Abstract
Heme oxygenase-1 (HO-1) is considered to be the main protein in diseases arising as a result of oxidative and inflammatory insults. Tremendous research has been carried out on HO-1 since years, pertaining its cytoprotective effect against oxidative injury and other cellular stresses. HO-1, by regulating intracellular levels of pro-oxidant heme, or by other benefits of its by-products such as carbon monoxide (CO) and biliverdin (BV) had become an important candidate protein to be up-regulated to combat diverse stressful events. Although the beneficial effects of HO-1 induction have been reported in a number of cells and tissues, a growing body of evidence indicates that this increased HO-1 expression may lead to the progression of several diseases such as neurodegeneration, carcinogenesis. But it is not clear, what accounts for the increased expression of HO-1 in cells and tissues. The observed friendly role of HO-1 in a wide range of stress conditions since times is now doubtful. Therefore, more studies are needed to elucidate the exact role of HO-1 in various stressful events. Being more concise, elucidating the effect of HO-1 up-regulation on critical genes involved in particular diseases such as cancer will help to a larger extent to comprehend the exact role of HO-1. This review will assist in understanding the dual role (protective and detrimental) of HO-1 and the signaling pathway involved and will help in unraveling the doubtful role of HO-1 induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heme oxygenase (HO) plays a vital role in the catabolism of heme and yields equimolar amounts of BV, CO and free iron [1]. Heme oxygenase is responsible for cleaving at the alpha-methene bridge of the heme ring to form biliverdin, which is acted upon by the biliverdin reductase to form bilirubin (BR). But if after cleavage, the heme remains still attached to the globin molecule, then verdoglobin is formed.
To date, two isoforms of HO (HO-1 and HO-2) have been reported in mammals and a third one, i.e., HO-3 has been proposed by Hayashi and his colleagues, while as four HO isoforms (HO-1, HO-2, HO-3, and HO-4) have been identified in plants. Among different HO isoforms, HO-1 is considered to be of keen research interest as its expression level is induced during various pathophysiological conditions [2].
Human HO-1 has a molecular weight of 32.8 kDa (kilodalton), composed of 288 amino acids and shares about 80% amino acid homology with rat HO-1. HO-1 has emerged as an ideal cytoprotective agent and modulation in its expression and activity levels could provide a potential therapeutic value [3]. HO-1 is associated with smooth endoplasm reticulum (SER), but is also localized in mitochondria nuclei and caveoli [4]. It has been found that mitochondrial HO-1 fraction is increased in lung epithelial cells when exposed with hemin, lipopolysaccharide (LPS) and cigarette smoke [5]. The increased translocation of HO-1 to mitochondria protects epithelial cells against mitochondria associated cell death [6]. Another study in rats, also reported enhanced translocation of HO-1 to the mitochondria of the gastric mucosa after indomethacin treatment [7]. This enhanced translocation of HO-1 to mitochondria has been associated with the decline in lipid peroxidation and also ameliorates gastric mucosal injury [7]. Under stress conditions, HO-1 gets translocated to nucleus where it regulates its own expression [8]. Nuclear localization of HO-1 has been found in different cancerous tissues such as oral, lung and prostate, and has been linked with the tumor progression [2]. Translocation of HO-1 to nucleus is mediated by its proteolytic cleavage, which releases HO-1 from SER and subsequently allows its entry to nucleus [4]. Nuclear HO-1 has been linked to tumor growth and protection against oxidative stress by up-regulating antioxidant genes [9] (Table 1).
Induction of HO-1
HO-1 is induced in response to panoply of stimuli such as hypoxia, oxidative stress, cytokines, LPS, heavy metals, in biological systems. Since HO-1 induction is a widely accepted strategy used by cells to neutralize a variety of stress conditions [10], the targeted induction of this powerful enzyme may be a beneficial therapeutic strategy against different diseases arising as a result of inappropriate immune response and oxidative dysregulation. Particularly, the identification of non-cytotoxic HO-1 inducers may represent a novel approach to combat various oxidative and inflammatory responses [11]. Many natural compounds have been confirmed to be effective non-cytotoxic HO-1 inducers in hepatic cellular models [12]. A majority of HO-1 inducers are present in plants that are being widely used as flavoring agents, food, spices and traditional medicinal plants. Besides toxicity, a possible concern with the use of pharmacological inducers of HO-1 relates to the GT dinucleotide repeat length in the HO-1 promoter [13].
Lipopolysaccharide is an important molecule present in the outer membrane of Gram-negative bacteria. LPS is known to activate toll-like receptor 4 (TLR4) which leads to a signaling cascade, thereby activating transcription factor nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs) and interferon response factors (IRFs) [14]. The cascade in turn causes release of pro-inflammatory mediators including interleukin-1 (IL-1), IL-6, IL-12, tumor necrosis factor-α (TNF), interferon (IFN)-γ, β and nitric oxide [15]. LPS-induced HO-1 induction is cytoprotective against pulmonary inflammation and decreases migration of polymorphonuclear leukocytes (PMNs) to lungs in response [16]. LPS can activate all the three mitogen-activated protein kinase (MAPK) pathways. However, it is not necessary that these pathways activated may be involved in HO-1 induction by LPS. Moreover, HO-1 induction by these MAPK pathways shows inducer-dependent specificity. Exogenous treatment of PGE2 has been found to suppress the HO-1 expression and enhances the LPS-induced cyclooxygenase-2 (COX-2) expression in RAW 264.7 macrophages [17]. Moreover, LPS has been found to induce the expression of HO-1 in monocytes and thereby provides protection against the excessive inflammatory responses [18]. It has been found that two enhancer regions in HO-1 gene mediate its activation in response to LPS in mouse [19].
Several chemicals and drugs from plant origin have been reported to induce HO-1 expression [20]. Among them is curcumin (a polyphenolic compound isolated from the rhizome of Curcuma longa), which exerts a significant anti-inflammatory activity [21] and a well-characterized non-cytotoxic HO-1 inducer in endothelial cells, astrocytes, macrophages and muscle cells [22]. Carnosol is a naturally occurring bioactive phenolic compound that has been associated with the stimulation of HO-1 expression in a time-dependent manner [23]. Thus, active constituents from different medicinal plants may prove to be potent inducers of HO-1 enzyme, and provide many therapeutic agents for the amelioration of inflammation and oxidative stress. Fraxetin (a coumarin derivative) induces HO-1 expression via activation of AMPKα/Nrf2 or Akt/Nrf2 pathway [24].
Signaling cascades leading to HO-1 activation
Earlier studies indicate that different HO-1 inducers activate diverse protein phosphorylation-dependent signaling pathways that ultimately regulate the HMOX1 gene expression by activating a wide variety of transcription factors. Different intracellular signaling molecules and transcription factors are associated with HO-1 expressions such as Nrf2, MAPK, Bric a brac, Tramtrack and Broad complex (BTB) and cap ‘n’ collar (CNC) homologue 1 (Bach1), AP-1 and NF-κB [25]. Mitogen-activated protein kinase (MAPK) is one of the most essential signaling molecule associated with HO-1 induction by non-toxic phytochemicals and drugs [26]. Other signaling molecules such as phosphatidylinositol 3-kinase (PI3K), tyrosine kinases and protein kinases A, B, C, and G are known to play role in HO-1 induction [27]. But unfortunately, a little work has been done on the role of these kinases in HO-1 induction. HO-1 expression is primarily regulated at the transcription level and different cis-acting regulatory elements (REs) are associated to mediate the basal and inducible expression levels of HO-1 gene. Two upstream enhancer regions (E1 and E2) play major functional roles for redox-dependent induction of HO-1. Both E1 and E2 enhancer regions contain several antioxidant response elements (AREs). For further details see refer to Fig. 1.
Showing different HO-1 induction pathways: transcription factors suc as AP-1, Nrf2 and NF-κB remain localized in cytosol under normal conditions. Under external stimuli, these transcriptional factors get activated and tranlocate to the nucleus. Within the nucleus they bind specifically to the DNA sequence and leads to the HO-1 transcription
Consequences of heme degradation by HO-1
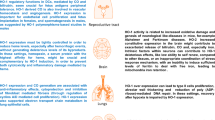
Several studies have reported the protective effects conferred by HO-1 through its ability to degrade heme. However, the actual mechanism of its action has not been yet completely revealed. The most accepted mechanism suggests that the by-products of the heme degradation [mostly carbon monoxide (CO) and bilirubin (BR)] mediate its cytoprotective effects [10]. However, these metabolic by-products have been seen to either ameliorate or exacerbate stress-related diseases depending upon particular disease models employed, the intensity of HO-1 expression and the existing redox microenvironment (as shown in Fig. 2).
Protective effects of HO-1 induction implicated in several diseases
The mechanism of heme degradation employed by HO-1 has proven to be an effective method of suppressing oxidative dysregulation, inappropriate immune response and related disorders. So the targeted induction of HO-1, particularly by non-toxic inducers, has been established as a potent therapeutic means of curing stress-related diseases.
HO-1 as an anti-inflammatory agent
HO-1 has been regarded as a potent anti-inflammatory enzyme, as evidenced by a large number of studies. The significance of HO-1 is illustrated by HO-1 knockdown studies (using HO-1−/−mice), which develop the progressive inflammatory disease and show a robust increase in the levels of pro-inflammatory cytokines (IL-1β, interferon-γ, TNF, and interleukin-6) [6, 28].
The actual underlying mechanism responsible for the anti-inflammatory properties of HO-1 is not fully known. However, the signaling action of CO together with the antioxidant properties shown by biliverdin/bilirubin and iron sequestration by ferritin could all contribute in the amelioration of inflammation (as described above). CO has been seen to act as an efficient anti-inflammatory agent in several in vitro and in vivo models of inflammation. CO has been reported to significantly impair the nitric oxide (NO) generation and TNF or IL-6 production in lipopolysaccharide-stimulated macrophages [29]. Further, Morse and his coworkers observed that these effects of CO might be caused by interfering of CO with AP-1 activity via a c-Jun N-terminal kinase (JNK)-dependent pathway [30]. CO also increases production of anti-inflammatory cytokine, IL-10, in macrophages [31]. BV/BR production and Fe2+ chelation reduce adhesion of leukocytes to vascular endothelium by causing certain changes in expression of various adhesion molecules such as E-selectin, vascular cell adhesion molecule-1 (VCAM-1) and thus reduce inflammation [32]. The HO-1 promoter contains binding sites for various transcription factors such as NF-kB, AP-1, and AP-2 as well as motifs for glucocorticoid-responsive elements. HO-1 has been seen to be capable of modulating activities of these transcription factors leading to different protective properties. For instance, it has been observed that upon up-regulating the HO enzyme with hemin, NF-kB expression was down-regulated in different in vivo models of type 2 diabetes [33], as well as different hypertensive models. Likewise, HO-1 has been found to be dispensable for the anti-inflammatory activity of intravenous immunoglobulin G (IVIG) [34].
Neuroprotective role of HO-1
The functioning of the brain is largely dependent on the constitutive supply of oxygen, as brain consumes an ample percentage (up to 50%) of total oxygen supplied to the body. However, under normal physiological condition, 2–5% of total oxygen consumed by cells is transformed into reactive oxygen species (ROS). However, if there is excessive and unregulated ROS production in the central nervous system (CNS), it leads to several neurodegenerative diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) [35]. However, there is emerging evidence that HO-1 expression helps in preventing the pathogenesis of various neurodegenerative diseases. HO-1 induction has been seen to implicate a neuroprotective role on exposure to a variety of noxious stimuli, both in animal models as well as in tissue culture. HO-1 induction occurs in both neuronal and non-neuronal brain cells. Astrocytes show more robust HO-1 response than neurons [36]. Many studies illustrate the protective role of HO-1 against various neurodegenerative disorders. Cerebellar granule cells of transgenic mice designed to overexpress HO-1 in neurons were relatively resistant for oxidative stress-mediated cell death [37]. When HO-1 was overexpressed in neuronal cells, they were seen to be resistant to oxidative damage induced by glutamate and H2O2. Similarly, transfection of neuroblastoma cell lines with HO-1 cDNA was seen to decrease the susceptibility of the cells to oxidative damage caused by H2O2 [38]. Also, HO-1, when overexpressed, protects neurons from toxicity induced by 1-methyl-4-phenylpyridinium (MPP) by increasing the expression of glial cell-derived neurotrophic factor [39]. The flavonoid quercetin induces HO-1 expression and inhibits iNOS expression in BV-2 cells [40], however, when these cells were treated with HO-1 antisense oligodeoxynucleotide, inducible nitric oxide synthase (iNOS) expression was no more seen to be inhibited. HO-1 induction affects other parameters of inflammation as well. For instance, in rat cortical astrocytes, HO-1 activity seems to be linked to the level of prostaglandin E2 production [41]. Conversely, down-regulation or knocking out HO-1 increases the susceptibility to oxidative and other stress challenges. It has been reported that astrocytes taken from HO-1 knockout mice show more vulnerability to toxicity induced by hemin relative to wild-type cells [42]. Moreover, the protective role of HO-1 has been attributed not only to its antioxidant properties but also to its potential of degradation of tau and α-synuclein by proteasomes [43]. Furthermore, HO-1 localized in mitochondria has been reported to reduce oxidative damage in renal epithelial cells [44].
Protective role of HO-1 in cardiovascular diseases
The incidence of cardiovascular diseases is so high that only in the United States it accounted for 33.6% of all deaths in 2007 [45]. A primary function of HO-1 in maintaining cardiac homeostasis was first shown by Ewing and his coworkers by observing an increased HO-1 expression in the heart in response to hyperthermia [46]. Further, in HO-1 knockout (HO-1−/−) mice studies, hypoxia induces severe right ventricular dilatation and infarction in comparison to wild-type mice [47]. Besides, many studies using cardiac-specific HO-1 transgenic mice models revealed reduced myocardial infarct size following ischemia/reperfusion injury due to HO-1 overexpression [48]. Moreover, in the heart failure model, overexpressed HO-1 promotes neovascularization and ameliorates apoptosis [49]. To avoid superfluous side effects of constitutive overexpression of HO-1, hypoxia-regulated HO-1 gene vector was designed which could turn on only in myocardial ischemic condition [50]. Previous studies have found that QT interval prolongation is a risk factor for cardiovascular risk [51]. Intriguingly, particulate air pollution and iron metabolism genes (including heme oxygenase-1) have been involved in QT prolongation [52]. Also, HO-1 is required for angiogenic function of bone marrow-derived progenitor cells [53] and provides protection from cardiovascular diseases [54]. It has been further found that HO-1 plays an important role in endothelial repair and vascular protection [55] and thus providing intriguing aspects of endothelial progenitor cells (EPC)—therapeutic potential [56].
HO-1 in renal function
The kidney encounters different toxins, which includes both exogenous and endogenous ones. Free heme is the main molecule which is responsible for inducing oxidative stress and ultimately results in HO-1 induction [57]. It has been observed that HO-1 induction occurs in different substructures of kidney such as renal interstitium, proximal tubules, renal mononuclear phagocytes (RMPs) and glomeruli in response to injury [58]. HO-1 induction has been linked with the restoration of renal function in animals subjected to ischemia/reperfusion injury (IRI) [59]. It has been observed that expression level of HO-1 increased many folds in rats treated with angiotensin II (induces renal injury) and thus provides protection against hypertension [60]. Gentamicin (GM)-mediated renal dysfunction can be effectively improved by inducing HO-1 expression [61].
Hepatoprotective role of HO-1
HO-1 induction has been observed to play a hepatoprotective against different injuries [62]. A wide variety of hepatotoxic conditions (ischemia reperfusion, hemorrhagic shock, hypoxia and endotoxin) and chemicals (halothane isoflurane, heavy metals, acetaminophen and reactive oxygen species) have been observed to induce HO-1 expression, and thereby protects liver against damage [25]. Curcumin has been found to induce HO-1, and thereby protects hepatocytes against oxidative injury [63]. Similarly, treatment with Majoon-e-Dabeed-ul-Ward (a unani formulation) has been observed to protect lung cells against ethanol-induced cell death by inducing HO-1 [64]. Up-regulation of HO-1 expression either chemically or by adenoviral gene transfer method has been observed to rescue the mice against the apoptotic liver damage [65, 66]. Recently, polyphenol-enriched fraction from folium microcos has been found to show hepatoprotective effect against the liver damage associated with the acetaminophen treatment [67].
HO-1 and diabetes
It has been observed that up-regulation of HO-1 is capable of increasing insulin secretion, and thereby reduce extra blood glucose (hyperglycemia) [68]. A similar study observed an increased insulin secretion by CO (a product of HO-1) [69]. Since type-1 diabetes is coupled with excessive production of inflammatory molecules and ultimately leads to apoptosis, HO-1 induction has been found to have beneficial effects in such conditions [68]. Recently, it has been found that HO-1 induction is responsible for decrease in diabetic neuropathy and in turn increases antinociceptive effects of morphine [70].
Adverse effects associated with HO-1 induction
In spite of such a beneficial mechanism of cytoprotection imparted by HO-1 in diverse stress conditions, its induction has been related to the development of several diseases [71]. In its role of imparting protection to the cells, HO-1 has been observed to increase the survival and suppress the apoptotic pathways in these cells [72]. The increased survival and suppression of apoptosis in these cells may lead to their uncontrolled proliferation and can make them progress towards carcinogenesis, metastasis and other ailments such as neurodystrophic disorders [2].
HO-1 and carcinogenesis
HO-1 acts as a cytoprotective agent in normal tissues exposed to various stimuli. It is induced in response to panoply of stimuli including carcinogens, and its enhanced expression imparts protection to cells. Much data obtained till date report the increased expression of the HO-1 enzyme in tumors in comparison to the surrounding normal tissues. This increased expression of HO-1 was revealed in lymphosarcoma, adenocarcinoma, hepatoma, glioblastoma, melanoma, prostate cancers, Kaposi sarcoma, squamous carcinoma, pancreatic cancer and in brain tumors [2]. Several in vivo studies revealed that animals upon treatment with carcinogens show higher expression of HO-1. For example, HO-1 was seen to be up-regulated in rats which were exposed to alachlor, a known inducer of olfactory tumors [73]. Similarly, the expression of HO-1 was increased in response to dietary p-dimethylaminoazobenzene (DAB) inducing hepatic carcinogenesis. Moreover, induction of HO-1 can also take place in response to some chemopreventive compounds. For instance, diallyl sulfide (DAS) increases the expression of HO-1 by increased ROS generation and subsequent increase in activity of transcription factors Nrf2, extracellular signal-regulated kinase (ERK) and p38 kinases. The increase in HO-1 expression by DAB and diallyl sulfide (DAS) was hypothesized to play a key role in the anti-carcinogenic effects of these chemicals [74]. The pancreatic cancer cells treated with gemcitabine or radiation-induced HO-1, while knockdown of HO-1 showed reverse effect inhibiting pancreatic cancer [75]. Induction of HO-1 enzyme genetically or using pharmacological inducers/carcinogens has been suggested to provide a growing environment to tumor cells and confer resistance to chemotherapy and radiotherapy besides protecting these cells from oxidative stress by its anti-oxidative mechanism [2]. This enhanced survival is most probably due to the anti-apoptotic property of HO-1 and increased angiogenesis [76].
An anti-apoptotic aspect of HO-1 has previously been demonstrated both in in vitro and in vivo models of inflammation and tumors. HO-1 is known to prevent apoptosis by mainly activating p38 kinase pathway. However, in many carcinomas, apoptosis is prevented by the involvement of Akt/protein kinase B pathway [77]. One more study has shown that overexpression of HO-1 protected the renal cancer cells from apoptosis induced by rapamycin and sorafenib and helped the tumor cells grow by blocking their apoptotic and autophagic pathways. In addition, it has been reported that when HO-1 is overexpressed, it increases the expression of Bcl-xL (an anti-apoptotic protein), and decreases the Beclin-1 and LC3B-II expression (autophagic proteins). HO-1 is responsible for the induction of anti-apoptotic protein (Bcl-xL) and decreases the expression of Beclin-1 and LC3B-II (autophagic proteins), however, HO-1 knockdown decreases Bcl-xL expression and markedly enhances LC3B-II [78]. In addition, it has been observed that HO-1 induction does not always protect the cells against apoptosis as was thought earlier. A study showed that HO-1 was not able to prevent chemotherapy-induced apoptosis in breast carcinoma model [79].
Further, several studies evidently showed that HO-1 also plays a key role in angiogenesis. Angiogenesis is an essential process for the sustained growth and invasion of solid tumors. In cultured endothelial cells, elevated HO-1 levels have been shown to be involved in the up-regulation of vascular endothelial growth factor (VEGF) and VEGF receptors increased proliferation and migration of endothelial cell and promoted angiogenesis [80]. It has also been seen that when HO-1 was transfected in severe combined immune deficient mice, it enhanced the development of pancreatic tumor via the stimulation of angiogenesis. On the other hand, stannous mesoporphyrin, the inhibitor of HO-1, transiently delayed the growth of the tumor. HO-1 inhibition is an emerging target to fight against cancer [2]. For instance, in the chronic myeloid leukemia-derived cell line K562, Gleevec-induced apoptosis was counteracted via HO-1 overexpression [81]. The effectiveness of such treatments has also been confirmed in in vivo models. The adenocarcinomic mice which were treated with photodynamic therapy, regrowth of tumors were observed to a great extent due to the increased expression of HO-1 [82]. Thus, using zinc protoporphyrin IX (ZnPPIX), a specific inhibitor of HO-1, expansion of hepatoma and sarcoma or lung cancer in mice can be suppressed significantly [83]. Since induction of HO-1 expression has been linked with cancer invasion. It has been observed that curcumin induces HO-1 expression and thus attenuates its anti-invasive effect in cancer therapy [84].
The effect of HO-1 on the expression of the cancer critical genes (oncogenes and tumor suppressor genes) would significantly collaborate in unraveling the relation between HO-1 activation and pathogenesis of different diseases. But unfortunately, very little data are available about this matter. However, one report reveals that HO-1 does not affect the telomerase and telomerase reverse transcriptase (TERT), which play a major role in cancer progression by regulating telomerase [85]. Moreover, HO-1 knockdown studies or animal models treated with pharmacologic inhibitors of HO-1 will help in determining the actual effect of HO-1 activity on the progression of different diseases and cancers, and the vice versa, i.e., the effect of these diseases on HO-1 expression.
Earlier studies have linked HO-1 gene (GT)n repeat polymorphism with the cancer risk [86]. Shorter (GT) repeats have been linked with the low risk of different human cancers such as esophageal squamous cell carcinoma, lung adenocarcinoma, breast cancer, oral squamous cell carcinoma, gastric adenocarcinoma and malignant mesothelioma [86]. But some reports associate shorter (GT) repeats with higher cancer risk for pancreatic cancer, melanoma and gastric cancer [87, 88].
Neurodystrophic role of HO-1
Under certain conditions, HO-1 induction is responsible for providing neuroprotection (as described above). In a normal brain, HO-1 mRNA and protein expression are limited to few scattered neuroglia and neurons. Induction of HO-1 occurs in response to different stress-causing agents. In Alzheimer disease, overexpression of HO-1 protein occurs in neurons and astrocytes of the hippocampus and cerebral cortex [89]. Similarly, in Parkinson’s disease, HO-1 is overexpressed in astrocytes (present in the cerebral cortex) [90]. HO-1 is overexpressed in glial cells present in the vicinity of cerebral infarcts, within multiple sclerosis plaques, contusions and hemorrhages, and in other inflammatory and degenerative CNS disorders [91]. HO-1 hyperactivity (chronic expression) leads to the bioenergetic failure and pathological iron deposition as observed in Parkinson, Alzheimer and various other neurodevelopment diseases, by promoting mitochondrial associated non-transferrin iron sequestration and macroautophagy [92]. Moreover, it has been reported that irreversible neurological injury caused by excessive hyperbilirubinemia in untreated neonatal jaundice children, can be prevented by the introduction of synthetic metalloporphyrins (competitive inhibitors of HO activity) [93]. Metalloporphyrin treatment has been shown to confer neuroprotection in the intracerebral hemorrhage experimental model [94] and is associated with diminished edema formation and tissue necrosis in the focal cerebral ischemia rats [95]. HO-1 has been found to facilitate dopaminergic cell injury following polychlorinated biphenyls exposure, which may be of possible relevance to Parkinson’s disease [96]. Moreover, it is suggested that differences in experimental models, therapeutic protocols and species may be responsible for the disparate data concerning the role of HO-1 induction in neurological diseases.
HO-1 and other disorders
It has been reported by Ursu et al. that at later stages of myocarditis, HO-1 is associated with apoptosis of heart muscle cells [97]. Additionally, HO-1 overexpression can lead to muscle damage in in vitro and in vivo models, causing skeletal muscle atrophy. However, the lack of HO-1 considerably attenuates muscle atrophy [98].
Conclusion
A large number of studies have been carried out to delineate the diverse roles of HO-1 in inflammatory, neurodegenerative and other stress conditions. However, the actual role of HO-1 in diverse stresses has not been elucidated yet. Several studies have suggested the beneficial roles of HO-1 in humans, e.g., protect tissues against different stresses. While as others have reported the involvement of HO-1 in disease progression. It seems that HO-1 in an attempt to protect the cells from stress involuntarily leads to several detrimental conditions including neurodegenerative diseases and cancer. Thus, HO-1 induction in diverse stress conditions seems to be a necessary evil. So, there is a need to search for the link between the HO-1 and the candidate genes (such as oncogenes and tumor suppressor genes in cancers) involved in various diseases to identify the actual role of HO-1. This will significantly collaborate in unraveling the relation between HO-1 activation and etiology of such diseases. Furthermore, it will eventually help in making HO-1 a potential therapeutic target for the amelioration of various stress-related diseases. In conclusion, unraveling the crosstalk between HO-1 and different candidate genes involved in diverse diseases will facilitate to comprehend the exact role of HO-1 in different diseases.
References
Maines MD, Gibbs PE. 30 some years of heme oxygenase: from a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem Biophys Res Commun. 2005;338:568–77.
Nitti M, Piras S, Marinari UM, Moretta L, Pronzato MA, Furfaro AL. HO-1 induction in cancer progression: a matter of cell adaptation. Antioxidants. 2017;6:29.
Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–50.
Lin Q, Weis S, Yang G, Weng YH, Helston R, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–33.
Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, et al. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol. 2007;36:409–17.
Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, et al. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–53.
Bindu S, Pal C, Dey S, Goyal M, Alam A, et al. Translocation of heme oxygenase-1 to mitochondria is a novel cytoprotective mechanism against non-steroidal anti-inflammatory drug-induced mitochondrial oxidative stress, apoptosis, and gastric mucosal injury. J Biol Chem. 2011;286:39387–402.
Sacca P, Meiss R, Casas G, Mazza O, Calvo JC, et al. Nuclear translocation of haeme oxygenase-1 is associated to prostate cancer. Br J Cancer. 2007;97:1683–9.
Hsu FF, Yeh CT, Sun YJ, Chiang MT, Lan WM, et al. Signal peptide peptidase-mediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene. 2015;34:2360–70.
Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650.
Son Y, Lee JH, Chung HT, Pae HO. Therapeutic roles of heme oxygenase-1 in metabolic diseases: curcumin and resveratrol analogues as possible inducers of heme oxygenase-1. Oxid Med Cell Longev. 2013;2013:639541.
Li Volti G, Sacerdoti D, Di Giacomo C, Barcellona ML, Scacco A, et al. Natural heme oxygenase-1 inducers in hepatobiliary function. World J Gastroenterol. 2008;14:6122–32.
Doberer D, Haschemi A, Andreas M, Zapf TC, Clive B, et al. Haem arginate infusion stimulates haem oxygenase-1 expression in healthy subjects. Br J Pharmacol. 2010;161:1751–62.
Guijarro-Munoz I, Compte M, Alvarez-Cienfuegos A, Alvarez-Vallina L, Sanz L. Lipopolysaccharide activates toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 2014;289:2457–68.
Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491.
Konrad FM, Knausberg U, Hone R, Ngamsri KC, Reutershan J. Tissue heme oxygenase-1 exerts anti-inflammatory effects on LPS-induced pulmonary inflammation. Mucosal Immunol. 2016;9:98–111.
Lee JH, Jung NH, Lee BH, Kim SH, Jun JH. Suppression of heme oxygenase-1 by prostaglandin E2-protein kinase A-A-kinase anchoring protein signaling is central for augmented cyclooxygenase-2 expression in lipopolysaccharide-stimulated RAW 264.7 macrophages. Allergy Asthma Immunol Res. 2013;5:329–36.
Rushworth SA, MacEwan DJ, O’Connell MA. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J Immunol. 2008;181:6730–7.
Camhi SL, Alam J, Wiegand GW, Chin BY, Choi AM. Transcriptional activation of the HO-1 gene by lipopolysaccharide is mediated by 5′ distal enhancers: role of reactive oxygen intermediates and AP-1. Am J Respir Cell Mol Biol. 1998;18:226–34.
Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid Med Cell Longev. 2015;2015:504253.
Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune modulation by curcumin: the role of interleukin-10. Crit Rev Food Sci Nutr. 2017:1–13. https://doi.org/10.1080/10408398.2017.1358139
Pae HO, Jeong GS, Jeong SO, Kim HS, Kim SA, et al. Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp Mol Med. 2007;39:267–77.
Kim SY, Park E, Park JA, Choi BS, Kim S, et al. The plant phenolic diterpene carnosol suppresses sodium nitroprusside-induced toxicity in c6 glial cells. J Agric Food Chem. 2010;58:1543–50.
Kundu J, Chae IG, Chun KS. Fraxetin induces heme oxygenase-1 expression by activation of Akt/Nrf2 or AMP-activated protein kinase alpha/Nrf2 pathway in HaCaT cells. J Cancer Prev. 2016;21:135–43.
Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–91.
Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–74.
Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60:1121–8.
Chen HG, Xie KL, Han HZ, Wang WN, Liu DQ, Wang GL, Yu YH. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. Int J Surg. 2013;11:1060–6.
Otterbein LE, Bach FH, Alam J, Soares M, Tao H, Lu, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–8.
Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–8.
Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–6.
Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, et al. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;85:663–71.
Ndisang JF, Jadhav A. Heme oxygenase system enhances insulin sensitivity and glucose metabolism in streptozotocin-induced diabetes. Am J Physiol Endocrinol Metab. 2009;296:E829–41.
Galeotti C, Hegde P, Das M, Stephen-Victor E, Canale F, et al. Heme oxygenase-1 is dispensable for the anti-inflammatory activity of intravenous immunoglobulin. Sci Rep. 2016;6:19592.
Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017:2525967.
Chen J. Heme oxygenase in neuroprotection: from mechanisms to therapeutic implications. Rev Neurosci. 2014;25:269–80.
Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–13.
Takeda A, Perry G, Abraham NG, Dwyer BE, Kutty RK, et al. Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. J Biol Chem. 2000;275:5395–9.
Hung SY, Liou HC, Kang KH, Wu RM, Wen CC, et al. Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol Pharmacol. 2008;74:1564–75.
Sun GY, Chen Z, Jasmer KJ, Chuang DY, Gu Z, Hannink M, Simonyi A. Quercetin attenuates inflammatory responses in BV-2 microglial cells: role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PloS One. 2015;10:e0141509.
Vairano M, Dello Russo C, Pozzoli G, Tringali G, Preziosi P, Navarra P. A functional link between heme oxygenase and cyclo-oxygenase activities in cortical rat astrocytes. Biochem Pharmacol. 2001;61:437–41.
Chen-Roetling J, Regan RF. Effect of heme oxygenase-1 on the vulnerability of astrocytes and neurons to hemoglobin. Biochem Biophys Res Commun. 2006;350:233–7.
Song W, Patel A, Han D, Paudel HK, Schipper HM. Heme oxygenase-1 promotes proteosomal degradation of tau and alpha-synuclein in human neuroblastoma cells. Alzheimer Assoc. 2008; 4(Supplement):T410.
Bolisetty S, Traylor A, Zarjou A, Johnson MS, Benavides GA, et al. Mitochondria-targeted heme oxygenase-1 decreases oxidative stress in renal epithelial cells. Am J Physiol Renal Physiol. 2013;305:F255–64.
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. American Heart Association Statistics, S. Stroke Statistics. Heart disease and stroke statistics–2011 update: a report from the. American Heart Association. Circulation. 2011;123:e18–e209.
Ewing JF, Raju VS, Maines MD. Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3′:5′-guanosine monophosphate. J Pharmacol Exp Ther. 1994;271:408–14.
Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, et al. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–29.
Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–73.
Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, et al. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–25.
Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J Cardiovasc Pharmacol Ther. 2005;10:251–63.
Schillaci G, Pirro M, Ronti T, Gemelli F, Pucci G, et al. Prognostic impact of prolonged ventricular repolarization in hypertension. Arch Int Med. 2006;166:909–13.
Mordukhovich I, Kloog I, Coull B, Koutrakis P, Vokonas P, Schwartz J. Association between particulate air pollution and QT interval duration in an elderly cohort. Epidemiology. 2016;27:284–90.
Grochot-Przeczek A, Kotlinowski J, Kozakowska M, Starowicz K, Jagodzinska J, et al. Heme oxygenase-1 is required for angiogenic function of bone marrow-derived progenitor cells: role in therapeutic revascularization. Antioxid Redox Signal. 2014;20:1677–92.
Pirro M, Schillaci G, Romagno PF, Mannarino MR, Bagaglia F, et al. Influence of short-term rosuvastatin therapy on endothelial progenitor cells and endothelial function. J Cardiovasc Pharmacol Ther. 2009;14:14–21.
Lin HH, Chen YH, Yet SF, Chau LY. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost JTH. 2009;7:1401–8.
Bianconi V, Sahebkar A, Kovanen P, Bagaglia F, Ricciuti B, et al. Endothelial and cardiac progenitor cells for cardiovascular repair: a controversial paradigm in cell therapy. Pharmacol Ther. 2018;181:156–68.
Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol. 2004;286:F425–441.
Lever JM, Boddu R, George JF, Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal. 2016;25:165–83.
Feitoza CQ, Goncalves GM, Bertocchi AP, Wang PW, Damiao MJ, et al. A role for HO-1 in renal function impairment in animals subjected to ischemic and reperfusion injury and treated with immunosuppressive drugs. Transp Proc. 2007;39:424–6.
Aizawa T, Ishizaka N, Taguchi J, Nagai R, Mori I, et al. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats: possible role in renoprotection. Hypertension. 2000;35:800–6.
Aycan-Ustyol E, Kabasakal M, Bekpinar S, Alp-Yildirim FI, Tepe O, et al. Vascular function and arginine and dimethylarginines in gentamicin-induced renal failure: a possible effect of heme oxygenase 1 inducer hemin. Can J Physiol Pharmacol. 2017;95:1406–13.
Origassa CS, Camara NO. Cytoprotective role of heme oxygenase-1 and heme degradation derived end products in liver injury. World J Hepatol. 2013;5:541–9.
McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase-1 in hepatocytes and is protective in simulated cold preservation and warm reperfusion injury. Transplantation. 2006;81:623–6.
Waza. AA, Hamid Z. Majoon-e-Dabeed-ul-Ward protects lung cells against ethanol-induced cell death and activates Nrf2/HO-1 signaling pathway. Int J Res BioSci. 2018;7:1–7.
McCarter SD, Badhwar A, Scott JR, Akyea TG, Bihari A, et al. Remote liver injury is attenuated by adenovirus-mediated gene transfer of heme oxygenase-1 during the systemic inflammatory response syndrome. Microcirculation. 2004;11:587–95.
Sass G, Soares MC, Yamashita K, Seyfried S, Zimmermann WH, et al. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology. 2003;38:909–18.
Wu H, Zhang G, Huang L, Pang H, Zhang N, Chen Y, Wang G. Hepatoprotective effect of polyphenol-enriched fraction from folium microcos on oxidative stress and apoptosis in acetaminophen-induced liver injury in mice. Oxid Med Cell Longev. 2017;2017:3631565.
Tiwari S, Ndisang JF. The heme oxygenase system and type-1 diabetes. Curr Pharm Des. 2014;20:1328–37.
Lee EM, Lee YE, Lee E, Ryu GR, Ko SH, et al. Protective effect of heme oxygenase-1 on high glucose-induced pancreatic beta-cell injury. Diabetes Metab J. 2011;35:469–79.
Castany S, Carcole M, Leanez S, Pol O. The induction of heme oxygenase 1 decreases painful diabetic neuropathy and enhances the antinociceptive effects of morphine in diabetic mice. PloS One. 2016;11:e0146427.
Prawan A, Kundu JK, Surh YJ. Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Antioxid Redox Signal. 2005;7:1688–703.
Abdalla MY, Ahmad IM, Switzer B, Britigan BE. Induction of heme oxygenase-1 contributes to survival of Mycobacterium abscessus in human macrophages-like THP-1 cells. Redox Biol. 2015;4:328–39.
Genter MB, Burman DM, Vijayakumar S, Ebert CL, Aronow BJ. Genomic analysis of alachlor-induced oncogenesis in rat olfactory mucosa. Physiol Genom. 2002;12:35–45.
Gong P, Hu B, Cederbaum AI. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch Biochem Biophys. 2004;432:252–60.
Nuhn P, Kunzli BM, Hennig R, Mitkus T, Ramanauskas T, et al. Heme oxygenase-1 and its metabolites affect pancreatic tumor growth in vivo. Mol Cancer. 2009;8:37.
Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–41.
Goswami A, Ranganathan P, Rangnekar VM. The phosphoinositide 3-kinase/Akt1/Par-4 axis: a cancer-selective therapeutic target. Cancer Res. 2006;66:2889–92.
Banerjee P, Basu A, Wegiel B, Otterbein LE, Mizumura K, et al. Heme oxygenase-1 promotes survival of renal cancer cells through modulation of apoptosis- and autophagy-regulating molecules. J Biol Chem. 2012;287:32113–23.
Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–40.
Jozkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal. 2003;5:155–62.
Mayerhofer M, Florian S, Krauth MT, Aichberger KJ, Bilban M, et al. Identification of heme oxygenase-1 as a novel BCR/ABL-dependent survival factor in chronic myeloid leukemia. Cancer Res. 2004;64:3148–54.
Nowis D, Legat M, Grzela T, Niderla J, Wilczek E, et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006;25:3365–74.
Hirai K, Sasahira T, Ohmori H, Fujii K, Kuniyasu H. Inhibition of heme oxygenase-1 by zinc protoporphyrin IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int J Cancer. 2007;120:500–5.
Momtazi AA, Shahabipour F, Khatibi S, Johnston TP, Pirro M, Sahebkar A. Curcumin as a microRNA regulator in cancer: a review. Rev Physiol Biochem Pharmacol. 2016;171:1–38.
Ghattas MH, Chuang LT, Kappas A, Abraham NG. Protective effect of HO-1 against oxidative stress in human hepatoma cell line (HepG2) is independent of telomerase enzyme activity. Int J Biochem Cell Biol. 2002;34:1619–28.
Murakami A, Fujimori Y, Yoshikawa Y, Yamada S, Tamura K, et al. Heme oxygenase-1 promoter polymorphism is associated with risk of malignant mesothelioma. Lung. 2012;190:333–7.
Kikuchi A, Yamaya M, Suzuki S, Yasuda H, Kubo H, et al. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase-1 gene promoter polymorphism. Hum Genet. 2005;116:354–60.
Sawa T, Mounawar M, Tatemichi M, Gilibert I, Katoh T, Ohshima H. Increased risk of gastric cancer in Japanese subjects is associated with microsatellite polymorphisms in the heme oxygenase-1 and the inducible nitric oxide synthase gene promoters. Cancer Lett. 2008;269:78–84.
Schipper HM, Cisse S, Stopa EG. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann Neurol. 1995;37:758–68.
Yu X, Song N, Guo X, Jiang H, Zhang H, Xie J. Differences in vulnerability of neurons and astrocytes to heme oxygenase-1 modulation: implications for mitochondrial ferritin. Sci Rep. 2016;6:24200.
Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem. 2009;110:469–85.
Schipper HM, Song W. A heme oxygenase-1 transducer model of degenerative and developmental brain disorders. Int J Mol Sci. 2015;16:5400–19.
Qato MK, Maines MD. Prevention of neonatal hyperbilirubinaemia in non-human primates by Zn-protoporphyrin. Biochem J. 1985;226:51–7.
Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–52.
Kadoya C, Domino EF, Yang GY, Stern JD, Betz AL. Preischemic but not postischemic zinc protoporphyrin treatment reduces infarct size and edema accumulation after temporary focal cerebral ischemia in rats. Stroke. 1995;26:1035–8.
Lee DW, Gelein RM, Opanashuk LA. Heme-oxygenase-1 promotes polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress and dopaminergic cell injury. Toxicol Sci. 2006;90:159–67.
Ursu ON, Sauter M, Ettischer N, Kandolf R, Klingel K. Heme oxygenase-1 mediates oxidative stress and apoptosis in coxsackievirus B3-induced myocarditis. Cell Physiol Biochem. 2014;33:52–66.
Kang J, Jeong MG, Oh S, Jang EJ, Kim HK, Hwang ES. A FoxO1-dependent, but NRF2-independent induction of heme oxygenase-1 during muscle atrophy. FEBS Lett. 2014;588:79–85.
Acknowledgements
Council of Scientific and Industrial Research (CSIR) GOI, New Delhi is acknowledged for providing fellowship to Ajaz Ahmad Waza (CSIR-RA fellow) (9/251 (0077)/2k17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no competing interests exist.
Additional information
Responsible Editor: Yoshiya Tanaka.
Rights and permissions
About this article
Cite this article
Waza, A.A., Hamid, Z., Ali, S. et al. A review on heme oxygenase-1 induction: is it a necessary evil. Inflamm. Res. 67, 579–588 (2018). https://doi.org/10.1007/s00011-018-1151-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1151-x