Abstract
Introduction
Food intolerance/malabsorption is caused by food ingredients, carbohydrates (mainly lactose and fructose), proteins (gluten), and biogenic amines (histamine) which cause nonspecific gastrointestinal and extra-intestinal symptoms. Here we focus on possible etiologic factors of intolerance/malabsorption especially in people with non-celiac gluten sensitivity (NCGS) or the so-called people without celiac disease avoiding gluten (PWCDAG) and histamine intolerance.
Methods
Recognizing the recently described symptoms of NCGS (PWCDAG) we review correlations and parallels to histamine intolerance (HIT).
Results
We show that intestinal and extra-intestinal NCGS (PWCDAG) symptoms are very similar to those which can be found in histamine intolerance.
Conclusions
After a detailed diagnostic workup for all possible etiologic factors in every patient, a targeted dietary intervention for single or possibly combined intolerance/malabsorption might be more effective than a short-term diet low in fermentable oligo-, di- and monosaccharides and polyols (FODMAP) or the untargeted uncritical use of gluten-free diets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food malabsorption syndromes and non-immunological food intolerances are reported in approximately 20% of the population in westernized countries and they cause nonspecific gastrointestinal symptoms and several extra-intestinal symptoms [1, 2]. Food intolerance/malabsorption is caused by certain food ingredients, carbohydrates (mainly lactose and fructose), proteins (gluten), and biogenic amines (histamine) which impair digestion [3]. Here we describe possible etiologic factors of intolerance/malabsorption, especially in people with non-celiac gluten sensitivity (NCGS) and people without celiac disease avoiding gluten (PWCDAG), and their link to histamine intolerance. Recognizing the recently described symptoms of NCGS (PWCDAG) we discuss correlations and parallels to histamine intolerance (HIT). Food malabsorption or intolerance requires personalized treatment and an individual dietary intervention for sustained relief of symptoms. After a detailed diagnostic workup for all possible etiologic factors in each patient, a targeted dietary intervention for single or possibly combined malabsorption might help more than an untargeted diet low in fermentable oligo-, di- and monosaccharides and polyols (FODMAP) or the widespread uncritical use of gluten-free diets.
Non-celiac gluten sensitivity: people without celiac disease avoiding gluten
Celiac disease or gluten malabsorption is an autoimmune disorder that occurs in genetically predisposed persons as a reaction to ingested gluten proteins, which are mainly found in wheat, rye, and barley. Celiac disease can be diagnosed with serologic testing and histologic confirmation, and has an estimated global prevalence of up to 5% in the general population. A gluten-free diet is the only effective treatment [4].
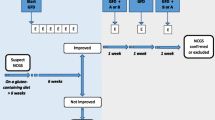
The use of gluten-free diets has rapidly increased in popularity and are followed by up to 20% of the population in Europe and the United States, mainly by people without celiac disease [5]. Therefore, a new symptom-based disorder related to the consumption of gluten-containing products, namely non-celiac gluten sensitivity (NCGS), is under discussion [6,7,8,9]. Since there are no diagnostic criteria available for these people they may be called “people without celiac disease avoiding gluten” (PWCDAG) [10]. So far, the diagnosis of NCGS (PWCDAG) solely relies on self-reported symptoms by patients and is self-diagnosed and then self-treated [11]. Although symptoms alone or symptom complexes are rarely, if ever, diagnostic [12], NCGS (PWCDAG) is characterized by a combination of a wide variety of intestinal and extra-intestinal symptoms (Table 1). However, since no specific blood test or radiological and/or endoscopic examination is known for NCGS (PWCDAG), factors other than gluten are suspected to cause NCGS (PWCDAG). A recent study demonstrated that gluten-containing cereals have a high content of amylase–trypsin inhibitors and this increased intestinal inflammation via activation of the toll-like receptor of myeloid cells [13]. There is some evidence that other factors, for example, the oligosaccharides fructane or galactane in wheat, might cause the reported symptoms [14]. It is also suspected that people with NCGS (PWCDAG) constitute a group of patients with irritable bowel syndrome (IBS) who are self-diagnosed and perform self-treatment by adhering to a gluten-free diet [15].
Withdrawal of wheat products reduces the symptom severity and improves the quality of life in persons with NCGS (PWCDAG) [16] and novel plant breeding strategies are being used to produce gluten-reduced grains. However, a gluten-free diet is expensive, usually high in fat and low in fibre [17], and is therefore, not recommended for healthy people because it may even lead to adverse health effects, as it may be associated with a higher risk of type 2 diabetes mellitus [18]. So far, it seems that gluten-containing food triggers the symptoms in NCGS (PWCDAG) despite the fact that NCGS (PWCDAG) is not well defined.
Gluten-containing grains are found in many foods, including bread, pasta, pizza, bulgur, couscous, and drinks such as beer [4]. However, most of these foods and drinks also contain the biogenic amine histamine [19] and/or they are usually consumed with additional histamine-containing seasonings. Many gluten-containing bakery goods and beers contain yeast [20, 21], and bulgur, pasta and pizza [22] are consumed regularly with tomatoes and other seasonings which because of their high histamine content are not digested and metabolized properly in people with histamine intolerance (HIT) [19, 23, 24]. Here we show that intestinal and extra-intestinal NCGS (PWCDAG) symptoms are very similar to those which can be found in histamine intolerance [25, 26]. Gastrointestinal nonspecific symptoms in HIT include postprandial fullness, flatulence, bloating, abdominal pain, loose stools, diarrhea and/or obstipation. Extra-intestinal symptoms include headache, migraine [27], foggy mind, chronic fatigue [28], joint and muscle pain, tingling of extremities, leg or arm numbness [29], eczema [30], asthma [31] and depression [28]. All of these may be associated with NCGS (PWCDAG), too (Table 1). However, withdrawal of gluten-containing wheat products reduces the overall quantity of the parallel consumption of histamine, which also reduces HIT-associated symptoms and this may explain the current wide spread popularity of gluten-free diets.
The only symptom which is described with NCGS (PWCDAG) that was not included in the table of symptoms (Table 1) is anaemia, because there is no correlation to histamine receptors. Additionally, co-incidence data on patients infected with Helicobacter pylori, the Gram-negative pathogen in the stomach frequently causing ion-deficiency anaemia [32, 33], are neither known for NCGS (PWCDAG) nor for HIT.
Histamine intolerance
The discovery of histamine [2-(4-imidazolyl)-ethylamine] dates back more than 100 years and histamine plays an important role as a mediator in allergic reactions including inflammation. New receptors of histamine were discovered and its importance in immune regulation leads to the development of selective antihistamine medications. The histamine pathway in allergic disease is described with several pharmacogenetic variations influencing disease expression and a response to treatment [34].
The name “histamine intolerance” is used due to postulated diamine oxidase (DAO) enzyme deficiency with reference to the term lactose intolerance. A scombroid poisoning is caused by consumption of fish contaminated with bacteria that cause high concentrations of histamine. Ingested histamine, even in small quantities, clearly below the dose causing scombroid poisoning, is suspected to cause HIT-related symptoms in affected people [35].
A disproportionate amount of histamine in the body is suspected to result from the consumption of histamine-containing food or drinks, and a reduced ability of enzymes (diamine oxidase and histamine N-methyl transferase) to digest histamine. Histamine is widely distributed throughout the body and within the gastrointestinal tract DAO appears to be the primary enzyme for the degradation of ingested histamine [36]. DAO is synthesized by mature apical enterocytes located in the upper intestinal villi and is continuously released from the intestinal mucosa for digestion and into the blood circulation [37]. Mucosal damage in the small intestine caused by, e.g., gastroenteritis, short bowel syndrome, gastrointestinal surgery and various drugs may also reduce DAO activity [38].
There are polymorphisms identified in the genes coding for DAO [39, 40] and for the known histamine receptors [41] which may help to explain the wide individual variability of symptoms observed in multiple organs. These polymorphisms may also influence disease expression and potentially the response to diets or treatment. Although these polymorphisms have been identified, their functional and clinical significance has not yet been determined. The clinical diagnosis of histamine intolerance (HIT) is difficult [25], and standardized in vitro diagnostic tests for HIT testing are still lacking, but the diagnosis of HIT may be supported with the measurement of diamine oxidase in serum [42]. Although serum DAO values have not been shown to correlate with gastrointestinal DAO activity, patients with reduced serum DAO activity (< 10 U/mL), two or more typical gastrointestinal symptoms described for HIT, and a reduction of abdominal complaints after following a histamine reduced diet, may be diagnosed with HIT [25, 43]. Concerning all these limitations determinations of serum DAO in well-defined patients with NCGS (PWCDAG) are needed to evaluate if their DAO activity is reduced [44].
Recently a high prevalence of low serum DAO values in patients with carbohydrate (lactose and fructose) intolerance/malabsorption was described. In patients with nonspecific gastrointestinal symptoms seven combinations of intolerance/malabsorption were described and more than 55% of these patients demonstrated DAO values < 10 U/ml [3]. It was reported that an individual and subjective perception of nonspecific gastrointestinal symptoms, which is often associated with carbohydrate malabsorption, also needs to be considered [45, 46]. Additionally, a so-called visceral hypersensitivity seems to play an essential role in gastrointestinal symptoms, but the triggers of these symptoms are still not understood and HIT may also play a role. Another factor to be considered is that the histamine content in food varies considerably depending on ripeness, storage time, and processing [47]. All of these variables need to be considered and may help to explain each person’s unique and even sometimes changing tolerance levels of food intolerance/malabsorption.
Discussion
There is increasing interest in food intolerance/malabsorption syndromes causing nonspecific abdominal complaints and what dietary triggers may be responsible. In recent years advances have led to a better understanding of the role of certain nutrients and food components. The gastrointestinal bacteria use various catabolic enzymes to degrade and ferment carbohydrates and proteins from food [48]. However, several experiments suggest that the amount and type of dietary carbohydrates and proteins affect the metabolic output of these microbes [49]. Food intolerance/malabsorption causes gastrointestinal nonspecific and extra-intestinal symptoms when a particular nutrient or a combination of certain nutrients cannot be absorbed and digested properly.
In fructose intolerance or malabsorption the sugar fructose is not absorbed adequately by glucose transporters in the small intestine [50]. Lactose intolerance is the inability to digest lactose found in milk and dairy products due to a deficiency of the lactose degrading enzyme lactase. In patients with intolerance/malabsorption, these carbohydrates, fructose and/or lactose, reach the large intestine where they are metabolized by intestinal bacteria, resulting in fermentation and hydrogen production. Therefore, for the clinical testing and diagnosis of lactose and fructose intolerance/malabsorption, using a hydrogen breath test is helpful in patients with nonspecific abdominal complaints [51]. Studies reported combined lactose and fructose intolerance/malabsorption in patients with functional gastrointestinal disorders [52] and demonstrated variable combinations of lactose, fructose and histamine intolerance/malabsorption [3].
One suggested diet for nonspecific abdominal complaints is a diet low in fermentable oligo-, di- and monosaccharides and polyols (FODMAP) which restricts a wide variety of foods from different food groups (cereals, fruits and vegetables, milk and dairy products) [53]. It is stated that patients with food malabsorption should be advised to avoid dietary triggers for as short a time as possible, usually 3–4 weeks, to induce symptom improvement [15]. Since there are several known genetic polymorphisms for lactase [54], celiac disease [55], DAO and histamine receptors [39,40,41], some genetic background might also exist for fructose malabsorption [56]. There are various combinations of intolerance/malabsorption [3], and a gradual food reintroduction within a short time period may cause the symptoms to return. Some clinical evidence supports the use of a low FODMAP diet for a limited time in clinical practice, but due to the genetic background and various combinations of intolerance/malabsorption, the short-term dietary changes with a low FODMAP diet can have only modest and rapidly reversible effects.
After a detailed individual diagnosis of single or combined food intolerance/malabsorption, a registered dietician is needed to develop an individually tailored diet to ensure nutritional adequacy [57, 58]. Since changes to diet clearly have an effect on the gastrointestinal microbiome, the effect of long-term removal of specific foods or food components is unknown [59]. It also seems important to consider the individual’s tolerance level when recommending dietary restrictions to reduce symptoms for a long time, to enlarge dietary variety, ensure nutritional adequacy and cause a minimal impact on the gastrointestinal microbiota [23].
Conclusions
Nonspecific abdominal complaints need to be assessed individually. All etiologic factors of intolerance/malabsorption including fructose, gluten, histamine, lactose, and Helicobacter pylori infection must be evaluated. Intolerance/malabsorption can then be treated by reducing the ingestion of the triggering nutrients and/or treating the Helicobacter pylori infection based on a detailed diagnosis and the individual tolerance level to the symptomatology.
References
Ortolani C, Pastorello EA. Food allergies and food intolerances. Best Pract Res Clin Gastroenterol. 2006;20:467–83.
Lomer MCE. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther. 2015;41:262–75.
Enko D, Meinitzer A, Mangge H, Kriegshäuser G, Halwachs-Baumann G, Reininghaus EZ, et al. Concomitant prevalence of low serum diamine oxidase activity and carbohydrate malabsorption. Can J Gastroenterol Hepatol. 2016;2016:4893501.
Valenti S, Corica D, Ricciardi L, Romano C. Gluten-related disorders: certainties, questions and doubts. Ann Med. 2017. https://doi.org/10.1080/07853890.2017.1325968.
Fasano A, Sapone A, Zevallos V, Schuppan D. Non-celiac gluten sensitivity. Gastroenterology. 2015;148:1195 – 204.
Czaja-Bulsa G. Non coeliac gluten sensitivity—a new disease with gluten intolerance. Dig Dis Sci. 2015;34:189 – 94.
Tavakkoli A, Lewis SK, Tennyson CA, Lebwohl B, Green PHR. Characteristics of patients who avoid wheat and/or gluten in the absence of celiac disease. Dig Dis Sci. 2014:59:1255–61.
van Gils T, Nijeboer P, IJssennagger CE, Sanders DS, Mulder CJJ, Bouma G. Prevalence and characterization of self-reported gluten sensitivity in the Netherlands. Nutrients. 2016;8:714.
Ontiveros N, López-Gallardo JA, Vergara-Jiménez MJ, Cabrera-Chávez F. Self-reported prevalence of symptomatic adverse reactions to gluten and adherence to gluten-free diet in an adult Mexican population. Nutrients. 2015;7:6000–15.
Choung RS, Unalp-Arida A, Ruhl MD, Brantner CE, Everhart TL, Murray JE. JA. Less hidden celiac disease but increased gluten avoidance without a diagnosis in the United States: Findings from the National Health and Nutrition Examination Surveys from 2009 to 2014. Mayo Clin Proc. 2017;92:30 – 3.
El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. The relation between celiac disease, nonceliac gluten sensitivity and irritable bowel syndrome. Nutr J. 2015;14:92.
Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–94.
Zevallos VF, Raker V, Tenzer S, Jimenez-Calvente C, Ashfaq-Khan M, Rüssel N, et al. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology. 2017;152:1100–13.
Elli L, Branchi F, Tomba C, Villalta D, Norsa L, Ferretti F, et al. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J Gastroenterol. 2015;21:7110–9.
De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. 2016;65:169–78.
Zanwar VG, Pawar SV, Gambhire PA, Jain SS, Surude RG, Shah VB, et al. Symptomatic improvement with gluten restriction in irritable bowel syndrome: a prospective, randomized, double blinded placebo controlled trial. Intest Res. 2016;14:343 – 50.
Tanner GJ, Blundel MJ, Colgrave ML, Howitt CA. Creation of the first ultra-low gluten barley (Hordeum vulgare L.) for coeliac and gluten-intolerant populations. Plant Biotechnol J. 2016;14:1139–50.
Low gluten diets may be associated with higher risk of type 2 diabetes. American Heart Association Meeting Report Presentation 11. http://newsroom.heart.org/news/low-gluten-diets-may-be-associated-with-higher-risk-of-type-2-diabetes?preview=b2db. Accessed Nov 2017.
Kovacova-Hanuskova E, Buday T, Gavliakova S, Plevkova J. Histamine, histamine intoxication and intolerance. Allergol Immunopathol (Madr). 2015;43:498–506.
Qi W, Hou LH, Guo HL, Wang CL, Fan ZC, Liu JF, et al. Effect of salt-tolerant yeast of Candida versatilis and Zygosaccharomyces rouxii on the production of biogenic amines during soy sauce fermentation. J Sci Food Agric. 2014;94:1537–42.
Verheyen C, Albrecht A, Herrmann J, Strobl M, Jekle M, Becker T. The contribution of glutathione to the destabilizing effect of yeast on wheat dough. Food Chem. 2015;173:243–9.
Chefkoch.de. http://www.chefkoch.de/rs/s60/bulgur/Rezepte.html; 593 Rezepte mit Bulgur. Accessed June 2017.
Kohn JB. Is there a diet for histamine intolerance? J Acad Nutr Diet. 2014;114:1860.
San Mauro Martin I, Brachero S, Garicano Vilar E. Histamine intolerance and dietary management: a complete review. Allergol Immunopathol (Madr). 2016;44:475 – 83.
Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185–96.
Smolinska S, Jutel M, Crameri R, O’Mahony L. Histamine and gut mucosal immune regulation. Allergy. 2014;69:273–81.
García-Martín E, Martínez C, Serrador M, Alonso-Navarro H, Ayuso P, Navacerrada F, et al. Diamine oxidase rs10156191 and rs2052129 variants are associated with the risk for migraine. Headache. 2015;55:276 – 86.
Cacabelos R, Torrellas C, Fernández-Novoa L, López-Muñoz F. Histamine and immune biomarkers in CNS disorders. Mediators Inflamm. 2016;2016:1924603.
Romero SA, Hocker AD, Mangum JE, Luttrell MJ, Turnbull DW, Struck AJ, et al. Evidence of a broad histamine footprint on the human exercise transcriptome. J Physiol. 2016;594:5009–23.
Worm M, Fiedler EM, Dölle S, Schink T, Hemmer W, Jarisch R, et al. Exogenous histamine aggravates eczema in a subgroup of patients with atopic dermatitis. Acta Derm Venerol. 2009;89:52–6.
Neumann D. Role of the histamine H4-receptor in bronchial asthma. Handb Exp Pharmacol. 2017;241:347 – 59.
Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter. 2017;22:e12330.
Mentis A, Lehours P, Francis Megraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2015;20(Suppl. 1):1–7.
Jones BL, Kearns GL. Histamine. New thoughts about a familiar mediator. Clin Pharm Therap. 2011;89:189 – 97.
Schwelberger HG. Histamine intolerance: a metabolic disease? Inflamm Res. 2010;59(Suppl 2):S219–21.
Ji Y, Sakata Y, Li X, Zhang C, Yang Q, Xu M, et al. Lymphatic diamine oxidase secretion stimulated by fat absorption is linked with histamine release. Am J Physiol Gastrointest Liver Physiol. 2013;304:G732-G740.
Wollin A, Wang X, Tso P. Nutrients regulate diamine oxidase release from intestinal mucosa. Am J Physiol. 1988;275:R969-R975.
Elsenhans B, Hunder G, Strugala G, Schümann K. Longitudinal pattern of enzymatic and absorptive functions in the small intestine of rats after short-term exposure to dietary cadmium chloride. Arch Environ Contam Toxicol. 1999;36:341–6.
Maintz L, Yu CF, Rodríguez E, Baurecht H, Bieber T, Illig T, et al. Association of single nucleotide polymorphisms in the diamine oxidase gene with diamine oxidase serum activities. Allergy. 2011;66:893–902.
Petersen J, Raithel M, Schwelberger HG. Characterisation of functional polymorphisms of the human diamine oxidase gene. Inflamm Res. 2005;54(Suppl 1):S58–9.
Sadek B, Stark H. Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology. 2015;106:56–73.
Mušič E, Korošec P, Šilar M, Adamič K, Košnik M, Rijavec M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien Klin Wochenschr. 2013;125:239 – 43.
Rosell-Camps A, Zibetti S, Pérez-Esteban G, Vila-Vidal M, Ferrés-Ramis L, García-Teresa-García E. Histamine intolerance as a cause of chronic digestive complaints in pediatric patients. Rev Esp Enferm Dig. 2013;105:201–6.
Lionetti E, Pulvirenti A, Vallorani M, Catassi G, Verma AK, Gatti S, Catassi C. Re-challenge studies in non-celiac gluten sensitivity: a systematic review and meta-analysis. Front Physiol. 2017;8:621.
Casellas F, Aparici A, Casaus M, Rodríguez P, Malagelada JR. Subjective perception of lactose intolerance does not always indicate lactose malabsorption. Clin Gastroenterol Hepatol. 2010;8:581–6.
Enko D, Rezanka E, Stolba R, Halwachs-Baumann G. Lactose malabsorption testing in daily clinical practice: a critical retrospective analysis and comparison of the hydrogen/methane breath test and genetic test (c/t-13910 polymorphism) results. Gastroenterol Res Pract. 2014;2014:464382.
Reese I, Ballmer-Weber B, Beyer K, Fuchs T, Kleine-Tebbe J, Klimek L, et al. German guideline for the management of adverse reactions to ingested histamine. Allergo J Int. 2017;26:72–9.
Pimentel M, Mathur R, Chang C. Gas and the microbiome. Curr Gastroenterol Rep. 2013;15:356.
Scott KP, Gratz SW, Sheridon PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60.
Gibson PR, Newnham E, Barrett JS, Shepherd SJ, Muir JG. Review article: fructose malabsorption and the bigger picture. Aliment Pharm Ther. 2007;25:349 – 63.
Hovde O, Farup PG. A comparison of diagnostic tests for lactose malabsorption—which one is the best? BMC Gastroenterol. 2009;9:82.
Goebel-Stengel M, Stengel A, Schmidtmann M, van der Voort I, Kobelt P, Mönnikes H. Unclear abdominal discomfort: Pivotal role of carbohydrate malabsorption. J Neurogastroenterol Motil. 2014;20:228–35.
Catassi G, Lionetti E, Gatti S, Catassi C. The low FODMAP diet: many question marks for a catchy acronym. Nutrients. 2017;9:292.
Järvelä I, Torniainen S, Kolho KL. Molecular genetics of human lactase deficiencies. Ann Med. 2009;41:568 – 75.
Hunt KA, van Heel DA. Recent advances in coeliac disease genetics. Gut. 2009;58:473–6.
Patel C, Douard V, Yu S, Tharabenjasin P, Gao N, Ferraris RP. Fructose-induced increases in expression of intestinal fructolytic and gluconeogenic genes are regulated by GLUT5 and KHK. Am J Physiol Regul Integr Comp Physiol. 2015;309:R499–R509.
Schnedl WJ, Enko D, Mangge H, Schenk M, Lackner S, Holasek SJ. Bening pancreatic hyperenzymemia (Gullo syndrome), histamine intolerance, and carbohydrate malabsorption. Proc (Bayl Univ Med Cent). 2017;30:177–8.
Schnedl WJ, Lipp RW, Wallner-Liebmann S, Kalmar P, Szolar DH, Mangge H. Primary epiploic appendagitis and fructose intolerance. Eur J Clin Nutr. 2014;68:1359–61.
Albenberg LG, Wu GD. Diet and the intestinal microbiome: Associations, functions and implications for health and disease. Gastroenterology. 2014;146:1564–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Wolfgang J. Schnedl received speaking honoraria from Sciotec. The other authors declare no competing interests.
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Schnedl, W.J., Lackner, S., Enko, D. et al. Non-celiac gluten sensitivity: people without celiac disease avoiding gluten—is it due to histamine intolerance?. Inflamm. Res. 67, 279–284 (2018). https://doi.org/10.1007/s00011-017-1117-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1117-4