Abstract
Purpose
Splanchnic artery occlusion (SAO) shock is a severe form of circulatory shock produced by ischemia and reperfusion of the splanchnic organs. The occlusion and reperfusion of the splanchnic arteries causes activation and adhesion of polymorphonuclear neutrophils (PMNs), release of proinflammatory substances and the formation of both species of oxygen and nitrogen derivatives free radicals. Olprinone is a specific phosphodiesterase-III inhibitor that has many properties; one of which is anti-inflammatory actions at therapeutic concentrations clinically used for heart failure. In this study, we wanted to evaluate the pharmacological action of olprinone (a PDEIII inhibitor) on SAO shock in mice.
Methods
SAO shock was induced by clamping both the superior mesenteric artery and the celiac trunk, resulting in a total occlusion of these arteries for 30 min. After this period of occlusion, the clamps were removed. Olprinone was given at a dose of 0.2 mg/kg i.p. 15 min before reperfusion.
Results
Our results indicated that olprinone up-regulated cAMP in injured ileum tissue, and decreased the ileum tissue damage after 1 h of reperfusion in SAO shock mice. Moreover, olprinone decreased NF-κB expression; the nitration of tyrosine residues; the phosphorylation of p38 MAPK and JNK; cytokine production (TNF-α and IL-1β); ICAM-1 and P-selectin expression and apoptosis in the injured ileum.
Conclusions
These results could imply a future use of olprinone in the therapy of ischemia and reperfusion shock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olprinone hydrochloride is a specific phosphodiesterase-III inhibitor developed in Japan and was originally developed as a cardiotonic agent, having positive inotropic and vasodilator actions. It improves myocardial mechanical efficiency [1] via elevation of intracellular cAMP levels in both cardiomyocytes and vascular smooth muscle cells. It also increases myocardial contractility and reduces vascular resistance, leading to an improvement of haemodynamic status [2]. Moreover, olprinone augments cerebral blood flow by its direct vasodilatory effect on the cerebral arteries. The cerebrovascular reactivity to olprinone is markedly observed especially in patients with impaired cerebral circulation [3]. These effects may be mediated by an increase in cyclic AMP content [4]. In addition, olprinone has anti-inflammatory actions at therapeutic concentrations clinically used for heart failure [5].
Splanchnic artery occlusion (SAO) shock is a severe form of circulatory shock produced by ischemia and reperfusion of the splanchnic organs [6–9]. Ischemia progressively damages the cell structures and, following the restoration of blood flow, lesions produced are further exacerbated [10, 11]. Moreover, it is believed that several mediators, such as reactive oxygen species (ROS) [12], pro inflammatory cytokines [13], chemokines [14], adhesion molecules [15], excess nitric oxide, contribute to this injury [16, 17].
Recently, some investigators have reported that olprinone reduces the ischemia and reperfusion (I/R)-induced acute renal injury [18], and decreases elevated concentrations of cytokine-induced neutrophil chemoattractant-1 in septic rats [19]. The present study was performed in order to determine the pharmacological effects of olprinone on IR-induced intestinal injury in mice.
Materials and methods
Animals
The study was carried out in 6 to 8-week-old (20–25 g) male mice CD1 (Harlan Nossan, Italy). The animals were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (DM 116192) as well as with the EEC regulations (OJ of ECL 358/1, 18 December 1986).
Surgical procedures
SAO shock was induced as previously described [20] (see supplemental materials).
Experimental groups
Mice were randomly allocated into the following groups: (1) Sham + vehicle group. Mice were treated with 10% dimethyl sulfoxide (DMSO, 1 ml/kg i.p.), and subjected to the surgical procedure alone, except that the blood vessels were not occluded and the mice were maintained under anaesthesia for the duration of the experiment (N = 10), (2) Sham + olprinone group. Identical to Sham + vehicle group, except for the administration of olprinone (0.2 mg/kg i.p.) 15 min prior to identical surgical procedures (N = 10), (3) I/R + vehicle group. Mice were subjected to SAO shock and were treated with DMSO (N = 10), (4) Olprinone group. Identical to the I/R + vehicle group but were administered with olprinone (0.2 mg/kg i.p.) 15 min prior to reperfusion (N = 10).
In the experiments investigating the survival rates and survival times, the mice (n = 15) from each group) were monitored for 24 h after reperfusion.
The doses of olprinone 1 used here to reduce ischemia/reperfusion injury in the gut were based on previous in vivo studies [21].
Western blot analysis for IκB-α, NF-κB p65, p-ERK, p-JNK, phosho-p38, ERK, cleaved Caspase-3 PDEIIIA, Bax, and Bcl-2
The western blot evaluation was performed as previously described [22] (see supplemental materials).
Histological assessment of damage after SAO shock
Ileum biopsies were taken at 60 min after reperfusion as previously described [20] (see supplemental materials).
Myeloperoxidase activity
Assessment of neutrophil infiltration in the intestinal tissues was performed, as described previously [23, 24].
Malondialdehyde (MDA) measurement
MDA levels in the ileum tissue were determined as an indicator of lipid peroxidation as previously described [25].
Measurement of cAMP in ileum tissue
For measuring cAMP levels, intestine extracted protein was analysed with a cAMP assay kit (R&D System). cAMP levels in ileum tissue were expressed as mg/tissue.
Measurement of intestinal permeability
Intestinal permeability (lumen to plasma) was measured using a 4,000 Da fluorescent dextran (FD4) according to previously described methods [26] (see supplemental materials).
Measurement of cytokines
TNFα and IL-1β levels were evaluated in plasma samples at 60 min after reperfusion. The assay was carried out by using a colorimetric commercial kit (Calbiochem-Novabiochem Corporation, USA).
Immunohistochemical localization of P-selectin, ICAM-1, IL-1β, TNF-α, nitrotyrosine, PAR, Fas ligand, Bax and Bcl-2
The immunohistochemical localization performed as previously described [20] (see supplemental materials).
Terminal Deoxynucleotidyltransferase-Mediated UTP End Labelling (TUNEL) Assay
TUNEL assay was conducted by using a TUNEL detection kit according to the manufacturer’s instructions (Apotag, HRP kit DBA, Milan, Italy, see supplemental materials).
Materials
Unless otherwise stated, all compounds were obtained from the Sigma-Aldrich Company Ltd. (Poole, Dorset, UK). All stock solutions were prepared in non-pyrogenic saline (0.9% NaCl; Baxter, Italy, UK).
Statistical evaluation
All values in the figures and text are expressed as mean ± standard error (SEM) of the mean of n observations. In the experiments involving histology or immunohistochemistry, the figures shown are representative of at least three experiments on the tissue sections collected from all the animals in each group. The results were analysed by one-way ANOVA followed by a Bonferroni post-hoc test for multiple comparisons. A P value less than 0.05 were considered significant and individual group means were then compared with Student’s unpaired t test. A P value of less than 0.05 was considered significant.
Results
Effects of SAO shock on PDEIII expression in gut
Previous studies have demonstrated an important role for PDEIII during ischemia and reperfusion [27]. Thus, we have evaluated PDEIIIA, which is known to be expressed in the mesenteric artery [28] expression in the ileum tissues by western blot analysis. A basal level of PDEIIIA was detected in ileum tissues from sham-operated mice, whereas PDEIIIA levels were substantially increased in the ileum tissues from SAO-shocked mice (Fig. 1a, see densitometry analysis a1).
PDEIIIA expression and effect of olprinone on cAMP level and MAPK signal-transduction pathway after SAO shock. By western blot analysis, a basal level of PDEIIIA was detected in ileum tissues from sham-operated mice, whereas PDEIIIA levels were substantially increased in the ileum tissues from SAO-shocked mice (a, a1). Moreover, in the ileum tissues after 60 min of reperfusion, the levels of cAMP in SAO-shocked mice was significantly reduced when compared with sham-operated mice, while they are increased by olprinone treatment (b). A significant increase of phosphorylated ERK (c, c1), of phosphorylated JNK (d, d1) and of phosphorylated p38 expression (e, e1) was observed in ileum tissues obtained from vehicle-treated animals at 60 min after reperfusion, compared to the sham-treated mice. Olprinone (0.2 mg/kg) treatment resulted in a significant decrease of phosphorylated ERK (c, c1), of phosphorylated JNK (d, d1) and of phosphorylated p38 expression (e, e1). The results in a1, c1, d1, and e1 are expressed as mean ± SEM from n = 5/6 ileum tissues for each group. * P < 0.01 versus sham group. ° P < 0.01 versus SAO shock. ND: not detectable
Effect of olprinone on the cAMP levels in SAO shock
Olprinone has been demonstrated to elevate cAMP levels in liver tissues after IR-induced hepatic injury [21]. To investigate the effect of olprinone on SAO shock, we evaluated the cAMP concentration in the ileum tissues after 60 min of reperfusion. As shown in the Fig. 1b, the reduced levels of cAMP in the ileum from SAO-shocked mice were significantly elevated by olprinone treatment.
Effect of olprinone on MAPK signal-transduction pathway
A significant increase of phosphorylated ERK (p-ERK Fig. 1c, see densitometry analysis c1) as well as of phosphorylated JNK expression (Fig. 1d, see densitometry analysis d1) was observed in ileum tissues obtained from vehicle-treated animals at 60 min after reperfusion. Olprinone (0.2 mg/kg) treatment resulted in a significant decrease of phosphorylated ERK (p-ERK) (Fig. 1c, see densitometry analysis c1) and the of phosphorylated JNK (Fig. 1d, see densitometry analysis d1). In addition, a significant increase of phosphorylated p38 expression was also observed in ileum tissues from vehicle-treated animals (Fig. 1e, see densitometry analysis e1). Olprinone (0.2 mg/kg) treatment decreased the expression of phosphorylated p38 in ileum tissues from vehicle-treated animals (Fig. 1e, see densitometry analysis e1). No expression of phosphorylated ERK, JNK and p38 was detected in ileum samples from sham-treated animals (Fig. 1c, 1d, 1e, see densitometry analysis c1, d1, e1).
Effect of olprinone on IκB-α degradation and NF-κB p65 activation
Basal expression of IκB-α was detected in ileum samples from sham-treated animals, whereas IκB-α levels were substantially reduced in ileum tissues obtained from vehicle-treated animals at 60 min after reperfusion (Fig. 2a, see densitometry analysis a1). Olprinone (0.2 mg/kg) treatment prevented SAO-induced IκB-α degradation (Fig. 2a, see densitometry analysis a1). Moreover, NF-κB p65 levels in the ileum nuclear fractions were also significantly increased at 60 min after reperfusion compared to the sham-treated mice (Fig. 2b, see densitometry analysis b1). Olprinone treatment significantly reduced the levels of NF-κB p65, as shown in Fig. 2b (see densitometry analysis b1).
Effect of olprinone on IκB-α degradation, nuclear NF-κB p65 expression and pro-inflammatory cytokine release in the ileum after SAO shock. Basal expression of IκB-α was detected in ileum samples from sham-treated animals, whereas IκB-α levels were substantially reduced in ileum tissues obtained from vehicle-treated animals at 60 min after reperfusion (a, a1). Olprinone (0.2 mg/kg) treatment prevented SAO shock-induced IκB-α degradation (a, a1). NF-κB p65 levels in the ileum nuclear fractions were also significantly increased at 60 min after reperfusion compared to the sham-treated mice (b, b1). Olprinone treatment significantly reduced the levels of NF-κB p65 (b, b1). A representative blot of lysates obtained from five animals per group is shown and densitometry analysis of all animals is reported. Moreover, tissue levels of cytokines TNF-α (c) and IL-1β (d) increased in samples obtained from SAO mice and when compared with sham-operated mice. Olprinone treatment reduced the tissue levels of TNF-α (a) and IL-1β (b). Moreover, ileum sections taken from SAO shock-treated mice pre-treated with vehicle showed a positive staining for TNF-α (e and i) and IL-1β (g and i). There was a marked reduction in the immunohistochemical localization of TNF-α (f and I) and IL-1β (h and I) in the ileum of SAO shock-treated mice pre-treated with 0.2 mg/kg olprinone. The figure is representative of at least 3 experiments performed on different experimental days. The results in a1, b1 are expressed as mean ± SEM from n = 5/6 ileum tissues for each group. The results in i are expressed as mean ± SEM from n = 10 mice for each group. * P < 0.01 versus sham group. ° P < 0.01 versus SAO shock plus vehicle. ND: not detectable
Effects of olprinone on the release of pro-inflammatory cytokine induced by SAO shock
When compared to sham animals, SAO mice resulted in an increase in the levels of TNF-α and IL-1β in the tissue homogenates (Fig. 2c, d, respectively). The release of TNF-α and IL-1β was significantly attenuated by treatment with olprinone (0.2 mg/kg) (Fig. 2c, d, respectively). Therefore, tissue sections obtained from vehicle-treated animals at 60 min after reperfusion demonstrate positive staining for TNF-α (Fig. 2e, see densitometry analysis i). In contrast, no staining for TNF-α was found in the ileum of SAO mice that had been treated with olprinone (Fig. 2f, see densitometry analysis i). Similarly, at 60 min after reperfusion, positive staining for IL-1β was observed in ileum tissue sections obtained from vehicle-treated animals (Fig. 2g, see densitometry analysis i). Olprinone treatment reduced the degree of IL-1β expression (Fig. 2h, see densitometry analysis i). No staining for either TNF-α (data not shown, see densitometry analysis Fig. 2i) or IL-1β (data not shown, see densitometry analysis Fig. 2I) in ileum tissues obtained from the sham group of mice.
Effect of olprinone on the expression of adhesion molecules and neutrophil infiltration
MPO activity in homogenates of the ileum was significantly elevated after SAO shock in vehicle-treated mice (Fig. 3a). A decrease of MPO activity was observed in the ileum of mice treated with olprinone (0.2 mg/kg) after 60 min of reperfusion (Fig. 3a). Moreover, following 60 min reperfusion, a positive immunohistochemical staining for ICAM-1 (Fig. 3c, see densitometry analysis e) and for P-selectin (Fig. 3g, see densitometry analysis e) was found mainly localised around the vessels in the ileum from SAO-shocked mice, compared to sham-operated mice (Fig. 3b, f, respectively, see densitometry analysis e). The positive immunostaining for ICAM-1 (Fig. 3d, see densitometry analysis e) and for P-selectin (Fig. 3h, see densitometry analysis e) was significantly decreased in sections from mice treated with olprinone (0.2 mg/kg).
Effect of olprinone on the expression of adhesion molecules and neutrophil infiltration. MPO activity, index of PMN infiltration, was significantly elevated at 60 min after SAO shock in vehicle-treated mice (a). Olprinone (0.2 mg/kg i.p.) significantly reduced MPO activity in the ileum (a). Moreover, ileum sections taken from SAO shock-treated mice pre-treated with vehicle showed positive staining for ICAM-1 (c, e), and for P-Selectin (g, e), compared to sham-operated mice (b, f, respectively, and e). The degree of positive staining for adhesion molecules was markedly reduced in tissue sections obtained from mice pre-treated with 0.2 mg/kg olprinone (d, h, respectively, and e). The figure is representative of at least three experiments performed on different experimental days. Data are expressed as mean ± SEM from n = 10 mice for each group. * P < 0.01 versus sham group. ° P < 0.01 versus SAO shock plus vehicle. ND: not detectable
Effects of olprinone on nitrotyrosine formation, lipid peroxidation and PARP activation associated with SAO shock
Immunohistochemical analysis of ileum sections obtained from vehicle-treated mice after SAO shock revealed positive staining for nitrotyrosine (Fig. 4b, see densitometry analysis h). In contrast, no positive staining for nitrotyrosine was found in the ileum of mice, which had been treated with olprinone (0.2 mg/kg) (Fig. 4c, see densitometry analysis h). As shown in Fig. 4g, MDA levels were significantly increased in the ileum of I/R-treated mice, compared to sham-operated mice (Fig. 4g). Lipid peroxidation was significantly attenuated by the intraperitoneal injection of olprinone (Fig. 4g). A positive staining for the PAR, an indicator of PARP activation, (Fig. 4e, see densitometry analysis h) was found primarily localized in the inflammatory cells present in the ileum tissue from SAO mice. Olprinone treatment (0.2 mg/kg) reduced the degree of PARP activation (Fig. 4f, see densitometry analysis h). No staining for either nitrotyrosine (Fig. 4a, see densitometry analysis h) or PAR (Fig. 4d, see densitometry analysis h) in ileum tissues were obtained from the sham group of mice.
Effect of olprinone on SAO-induced nitrotyrosine formation and lipid peroxidation and PARP activation in the ileum. Ileum sections taken from SAO shock-treated mice pre-treated with vehicle showed positive staining for nitrotyrosine, localized mainly in inflammatory cells (b, h), compared to sham-operated mice (a, h). There was a marked reduction in the immunostaining for nitrotyrosine in the ileum of SAO shock-treated mice pre-treated with 0.2 mg/kg olprinone (c, h). Malonyldialdeide (MDA) levels, an index of lipid peroxidation, were significantly increased in ileum tissues 60 min after SAO shock (g). Olprinone (0.2 mg/kg i.p.) significantly reduced the SAO shock-induced elevation of MDA tissues levels (g). Ileum sections taken from SAO shock-treated mice pre-treated with vehicle showed positive staining for PAR (e, h), compared to sham-operated mice (d, h). There was a marked reduction in the immunostaining for PAR in the ileum of SAO shock-treated mice pre-treated with 0.2 mg/kg olprinone (f, h). The figure is representative of at least three experiments performed on different experimental days. Data are expressed as mean ± SEM from n = 10 mice for each group. * P < 0.01 versus sham group. ° P < 0.01 versus SAO shock plus vehicle. ND: not detectable
Olprinone modulates expression of Caspase-3 after SAO shock
No cleaved Caspase-3 expression was detected in ileum tissues obtained from sham-operated animals (Fig. 5a, see densitometry analysis a1). Cleaved Caspase-3 levels were substantially increased in the ileum tissues from SAO shock mice (Fig. 5a, see densitometry analysis a1). On the contrary, olprinone (0.2 mg/kg) treatment prevented the SAO-induced Caspase-3 activation (Fig. 5a, see densitometry analysis a1).
Effect of olprinone on SAO shock-induced Fas ligand expression Caspase-3 activation and on apoptosis as measured by TUNEL-like staining and Bax and Bcl-2 expression in the ileum. Representative western blots showing no cleaved Caspase-3 expression in ileum tissues obtained from sham-treated animals (a, a1). Cleaved Caspase-3 levels were increased in the ileum tissues from SAO shock-treated mice (a, a1). Olprinone (0.2 mg/kg) treatment prevented the SAO shock-induced Caspase-3 activation (a, a1). Positive staining for Fas ligand was observed in ileum sections taken from SAO shock-treated mice pre-treated with vehicle (c), compared to sham-operated mice (b). In contrast, olprinone (0.2 mg/kg) treatment reduced the degree of positive staining for Fas ligand in the ileum tissues (d). Almost no apoptotic cells were observed in the ileum of sham mice (e). Positive TUNEL staining was observed in ileum sections taken from SAO shock-treated mice pre-treated with vehicle (f). In contrast, tissue obtained from SAO shock-treated mice pre-treated with olprinone (0.2 mg/kg) demonstrated no apoptotic cells or fragments (g). Moreover, representative western blots showing no Bax expression in ileum tissues obtained from sham-treated animals (h, h1). Bax levels were increased in the ileum tissues from SAO shock-treated mice (h, h1). Olprinone (0.2 mg/kg) treatment prevented the SAO shock-induced Bax expression (h, h1). A basal level of Bcl-2 expression was detected in ileum tissues from sham-treated mice (i, i1). At 60 min after reperfusion, Bcl-2 expression was significantly reduced (i, i1). Treatment of mice with olprinone (0.2 mg/kg) significantly attenuated SAO shock-induced inhibition of Bcl-2 expression (i, i1). Ileum sections taken from SAO shock-treated mice pre-treated with vehicle showed positive staining for Bax (j) localised mainly in the inflammatory cells. The degree of positive staining for Bax was markedly reduced in ileum sections obtained from mice pre-treated with 0.2 mg/kg olprinone (k). No staining for Bax was observed in ileum tissues obtained from sham-treated animals (l). Positive staining for Bcl-2 was observed in ileum sections taken from sham mice (m). The degree of positive staining for Bcl-2 was markedly reduced in ileum sections obtained from SAO shock mice treated with vehicle (n). Pre-treatment with olprinone 0.2 mg/kg significantly attenuated the reduction in Bcl-2 expression caused by SAO shock (o). The figures are representative of at least three experiments performed on different experimental days. A representative blot of lysates obtained from five animals per group is shown and densitometry analysis of all animals is reported. The results in a1, h1, and i1 are expressed as mean ± SEM from n = 5/6 ileum tissues for each group. * P < 0.01 versus sham group. ° P < 0.01 versus SAO shock. ND: not detectable
Olprinone modulates expression of Fas ligand after SAO shock
Ileum sections from sham-treated mice did not stain for Fas ligand (Fig. 5b, for densitometry analysis see supplemental materials A), whereas ileum sections obtained from SAO shock mice exhibited positive staining for Fas ligand (Fig. 5c, for densitometry analysis see supplemental materials A). Olprinone (0.2 mg/kg) treatment reduced the degree of positive staining for Fas Ligand in the ileum tissues (Fig. 5d, for densitometry analysis see supplemental materials A).
Effects of olprinone on apoptosis in ileum tissues after SAO shock
At 60 min after reperfusion, ileum tissues demonstrated a marked appearance of dark brown apoptotic cells and intercellular apoptotic fragments (Fig. 5e, for cell count see supplemental materials B). In contrast, no apoptotic cells or fragments were observed in the tissues obtained from mice treated with olprinone (0.2 mg/kg) (Fig. 5f, for cell count see supplemental materials B). Similarly, no apoptotic cells were observed in ileum of sham-treated mice (Fig. 5g, for cell count see supplemental materials B).
Effect of olprinone on Bax and Bcl-2 expression
No Bax expression was detected in ileum tissues obtained from sham-operated animals (Fig. 5h, see densitometry analysis h1). Bax levels were substantially increased in the ileum tissues from SAO shock mice (Fig. 5h, see densitometry analysis h1). On the contrary, olprinone (0.2 mg/kg) treatment prevented the SAO-induced Bax expression (Fig. 5h, see densitometry analysis h1). Moreover, a basal level of Bcl-2 expression was detected in ileum tissues from sham-treated mice (Fig. 5i, see densitometry analysis i1). At 60 min after reperfusion, Bcl-2 expression was significantly reduced (Fig. 5i, see densitometry analysis i1). Treatment of mice with olprinone (0.2 mg/kg) significantly attenuated SAO-induced inhibition of Bcl-2 expression (Fig. 5i, see densitometry analysis i1). Therefore, ileum tissues taken from sham-treated mice did not stain for Bax (Fig. 5j, for densitometry analysis see supplemental materials C) whereas ileum sections obtained from SAO shock mice exhibited positive staining for Bax (Fig. 5k, for densitometry analysis see supplemental materials C). Olprinone (0.2 mg/kg) treatment reduced the degree of positive staining for Bax in the ileum of mice subjected to SAO shock (Fig. 5l, for densitometry analysis see supplemental materials C). In addition, ileum sections from sham-treated mice demonstrated positive staining for Bcl-2 (Fig. 5m, for densitometry analysis see supplemental materials C) whereas in SAO treated mice Bcl-2 staining was significantly reduced (Fig. 5n, for densitometry analysis see supplemental materials C). Olprinone (0.2 mg/kg) treatment significantly attenuated the loss of positive staining for Bcl-2 in mice subjected to SAO shock (Fig. 5o, for densitometry analysis see supplemental materials C).
Effect of olprinone on epithelial permeability
There was a massive increase in the intestinal epithelial permeability at 60 min of reperfusion after SAO, as evidenced by a marked increase in the lumen to plasma flux of the fluorescent dye FD 4 (Fig. 6a). Treatment with olprinone reduced the increase in the epithelial permeability during SAO and reperfusion (Fig. 6a).
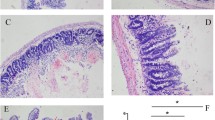
Effect of olprinone on survival and on histological alterations of ileum tissue 60 min after reperfusion. An increase in the intestinal epithelial permeability at 60 min of reperfusion after SAO is evidenced by a marked increase in the lumen to plasma flux of the fluorescent dye FD 4 (a). Treatment with olprinone reduced the increase in the epithelial permeability during SAO and reperfusion (a). Distal ileum section from a sham animal demonstrating the normal architecture of the intestinal epithelium and wall (b). Distal ileum section from a SAO-shocked mouse demonstrating oedema of the distal portion of the villi (c). Distal ileum from olprinone-treated mice shows reduced SAO-induced organ injury (d). See histological score (e). Survival was monitored for 24 h after SAO shock (f). The figure is representative of at least three experiments performed on different experimental days. * P < 0.01 versus sham group. ° P < 0.01 versus SAO shock plus vehicle. ND: not detectable
Effect of olprinone on intestinal injury associated with SAO shock
In vehicle-treated mice, SAO shock resulted in tissue injury mainly localized to the small intestine (Fig. 6c, e) compared to sham-operated mice (Fig. 6b, e). Further histological examination of the tissue demonstrated damage localised to the villi and associated with infiltration of inflammatory cells in the mucosa as well as tissue haemorrhage (Fig. 6c, e). Treatment with olprinone (0.2 mg/kg) given i.p. 15 min before reperfusion, significantly decreased the extent and severity of the histological signs of ileum (Fig. 6d, e).
Effect of olprinone on mortality after SAO shock
To study the clinical situation of mesenteric infarction, mice were subjected to 30 min occlusion followed by reperfusion of the superior mesenteric artery and celiac trunk. At 24 h after reperfusion 90% of the animals had died (Fig 6f). Treatment with olprinone (0.2 mg/kg) reduced the SAO-induced mortality (Fig. 6f).
Discussion
We report here that the pharmacological (mice treated with olprinone) inhibition of PDEIII exerts a protective effect against the pathological changes caused by ischemia/reperfusion injury of the gut. Thus, we propose that PDEIII contributes to the pathophisiology of ischemia/reperfusion injury. What is then the mechanism by which inhibition of PDEIII decreases the intestine inflammation caused by ischemia/reperfusion injury of the gut? First, it is well known that olprinone inhibits PDEIII, the enzyme which is responsible for the degradation of cAMP, leading to an increase in cAMP [27]. In the present study, we have clearly demonstrated that the intestinal ischemia and reperfusion (I/R) induced the expression of PDEIIIA along with decreased cAMP in the ileum. The olprinone treatment attenuated the decrease of cAMP in the ileum tissues.
The second possible mechanism by which olprinone may protect the ileum is as an anti-inflammatory. We found that the levels of TNF-α and IL-1β had significantly decreased in the olprinone-treated groups. This observation is in agreement with previous studies in which have demonstrated that olprinone treatment reduced the generation and release of proinflammatory cytokines [27, 29], it is well known that TNF-α increases endothelial PDE activity and decreases intracellular cAMP [30]. Olprinone inhibits PDEIII, which results in an increase in cAMP. The elevation of endothelial cell cAMP levels inhibits NF-κB activation by targeting p38 mitogen activated protein kinases (MAPK) [31]. In the present study, we have observed an increase of phosphorilated MAPKs (ERK, p38, and JNK) in the ileum from SAO-shocked mice which are significantly reduced by the treatment with olprinone. Furthermore, we report here that SAO shock caused a significant increase in the nuclear expression of p65 in the ileum tissues, whereas treatment with olprinone significantly reduced the p65 expression. Moreover, we also demonstrate that olprinone inhibited IκB-α degradation. Thus, the activity of olprinone on the cAMP levels might account for its effect on NF-κB activation, since it has been shown that cAMP also activates protein kinase A, which inhibits NF-κB [32].
Furthermore, we observed that SAO shock induced the expression of P-selectin and ICAM-1 on endothelial cells. Treatment with olprinone abolished the expression of P-selectin and ICAM-1. These results demonstrate that inhibition of the PDEIII pathway may interrupt the interaction of neutrophils and endothelial cells both at the early rolling phase mediated by P-selectin and at the late firm adhesion phase mediated by ICAM. The absence of an increased expression of the adhesion molecule in the ileum tissue of SAO-shocked rats treated with olprinone correlated with the reduction of leukocyte infiltration and with the attenuation of the ileum tissue damage. Activation and accumulation of leukocytes is one of the initial events of tissue injury due to the release of oxygen free radicals [33]. In the present study, the increased levels of MDA, which is the product of lipid peroxidation, by ischemia/reperfusion were significantly reduced in the olprinone-treated animals probably in part dependent on the observed reduction of neutrophil infiltration into the ileum. Reduction of lipid peroxidation was also paralleled with the inhibition of nitrotyrosine immunoreactivity as an index of nitrosative stress.
Therefore, various studies have demonstrated that PARP activation after single DNA strand breakage induced by reactive oxygen species (ROS) plays an important role in the process of I/R [34, 35]. In this study we confirm the increase of PAR formation in the ileum from SAO-shocked mice as well as that olprinone treatment attenuates PARP activation. Recent studies have also demonstrated that the process of ischemia/reperfusion induces apoptosis in various tissues [36–38]. In this regard, various evidence have demonstrated that the expression of caspase-3 and the interaction of Fas/FasL leads to apoptosis after SAO shock [39]. In this study, we show that SAO shock leads to a substantial expression of caspase-3 and FasL in the ileum tissues which is significantly reduced in mice treated with olprinone. Moreover, we have also demonstrated that treatment with olprinone attenuates the degree of apoptosis, measured by TUNEL, in the ileum. Furthermore, in this study we have identified pro-apoptotic transcriptional changes, including up-regulation of pro-apoptotic Bax and down-regulation of anti-apoptotic Bcl-2. We report in the present study for the first time that the treatment with olprinone significantly reduced the apoptotic cell death after SAO shock, suggesting that protection from apoptosis may be a prerequisite for anti-inflammatory approaches. In particular, we demonstrated that the treatment with olprinone lowers the signal for Bax in treated group when compared with ileum sections obtained from SAO-shocked rats, while on the contrary, the signal is much more express for Bcl-2 in olprinone treated rats than in SAO-shocked mice. Taken together, the results of the present study enhance our understanding of the role of PDEIII in the pathophysiology of ischemia and reperfusion. Our results imply that inhibitors of the activity of PDEIII may be useful in the therapy of ischemia and reperfusion.
References
Mizushige K, Ueda T, Yukiiri K, Suzuki H (2002) Olprinone: a phosphodiesterase III inhibitor with positive inotropic and vasodilator effects. Cardiovasc Drug Rev 20:163–174
Sanada S, Kitakaze M, Papst PJ, Asanuma H, Node K, Takashima S, Asakura M, Ogita H, Liao Y, Sakata Y, Ogai A, Fukushima T, Yamada J, Shinozaki Y, Kuzuya T, Mori H, Terada N, Hori M (2001) Cardioprotective effect afforded by transient exposure to phosphodiesterase III inhibitors: the role of protein kinase A and p38 mitogen-activated protein kinase. Circulation 104:705–710
Ueda T, Mizushige K, Yukiiri K, Nishiyama Y, Kohno M (2004) The cerebrovascular dilatation effects of olprinone, a phosphodiesterase III inhibitor, in comparison with acetazolamide––a pilot study. Clin Neurol Neurosurg 106:284–288
Tajimi M, Ozaki H, Sato K, Karaki H (1991) Effect of a novel inhibitor of cyclic AMP phosphodiesterase, E-1020, on cytosolic Ca++ level and contraction in vascular smooth muscle. Naunyn Schmiedebergs Arch of Pharmacol 344:602–610
Okuda K, Kudo H, Ohishi K, Kitano T, Imasaka H, Noguchi T (1997) Effects of olprinone on IL-6 and IL-10 production during and after cardiac surgery. Masui 46:1580–1584
Altura BM, Gebrewold A, Burton RW (1985) Reactive hyperemic responses of single arterioles are attenuated markedly after intestinal ischemia, endotoxemia and traumatic shock: possible role of endothelial cells. Microcirc Endothelium Lymphatics 2:3–14
Carey C, Siegfried MR, Ma XL, Weyrich AS, Lefer AM (1992) Antishock and endothelial protective actions of a NO donor in mesenteric ischemia and reperfusion. Circ Shock 38:209–216
Zingarelli B, Squadrito F, Ioculano M, Altavilla D, Bussolino F, Campo GM, Caputi AP (1992) Platelet activating factor interaction with tumor necrosis factor and myocardial depressant factor in splanchnic artery occlusion shock. Eur J Pharmacol 222:13–19
Lefer AM, Lefer DJ (1993) Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu Rev Pharmacol Toxicol 33:71–90
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
Parks DA, Granger DN (1986) Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol 250:G749–G753
Masini E, Cuzzocrea S, Mazzon E, Muia C, Vannacci A, Fabrizi F, Bani D (2006) Protective effects of relaxin in ischemia/reperfusion-induced intestinal injury due to splanchnic artery occlusion. Br J Pharmacol 148:1124–1132
Husted TL, Lentsch AB (2006) The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury. Curr Pharm Des 12:2867–2873
Frangogiannis NG (2007) Chemokines in ischemia and reperfusion. Thromb Haemost 97:738–747
Martinez-Mier G, Toledo-Pereyra LH, Ward PA (2000) Adhesion molecules in liver ischemia and reperfusion. J Surg Res 94:185–194
Serracino-Inglott F, Habib NA, Mathie RT (2001) Hepatic ischemia-reperfusion injury. Am J Surg 181:160–166
Ayub K, Serracino-Inglott F, Williamson RC, Mathie RT (2001) Expression of inducible nitric oxide synthase contributes to the development of pancreatitis following pancreatic ischaemia and reperfusion. Br J surg 88:1189–1193
Mizutani A, Murakami K, Okajima K, Kira S, Mizutani S, Kudo K, Takatani J, Goto K, Hattori S, Noguchi T (2005) Olprinone reduces ischemia/reperfusion-induced acute renal injury in rats through enhancement of cAMP. Shock 24:281–287
Miyakawa H, Kira S, Okuda K, Takeshima N, Mori M, Noguchi T (2008) Olprinone decreases elevated concentrations of cytokine-induced neutrophil chemoattractant-1 in septic rats. J Anesth 22:27–31
Roviezzo F, Cuzzocrea S, Di Lorenzo A, Brancaleone V, Mazzon E, Di Paola R, Bucci M, Cirino G (2007) Protective role of PI3-kinase-Akt-eNOS signalling pathway in intestinal injury associated with splanchnic artery occlusion shock. Br J Pharmacol 151:377–383
Yamaguchi K, Kawahara T, Kumakura S, Hua J, Kugimiya T, Nagaoka I, Inada E (2009) Effect of olprinone a phosphodiesterase III inhibitor, on hepatic ischemiareperfusion injury in rats. Shock, Augusta
Genovese T, Esposito E, Mazzon E, Crisafulli C, Paterniti I, Di Paola R, Galuppo M, Bramanti P, Cuzzocrea S (2009) PPAR-? modulate the anti-inflammatory effect of glucocorticoids in the secondary damage in experimental spinal cord trauma. Pharmacol Res 59:338–350
Cuzzocrea S, Zingarelli B, Caputi AP (1998) Role of peroxynitrite and poly (ADP-ribosyl) synthetase activation in cardiovascular derangement induced by zymosan in the rat. Life Sci 63:923–933
Cuzzocrea S, Zingarelli B, Caputi AP (1998) Role of constitutive nitric oxide synthase and peroxynitrite production in a rat model of splanchnic artery occlusion shock. Life Sci 63:789–799
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Cuzzocrea S, Mazzon E, De Sarro A, Caputi AP (2000) Role of free radicals and poly (ADP-ribose) synthetase in intestinal tight junction permeability. Mol Med 6:766–778
Anas C, Ozaki T, Maruyama S, Yamamoto T, Zu Gotoh M, Ono Y, Matsuo S (2007) Effects of olprinone, a phosphodiesterase III inhibitor, on ischemic acute renal failure. Int J Urol 14:219–225
Matsumoto T, Kobayashi T, Kamata K (2003) Alterations in EDHF-type relaxation and phosphodiesterase activity in mesenteric arteries from diabetic rats. Am J Physiol Heart Circ Physiol 285:H283–H291
Zager RA, Johnson AC, Hanson SY, Lund S (2005) Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-alpha generation and systemic release. Am J Physiol 289:F289–F297
Koga S, Morris S, Ogawa S, Liao H, Bilezikian JP, Chen G, Thompson WJ, Ashikaga T, Brett J, Stern DM et al (1995) TNF modulates endothelial properties by decreasing cAMP. Am J Physiol 268:C1104–C1113
Rahman A, Anwar KN, Minhajuddin M, Bijli KM, Javaid K, True AL, Malik AB (2004) cAMP targeting of p38 MAP kinase inhibits thrombin-induced NF-kappaB activation and ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol 287:L1017–L1024
Aizawa T, Wei H, Miano JM, Abe J, Berk BC, Yan C (2003) Role of phosphodiesterase 3 in NO/cGMP-mediated antiinflammatory effects in vascular smooth muscle cells. Circ Res 93:406–413
Salvemini D, Muscoli C, Riley DP, Cuzzocrea S (2002) Superoxide dismutase mimetics. Pulm Pharmacol Ther 15:439–447
Hassa PO, Hottiger MO (2002) The functional role of poly (ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci 59:1534–1553
Giovannelli L, Cozzi A, Guarnieri I, Dolara P, Moroni F (2002) Comet assay as a novel approach for studying DNA damage in focal cerebral ischemia: differential effects of NMDA receptor antagonists and poly (ADP-ribose) polymerase inhibitors. J Cereb Blood Flow Metab 22:697–704
Itoh G, Tamura J, Suzuki M, Suzuki Y, Ikeda H, Koike M, Nomura M, Jie T, Ito K (1995) DNA fragmentation of human infarcted myocardial cells demonstrated by the nick end labeling method and DNA agarose gel electrophoresis. Am J Pathol 146:1325–1331
Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY (1998) Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol 274:G270–G276
Fukuda K, Kojiro M, Chiu JF (1993) Induction of apoptosis by transforming growth factor-beta 1 in the rat hepatoma cell line McA-RH7777: a possible association with tissue transglutaminase expression. Hepatology 18:945–953
Masini E, Cuzzocrea S, Bani D, Mazzon E, Muja C, Mastroianni R, Fabrizi F, Pietrangeli P, Marcocci L, Mondovi B, Mannaioni PF, Federico R (2007) Beneficial effects of a plant histaminase in a rat model of splanchnic artery occlusion and reperfusion. Shock 27:409–415
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crisafulli, C., Mazzon, E., Galuppo, M. et al. Olprinone attenuates the development of ischemia/reperfusion injury of the gut. Intensive Care Med 36, 1235–1247 (2010). https://doi.org/10.1007/s00134-010-1798-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1798-4