Abstract
Background
Chronic kidney disease (CKD) is linked to immunity and inflammation. Systemic immune-inflammation index (SII) and systemic inflammation response index (SIRI) are novel measures for gauging an individual’s systemic inflammatory activity. We aim to investigate the potential associations between them.
Methods
This study encompassed a cohort of 40,937 adults from the National Health and Nutrition Examination Survey (NHANES) 1999–2018. SII and SIRI were log2-transformed before conducting regression analysis, considering that these inflammatory markers were right skewed distributed. Weighted logistic regression models assessed the association of log2-SII and log2-SIRI levels with CKD prevalence. Weighted Cox regression models were utilized to estimate the risk of death. Subgroup analyses were performed to further clarify the effects of other covariates on the associations. Sensitivity analyses were performed to assess the robustness of our results.
Results
6986 participants with CKD were recorded, and 2818 patients died during a mean follow-up time of 100 months. After adjusting for all covariates, the highest level of log2-SII increased the CKD incidence (odds ratio [OR]: 1.47, 95% confidence intervals [CI]: 1.32–1.65, P < 0.001), as well as log2-SIRI (OR: 1.79, 95% CI 1.60–2.01, P < 0.001) when compared with the lowest level reference group. The highest level of log2-SII significantly increased all-cause mortality (hazard risk [HR]: 1.29; 95% CI 1.13–1.48, P < 0.001), cardiovascular mortality (HR: 1.61, 95% CI 1.25–2.09, P < 0.001), and hypertension mortality (HR: 1.73, 95% CI 1.23–2.42, P = 0.001) in CKD patients. Additionally, the positive associations were also found between log2-SIRI and all cause (HR: 1.54, 95% CI 1.35–1.76, P < 0.001), cardiovascular (HR: 1.90, 95% CI 1.38–2.60, P < 0.001), and hypertension mortality (HR: 2.15, 95% CI 1.56–2.94, P < 0.001). Subgroup analyses unveiled variations in these effects among different populations.
Conclusion
There existed a substantial association of SII and SIRI levels with CKD prevalence, as well as mortality in patients with CKD in the U.S. population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 10% of the world’s population is affected by chronic kidney disease (CKD), which is one of the leading causes of death worldwide [1]. It is anticipated that by 2040, it will be the fifth most significant contributor to the decrease in life expectancy around the world, creating a primary health concern on a global scale [2]. Consequently, it is critical to thoroughly comprehend the fundamental elements related to the development and outlook of CKD to devise successful treatment plans to stop the start and advancement of CKD.
The development of CKD is complicated, and several risk factors, including diabetes mellitus, hypertension, and excessive salt consumption, can affect its progression [3,4,5]. Recent research has demonstrated that immune and inflammatory elements are essential in the development of CKD, which could be linked to the stimulation of oxidative stress, changes in tissue metabolism, endothelial cell harm, and other processes [6]. The combination of inflammation and oxidative stress in CKD progression can increase reactive oxygen species (ROS) production, resulting in apoptosis of renal vascular endothelial cells and necrosis, which can cause renal damage by disrupting microcirculatory regulation and perfusion distribution within the kidney [7, 8]. Irrespective of the cause of CKD, inflammation can contribute to glomerular and tubulointerstitial lesions.
Systemic immune-inflammation index (SII) and systemic inflammation response index (SIRI) can measure an individual’s systemic inflammatory activity, demonstrating the equilibrium between inflammation and immune response [9]. Previous research has shown that SII and SIRI can be employed to evaluate local inflammation and systemic immune response, and they have been extensively utilized in the prediction of numerous illnesses [9,10,11], thus enhancing the effectiveness of personalized treatment [12]. Despite this, there is a need for more research into the relationship between SII and SIRI and the occurrence of CKD in the general U.S. population, as well as mortality in patients with CKD.
Materials and methods
Study design and study population
We conducted two observational studies. The associations of SII and SIRI with the occurrence of CKD in the general U.S. population were assessed by a cross-sectional study, and the correlations between SII and SIRI and the predicted risk of death in patients with CKD were investigated by a prospective cohort study, respectively.
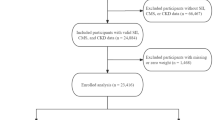
This study incorporated National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2018, with an initial enrollment of 101,316 participants. Simultaneously, we employed a set of exclusion criteria, and individuals who fulfilled any of the subsequent criteria were disqualified from participating in this study: (I) those with incomplete data in the SII and SIRI calculations; (II) those with incomplete data necessary for defining CKD; (III) pregnant [13]; (IV) below the age of 20; (V) those with incomplete data on significant covariates. In the end, the study encompassed a total of 40,937 participants. The sample’s screening process was shown in detail in Fig. 1.
Definition of CKD
As per the Kidney Disease Improving Global Outcomes (KDIGO) Guidelines [14], CKD was characterized by a diminished estimated glomerular filtration rate (eGFR, < 60 mL/min per 1.73 m2) or an increased albumin-creatinine ratio (ACR, ≥ 30 mg/g). The eGFR was determined using the Chronic Kidney Disease Epidemiology Collaborative (2021 CKD-EPI) equation [15], taking into account serum creatinine (SCr) (mg/dL) data obtained through the Jaffe rate method and adjusted for gender and age.
Exposure variables
Participants’ data were collected between 1999 and 2018. SII was defined as (neutrophil count) * (platelet count)/(lymphocyte count). SIRI was defined as (neutrophil count) * (monocyte count)/(lymphocyte count). Wide time interval makes the error of hematology variables because of detecting instrument. We chose Mobile Examination Centers (MEC) weights to eliminate this error. The weight calculation formula for 1999–2000 and 2001–2002 was 2/10* wtmec4yr, and the weight calculation formula for 2003–2018 was 1/10* wtmec2yr. Besides, SII and SIRI were log2-transformed before conducting regression analysis, considering that these inflammatory markers were right skewed distributed [10, 16, 17] (Figure S1).
Outcome ascertainment
The objective of the cross-sectional study was to determine the occurrence of CKD. The ultimate goal of the prospective cohort study was to assess patients’ CKD progress over time. The study involved participants with CKD connected to follow-up records in the National Death Index (NDI) until December 31, 2019, and matched by NHANES respondent serial number (SEQN) to ascertain their crucial condition and the length of their follow-up.
Covariates
In light of prior research concerning the prevalence and outlook of CKD [18, 19], we ultimately examined for the incorporation of pertinent potential confounding factors. Covariates included demographic information: age (years), gender (male/female), race (Mexican American/other Hispanic/non-Hispanic white/non-Hispanic black/other race), marital status (married/living with a partner/widowed/divorced/separated), health insurance (presence/absence), body mass index (BMI) measured as body weight (kilograms) divided by height squared (square meters) determined, smoking (yes/no, defined as smokers smoking more than 100 cigarettes in their lifetime); laboratory test data: serum calcium (mmol/L), serum iron (µmol/L), serum phosphorus (mmol/L), serum sodium (mmol/L); health information: diabetes (yes/no, defined as a diagnosis of diabetes mellitus reported by a doctor/specialist), hypertension (yes/no, expressed as a diagnosis of hypertension reported by a doctor/specialist), Cardiovascular diseases (CVD, yes/no, defined as a physician/specialist, including coronary artery disease, congestive heart failure, stroke, angina, and heart attack), cancer (yes/no, defined as a physician/specialist-reported diagnosis of cancer or malignancy).
Statistical analyses
The participants were divided into four categories according to log2-SII and log2-SIRI quaternary groupings, with the lowest levels of log2-SII and log2-SIRI acting as the benchmark. We computed the odds ratios (ORs) and 95% confidence intervals (CIs) for the effects of log2-SII and log2-SIRI on CKD morbidity and the hazard ratios (HR) and 95% confidence intervals (CIs) for the impact of log2-SII and log2-SIRI on mortality in patients with CKD for each subgroup and calculated P values before and after adjustment. We utilized the outcomes of model 3, taking into account all covariates, as the primary model. The model examined the linear trend of each subgroup’s median value as a continuous variable. We used Cox proportional hazard regression models to create Kaplan–Meyer survival curves for patients, taking into account the four log2-SII and log2-SIRI subgroups.
Furthermore, we conducted distinct subgroup analyses according to the participants’ initial attributes. Age (< 60, ≥ 60) and BMI (< 25, 25–30, > 30) [20] were converted to categorical variables. The interaction tests were performed across the stratified factors. This study also employed sensitivity analyses to evaluate the reliability of the results. Introducing classification for model prediction based on quartiles, as log2-SII and log2-SIRI were continuous variables, could lead to a significant loss of information and potentially diminish the model’s predictive capability. Therefore, we investigated the correlation between constant log2-SII and log2-SIRI and the mortality risk in patients with CKD by utilizing smoothed curve fitting. Logistic and Cox regression models were utilized to evaluate the correlations between log2-SII and log2-SIRI and the likelihood of CKD morbidity and mortality, respectively.
Statistical analyses were performed using the R software package (http://www.r-project.org., the R Foundation), the IBM SPSS Statistics statistical package [IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, N.Y., USA)] and Empower-Stats (http://www.empowerstats.com., X&Y Solutions, Inc., Boston, MA). Statistical significance was determined by a two-tailed test P < 0.05.
Results
Participant baseline characteristics
The baseline characteristics of the 40,937 participants enrolled were shown in Table 1. It was discovered that individuals with elevated log2-SII levels exhibited a higher likelihood of being older, female, non-Hispanic white, widowed/divorced, higher BMI levels, smokers, diabetes, hypertensive, cancer, higher UACR, and those with CVD. Additionally, elevated levels of log2-SII were linked to decreased serum iron, sodium concentrations, and eGFR levels. Individuals with elevated log2-SIRI levels exhibited a higher likelihood of being of advanced age, male, non-Hispanic white, widowed/divorced, possessing health insurance, higher BMI levels, smokers, diabetes, hypertensive, cancer, higher urine albumin-creatinine ratio (UACR), and those with CVD. Additionally, elevated levels of log2-SIRI were linked to decreased levels of serum iron, sodium, and phosphorus concentrations, as well as eGFR.
Association of log2-SII, log2-SIRI, and CKD prevalence risk
Table 2 displayed the outcomes of the logistic regression analyses. After adjusting for all covariates, it was observed that the likelihood of CKD prevalence rose proportionally with the escalation of log2-SII levels (ORQuartile4 = 1.47; 95% CI 1.32–1.65), and all subgroups with elevated concentrations displayed an elevated risk of CKD prevalence (Ptrend < 0.001). The likelihood of CKD prevalence rose as log2-SIRI levels increased (ORQuartile4 = 1.79; 95% CI 1.60–2.01), and all subgroups of higher concentrations exhibited a higher risk of CKD prevalence (Ptrend < 0.001).
Subgroup analyses and interaction tests
Table S1 displayed the outcomes of the stratified and interaction analyses based on age, gender, BMI, smoking status, diabetes, hypertension, cancer, and CVD. The interaction test revealed a noteworthy correlation between log2-SIRI and CKD in the subgroups divided by age, tobacco use, hypertension and CVD (P for interaction < 0.05), and the impact of increased log2-SIRI on the likelihood of developing CKD was more pronounced in elderly, smokers, hypertensive patients and cardiovascular patients. There was also a noteworthy correlation between smoking for log2-SII and CKD (P for interaction < 0.05), with the effect of increased log2-SII on the likelihood of developing CKD being more pronounced in smokers (Figure S2). No significant correlation was demonstrated in other strata (P for interaction tests were all > 0.05).
Baseline characteristics of patients with CKD
The baseline characteristics of the 6986 patients with CKD were summarized in Table S2 and Table S3, which were based on the four subgroup levels of log2-SII and log2-SIRI. Individuals with elevated log2-SII levels exhibited a higher likelihood of being female, non-Hispanic white, widowed, having lower serum iron and serum sodium concentrations, smoking, cancer, and a higher UACR. Individuals with elevated log2-SIRI levels exhibited a higher likelihood of being elderly, male, non-Hispanic white, widowed, possessing health insurance, having lower serum iron and serum sodium concentrations, and displaying reduced eGFR.
Association of log2-SII, log2-SIRI, and mortality in patients with CKD
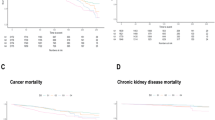
The Kaplan–Meier survival curves for all-cause, CVD, and hypertension mortality in patients with CKD based on the 4 groupings of log2-SII and log2-SIRI were shown in Fig. 2. The curves all showed that participants in the Q4 group had a worse prognosis during the follow-up period (P-values for log-rank test < 0.05). Overall, these findings suggested that higher levels of log2-SII and log2-SIRI tended to have worse prognosis.
Cox regression models with stepwise adjustment for covariates were used to explore the effects of log2-SII and log2-SIRI levels in all cause mortality, cardiovascular mortality, hypertension mortality, respectively (Table 3). After adjusting for all confounders, the highest level of log2-SII significantly increased all-cause mortality (HR: 1.29; 95% CI 1.13–1.48, P < 0.001), cardiovascular mortality (HR: 1.61, 95% CI 1.25–2.09, P < 0.001), and hypertension mortality (HR: 1.73, 95% CI 1.23–2.42, P = 0.001) in CKD patients. Additionally, the positive associations were also found between log2-SIRI and all cause (HR: 1.54, 95% CI 1.35–1.76, P < 0.001), cardiovascular (HR: 1.90, 95% CI 1.38–2.60, P < 0.001), and hypertension mortality (HR: 2.15, 95% CI 1.56–2.94, P < 0.001).
Subgroup analyses and interaction tests
The results of stratified and interaction analyses were shown in Table 4. The results of the interaction test showed that elevated log2-SII level was more significant for increasing the risk of all-cause mortality among those obese (BMI > 30), smoked, and those with cancer. For CVD mortality, the effect of elevated log2-SII levels was more pronounced among those obese (BMI > 30). For hypertension mortality, elevated log2-SII level was more significant for increasing the risk of death in smokers. For all-cause mortality, elevated log2-SIRI level was more significant in increasing the risk of all cause and CVD mortality in obese individuals. For hypertension mortality, the effect of elevated log2-SIRI levels was more significant among smokers (P for interaction < 0.05) (Figure S3).
Sensitivity analysis
Smooth curves were shown in Figures S4 and S5. The results showed that log2-SII and log2-SIRI levels positively correlated with CKD incidence. Within a specific range, higher log2-SII and log2-SIRI levels tended to have higher all-cause mortality, CVD mortality, and hypertension mortality. Sensitivity analyses demonstrated the robustness of the findings, i.e., significant positive association of SII and SIRI levels with CKD prevalence, as well as mortality in patients with CKD in the U.S. population (Tables 2, 3).
Discussion
The present study provided comprehensive epidemiologic insights into the relationship between new metrics for assessing an individual’s systemic inflammatory activity on the impact of CKD progression and prognostic outcomes. Our findings suggested that elevated levels of SII and SIRI were significantly associated with CKD prevalence, as well as mortality in patients with CKD in the U.S. population.
As indicators for assessing an individual’s systemic inflammatory activity, the higher the values of SII and SIRI, the more active the body’s immune system and inflammatory state [9]. A study showed that high levels of SII increased all-cause and cardiovascular mortality in patients with CKD, with more than half of the deaths in patients attributed to cardiovascular diseases [21]. The authors suggest that a possible explanation is that inflammation accelerates the progression of atherosclerosis in patients with CKD, leading to coronary artery damage, which exacerbates myocardial ischemia and strikes a blow to patients’ cardiac function, ultimately leading to death.
Despite the lack of systematic studies on the association of SII and SIRI with CKD, several previous studies have demonstrated that inflammation and inflammatory responses can contribute to the progression of CKD by altering or interfering with intrarenal microcirculatory regulation and perfusion distribution, causing renal damage [22,23,24]. Inflammatory cells such as monocytes, neutrophils, lymphocytes, and inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-a (TNF-a) are involved in the progression of CKD by a variety of mechanisms, including an increase in pro-inflammatory cytokines, activation of oxidative stress, chronic and recurrent infections, altered adipose tissue metabolism, and dysregulation of the gut microbiota and many other mechanisms [25]. Previous studies have shown that monocytes play an essential role in the progression of CKD and that NLRP3 inflammasomes associated with renal disease when stimulated by substances such as reactive oxygen species (ROS), promote the release of IL-1 from monocytes, which in turn activates the classical pro-inflammatory signaling pathways of the intrinsic immune system, NF-kB (nuclear factor-kB) and AP-1 (activator protein-1), destroying renal units and leading to intrarenal microcirculatory dysregulation [22, 26]. In addition, neutrophils contribute to the progression of CKD through migration, production of ROS, secretion of neutrophil serine proteases (NSPs), and release of neutrophil extracellular traps (NETs) [26, 27]. Patients with CKD also experience premature aging of their T lymphocytes, which express high levels of pro-inflammatory cytokines that interact with the chronic inflammatory milieu of the patients with CKD, exacerbating the progression of the CKD and increasing the patient’s susceptibility to atherosclerosis and ischemic organ damage [28,29,30]. In addition, the decrease in lymphocyte count also reflects patients’ impaired immune resistance, making them more prone to infections and deterioration of renal function [25]. It has been demonstrated in animal studies that T lymphocytes infiltrating the kidneys increase free radicals to participate in the development of CKD and hypertension [31]. This finding is supported by observational studies showing that higher monocytes, neutrophils, and low lymphocytes are associated with a higher risk of CKD [32]. Meanwhile, platelets are involved in thrombus formation at the site of vascular injury, and changes in the PLT index reflect the severity of endothelial injury in renal vessels to some extent [33].
We found that high BMI increased all cause mortality and cardiovascular mortality in patients. This may be because abdominal fat deposition that may accompany obesity increases the level of inflammation and brings higher mortality in patients with CKD [34]. Our results showed that smoking interacted with SII and SIRI and increased the risk of CKD morbidity and mortality. It may be because smoking-induced endothelial dysfunction may modulate immune and inflammatory cell responses, resulting in elevated levels of inflammation [35]. In addition, smoking negatively affects many processes closely related to the development of renal fibrosis [36].
In addition, we observed that SIRI levels were associated with the incidence of CKD in the U.S. populations with different underlying diseases, including hypertension and cardiovascular disease. In terms of the effect of hypertension on the development of CKD, it may be due to the fact that hypertension is usually accompanied by additional inflammation, with elevated biomarkers of inflammation in hypertensive patients [37]. Therefore, the increased incidence of hypertension would be more pronounced. Our subgroup analysis showed that cardiovascular disease increased the risk of developing chronic kidney disease. This may be related to impaired vascular reactivity, endothelial dysfunction and increased arterial stiffness in patients with CKD [38].
Our study has several strengths. First, this study is based on the large sample size and appropriate covariates adjustment, enhancing the reliability and representativeness of our findings. In addition, we performed sensitivity analyses to assess the robustness of our results. To the best of our knowledge, this is the first study to systematically report the association between these inflammatory indices and the incidence and prognosis of CKD.
We also note some limitations of this study. First, the blood cell-based test was performed once, and the concentrations of these blood cells may change during the follow-up period, not fully reflecting the long-term inflammatory index levels in the population. In addition, single measurements of blood cell counts may be affected by other factors, which may lead to residual confounding and require attention when interpreting results. We agree that the observational nature of this study means that causality cannot be established. Going forward, more research methods as well as large-sample, multicenter cohort studies involving a larger number of participants are needed to examine these associations to provide more detailed and reliable clinical guidance. At the same time, while this study deepened the academic understanding of chronic kidney disease, it did not provide direct guidance for clinical practice or patient management. Future research could focus on how to apply these findings to real-world settings.
Conclusion
This observational study provided valuable insights into the role of systemic inflammation and immune responses in CKD. Our findings suggested that there existed a substantial association of SII and SIRI levels with CKD prevalence, as well as mortality in patients with CKD in the U.S. population. SII and SIRI may be considered effective predictors for assessing the risk of CKD morbidity in the general U.S. population and mortality risk in the CKD population. Going forward, more research methods as well as large-sample, multicenter cohort studies involving a larger number of participants are needed to examine these associations to provide more detailed and reliable clinical guidance.
Data availability
The original data used in this study are nationally representative and publicly available, and can be directly obtained through application to NHANES, which is an ongoing periodic survey of a nationally representative sample of the U.S. noninstitutionalized civilian population using a complex multistage whole-population probability sampling strategy. More details about the survey can be found on the publicly available NHANES website.
References
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11.
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–90.
Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25(2):121–32.
Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74(1):120–31.
Malta D, Petersen KS, Johnson C, Trieu K, Rae S, Jefferson K, et al. High sodium intake increases blood pressure and risk of kidney disease. From the science of salt: a regularly updated systematic review of salt and health outcomes (August 2016 to March 2017). J Clin Hypertens. 2018;20(12):1654–65.
Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39(1–3):84–92.
Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10(9):493–503.
Ebert T, Neytchev O, Witasp A, Kublickiene K, Stenvinkel P, Shiels PG. Inflammation and oxidative stress in chronic kidney disease and dialysis patients. Antioxid Redox Signal. 2021;35(17):1426–48.
Dziedzic EA, Gąsior JS, Tuzimek A, Paleczny J, Junka A, Dąbrowski M, et al. Investigation of the associations of novel inflammatory biomarkers-systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. 2022;23(17):9553.
Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25(1):34.
Luo J, Qin X, Zhang X, Zhang Y, Yuan F, Shi W, et al. Prognostic implications of systemic immune-inflammation index in myocardial infarction patients with and without diabetes: insights from the NOAFCAMI-SH registry. Cardiovasc Diabetol. 2024;23(1):41.
Pacheco-Barcia V, Mondéjar Solís R, France T, Asselah J, Donnay O, Zogopoulos G, et al. A systemic inflammation response index (SIRI) correlates with survival and predicts oncological outcome for mFOLFIRINOX therapy in metastatic pancreatic cancer. Pancreatology. 2020;20(2):254–64.
Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–15.
Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, Cook HT, Fervenza FC, Gibson KL, Glassock RJ, Jayne DR. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4s):S1–276.
Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. 2021;32(12):2994–3015.
Feng C, Wang H, Lu N, Chen T, He H, Lu Y, et al. Log-transformation and its implications for data analysis. Shanghai Arch Psychiatry. 2014;26(2):105–9.
Curran-Everett D. Explorations in statistics: the log transformation. Adv Physiol Educ. 2018;42(2):343–7.
Li J, Liu Z, Pu Y, Dai H, Peng F. Association between dietary vitamin E intake and chronic kidney disease events in US adults: a cross-sectional study from NHANES 2009–2016. Clin Kidney J. 2023;16(12):2559–66.
Mazidi M, Gao HK, Kengne AP. Food patterns are associated with likelihood of CKD in US adults. Sci Rep. 2018;8(1):10696.
Weir CB, Jan A. BMI classification percentile and cut off points. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Arif Jan declares no relevant financial relationships with ineligible companies. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
Lai W, Xie Y, Zhao X, Xu X, Yu S, Lu H, et al. Elevated systemic immune inflammation level increases the risk of total and cause-specific mortality among patients with chronic kidney disease: a large multi-center longitudinal study. Inflamm Res. 2023;72(1):149–58.
Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018:2180373.
Stenvinkel P, Chertow GM, Devarajan P, Levin A, Andreoli SP, Bangalore S, et al. Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int Rep. 2021;6(7):1775–87.
Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269–88.
Syed-Ahmed M, Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26(1):8–15.
Bronze-da-Rocha E, Santos-Silva A. Neutrophil elastase inhibitors and chronic kidney disease. Int J Biol Sci. 2018;14(10):1343–60.
Kim IS, Kim DH, Lee HW, Kim SG, Kim YK, Kim JK. Role of increased neutrophil extracellular trap formation on acute kidney injury in COVID-19 patients. Front Immunol. 2023;14:1122510.
Meijers RW, Betjes MG, Baan CC, Litjens NH. T-cell ageing in end-stage renal disease patients: assessment and clinical relevance. World J Nephrol. 2014;3(4):268–76.
Vicente R, Mausset-Bonnefont AL, Jorgensen C, Louis-Plence P, Brondello JM. Cellular senescence impact on immune cell fate and function. Aging Cell. 2016;15(3):400–6.
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol. 2011;300(3):F734–42.
Yoshitomi R, Nakayama M, Sakoh T, Fukui A, Katafuchi E, Seki M, et al. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren Fail. 2019;41(1):238–43.
Xu B, Zhang Y, Chen G, Feng J, Gan L. Association of mean platelet volume/lymphocyte ratio with inflammation in non-dialysis patients with chronic kidney disease stages 1–4: a retrospective study. Front Immunol. 2022;13:1041356.
Cordeiro AC, Qureshi AR, Stenvinkel P, Heimbürger O, Axelsson J, Bárány P, et al. Abdominal fat deposition is associated with increased inflammation, protein-energy wasting and worse outcome in patients undergoing haemodialysis. Nephrol Dial Transplant. 2010;25(2):562–8.
Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–15.
Alkhatib L, Velez Diaz LA, Varma S, Chowdhary A, Bapat P, Pan H, et al. Lifestyle modifications and nutritional and therapeutic interventions in delaying the progression of chronic kidney disease: a review. Cureus. 2023;15(2): e34572.
Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. 2020;36(5):635–47.
Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97.
Acknowledgements
We thank Dr. Dachuan Guo, National Key Laboratory for Innovation and Transformation of Luobing Theory; The Key Laboratory of Cardiovascular Remodeling and Function Research, Department of Cardiology, Chinese Ministry of Education, Chinese National Health Commission and Chinese Academy of Medical Sciences, Qilu Hospital of Shandong University, for his guidance on the NHANES data weighting processing R code as well as methodology, and we thank Ying-Long Peng, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), School of Medicine, South China University of Technology, for guidance on this public database clinical study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Peixian Huang: conceptualization; methodology; formal analysis; writing—original draft. Yanpei Mai: methodology; formal analysis; writing—original draft; writing—review and editing. Jun Zhao, Yushan Yi: formal analysis; writing—original draft. Yaqing Wen: data curation; writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no relationships or activities might bias their work or be perceived as biased.
Ethical approval
The survey protocol for the NHANES was approved by CDC’s National Center for Health Statistics Institutional Research Ethics Review Board.
Consent for publication
We confirm that the manuscript has been read and approved for publication by all of the named authors, and that there are no other persons who meet the criteria for authorship but are not listed. We also confirm that the order of authorship listed in the manuscript has been approved by all of us.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanpei Mai is tagged as co-first author. Peixian Huang and Yanpei Mai share the first authorship and have equal status.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, P., Mai, Y., Zhao, J. et al. Association of systemic immune-inflammation index and systemic inflammation response index with chronic kidney disease: observational study of 40,937 adults. Inflamm. Res. 73, 655–667 (2024). https://doi.org/10.1007/s00011-024-01861-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-024-01861-0