Abstract
Objective
The aim of this review is to examine the role of polymorphonuclear neutrophils (PMNs) in the evolution of atherosclerosis.
Introduction
While the role of PMNs in the evolution of atherosclerosic process has failed until recently to attract much attention, a body of research carried out over the last decade has disclosed the unexpectedly complex behavior of these cells, unraveling an unexpected key role for PMNs in the onset and progression of atheroma.
Methods
A PubMed database search was performed for studies providing evidences on the role of PMNs in the development and progression of atherosclerotic lesion.
Results and Conclusions
Activated PMNs were shown to produce and release reactive oxygen species, inflammatory leukotrienes and proteolytic lysosomal enzymes, directly inducing vascular damage. Activated PMNs also secrete myeloperoxidase, involved in lipoprotein oxidation. PMNs have a finite lifespan and typically die through apoptosis, which thus represents a counter-regulatory mechanism limiting the toxic potential of these short-lived, terminally differentiated cells. Dysregulation of this process probably contributes to the pathogenesis and progression of several inflammatory diseases. Moreover, high circulating levels of PMN–platelet aggregates have been reported in patients with clinical atherosclerosis, and recent studies suggest that these aggregates may play a role in vascular response to injury. It has been suggested that this heterotypic interaction between platelets and leukocytes might represent a link between hemostasis/thrombosis and the inflammatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade, much effort has been devoted to elucidating the role of specific cells involved in the inflammatory and immune responses that occur in every phase of the atherosclerotic process [1]. In particular, macrophages have been shown to account for the majority of leukocytes entering the plaques, and macrophage colony-stimulating factor produced by the inflamed intima was demonstrated to induce monocytes entering the plaque to differentiate into macrophages. This step is associated with upregulation of pattern-recognition receptors for innate immunity, including scavenger receptors and Toll-like receptors, and plays a crucial role in the development of atherosclerosis [2–4]. Different macrophage subpopulations identified by specific gene expression patterns have been observed in coronary artery lesions and associated with pro-thrombotic activities [5]. Moreover, the high prevalence of oligoclonal T-cell expansion in unstable coronary plaques [6, 7] suggests that adaptive immunity might be related to coronary instability. Accordingly, an increased frequency of autoaggressive CD4+CD28null T cells was observed in patients with acute coronary syndrome (ACS) [6, 8]. CD4+CD28null T cells infiltrate unstable coronary plaques where they undergo clonal expansion [9] and release large amounts of pro-inflammatory cytokines, in particular interferon-γ (IFN-γ), thus activating monocytes and macrophages. Direct cytolytic effects of CD4+CD28null T cells on endothelial cells [10] and on vascular smooth muscle cells [11] have been demonstrated.
Recently, a T-cell repertoire perturbation involving two other T-cell subsets, type 17 helper T cells (Th17) and CD4+CD25+ regulatory T cells (Treg) has been proven [12, 13].
Conversely, the role of polymorphonuclear neutrophils (PMNs) in atherosclerotic plaque evolution has attracted little attention until recently [14]. Given their limited lifespan, PMN infiltrates are seldom observed within human atherosclerotic plaques in comparison with other inflammatory cells. However, studies carried out during the last decade have shed light on the important role of PMNs in the onset, progression and instability of atherosclerotic plaque, which will be the subject of this review.
Neutrophils and inflammatory response
Endothelial dysfunction, triggered by irritative stimuli such as hyperlipidemia, high shear, and pro-inflammatory cytokines, is considered to be the initial stage of atherosclerosis [15]. The subsequent expression of adhesion molecules, such as E-selectin, P-selectin and intercellular adhesion molecule-1, triggers the recruitment and adhesion of PMNs [16]. The adhesion of PMNs to endothelial cells via β2-integrin ligation in turn induces the mobilization of cytoplasmic granules and secretory vesicles, containing a wide variety of membrane-bound receptors for endothelial adhesion molecules and extracellular matrix proteins, as well as antimicrobial proteins, proteases, components of the respiratory burst oxidase, and soluble mediators of inflammation that amplify the inflammatory response [17–20] (Table 1). The extravasation of PMNs to the site of inflammation is promptly followed by a second wave of migrating monocytes [21] (Fig. 1a). PMNs are also able to generate leukotriene B4 (LTB4) [22], a lipid mediator that, besides stimulating the generation of reactive oxygen species (ROS) and the release of granular enzymes by PMNs, represents a chemotactic factor for PMNs themselves and for other leukocytes, and augments endothelial adhesiveness and vascular permeability [23].
Neutrophils in inflammatory response. a Adhesion of PMNs to endothelial cells triggers the mobilization of cytoplasmic granules and secretory vesicles, containing a wide variety of membrane-bound receptors for endothelial adhesion molecules and extracellular matrix proteins, as well as antimicrobial proteins, proteases, components of the respiratory burst oxidase, and soluble mediators of inflammation that amplify the inflammatory response. The extravasation of PMNs to the site of inflammation is followed by a second wave of migrating monocytes; granule proteins released by PMNs on the endothelium at the site of inflammation induce adhesion and recruitment of inflammatory monocytes. In particular, azurocidin, LL-37 and cathepsin G released from secretory vesicles enhance the expression of vascular cell-adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM), resulting in enhanced adhesion of monocytes to endothelial cells. b Furthermore, PMN granule proteins promote de-novo synthesis of monocyte-attracting chemokines, such as MCP-1 and MIP-1α, from endothelial cells and macrophages, favoring the extravasation of monocytes. c PMN granule proteins, such as LL37 and α-defensin, play an important role in the activation of dendritic cells which internalize, process and present antigen, resulting in the activation of T-lymphocytes and enhancing the release of pro-inflammatory cytokines, such as INF-γ, TNF-α and IL-2. Abbreviations: APC-DC antigen-presenting cell-dendritic cell, ICAM intercellular adhesion molecule; IL interleukin, INF-γ interferon-γ, MCP-1 monocyte chemotactic protein-1, MHC major histocompatibility complex, MIP-1α macrophage inflammatory protein-1α, PMNs polymorphonuclear neutrophils, TCR T-cell receptor, TLR Toll-like receptor, TNF-α tumor necrosis factor-α, VCAM vascular cell-adhesion molecule
Azurocidin and proteinase-3 released from the secretory vesicles enhance the expression of vascular cell-adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), resulting in adhesion of monocytes to endothelial cells [24, 25].
Other antimicrobial peptides known to possess monocyte-attracting activity are cathepsin G [26] and cathelicidins (LL-37 in humans [27]), both of which can be found in plaques [28]. Furthermore, modification of the chemokine network by PMNs creates a milieu favoring extravasation of inflammatory monocytes. Azurocidin and related granule proteins, stimulating the release of chemokine from monocytes and macrophages, also favor the extravasation of monocytes (Fig. 1b). Finally, emigrated PMNs rapidly undergo apoptosis, leading to exposure of granule proteins and entrapment within a net of DNA that creates a gradient of chemotactic granule components relevant to monocyte recruitment [21].
The influence of PMNs on macrophages also seems to contribute significantly to more advanced stages of atherosclerosis. PMNs may activate macrophages by secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-8 and IFN-γ. Furthermore, myeloperoxidase (MPO) released from granules can induce the formation of ROS, along with the release of other pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8 and granulocyte–macrophage-colony-stimulating factor (GM-CSF) from macrophages [29]. ROS in turn modify extravasated low-density lipoprotein (LDL) into oxidized LDL, thereby supplying the substrate for foam cell development [30].
Data from studies investigating the role of inflammatory cells in chronic inflammatory disease suggest that PMNs may contribute to adaptive immunity by direct or mediated interaction with T cells and dendritic cells. Cathepsin G and azurocidin may work as a chemoattractant for T lymphocytes [26], recruiting them to the sites of inflammation. Cathepsin G furthermore stimulates IFN-γ secretion by T lymphocytes [31]. Other data suggest that human PMN granule proteins, such as LL-37 (or mouse Cramp) and α-defensin, play an important role in the activation of dendritic cells, which internalize, process and present the antigen, resulting in the activation of T lymphocytes [32] (Fig. 1c). While complexes of DNA and LL-37 trigger plasmocytoid dendritic cell activation and subsequent type I interferon release [33], complexes of RNA and LL-37 also induce plasmocytoid dendritic cell maturation [34]. LL-37 [33] and α-defensin [32], moreover, play an important role in the recruitment and activation of dendritic cells.
Polymorphonuclear neutrophils are also capable of producing several cytokines which promote systemic inflammatory responses [20, 35], modulate endothelial permeability [36], and affect endothelial and smooth muscle cell responses [37]. PMNs actively release ATP at sites of hypoxia or ongoing inflammation [38]. PMN-derived pro-oxidative enzymes such as NADPH oxidase and MPO not only mediate acute inflammatory responses, but also catalyze reactions that consume vascular nitric oxide (NO), resulting in impaired endothelial function [39]. On the other hand, the effect of ATP accumulation on endothelial function has not been fully elucidated [40].
Finally, in addition to influencing the inflammatory response through direct cell–cell interactions with other immune and non-immune cells [41], PMNs are also the major cellular source of anti-inflammatory and pro-resolving lipid-derived mediators (such as lipoxins, resolvins, and protectins), which promote the return of inflamed tissues to homeostasis by halting leukocyte traffic, stimulating the removal of apoptotic leukocytes, and redirecting the chemokine–cytokine axis towards anti-inflammatory pathways [42]. In-depth recognition of these paradoxical anti-inflammatory mechanisms is very important, since uncontrolled inflammation may represent an important step in the pathogenesis of cardiovascular disease [43].
Neutrophils in experimental animal models of atherosclerosis
The systematic investigation of the mechanisms that initiate atherosclerosis and mediate its clinical manifestation relies on animal models [14, 44].
An early influx of PMNs has been described in several experimental models of vascular injury [45–53]. Soehnlein et al. [19] highlighted the multiple roles of PMNs in animal models of plaque formation and destabilization. In a mouse model of plaque vulnerability and rupture, Sasaki et al. [47] observed PMN infiltrates 4 days after collar placement in previously ligated carotid arteries. The evoked intraplaque hemorrhage was associated with decreased collagen content and increased apoptosis in the intima; in addition, abundant accumulation of PMNs was detected after cuff placement, suggesting a contribution of infiltrating PMNs to plaque instability and rupture. Van Leeuwen et al. [48] investigated the presence and the distribution of PMNs and MPO during the development and progression of atherosclerosis in LDL-receptor-deficient (LDLR–/–) mice fed with a high-fat diet for different time periods. Immunohistochemistry revealed marked accumulation of PMNs close to the fibrous cap and in the arterial adventitia. Zernecke et al. [49] studied the CXC ligand (CXCL)-12/CXC receptor (CXCR)-4 chemokine-receptor axis in apolipoprotein-E-deficient (ApoE–/–) or LDLR–/– mice and demonstrated that the chronic blockade of CXCR4 caused leukocytosis and expansion of PMNs, with increased PMNs in plaques. The depletion of circulating PMNs attenuated plaque formation in ApoE–/– mice and prevented CXCR4 antagonist-induced lesion progression. Drechsler et al. [50], in ApoE–/– mice, showed that the hypercholesterolemia-induced increased peripheral PMN count was closely correlated with the extent of early atherosclerosis. Rotzius et al. [51], in a murine model of ApoE-deficient mice carrying a knock-in mutation for enhanced green fluorescent protein (EGFP), which allows for sensitive detection of PMNs in atherosclerotic plaques, found that the number of PMNs was higher in the shoulder regions of plaques, especially at sites of high inflammatory activity. Recently, Döring et al. [52] have demonstrated that self-DNA, released from dying cells or by PMN extracellular traps, and elevated expression of the antimicrobial peptide Cramp/LL-37 in atherosclerotic plaque stimulate a plasmacytoid dendritic cell-driven pathway of autoimmune activation, with a consequent aggravation of atherosclerotic lesion formation.
In contrast, in an experimental study investigating the involvement of PMN-derived granule proteins in arterial healing, Soehnlein et al. [53] identified a prominent role for PMN-borne Cramp/LL-37 in promoting re-endothelization and limiting neointima formation after stent implantation by a variety of mechanisms, including angiogenic early outgrowth cell recruitment and endothelial recovery by paracrine effects (LL-37 stimulates the release of the pro-angiogenic factors VEGF and EGF). Moreover, the authors engineered a biofunctionalized stent coated with LL-37, and demonstrated that this PMN-instructing device can reduce in-stent restenosis in mice [45].
Polymorphonuclear neutrophils can thus be considered as first-line immune cells, recruited to sites of arterial injury. The rapid release of their granule proteins critically shapes the vascular inflammatory response, leading to amplification of the inflammatory injury during the early healing process. These data underline the complexity of action of PMNs and support their importance in atherosclerosis.
Neutrophils in human atherosclerosis and its clinical manifestation
Observational epidemiologic studies have documented a relationship between elevated circulating white blood cell count and increased cardiovascular risk [54, 55]. Among white blood cell subtypes, a greater predictive ability was found for the PMN count [56], which correlated with circulating levels of pro-inflammatory cytokines [57]. Furthermore, angiographic studies in patients with chronic stable angina (SA) [58] or ACS [59] showed that PMN count was an independent predictor of the presence of multiple complex coronary stenoses. Several clinical studies also demonstrated a marked in-vivo activation of PMNs in patients with unstable angina (UA) or acute myocardial infarction (AMI) [60, 61]. Mehta et al. [60] observed in UA patients a 15-fold increase in levels of peptide Bβ, a marker of elastase release and a constituent of primary granules such as MPO. Biasucci et al. [62] found that circulating PMNs from patients with UA and AMI had low MPO content, indicative of PMN activation. Moreover, in patients with resolving UA, PMN MPO content returned to levels similar to those in patients with SA and in normal subjects, suggesting that PMN activation was confined to the active phase of UA. By measuring, in aortic and coronary sinus blood, leukocyte expression of CD11b and CD18, cell surface glycoproteins upregulated in response to chemotactic factors and involved in the leukocyte adhesion process, Mazzone et al. [63] demonstrated a transcoronary activation of PMNs in UA patients. Buffon et al. [61] confirmed the transcoronary PMN activation in patients with UA by measuring their MPO content. This activation was not confined to the coronary bed perfused by the artery in which the culprit stenosis was located, suggesting a widespread inflammatory process.
Naruko et al. [64] showed that all culprit lesions of patients who had died of AMI had PMNs within the plaques, while PMNs were extremely rare in coronary lesions obtained from patients who had died of non-cardiovascular diseases. Similarly, more PMNs were found in the culprit lesions of patients with UA than in plaques extracted from patients with stable disease. Ionita et al. [65], in 355 human carotid plaques, showed that PMNs were strongly associated with IL-8 and matrix metalloproteases 8 and 9 plaque levels, and with histopathologic features of rupture-prone lesions, such as large lipid core, heavy macrophage influx, low collagen amount and smooth muscle cell numbers. Furthermore, Ferrante et al. [66] reported a significant elevation in serum MPO in ACS patients presenting with culprit plaque erosion compared with those with culprit plaque rupture. Additionally, in postmortem specimens obtained from an unrelated sample, luminal thrombi superimposed on eroded plaque contained more MPO-positive cells than thrombi superimposed on ruptured plaques, suggesting a role for PMNs in plaque erosion [66].
In summary, data from human studies show a positive correlation between increased PMN count and cardiovascular risk, and document increased numbers and/or activation of PMNs in patients with overt cardiovascular disease. PMNs are present at sites of plaque rupture and, perhaps more importantly, might directly contribute to plaque erosion.
Plaque instability and neutrophils: myeloperoxidase
The function of PMNs at the site of tissue lesion can be summarized as endocytosis of foreign material and secretion of intracellular enzymes (such as elastase, endopeptidase and MPO) [20].
Myeloperoxidase (MPO) is the main component of the azurophilic granules of the PMNs; this cationic protein is promptly liberated after activation by different antagonists contributing to the innate immune response of the organism [67]. Through reaction with hydrogen peroxide, MPO forms free radicals and diffusible oxidative substances with antimicrobial activity. It also exercises pleiotropic effects in the vascular system with potential impact on atherosclerosis development, endothelial dysfunction, plaque destabilization and ventricular remodeling after ischemic injury [68]. Oxidative modification of LDL leads to its uptake and degradation by macrophages, resulting in cholesterol deposit and formation of foam cells [69]. MPO is capable of promoting oxidation of lipoproteins in vivo [70]. Macrophages use NADPH oxidase to produce superoxide (O2 −) that can dismutate and form hydrogen peroxide (H2O2). MPO catalyzes reactions with H2O2 to generate more potent cytotoxic oxidizers like hypochlorous acid (HOCl) and tyrosyl radical, the only human enzyme capable of generating HOCl. Several stable final products generated by these species have been detected in atherosclerotic plaques [70, 71].
Myeloperoxidase as catalyst of lipid oxidation: effects on LDL and HDL
Myeloperoxidase has the ability to modify the amino-acid tyrosine of the apolipoprotein B-100 (apo B-100) using H2O2 and Cl− ions to generate 3-chlorotyrosine. It can also generate 3-nitrotyrosine using nitrite (NO2 −), the final product of the metabolism of the NO [72, 73]. Podrez et al. [72] characterized the MPO–H2O2–NO2 system as the preferential pathway used by monocytes to convert LDL into their atherogenic form (Fig. 2). HDL are also susceptible to oxidative modifications mediated by MPO, through nitration or halogenation of tyrosine residues in apolipoprotein A-I (Apo A-I), harming the ability of the protein to promote the ABCA-1-dependent reverse transport of cholesterol [70, 74] (Fig. 2).
MPO and the evolution of atherosclerosis. MPO participates in the initiation and progression of atheroma, favoring lipid peroxidation and generation of atherogenic lipoproteins. Modified low-density lipoproteins (LDL), predominantly oxidized, play a central role in promoting the atherogenic process. The oxidized form of LDL is selectively recognized by the scavenger receptor CD36, a major participant in fatty streak and lesion development. MPO also generates dysfunctional high-density lipoprotein (HDL), by nitration or halogenation of tyrosine residues in the apolipoprotein A-I. These molecules harm the ability of the protein to promote the ABCA-1-dependent reverse transport of cholesterol, thus contributing to the formation of atherosclerotic lesions. MPO also participates in ischemic complications of atherosclerosis via activation of protease cascades, leading to breakdown of the fibrous cap. Culprit lesions are typically macrophage-rich atheroma containing large amounts of matrix metalloproteinases and prothrombotic material. These plaques are more likely to undergo thinning and subsequent breakdown of the overlying fibrous cap. This exposes circulating blood to the thrombogenic core of the plaque, resulting in thrombus formation, luminal compromise and ischemia. MPO-derived lipid oxidation products enriched in atheroma activate endothelial cells, promoting the surface expression of P-selectin, favoring platelet adhesion. MPO-expressing macrophages result in increased expression and activity of tissue factor. Latent tissue factor pathway activity is also activated by lipid hydroperoxides which are generated by the activity of MPO. Abbreviations: ABCA-1 ATP-binding cassette a-1, HDL high-density lipoproteins, LDL low-density lipoproteins, MPO myeloperoxidase
Myeloperoxidase and nitric oxide metabolism: contribution to endothelial dysfunction
Nitric oxide (NO), produced by endothelial nitric oxide synthase (NOS), is a powerful vasodilator. NO suppresses the binding of circulating cells to the endothelium and inhibits the proliferation of smooth muscle cells in the vascular wall. There are strong indications that MPO may reduce the bioavailability of NO by several mechanisms [75]. Firstly, NO serves as a substrate for peroxidases and MPO may thus serve as a catalytic sink for NO [75]. Secondly, scavenging of NO by MPO-derived reactive substances may further reduce the bioavailability of NO. Thirdly, HOCl can react with nitrogen atoms of the NOS substrate arginine to produce chlorinated arginine species, which are inhibitors of all isoforms of NOS and have been shown to impair endothelium-dependent relaxation of rat aortic rings [76]. Finally, it has been demonstrated that HOCl is a potent inducer of uncoupling of endothelial NOS, thereby turning NOS into a superoxide-producing enzyme [77]. Although the relative impact of these mechanisms is currently unknown, it is clear that MPO, by catalytic as well as noncatalytic processes, depletes NO in the vascular wall. In a study by Baldus et al. [78], release of vascular MPO from the subendothelial space by intravenous administration of heparin resulted in an improvement of endothelium-dependent vascular function.
Myeloperoxidase and plaque vulnerability
Recent studies emphasize the modulating action of MPO over the protease cascade involved in the acute complications of atherosclerosis [79]. Serum MPO is considered a marker of PMN activation [80], although the presence of circulating monocytes with high or low MPO expression suggests the existence of other possible sources [81]. MPO catalyzes lipid peroxidation in vivo [82], leading to tissue factor activation [83] and tissue factor pathway inhibitor inactivation [84]. Moreover, MPO reacts with hydrogen peroxide and Cl− to form an enzymatic substrate complex with strong oxidant activity and, as MPO is a highly basic protein, it can firmly bind to negatively charged glycosaminoglycans and proteoglycans in the extracellular matrix [85]. Fu et al. [86] demonstrated generation of oxidative species of HOCl by MPO, through activation of pro-matrilysin (MMP-7), an enzyme capable of promoting degradation of the extracellular matrix and potentially contributing to atherosclerotic plaque instability. Additionally, MPO, by reducing NO bioavailability, is able to modify the endothelial surface, from antithrombotic to thrombogenic (see above).
Myeloperoxidase and its oxidation products have been detected in human atherosclerotic lesions [87, 88]. Sugiyama et al. [87] identified a novel subset of macrophages containing MPO and infiltrating the subendothelium at sites of coronary plaque erosion or rupture, with few PMNs in the same lesions. In a subsequent in-vitro study, the same group reported a pathogenic role for MPO in determining plaque erosion, in which they showed that HOCl may directly cause human endothelial cell death and detachment by both apoptotic and oncotic pathways, and that HOCl at sublethal concentrations increases the expression of tissue factor by endothelial cells [89]. Ferrante et al. [66] confirmed the presence of two subpopulations of MPO-positive cells, PMNs and macrophages, in thrombi superimposed onto eroded plaques, also reporting a significant elevation in serum MPO in patients with culprit plaque erosion (see above). Buffon et al. [61] documented a transcoronary gradient of leukocyte intracellular MPO depletion in patients with UA, which was independent of the location of the culprit lesion, suggesting widespread leukocyte activation in ACS (see above).
In summary, MPO may play an important role in the process of plaque instability. However, the difficulty of detecting PMNs within human atherosclerotic plaque, due to their limited life span, has not allowed clarification of whether plaque MPO derives from PMNs or from monocytes/macrophages. Nevertheless, PMNs may exert their pro-inflammatory functions via macrophage activation, thus contributing both directly and indirectly to MPO production.
Myeloperoxidase as marker of atherosclerosis
Using an enzyme assay, Zhang et al. [90] conducted a case–control study to determine the association between MPO levels and coronary artery disease (CAD) in 158 patients with established CAD and 175 patients without angiographically significant CAD. In multivariate models adjusted for traditional risk factors, Framingham risk score and leukocyte count, MPO levels were significantly associated with the presence of CAD. More recently, Meuwese et al. [91], in the European Prospective Investigation into Cancer and Nutrition (EPIC) Norfolk study, demonstrated that MPO levels were significantly higher in case subjects than in controls, and were correlated with C-reactive protein (CRP) and white blood cell count. Risk of future CAD increased in consecutive quartiles of MPO concentration, with an odds ratio (OR) of 1.49 in the top versus the bottom quartile. Serum MPO levels were associated with the risk of future development of CAD in apparently healthy individuals, but the association was weaker than that of traditional risk factors and CRP. However, MPO, unlike CRP, was largely independent of classical risk factors.
In ACS, MPO has consistently been associated with the presence of instability and the risk of future events. Biasucci et al. [62] first observed that circulating PMNs in patients with AMI and UA have a low MPO content, indicative of PMN activation. The lack of PMN activation in patients with variant angina and after stress test suggests that this phenomenon may occur independently of ischemia. Furthermore, in this study MPO did not correlate with creatine kinase-MB (CK-MB) and troponin T release, suggesting a role for MPO as a marker of instability.
Several studies have investigated the value of MPO in predicting long-term outcomes. Li et al. [92] studied 176 consecutive patients who underwent coronary angiography; the patients were divided into four groups according to quartiles of MPO level. They found that ACS prevalence in the fourth quartile of MPO levels was six times higher than that in the first quartile. The long-term prognostic value of MPO and of markers of protein oxidation was also evaluated by Mocatta et al. [29] in 512 AMI patients. MPO at 24–96 h after admission was higher in patients than in controls. Patients with high MPO levels in combination with high levels of NT-proBNP and reduced ejection fraction had significantly reduced survival.
Plaque instability and neutrophils: neutrophil apoptosis
Polymorphonuclear neutrophils are terminally differentiated and normally have a very short lifespan in circulation (8–20 h) and in tissue (1–4 days). Apoptosis plays an important role in eliminating PMNs from inflamed tissues without releasing hazardous intracellular contents [93]. Apoptotic PMNs are phagocytosed by other cells, a process that is associated with the release of anti-inflammatory mediators. Thus, PMN apoptosis controls the duration and the intensity of the inflammatory response and, therefore, the extent of PMN-mediated tissue damage [94]. In addition, PMN apoptosis is an important mechanism that maintains appropriate PMN numbers under physiologic conditions [95]. Thus, the death program in PMNs needs to be tightly controlled.
The relevance of PMN death to the pathogenesis of infectious and inflammatory diseases has just begun to be recognized. A growing body of evidence suggests that PMN death is mediated by a complex network of intracellular death/survival signaling pathways and can be modulated by a variety of extracellular stimuli such as pro-inflammatory cytokines.
Intracellular modulators
Reactive oxygen species, produced by NADPH oxidase in activated PMNs to facilitate bacterial killing, are recognized as one of the causal mediators of PMN apoptosis [96]. ROS may lead to DNA alteration and trigger p53 leading to genotoxic injury. Alternatively, ROS may directly alter the activity of intracellular signaling pathways involved in PMN death/survival such as nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK) and c-jun N-terminal kinase (JNK).
Among intracellular modulators of PMN apoptosis, a key role is recognized to be played by caspases, a family of cysteine proteases [97]. Caspase cascade can be activated by two independent mechanisms. The first, the extrinsic death receptor pathway, is mainly mediated by TNF receptors (TNFR) and Fas, which can subsequently trigger downstream caspase cascade via activation of caspase 8. The second is known as the intrinsic apoptosis pathway, and is a mitochondria-mediated cellular process characterized by loss of mitochondrial membrane potential and release of pro-apoptotic factors such as cytochrome c to cytosol, leading to the assembly of a multimolecular complex known as apoptosome and to the activation of caspase 9.
Polymorphonuclear neutrophils do not express anti-apoptotic factor Bcl-2, instead they express anti-apoptotic Bcl-2 family proteins Mcl-1, A1 and Bcl-xL; however, during PMN apoptosis the Mcl-1 protein level is gradually reduced. Constitutive PMN apoptosis is also associated with upregulation of death signaling and downregulation of survival signaling pathways. For example, the activity of protein kinase B (PKB)/Akt, a pro-survival and anti-apoptotic factor, is dramatically reduced during PMN death. On the other hand, treatment with GM-CSF, granulocyte-colony stimulating factor (G-CSF) or IFN-γ promotes cell survival [98].
Extracellular stimuli
Polymorphonuclear neutrophil death can be accelerated by many extracellular stimuli and can be induced in response to specific ligands that engage the so-called cell surface “death receptors”. PMNs constitutively express high levels of Fas and Fas ligand (FasL), and Fas engagement by agonistic anti-Fas antibody leads to enhanced mitochondrial permeability and caspase-8 and -3 activation [99]. PMNs also express other death receptors such as TRAIL-R2 and R3 and their ligand, TNF-related apoptosis-inducing ligand (TRAIL) [100]. Following Fas, TNF-α, and TRAIL receptor activation, small heterodimer partner-1 (SHP-1) interacts with an important tyrosine kinase known as Lyn, dephosphorylates it and transduces pro-apoptotic signals in PMNs [101].
Although PMNs undergo apoptosis even in the absence of any extracellular stimuli, their survival time increases significantly once they migrate out of the circulation and into the sites of inflammation, where they encounter pro-survival cytokines and chemokines, such as interferon, lipopolysaccharide (LPS), GM-CSF and G-CSF. Plasmocytoid dendritic cells activated by complexes of neutrophilic DNA and LL-37 release type I interferon [33] that, in turn, delays the PMN apoptosis via activation of signal transducer and activator of transcription 3 (STAT3) and upregulation of the cellular inhibitor of apoptosis 2 (cIAP2) expression [102]. LPS suppresses apoptosis of primary and HL-60-derived PMNs through activation of Toll-like receptor 4 (TLR4) and subsequent enhancement of Akt phosphorylation, thus increasing the levels of pro-survival factors Mcl-1 and A1.
Granulocyte–macrophage-colony-stimulating factor induces delay of apoptosis by coupling its receptor with the Src family tyrosine kinase, Lyn, and by subsequently upregulating anti-apoptotic pathways such as PI3K/Akt and downregulating pro-apoptotic factors [103]. Additionally, potassium released locally by damaged cells and a large amount of antioxidants produced by red blood cells can work as a survival signal and delay PMN apoptosis [ 93]. Finally, it has been reported that culturing PMNs in the presence of platelets dramatically inhibits PMN apoptosis [104], even if the underlying mechanisms have not been defined yet.
Neutrophil apoptosis and inflammation
A variety of autoimmune/rheumatic diseases, such as rheumatoid arthritis, are associated with decreased propensity to apoptosis by PMNs [105].
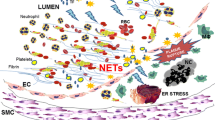
Apoptosis at the inflammation site appears to be delayed when survival factors are generated by neighboring cells or even by autocrine production of pro-inflammatory cytokines. Recently, a peculiar cell death, characterized by active release of nuclear chromatin with embedded granule proteins and termed NETosis, has been identified for PMNs; neutrophils expel nuclear DNA forming a scaffold of granule proteins and histones (neutrophil extracellular traps or NET). Interestingly, PMN extracellular traps have recently been identified in association with human and murine atherosclerotic lesions [106].
It has also been demonstrated that PMN apoptosis could have a relevant role in monocyte recruitment [21]. In recent years several apoptotic cell-derived “find me” signals have been identified, such as the S19 ribosomal protein dimer, split tyrosyl-tRNA synthetase, thrombospondin-1 and lysophosphatidylcholine [107].
An important question is how PMNs suddenly decide to undergo apoptosis, a scenario that occurs even in presence of survival factors. Under most conditions, PMNs will be exposed to both pro-apoptotic and anti-apoptotic factors. The net effect on PMN death and survival reflects a balance between the activities of such factors. One mechanism able to fine-tune this balance is the O2 tension at the site of inflammation. In the synovial fluid of rheumatoid arthritis patients, the pro-apoptotic pathways are predominantly activated, and O2 concentration is around 20 %. Under hypoxia (1 % O2 tension), the activity of the anti-apoptotic factors will predominate [108]. However, several fundamental questions still remain unanswered. For example, the initial trigger stimuli for constitutive death are elusive. Many internal modulations and external stimuli can delay constitutive PMN death, but none of them can completely prevent it.
Neutrophil apoptosis and acute coronary syndromes
Telomeres are specialized DNA–protein structures that contain non-coding TTAGGG repeats and associated proteins, essential for chromosome stability. Depending upon the cellular context, telomere shortening may lead to cell senescence or apoptosis. Telomere maintenance is primarily achieved by telomerase, a ribonucleoprotein with reverse transcriptase activity that uses its internal ribonucleic acid component as a template for the synthesis of telomeric DNA. In the absence of the enzyme, telomeres shorten with cell division, a process that may act as a mitotic clock and signal entry into senescence. Shortening of telomeres is thus held responsible for the limited lifespan of somatic cells in culture and has also been associated with organismal aging [109]. Indeed, inhibition of telomerase and the ensuing telomere shortening below a critical length result in apoptosis in various cell types, whereas induction of telomerase activity is associated with resistance to apoptosis [110].
Telomerase activity is normally absent in PMNs, but once these cells undergo ex-vivo expansion under mitogenic stimulation, telomerase activity is dramatically increased [111] and it could represent a way to overcome replicative senescence. Narducci et al. [112] demonstrated high telomerase activity in PMNs from coronary plaques of patients with UA, but not from patients with SA or in PMNs from peripheral blood. Notably, in patients with UA, the only predictor of telomerase activity in the coronary atherosclerotic plaque was a shorter time interval from symptom onset to PMN collection. Therefore, in unstable patients, the survival of locally activated PMNs could be prolonged by telomerase reactivation confined to coronary plaque PMNs. Specific mechanisms explaining PMN telomerase reactivation in patients with UA, however, are not known. Biffl et al. [113] reported that IL-6 delays PMN apoptosis in vitro. GM-CSF and G-CSF are also able to increase mature PMN survival [114]. All these soluble factors are expressed in atherosclerotic plaques [35]. The determination of the mechanisms of telomerase reactivation and apoptotic delay of PMNs in patients with UA may help in the understanding of the complex phenomenon of plaque instability, and may also potentially open new therapeutic strategies.
Normal human PMNs, like other somatic cells, divide a limited number of times before entering a non-dividing state called replicative senescence. Telomerase is normally inhibited in these inflammatory cells [111, 115].
Data from Narducci et al. [112] are in keeping with a previous study showing that telomerase activity is barely detectable in circulating PMNs, but that telomerase reactivation in these cells is possible [116], as typically observed in cell types that retain high proliferative potential [117, 118].
Moreover, Biasucci et al. [119] demonstrated a delayed apoptosis of peripheral PMNs in UA patients and the correlation of delayed PMN apoptosis with high-sensitivity CRP (hs-CRP) levels and with recurrence of symptoms at 48 h. Furthermore, they found a negative correlation between spontaneous PMN apoptosis and hs-CRP, suggesting that cytokines responsible for the induction of acute phase proteins, in particular IL-6, might also play a role in determining PMN survival. Finally, this study is in agreement with the significant correlation between time from symptom onset and PMN telomerase activity found by Narducci et al. [112].
Plaque instability and neutrophils: neutrophil–platelet interaction
Adhesive interactions between vascular cells play important roles in orchestrating the inflammatory response. Platelet adhesion to and leukocyte rolling on endothelium are the initial stages of a multistep process leading to extravasation of white blood cells to sites of inflammation, to platelet–leukocyte interaction and aggregation on a thrombogenic surface. Recruitment of circulating leukocytes to vascular endothelium requires adhesive and signaling events, including selectin-mediated attachment and rolling, leukocyte activation, and integrin-mediated firm adhesion and diapedesis [120].
Leukocyte recruitment and infiltration also occur at sites of vascular injury where the lining endothelial cells have been denuded and platelets and fibrin have been deposited. In-vivo studies show that leukocytes and platelets co-localize at sites of hemorrhage, within atherosclerotic and post-angioplasty restenosis lesions, and in areas of ischemia–reperfusion injury. This heterotypic interaction between platelets and leukocytes links the hemostatic/thrombotic and inflammatory responses [121].
The initial tethering and rolling of leukocytes on platelet P-selectin [122, 123] are followed by their firm adhesion and transplatelet migration, processes that are dependent on the leukocyte integrin (Mac-1) and platelet glycoprotein (GpIb) [124–126]. The binding of platelets to PMNs influences key cellular effector responses by inducing PMN activation, upregulating expression of cell adhesion molecules [127], and generating signals that promote integrin activation [125], chemokine synthesis [128, 129] and the respiratory burst [127]. Recently, Fuchs et al. [130] have demonstrated that PMN extracellular traps can cause platelet activation, adhesion and aggregation in vitro, suggesting a new potential mechanism linking the hemostatic/thrombotic and inflammatory responses. Interestingly, both PMN–platelet and monocyte–platelet aggregates have been identified in the peripheral blood of patients with CAD and may be markers of disease [127]. Moreover, the inhibition of platelet–PMN interactions had beneficial effects in an animal model of vascular injury [131]. A cross-talk between monocytes, PMNs and platelets may be responsible for the initiation and amplification of deep vein thrombosis [132].
Fuchs et al. [130] demonstrated that NETs can cause platelet activation, adhesion and aggregation in vitro. Ott et al. [127] observed an increased number of PMN–platelet aggregates and a strong correlation between the number of aggregates and the degree of activation of PMNs in peripheral blood of ACS patients, but not in SA patients. Moreover, PMNs from ACS patients showed a higher degree of activation compared with PMNs from stable patients, while no differences were found in the degree of platelet activation between unstable and stable patients, suggesting that platelets could activate PMNs through a mechanism requiring direct cell-to-cell interaction. Circulating PMN–platelet aggregates significantly increased 1–2 h after percutaneous coronary intervention (PCI), then returned to normal after 4 h. Their precocious but brief appearance in blood may suggest that these aggregates have a role in the early phases of plaque instability [133]. Garlichs et al. [134] demonstrated that co-culture of isolated PMNs with activated platelets induced a significant delay in PMN apoptosis, indicating a partial activation of platelets under culture conditions. Stimulation of platelets with ADP further delayed PMN apoptosis in co-culture. Enhanced leukocyte–platelet interactions in ACS patients resulted in enhanced cardiac tissue damage [135].
Recently, Maugeri et al. [136] have observed that, in the very early phase of AMI, only a subpopulation of PMNs is massively activated, with consequent massive MPO release, possibly via platelet–P-selectin interactions, contributing to thrombus formation. Previous experimental models supported the concept that P-selectin is able to induce PMN activation and degranulation [137]. The time course of P-selectin expression observed by Maugeri and the evidence that platelet P-selectin is able to prompt the degranulation of PMNs in vitro [137] suggest that PMN MPO complete depletion may occur when platelets express P-selectin rather abruptly, as in the very early phases of AMI. In contrast, platelets of patients with UA are characterized by a persistent, waxing and waning, P-selectin expression. The transient nature of PMN activation in patients with AMI could be explained by rapid loss of P-selectin as a consequence of active proteolysis by the inhibitory effect of pentraxin 3 released by PMNs as a consequence of activated platelet adhesion. Moreover, the clearance of activated platelets likely represents a further mechanism involved in the swift termination of PMN activation [138]. These observations may have diagnostic and pathogenic implications.
Conclusions
The role of PMNs in the evolution of atherosclerosis has attracted little attention until recently. Research carried out over the last decade, however, has revealed the hitherto unappreciated complexity of PMNs and has disclosed an unexpected role of PMNs in the onset and progression of atherosclerosis. Moreover, recent data show a potential role of PMNs in the processes of atherosclerotic plaque instability, while a growing body of evidence supports the role of PMNs as a link between inflammation and atherosclerotic/thrombotic processes.
Although more definitive data are required before a firm link can be established between PMNs and the evolution of atherosclerosis into ACS, the possibility that these cells may represent a potential target for new pharmacologic approaches in ACS patients is intriguing and worth the research efforts.
References
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92(18):8264–8.
Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14(1):123–8.
Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216.
Libby P, Nahrendorf M, Pittet MJ, Swirski FK. Diversity of denizens of the atherosclerotic plaque: not all monocytes are created equal. Circulation. 2008;117(25):3168–70.
Liuzzo G, Goronzy JJ, Yang H, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101(25):2883–8.
De Palma R, Del Galdo F, Abbate G, et al. Patients with acute coronary syndrome show oligoclonal T-cell recruitment within unstable plaque: evidence for a local, intracoronary immunologic mechanism. Circulation. 2006;113:640–6.
Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100(21):2135–9.
Zal B, Kaski JC, Arno G, et al. Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation. 2004;109(10):1230–5.
Nakajima T, Schulte S, Warrington KJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105(5):570–5.
Pryshchep S, Sato K, Goronzy JJ, Weyand CM. T cell recognition and killing of vascular smooth muscle cells in acute coronary syndrome. Circ Res. 2006;98(9):1168–76.
Han SF, Liu P, Zhang W, et al. The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin Immunol. 2007;124(1):90–7.
Cheng X, Yu X, Ding YJ, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127(1):89–97.
Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15.
Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92.
Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–94.
Soehnlein O, Weber C, Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30:538–46.
Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–5.
Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110(6):875–88.
Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–31.
Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114(21):4613–23.
Borgeat P, Hamberg M, Samuelsson B. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J Biol Chem. 1976;251:7816–20.
Di Gennaro A, Kenne E, Wan M, Soehnlein O, Lindbom L, Haeggström JZ. Leukotriene B4-induced changes in vascular permeability are mediated by neutrophil release of heparin-binding protein (HBP/CAP37/azurocidin). FASEB J. 2009;23:1750–7.
Taekema-Roelvink ME, Kooten C, Kooij SV, Heemskerk E, Daha MR. Proteinase 3 enhances endothelial monocyte chemoattractant protein-1 production and induces increased adhesion of neutrophils to endothelial cells by upregulating intercellular cell adhesion molecule-1. J Am Soc Nephrol. 2001;12(5):932–40.
Soehnlein O, Xie X, Ulbrich H, et al. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol. 2005;174(10):6399–405.
Chertov O, Ueda H, Xu LL, et al. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med. 1997;186(5):739–47.
Kai-Larsen Y, Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front Biosci. 2008;13:3760–7.
Soehnlein O, Weber C. Myeloid cells in atherosclerosis: initiators and decision shapers. Semin Immunopathol. 2009;31(1):35–47.
Mocatta TJ, Pilbrow AP, Cameron VA, et al. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007;49:1993–2000.
Tavakoli S, Asmis R. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid Redox Signal. 2012;17(12):1785–95.
Woloszynek JC, Hu Y, Pham CT. Cathepsin G-regulated release of formyl peptide receptor agonists modulate neutrophil effector functions. J Biol Chem. 2012;287(41):34101–9.
Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68(1):9–14.
Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–9.
Ganguly D, Chamilos G, Lande R, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206(9):1983–94.
Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81.
DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30:547–56.
Raines EW, Ferri N. Thematic review series: the immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. J Lipid Res. 2005;46:1081–92.
Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99(10):1100–8.
Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–4.
Gündüz D, Aslam M, Krieger U, et al. Opposing effects of ATP and adenosine on barrier function of rat coronary microvasculature. J Mol Cell Cardiol. 2012;52(5):962–70.
Drechsler M, Döring Y, Megens RT, Soehnlein O. Neutrophilic granulocytes—promiscuous accelerators of atherosclerosis. Thromb Haemost. 2011;106(5):839–48.
Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312.
Bengtsson E, To F, Hakansson K, et al. Lack of the cysteine protease inhibitor cystatin C promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2151–6.
Cullen P, Baetta R, Bellosta S, et al. Rupture of the atherosclerotic plaque: does a good animal model exist? Arterioscler Thromb Vasc Biol. 2003;23:535–42.
Soehnlein O, Wantha S, Simsekyilmaz S, et al. Neutrophil-derived cathelicidin protects from neointimal hyperplasia. Sci Transl Med. 2011;3(103):103ra98.
Yvan-Charvet L, Welch C, Pagler TA, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via Toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118(18):1837–47.
Sasaki T, Kuzuya M, Nakamura K, et al. A simple method of plaque rupture induction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1304–9.
Van Leeuwen M, Gijbels MJ, Duijvestijn A, et al. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR-/-mice. Arterioscler Thromb Vasc Biol. 2008;28:84–9.
Zernecke A, Bot I, Djalali-Talab Y, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–17.
Drechsler M, Megens R, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–45.
Rotzius P, Thams S, Soehnlein O, et al. Distinct infiltration of neutrophils in lesion shoulders in ApoE-/- mice. Am J Pathol. 2010;177(1):493–500.
Döring Y, Manthey HD, Drechsler M, et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125:1673–83.
Soehnlein O, Zernecke A, Eriksson EE, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112(4):1461–71.
Freidman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974;290:1275–8.
Furman MI, Becker RC, Yarzebski J, Savegeau J, Gore JM, Goldberg RJ. Effect of elevated leukocyte count on in-hospital mortality following acute myocardial infarction. Am J Cardiol. 1996;78:945–8.
Gillum RF, Mussolino ME, Madans JH. Counts of neutrophils, lymphocytes, and monocytes, cause-specific mortality and coronary heart disease: the NHANES-I epidemiologic follow-up study. Ann Epidemiol. 2005;15:266–71.
Nijm J, Wikby A, Tompa A, Olsson AG, Jonasson L. Circulating levels of proinflammatory cytokines and neutrophil-platelet aggregates in patients with coronary artery disease. Am J Cardiol. 2005;95:452–6.
Avanzas P, Arroyo-Espliguero R, Cosin-Sales J, Quiles J, Zouridakis E, Kaski JC. Multiple complex stenoses, high neutrophil count and C-reactive protein levels in patients with chronic stable angina. Atherosclerosis. 2004;175:151–7.
Avanzas P, Arroyo-Espliguero R, Cosin-Sales J, et al. Markers of inflammation and multiple complex stenoses (pancoronary plaque vulnerability) in patients with non-ST segment elevation acute coronary syndromes. Heart. 2004;90:847–52.
Mehta J, Dinerman J, Mehta P, et al. Neutrophil function in ischemic heart disease. Circulation. 1989;79:549–56.
Buffon A, Biasucci LM, Liuzzo G, D’Onofrio G, Crea F, Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002;347:5–12.
Biasucci LM, D’Onofrio G, Liuzzo G, et al. Intracellular neutrophil myeloperoxidase is reduced in unstable angina and acute myocardial infarction, but its reduction is not related to ischemia. J Am Coll Cardiol. 1996;27:611–6.
Mazzone A, De Servi S, Ricevuti G, et al. Increased expression of neutrophils and monocyte adhesion molecules in unstable coronary artery disease. Circulation. 1993;88:358–63.
Naruko T, Ueda M, Haze K, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106:2894–900.
Ionita MG, van den Borne P, Catanzariti LM, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2010;30(9):1842–8.
Ferrante G, Nakano M, Prati F, et al. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes. Circulation. 2010;122:2505–13.
Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharm Therap. 2006;111:16–26.
Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–11.
Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol. 2010;10(11):1325–34.
Malle E, Marsche G, Panzenboeck U, Sattler W. Myeloperoxidase-mediated oxidation of high-density lipoproteins: fingerprints of newly recognized potential proatherogenic lipoproteins. Arch Biochem Biophys. 2006;445:245–55.
Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–44.
Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–60.
Mohiuddin I, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Nitrotyrosine and chlorotirosine: clinical significance and biological functions in the vascular system. J Surg Res. 2006;133(143):149.
Zheng L, Nukuna B, Brennan ML, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004; 114: 529–541.
Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–32.
Yang J, Ji R, Cheng Y, Sun JZ, Jennings LK, Zhang C. l-Arginine chlorination results in the formation of a nonselective nitric-oxide synthase inhibitor. J Pharmacol Exp Ther. 2006;318:1044–9.
Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–95.
Baldus S, Rudolph V, Roiss M, et al. Heparins increase endothelial nitric oxide bioavailability by liberating vessel immobilized myeloperoxidase. Circulation. 2006;113:1871–8.
Hazen SL. Myeloperoxidase and plaque vulnerability. Arterioscler Thromb Vasc Biol. 2004;24(1143–6):26.
Koeffler HP, Ranyard J, Pertcheck M. Myeloperoxidase: its structure and expression during myeloid differentiation. Blood. 1985;65:484–91.
Owen CA, Campbell MA, Boukedes SS, Stockley RA, Campbell EJ. A discrete subpopulation of human monocytes expresses a neutrophil-like proinflammatory (P) phenotype. Am J Physiol. 1994;267(6 Pt 1):L775–85.
Zhang R, Shen Z, Nauseef WM, Hazen SL. Defects in leukocyte-mediated initiation of lipid peroxidation in plasma as studied in myeloperoxidase-deficient subjects: systematic identification of multiple endogenous diffusible substrates for myeloperoxidase in plasma. Blood. 2002;99(5):1802–10.
Penn MS, Patel CV, Cui MZ, DiCorleto PE, Chisolm GM. LDL increases inactive tissue factor on vascular smooth muscle cell surfaces: hydrogen peroxide activates latent cell surface tissue factor. Circulation. 1999;99(13):1753–9.
Lesnik P, Dentan C, Vonica A, Moreau M, Chapman MJ. Tissue factor pathway inhibitor activity associated with LDL is inactivated by cell- and copper-mediated oxidation. Arterioscler Thromb Vasc Biol. 1995;15(8):1121–30.
Rees MD, Pattison DI, Davies MJ. Oxidation of heparan sulphate by hypochlorite: role of N-chloro derivatives and dichloramine-dependent fragmentation. Biochem J. 2005;391(Pt 1):125–34.
Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7): a mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–87.
Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–91.
Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99(9):2075–81.
Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1309–14.
Zhang R, Brennan ML, Fu X, et al. Association between MPO levels and risk of coronary artery disease. JAMA. 2001;286:2136–42.
Meuwese MC, Stroes ESG, Hazen SL, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals. The EPIC-Norfolk prospective population study. JACC. 2007;50:159–65.
Li S-H, Xing Y-W, Li Z–Z, Bai SG, Wang J. Clinical implications of relationship between myeloperoxidase and acute coronary syndromes [in Chinese]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:241–4.
Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–95.
Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–10.
Cartwright GE, Athens JW, Wintrobe MM. The kinetics of granulopoiesis in normal man. Blood. 1964;24:780–803.
Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005; 120:649–61.
Daigle I, Simon HU. Critical role for caspases 3 and 8 in neutrophil but not in eosinophil apoptosis. Int Arch Allergy Immunol. 2001;126:147–56.
Gardai SJ, Hildeman DA, Frankel SK, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085–95.
Renshaw SA, Timmons SJ, Eaton V, et al. Inflammatory neutrophils retain susceptibility to apoptosis mediated via the Fas death receptor. J Leukoc Biol. 2000;67:662–8.
Kamohara H, Matsuyama W, Shimozato O, et al. Regulation of tumor necrosis factor related apoptosis inducing ligand (TRAIL) and TRAIL receptor expression in human neutrophils. Immunology. 2004;111:186–94.
Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors and SHP-1 and block cytokine-induced antiapoptotic signalling in neutrophils. Nat Med. 2002;8:61–7.
Sakamoto E, Hato F, Kato T, et al. Type I and II interferon delay human neutrophil apoptosis via activation of STAT3 and upregulation of cellular inhibitor of apoptosis 2. J Leukoc Biol. 2005;78:301–9.
Kobayashi SD, Voylch JM, Whithney AR, DeLeo FR. Spontaneous neutrophil apoptosis and regulation of cell survival by GM-CSF. J Leukoc Biol. 2005;78:1408–18.
Andonegui G, Trevani AS, Lopez DH, Raiden S, Giordano M, Geffner JR. Inhibition of human neutrophil apoptosis by platelets. J Immunol. 1997;158:3372–7.
Peng SL. Neutrophil apoptosis in autoimmunity. J Mol Med. 1996;84:122–5.
Megens RT, Vijayan S, Lievens D, Döring Y, van Zandvoort M, Grommes J, Weber C, Soehnlein O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost. 2012;107(3):597–8.
Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–60.
Cross A, Barnes T, Bucknall RC, Edwards SW, Moots RJ. Neutrophil apoptosis in rheumatoid arthritis is regulated by local oxygen tensions within joints. J Leukoc Biol. 2006;80:521–6.
Bailey SM, Murnane JP. Telomeres, chromosome instability and cancer. Nucleic Acids Res. 2006;34:2408–17.
Yang J, Chang E, Cherry AM, et al. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–8.
Hiyama K, Hirai Y, Kyoizumi S, et al. Activation of telomerase in human lymphocytes and haematopoietic cells. J Immunol. 1995;155:3711–5.
Narducci ML, Grasselli A, Biasucci LM, et al. High telomerase activity in neutrophils from unstable coronary plaques. J Am Coll Cardiol. 2007;50:2369–74.
Biffl WL, Moore EE, Moore FA, Barnett CC Jr, Carl VS, Peterson VN. IL-6 delays neutrophil apoptosis. Arch Surg. 1996;131:24–30.
Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–20.
Robertson JD, Gale RE, Wynn RF, et al. Dynamic of telomere shortening in neutrophils and T lymphocytes during ageing and the relationship to skewed X chromosome inactivation patterns. Br J Haematol. 2000;109:272–9.
Norrback KF, Enblad G, Erlanson M, Sundström C, Roos G. Telomerase activity in Hodgkin’s disease. Blood. 1998;92:566–73.
Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5.
Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362:983–8.
Biasucci LM, Liuzzo G, Giubilato S, et al. Delayed neutrophil apoptosis in patients with unstable angina: relation to C-reactive protein and recurrence of instability. Eur Heart J. 2009;30:2220–5.
Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: a multistep paradigm. Cell. 1994;76:301–14.
Marcus AJ. Thrombosis and inflammation as multicellular processes: significance of cell–cell interactions. Semin Hematol. 1994;31:261–9.
Larsen E, Celi A, Gilbert GE, et al. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–12.
Hamburger SA, McEver RP. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 1990;75:550–4.
Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–57.
Evangelista V, Manarini S, Rontondo S, et al. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and the b2 integrin CD11b/CD18. Blood. 1996;88:4183–94.
Wang Y, Sakuma M, Chen Z, et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993–3000.
Ott I, Neumann F, Gawaz M, Schmitt M, Schömig A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation. 1996;94:1239–46.
Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-a secretion. J Clin Invest. 1995;95:2297–303.
Weyrich AS, Elstad MR, McEver RP, et al. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–34.
Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–5.
Merhi Y, Provost P, Chauvet P, Théorêt JF, Phillips ML, Latour JG. Selectin blockade reduces neutrophil interaction with platelets at the site of deep arterial injury by angioplasty in pigs. Arterioscler Thromb Vasc Biol. 1999;19:372–7.
Von Brühl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819–35.
Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104(13):1533–7.
Garlichs CD, Eskafi S, Cicha I, et al. Delay of neutrophil apoptosis in acute coronary syndromes. J Leukoc Biol. 2004;75:828–35.
Lefer AM, Campbell B, Scalia R, Lefer DJ. Synergism between platelets and neutrophils in provoking cardiac dysfunction after ischemia and reperfusion: role of selectins. Circulation. 1998;98:1322–8.
Maugeri N, Rovere-Querini P, Evangelista V, et al. An intense and short-lasting burst of neutrophil activation differentiates early acute myocardial infarction from systemic inflammatory syndromes. PLoS ONE. 2012;7(6):e39484.
Maugeri N, Rovere-Querini P, Evangelista V, et al. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and ß2 integrin-dependent cell clearance program. Blood. 2009;113:5254–65.
Maugeri N, Malato S, Femia EA, et al. Clearance of circulating activated platelets in polycythemia vera and essential thrombocythemia. Blood. 2011;118:3359–66.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael Parnham.
Rights and permissions
About this article
Cite this article
Della Bona, R., Cardillo, M.T., Leo, M. et al. Polymorphonuclear neutrophils and instability of the atherosclerotic plaque: a causative role?. Inflamm. Res. 62, 537–550 (2013). https://doi.org/10.1007/s00011-013-0617-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0617-0