Abstract
Background
Chronic kidney disease (CKD) is inherently a complex immune-inflammatory condition, and heightened inflammation and immune dysfunction are closely related to an increased risk of death. However, evidence regarding the relationship between immune-inflammatory levels and all-cause, cardiovascular, and cancer mortality among patients with CKD is scarce.
Methods
Patients with non-dialysis dependent CKD undergoing coronary angiography (CAG) were included from five Chinese tertiary hospitals. Systemic immune inflammation index (SII) was calculated by multiplying peripheral platelet count with neutrophil-to-lymphocyte ratio, and patients were categorized into four groups by SII quartiles. Cox regression models and competing risk Fine and Gray models were used to examining the relationships between SII levels and all-cause, cardiovascular, and cancer mortality.
Results
A total of the 19,327 patients (68.8 ± 10.03 years, female 32.0%) were included in this study. During a median follow-up of 4.5 years, 5,174 deaths occurred, including 2,861 cardiovascular deaths and 375 cancer deaths. Controlling for confounders, all-cause mortality (Q2, Q3, Q4: hazard ratio(HR) [95 CI%] = 1.15 [1.06–1.26], 1.30 [1.19–1.42], 1.48 [1.35–1.62], respectively; p for trend < 0.001) and cardiovascular mortality (Q2, Q3, Q4: HR [95 CI%] = 1.16 [1.03–1.31], 1.40 [1.24–1.58], 1.64 [1.44–1.85], respectively; p for trend < 0.001) increased with higher SII levels, and SII levels was related to cancer mortality comparing last quartile to first quartile of SII (Q2, Q3, Q4: HR [95 CI%] = 1.12 [0.83–1.52], 1.22 [0.90–1.67], 1.50 [1.09–2.08], respectively; p for trend < 0.001).
Conclusion
Elevated immune inflammation level on admission was an independent risk factor for all-cause, cardiovascular, and cancer mortality among CKD patients. Further research is needed to validate the predictive value of SII for mortality risk among CKD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is recognized as a major public health burden with substantial morbidity and mortality [1]. CKD was related to an increased risk of all-cause and cardiovascular mortality [2, 4]. Cancer risk was increased in mild to moderate CKD and among transplant patients, and cancer-related mortality was significantly higher among patients with kidney disease [3]. Therefore, identifying the high-risk patients with CKD is needed in clinical practice.
Traditional cardiovascular disease (CVD) and cancer risk factors are prevalent among patients with CKD but do not fully explain the increased mortality rates among these subjects [3, 5]. Models based on traditional clinical risk factors are poor in predicting long-term prognostic among CKD patients, and there is still a lack of good tools for risk stratification [6]. Previous studies have demonstrated that extending traditional guideline-based clinical risk factors by adding inflammatory biomarkers may improve risk stratification in CVD prevention [7, 8]. In addition, CKD subjects are at a higher risk of developing cancer due to heightened inflammation and immune dysfunction [9,10,11]. Persistent low-grade inflammation and immune dysfunction are now considered a hallmark feature of CKD, being involved in the mortality of these patients [12]. Recently, systemic immune inflammation index (SII), which combines neutrophil counts, lymphocyte counts, and platelet counts, is recently proposed as a prognostic indicator comprehensively reflecting patients' inflammatory and immune status [13]. Previous studies have demonstrated that SII is not only associated with adverse outcomes in subjects with CVD [14], but also a potential superior marker in predicting death among different cancer patients [15]. However, the SII for CKD has not been reported to date, and little is known about its prognostic value for CKD.
Therefore, the objective of this multi-center research was to reveal the prognostic impact of immune inflammation levels on all-cause, cardiovascular, and cancer mortality in a large cohort, which may help clinicians early recognize patients at high risk for mortality and initiate the prompt, appropriate treatment for prevention.
Method
Data sources and patient selection
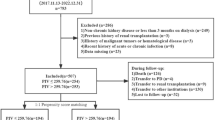
This cohort study analyzed data from the Cardiorenal Improvement II (CIN-II) study, which is a multi-center cohort of patients enrolled at five large tertiary hospitals (Cardiorenal Improvement II, ClinicalTrials.gov NCT05050877) in China between January 2007 and December 2020. A total of 22,898 patients diagnosed with CKD (estimated glomerular filtration rates [eGFR] < 60 mL/min/1.73m2) undergoing coronary angiography (CAG) on initial admission were enrolled in this multi-center cohort. The exclusion criteria were as follows: (a) patients missing laboratory parameters; (b) patients receiving dialysis or those who were unclear to have received it or not; (c) patients missing survival information; (d) patients aged < 18 years; (e) patients have known cancer (Supplementary Fig. 1). The Ethics Committee of the Guangdong Provincial People's Hospital approved the study (No.GDREC2019-555H-2). All participating sites received institutional review board approval from their own ethics committees. It was conducted in accordance with the principles of the Declaration of Helsinki.
Baseline data collection
The patient data were extracted from the electronic clinical management system (ECMS). The baseline data included demographic characteristics, complications, procedures, laboratory examinations, and medications at discharge. Biochemistry data including platelets, neutrophils, lymphocytes, and serum creatinine (Scr) were tested on initial admission. Survival information was obtained by cause-specific surveillance data from the Public Security System and Centers for Disease Control and Prevention, recorded and matched with the ECMS for all participant centers.
Clinical definition and endpoint
The endpoints were all-cause, cardiovascular and cancer mortality (the median follow-up period was 4.5 years [interquartile range: 2.4–7.5]). SII was calculated as total platelet counts × neutrophil-to-lymphocyte ratio. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [16], and CKD was defined as eGFR < 60 mL/min/1.73 m2 [17]. Congestive heart failure (CHF) was defined as New York Heart Association class > 2 or Killip class > 1. Anemia was defined as a hematocrit ≤ 39% for males or ≤ 36% for females. Acute myocardial infarction (AMI), diabetes mellitus (DM), and hypertension (HT) were defined according to the 10th Revision Codes of the International Classification of Diseases (ICD-10).
Statistical analysis
The population was categorized by SII quartiles: quartile (Q)1 > 420.09, Q2 > 420.09 and < 646.91, Q3 > 646.91 and < 1063.39, Q4 > 1063.39. For the baseline characteristics, continuous variables were summarized as median and quartiles or mean and standard deviation as appropriate. Categorical variables were described as frequencies and percentages. Student t tests and non-parametric tests (Kruskal–Wallis) were used to compare normally and non-normally distributed variables, respectively. Differences in categorical variables were compared using the chi-square test. To test the relationship between all-cause, cardiovascular, and cancer mortality and SII levels as a continuous variable, restricted cubic splines (RCS) analyses were conducted. Time-to-event data were presented graphically using Kaplan–Meier curves and compared with a log-rank test. The association between SII levels and all-cause mortality was assessed by multivariate Cox regression models, unadjusted and adjusted for age, gender, anemia, AMI, CHF, DM, HT, stroke, percutaneous interventions (PCI), eGFR, low-density lipoprotein cholesterol (LDL-C), angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ACEI_ARB), beta-blocker, calcium channel blocker (CCB), statins. A variance inflation factor ≥ 5 can be considered as a criterion for multicollinearity [18], suggesting there was no synteny problem among the variables. To better visually depict the influence of the competing risk, we generated cumulative incidence function (CIF) curves for cardiovascular and cancer mortality. Gray's tests were used to assess differences between cardiovascular and cancer mortality. For cause-specific mortality analyses, we further excluded individuals with unknown causes of mortality. The associations of cardiovascular and cancer mortality with SII levels were examined using competing risk Fine and Gray models, unadjusted and adjusted for the same confounders. Mortalities due to other causes were treated as competing risks. Subgroup analyses were also performed to explore the source of heterogeneity according to age [(≥ 65 versus < 65 years of age], gender, CAD, and anemia. Stratification and interaction analyses showed consistency with the main results. To assess whether the accuracy of predicting long-term prognosis would improve after adding SII to a traditional clinical risk factors model (age, gender, HT, DM, CAD, AMI, CHF, stroke, anemia, and low-density lipoprotein cholesterol (LDLC), the C-index, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated. NRI assesses the ability of a model including a new prognostic marker to more accurately reclassify individuals into higher or lower risk (of death) categories compared to traditional clinical risk factor models. The IDI was used to estimate the discrimination improvement in average sensitivity and any potential increase in average 1-specificity [19]. Missing values in the candidate predictor variables were imputed using multivariate imputations by the chained equation method. Data management and statistical analyses were performed by R version 4.0.3. We considered a two-sided P value < 0.05 statistically significant.
Results
Baseline characteristics
Among the 19,327 patients with CKD (the mean age 68.8 ± 10.03 years, 32.0%were women) were included. All subjects were categorized into four groups according to SII levels at baseline: Q1 group (n = 4831), Q2 group (n = 4832), Q3 group (n = 4832) and Q4 group (n = 4832). As baseline SII levels increase, patients are more likely to combine with AMI, CHF, and anemia. On the contrary, higher SII levels were related to lower levels of HGB and eGFR. More data on the baseline characteristics of the study population are shown in Table 1.
With a median follow-up of 4.5 years, 5174 (26.77%) participants died of all causes, of which, 2861 (14.80%) were cardiovascular-specific deaths, and 375 (1.94%) were cancer-specific deaths. The all-cause mortality of Q1 group vs. Q2 group vs. Q3 group vs. Q4 group was 22.9 vs. 24.9% vs. 26.8 vs. 32.4% (p for trend < 0.001). Correspondingly, the rates for cardiovascular and cancer mortality were 12.0 vs. 13.3% vs. 15.3 vs. 18.6% (p for trend < 0.001) and 1.8 vs. 1.9% vs. 1.9 vs. 2.2% (p for trend < 0.001) (Fig. 1).
Systemic immune inflammation index and clinical outcomes
When SII as a continuous variable, we performed multivariable regression analysis and it was significantly associated with all-cause, cardiovascular and cancer mortality (all-cause mortality: adjusted hazard ratio [aHR], 1.20, 95% confidence interval (CI): 1.14–1.26, p < 0.001; cardiovascular mortality: aHR, 1.27, 95% CI 1.19–1.36, p < 0.001; cancer mortality: aHR, 1.23, 95% CI 1.04–1.47, p < 0.001) (Table 2). Subsequently, we completed univariate and multivariate regression analysis as a categorical variable to further verify the association of SII levels with the increased risk of total or cause-specific death. After adjusting for confounders, all-cause mortality (Q2, Q3, Q4: HR [95 CI%] = 1.15 [1.06–1.26], 1.30 [1.19–1.42], 1.48 [1.35–1.62], respectively; p for trend < 0.001) and cardiovascular mortality (Q2, Q3, Q4: HR [95 CI%] = 1.16 [1.03–1.31], 1.40 [1.24–1.58], 1.64 [1.44–1.85], respectively; p for trend < 0.001) increased with higher levels of SII. And SII levels were related to an increased risk of cancer mortality comparing the highest quartile to the lowest quartile of SII (Q2, Q3, Q4: HR [95 CI%] = 1.12 [0.83–1.52], 1.22 [0.90–1.67], 1.50 [1.09–2.08], respectively; p for trend < 0.001) (Table 2). Kaplan–Meier curves for all-cause mortality and cumulative incidence function curves for cardiovascular mortality manifested that the highest quartile group displayed the worst all-cause and cardiovascular death in a significant SII levels-dependent manner between four groups. While the highest quartile group showed a significantly increased cancer mortality (Fig. 2). In addition, RCS analysis demonstrated a nearly linear increase in the risk of all-cause mortality (nonlinear p = 0.160) and cardiovascular mortality (nonlinear p = 0.229) as the SII increased. But there is a nonlinear relationship between cancer mortality and SII levels (nonlinear p = 0.032): SII evidently increased the risk of cancer death at relatively high levels (Fig. 3).
Discrimination and calibration analysis of SII added to traditional risk factors model
The addition of the continuous variable SII to the clinical risk factors model did contribute to a certain increase in C-statistics, which ranged from 0.613 to 0.626 (p = 0.0034) for all-cause mortality and 0.617 to 0.628 (p = 0.0194) for cardiovascular mortality. Reclassification adding SII also displayed an IDI of 0.0014 (p < 0.0001) with 8.3% improvement in NRI (p < 0.0001) in all-cause mortality and cardiovascular mortality (NRI; 0.0743, p = 0.0005; IDI: 0.0003, p = 0.0417), but no significant improvement in cancer mortality (C-statistics:0.618 to 0.620, p = 0.3017, NRI; 0.1162, p = 0.0291; IDI: 0.0001, p = 0.3715) (Table 3).
Subgroup analysis
When results were stratified by age, gender, anemia, and CAD, a similar prognostic impact on all-cause mortality of different SII levels was found for all the subgroup analyses except for female patients (Supplementary Fig. 2). As concerns the association of SII levels with cardiovascular and cancer mortality stratified by the aforementioned variables, the results demonstrated that there is a consistent effect in subjects (males, anemia, CAD, non-CAD, and regardless of age) for cardiovascular mortality (Supplementary Fig. 3), while there is a consistent effect in patients (youngers, males, anemia, and non-CAD) for cancer mortality. Whereas stratification of inflammation levels was not significantly associated with cancer death in CAD patients (Supplementary Fig. 4). Stratified and interaction analyses demonstrated the association of SII levels with cardiovascular and cancer mortality was evident in gender (p for interaction = 0.004), and age (p for interaction < 0.001), respectively.
Discussion
To our knowledge, this is the first largest multicenter, longitudinal cohort study to report the associations of immune inflammation levels on admission with the risk of all-cause, cardiovascular, and cancer mortality among CKD patients. The results indicated that the highest quartile group showed the worst all-cause and cardiovascular mortality in a significant SII levels-dependent manner among the four groups, while there was a significant impact on cancer mortality when SII was at a high level. Moreover, adding SII to a traditional risk factors model improved the clinical prediction of long-term prognosis. Our findings suggested it is important to account for SII levels in the assessment of mortality risk. Monitoring SII levels may be considered as one focus of reduced mortality efforts in subjects with CKD.
The Global Burden of Disease studies have shown that CKD has emerged as a leading cause of worldwide mortality [20, 21]. The mechanisms of increased mortality among CKD are complicated and involve multiple pathophysiologic alterations, of which, heightened inflammation and immune dysfunction may play an important role in this process [22, 23]. It has been reported that increased levels of inflammatory markers are associated with higher mortality. Elevated platelet levels are related to higher mortality and risk of cardiovascular events, and platelet count may be a useful marker for further stratification of cardiovascular risk, and especially of mortality [24]. In addition, previous research demonstrated that blood neutrophil count may play an important role in accelerating atherosclerosis, which is related to all-cause and cardiovascular mortality in apparently healthy men [25]. Meanwhile, Christelle et al. found that elevated lymphopenia levels are an independent prognostic factor for overall survival (OS), which is conducive to risk stratification when assessing OS among pancreatic cancer subjects [26]. SII, a new and widely available hematologic marker of inflammation, had a superior ability to predict the risk of poor outcomes among subjects with cancer and cardiovascular disease [14, 15]. Our previous studies also have displayed that elevated SII levels were an independent prognostic factor for all-cause mortality following CAG [27]. However, the relationship between SII levels and prognosis among the CKD population is not yet clear. This research indicated that higher SII levels were closely associated with the increased all-cause, cardiovascular, and cancer mortality among CKD subjects, and adding SII to the traditional risk factors model could improve predictive performance. Therefore, it is necessary to monitor SII levels among CKD patients, which could significantly improve long-term prognosis.
The findings in the current study demonstrated that more than half of deaths in patients with CKD are due to cardiovascular disease, and elevated SII levels were a significant risk factor for cardiovascular-specific deaths. CKD continually leads to chronic impairment of cardiac function, and the communication between the heart and kidney occurs through different mechanisms [28]. Of which, inflammatory response is involved in the progression of arterial stiffening among CKD subjects, which could lead to increasing cardiac workload, reducing coronary artery perfusion pressure, and microvascular cardiac ischemia [29]. Moreover, inflammation plays a key role in the progression of coronary artery atherosclerosis [30]. Consistent with previous research, anemia and stroke could significantly increase the risk of all-cause and cardiovascular death. Anemia contributes to increased cardiac output, the development of left ventricular hypertrophy, and congestive heart failure, and the presence of anemia is associated with worse outcomes among patients with CKD [31]. Moreover, CKD is strongly related to ischemic and hemorrhagic stroke, which may have severe neurological deficits and poor vital and functional outcomes because of the limitations of pharmacotherapies [32].
The prevalence of 5-year cancer mortality was 2.0% among patients with CKD in our study. Previous research has highlighted conflicting results about the association of CKD with overall cancer risk. A meta-analysis including 32,057 participants reported a non-significant relationship between reduced renal function and cancer risk, but did with urological cancers [33], Similarly, Guo et al. also found CKD is significantly associated only with genitourinary cancer among different types of cancer [4]. On the other hand, Kitchlu et al. reported that cancer risk was increased in mild to moderate CKD and among transplant recipients, but not in advanced kidney disease. And patients with kidney disease are related to higher cancer death in a large US cohort research [3]. On the other hand, previous studies showed that elevated levels of systemic inflammation markers are related to increased cancer risk and mortality, SII displayed the strong association with cancer risk in many sites evaluated including colorectal, kidney, liver, lung, lymphoma, and myeloma cancers [34]. Moreover, a body of studies showed that SII could be used as a potential prognostic marker among subjects with cancers, elevated SII levels were significantly related to adverse outcomes [14, 35]. Consistently, current study also found that elevated SII levels increased the risk of cancer deaths in patients with CKD.
SII could improve the predictive performance of the traditional risk factors model. Therefore, SII may be a useful tool to assess the risk of death among patients with CKD and to help clinicians identify high-risk population. Besides, based on the association between SII and the risk of mortality, it is important to monitor SII levels and control inflammation with certain anti-inflammatory therapies to reduce the risk of mortality among high-risk population. In addition, ACEI/ARB treatment could protect the heart and kidney function given its anti-inflammatory effects [36]. Moreover, managing comorbidities like stroke, CHF as well as anemia is essential to reduce the risk of mortality. Finally, further investigation is needed to prospectively validate the prediction of SII for all-cause, cardiovascular, and cancer mortality among CKD patients.
Limitation
First, this is a retrospective observational study with the subsequent disadvantages secondary to its nature, so our inferences did not reflect direct causality. Second, our study enrolled CKD patients undergoing CAG, but our research still included a number of patients without CAD, and subgroup analysis showed that our results were still stable in the non-CAD group. Third, despite adjusting for potential confounders, we cannot entirely exclude the possibility that residual, uncontrolled confounding may explain the associations. Fourth, we only evaluated SII levels on admission and did not record changes during the follow-up period. The post-discharge changes in SII level fail to be acquired, and the impacts on long-term mortality remained uncertain. Finally, well-designed and well-executed future research should be conducted to examine the predictive validity of the SII levels on admission for long-term prognosis.
Conclusions
Elevated immune inflammation level is an independent risk factor for all-cause, cardiovascular, and cancer mortality among CKD patients, and SII improved the risk prediction of long-term prognosis than traditional risk factors. Our study demonstrates that the SII levels may provide a more accurate risk stratification for the high-risk CKD population and may prompt an important reference for the subsequent improvement of long-term prognosis among CKD patients through monitoring SII levels.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to the institution policy but are available from the corresponding author on reasonable request.
References
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11. https://doi.org/10.1016/j.kisu.2021.11.003.
Gansevoort RT, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52. https://doi.org/10.1016/s0140-6736(13)60595-4.
Kitchlu A, et al. cancer risk and mortality in patients with kidney disease: a population-based cohort study. Am J Kidney Dis. 2022. https://doi.org/10.1053/j.ajkd.2022.02.020.
Saran R, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):A6-a7. https://doi.org/10.1053/j.ajkd.2019.09.003.
Longenecker JC, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE study. J Am Soc Nephrol. 2002;13(7):1918–27. https://doi.org/10.1097/01.asn.0000019641.41496.1e.
Weiner DE, et al. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50(3):217–24. https://doi.org/10.1016/j.jacc.2007.03.037.
Liu M, Dudley SC Jr. Magnesium, oxidative stress, inflammation, and cardiovascular disease. Antioxidants (Basel). 2020. https://doi.org/10.3390/antiox9100907.
García N, Zazueta C, Aguilera-Aguirre L. Oxidative stress and inflammation in cardiovascular disease. Oxid Med Cell Longev. 2017;2017:5853238. https://doi.org/10.1155/2017/5853238.
Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease—what have we learned in 10 years? Semin Dial. 2010;23(5):498–509. https://doi.org/10.1111/j.1525-139X.2010.00784.x.
Peired AJ, et al. From kidney injury to kidney cancer. Kidney Int. 2021;100(1):55–66. https://doi.org/10.1016/j.kint.2021.03.011.
Wegman-Ostrosky T, et al. The renin-angiotensin system meets the hallmarks of cancer. J Renin Angiotensin Aldosterone Syst. 2015;16(2):227–33. https://doi.org/10.1177/1470320313496858.
Mihai S, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018:2180373. https://doi.org/10.1155/2018/2180373.
Tosu AR, Biter H. Association of systemic immune-inflammation index (SII) with presence of isolated coronary artery ectasia. Arch Med Sci Atheroscler Dis. 2021;6:e152–7. https://doi.org/10.5114/amsad.2021.109253.
Yang R, et al. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–302. https://doi.org/10.7150/jca.25691.
Yang YL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5): e13230. https://doi.org/10.1111/eci.13230.
Aguiar-Souto P, et al. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. 2010;139(1):68–74. https://doi.org/10.1016/j.ijcard.2008.10.006.
Levey AS, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–70. https://doi.org/10.7326/0003-4819-130-6-199903160-00002.
O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673–90. https://doi.org/10.1007/s11135-006-9018-6.
Pencina MJ, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. https://doi.org/10.1002/sim.2929.
Rhee CM, Kovesdy CP. Epidemiology: Spotlight on CKD deaths—increasing mortality worldwide. Nat Rev Nephrol. 2015;11(4):199–200. https://doi.org/10.1038/nrneph.2015.25.
Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. https://doi.org/10.1016/s0140-6736(14)61682-2.
Leoni M, Gorini A. Atherosclerosis, chronic inflammation and oxidative stress in CKD. G Ital Nefrol. 2017;34(Suppl 69):142–9.
Machowska A, et al. Therapeutics targeting persistent inflammation in chronic kidney disease. Transl Res. 2016;167(1):204–13. https://doi.org/10.1016/j.trsl.2015.06.012.
Patti G, et al. Platelet indices and risk of death and cardiovascular events: results from a large population-based cohort study. Thromb Haemost. 2019;119(11):1773–84. https://doi.org/10.1055/s-0039-1694969.
Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–43. https://doi.org/10.1161/circresaha.116.309692.
d’Engremont C, et al. Additive value of pre-operative and one-month post-operative lymphocyte count for death-risk stratification in patients with resectable pancreatic cancer: a multicentric study. BMC Cancer. 2016;16(1):823. https://doi.org/10.1186/s12885-016-2860-6.
Jiang H, et al. Systemic immune-inflammation index predicts contrast-induced acute kidney injury in patients undergoing coronary angiography: a cross-sectional study. Front Med (Lausanne). 2022;9: 841601. https://doi.org/10.3389/fmed.2022.841601.
Clementi A, et al. Neurohormonal, endocrine, and immune dysregulation and inflammation in cardiorenal syndrome. Cardiorenal Med. 2019;9(5):265–73. https://doi.org/10.1159/000500715.
Zanoli L, et al. Arterial stiffness in the heart disease of CKD. J Am Soc Nephrol. 2019;30(6):918–28. https://doi.org/10.1681/asn.2019020117.
Oylumlu M, et al. Platelet-to-lymphocyte ratio is a predictor of in-hospital mortality patients with acute coronary syndrome. Anatol J Cardiol. 2015;15(4):277–83. https://doi.org/10.5152/akd.2014.5366.
Zadrazil J, Horak P. Pathophysiology of anemia in chronic kidney diseases: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(2):197–202. https://doi.org/10.5507/bp.2013.093.
Kelly DM, Kelleher EM, Sood MM. Stroke and chronic kidney disease. Contrib Nephrol. 2021;199:80–90. https://doi.org/10.1159/000517698.
Wong G, et al. Chronic kidney disease and the risk of cancer: an individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer. 2016;16:488. https://doi.org/10.1186/s12885-016-2532-6.
Nøst TH, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36(8):841–8. https://doi.org/10.1007/s10654-021-00752-6.
Zheng K, et al. The efficacy of different inflammatory markers for the prognosis of patients with malignant tumors. J Inflamm Res. 2021;14:5769–85. https://doi.org/10.2147/jir.s334941.
Wehbe Z, et al. Molecular insights into SARS COV-2 interaction with cardiovascular disease: role of RAAS and MAPK signaling. Front Pharmacol. 2020;11:836. https://doi.org/10.3389/fphar.2020.00836.
Funding
This research was funded and supported by the Beijing Lisheng Cardiovascular Health Foundation Pilot Fund (No. LHJJ20141751). The National Science Foundation of China (No. 81970311 and No. 82070360). Study on the function and mechanism of the potential target for early warning of cardiorenal syndrome after acute myocardial infarction based on transformism (DFJH201919, DFJH2020026). Clinical Medicine Research Fund of Guangdong Province (2019ZX01). Key Laboratory of Emergency and Trauma (Hainan Medical University), Ministry of Education (Grant. KLET-202116). NSFC Incubation Project of Guangdong Provincial People” Hospital (KY0120220041). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript, and the work was not funded by any industry sponsors.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—(I) Research idea and study design: WGL, JL, SQC, and YL. (II) Data acquisition: WGL, YX, XLZ, XYX and; (III) Data analysis/interpretation: WGL, SJY, and HYL; (IV) Statistical analysis: HZH, QL; (V) Supervision and mentorship: JYX; (VI) Writing guidance: JL, SQC, and YL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions on the accuracy or integrity of any portion of the work are appropriately investigated and resolved. The authors declare that there is no competing interest. All authors read and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial/non-financial relationships that could be construed as a potential conflict of interest, and all authors contributed to the article and approved the submitted version.
Ethics approval and informed consent
All traceable personal identifiers were removed from the analytic dataset to protect patients’ privacy. The study protocol was approved by Guangdong Provincial People’s Hospital ethics committee, and all participating sites received institutional review board approval from their own ethics committees. The study was performed according to the declaration of Helsinki. Since our research included retrospective cases, there was no additional intervention, and information of all patients was desensitized, and no informed consent was required.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lai, W., Xie, Y., Zhao, X. et al. Elevated systemic immune inflammation level increases the risk of total and cause-specific mortality among patients with chronic kidney disease: a large multi-center longitudinal study. Inflamm. Res. 72, 149–158 (2023). https://doi.org/10.1007/s00011-022-01659-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01659-y