-sensitive pathway in alveolar epithelial cells. J Pharmacol Exp Ther 300(2):567–576
Rao Ch V, Li X, Manna SK, Lei ZM, Aggarwal BB (2004) Human chorionic gonadotropin decreases proliferation and invasion of breast cancer MCF-7 cells by inhibiting NF-kappaB and AP-1 activation. J Biol Chem 279(24):25503–25510
Holden NS, Rider CF, Bell MJ, Velayudhan J, King EM, Kaur M, Salmon M, Giembycz MA, Newton R (2010) Enhancement of inflammatory mediator release by beta(2)-adrenoceptor agonists in airway epithelial cells is reversed by glucocorticoid action. Br J Pharmacol 160(2):410–420
Ghersa P, Hooft van Huijsduijnen R, Whelan J, Cambet Y, Pescini R, DeLamarter JF (1994) Inhibition of E-selectin gene transcription through a cAMP-dependent protein kinase pathway. J Biol Chem 269(46):29129–29137
Otsuki M, Saito H, Xu X, Sumitani S, Kouhara H, Kurabayashi M, Kasayama S (2001) Cilostazol represses vascular cell adhesion molecule-1 gene transcription via inhibiting NF-kappaB binding to its recognition sequence. Atherosclerosis 158(1):121–128
Newman WH, Zhang LM, Lee DH, Dalton ML, Warejcka DJ, Castresana MR, Leeper-Woodford SK (1998) Release of tumor necrosis factor-alpha from coronary smooth muscle: activation of NF-kappaB and inhibition by elevated cyclic AMP. J Surg Res 80(2):129–135
Ammit AJ, Hoffman RK, Amrani Y, Lazaar AL, Hay DW, Torphy TJ, Penn RB, Panettieri RA Jr (2000) Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth-muscle cells. Modulation by cyclic adenosine monophosphate. Am J Respir Cell Mol Biol 23(6):794–802
Kim NY, Pae HO, Kim YC, Choi CK, Rim JS, Lee HS, Kim YM, Chung HT (2000) Pentoxifylline potentiates nitric oxide production in interleukin-1beta-stimulated vascular smooth muscle cells through cyclic AMP-dependent protein kinase A pathway. Gen Pharmacol 35(4):205–211
Aizawa T, Wei H, Miano JM, Abe J, Berk BC, Yan C (2003) Role of phosphodiesterase 3 in NO/cGMP-mediated antiinflammatory effects in vascular smooth muscle cells. Circ Res 93(5):406–413
Kaur M, Holden NS, Wilson SM, Sukkar MB, Chung KF, Barnes PJ, Newton R, Giembycz MA (2008) Effect of beta2-adrenoceptor agonists and other cAMP-elevating agents on inflammatory gene expression in human ASM cells: a role for protein kinase A. Am J Physiol Lung Cell Mol Physiol 295(3):L505–L514
Aoki C, Hattori Y, Tomizawa A, Jojima T, Kasai K (2010) Anti-inflammatory role of cilostazol in vascular smooth muscle cells in vitro and in vivo. J Atheroscler Thromb 17(5):503–509
Woo MS, Jang PG, Park JS, Kim WK, Joh TH, Kim HS (2003) Selective modulation of lipopolysaccharide-stimulated cytokine expression and mitogen-activated protein kinase pathways by dibutyryl-cAMP in BV2 microglial cells. Brain Res Mol Brain Res 113(1–2):86–96
Satriano J, Schlondorff D (1994) Activation and attenuation of transcription factor NF-kB in mouse glomerular mesangial cells in response to tumor necrosis factor-alpha, immunoglobulin G, and adenosine 3′:5′-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J Clin Invest 94(4):1629–1636
Grassl C, Luckow B, Schlondorff D, Dendorfer U (1999) Transcriptional regulation of the interleukin-6 gene in mesangial cells. J Am Soc Nephrol 10(7):1466–1477
Combes P, Dickenson JM (2001) Inhibition of NF-kappaB-mediated gene transcription by the human A2B adenosine receptor in Chinese hamster ovary cells. J Pharm Pharmacol 53(8):1153–1156
Kamthong PJ, Wu FM, Wu MC (2000) cAMP attenuates interleukin-1-stimulated macrophage colony-stimulating factor (M-CSF) expression. Biochem J 350(Pt 1):115–122
Harbrecht BG, Taylor BS, Xu Z, Ramalakshmi S, Ganster RW, Geller DA (2001) cAMP inhibits inducible nitric oxide synthase expression and NF-kappaB-binding activity in cultured rat hepatocytes. J Surg Res 99(2):258–264
Batmunkh C, Krajewski J, Jelkmann W, Hellwig-Burgel T (2006) Erythropoietin production: Molecular mechanisms of the antagonistic actions of cyclic adenosine monophosphate and interleukin-1. FEBS Lett 580(13):3153–3160
Yamaguchi K, Kawahara T, Kumakura S, Hua J, Kugimiya T, Nagaoka I, Inada E (2010) Effect of olprinone, a phosphodiesterase III inhibitor, on hepatic ischemiareperfusion injury in rats. Shock 33(4):436–441
Dendorfer U, Oettgen P, Libermann TA (1995) Interleukin-6 gene expression by prostaglandins and cyclic AMP mediated by multiple regulatory elements. Am J Ther 2(9):660–665
Acknowledgments
This work was supported by the Fund for Scientific Research Flanders (FWO). K. Kolmus and A. Spooren are FWO predoctoral fellows and I. Beck is an FWO postdoctoral fellow. S. Gerlo is an FWO postdoctoral fellow and supported by the Ghent University GROUP-ID consortium.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerlo, S., Kooijman, R., Beck, I.M. et al. Cyclic AMP: a selective modulator of NF-κB action. Cell. Mol. Life Sci. 68, 3823–3841 (2011). https://doi.org/10.1007/s00018-011-0757-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0757-8