Abstract
Objectives
We compared the concentrations of the proinflammatory cytokine interleukin-2 (IL-2) with the anti-inflammatory cytokine interleukin-10 (IL-10) in serial serum samples from improved and expired acute ischemic stroke (AIS) patients to determine their prognostic usefulness.
Materials and methods
Blood from AIS patients (n = 17) admitted within 24 h of the onset of symptoms were collected at admission and 24, 48, 72, and 144 h after admission. Pro- and anti-inflammatory cytokines were measured by enzyme-linked immunosorbant assay.
Results
IL-2 levels were elevated in serum samples from AIS patients collected at 0 (0.97, P < 0.01) and 24 h (1.011, P < 0.01). IL-2 concentrations gradually decreased at subsequent time-points (48, 72, and 144 h samples, 0.324, P < 0.05) in patients who improved (n = 13), but not in those who expired (n = 4). Similarly, a decrease in IL-10 levels was observed in serum samples from AIS patients who improved at 24 h (0.180, P < 0.05), followed by significant increases at 72 h (0.97, P < 0.01 vs. control) and 144 h (1.38, P < 0.01).
Conclusion
The levels of IL-2 and IL-10 correlate well with outcome in AIS patients and may be useful in prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke continues to have a devastating impact on public health, and remain the third leading cause of death and disability after heart attack and cancer in India according to the World Health Organization. Approximately 77% of strokes are ischemic in nature [1]. The disease has also turned out to be the most common cause of disability and dependence, with the majority of stroke survivors remaining vocationally impaired and requiring assistance for daily living activities. In India, a substantial number of patients are in the younger age group. The loss of life and morbidity not only cause significant social deprivation, but also lead to direct and indirect financial burdens [2].
Inflammation plays an important role in acute ischemic stroke (AIS). Therefore, measuring inflammatory markers at regular intervals in AIS patients and comparing with clinical progression may provide prognostic clues. Cerebral ischemia induces an inflammatory response associated with the activation and release of various proinflammatory cytokines and additional acute-phase reactants that aggravate tissue damage [3, 4]. Previously, Fassbender et al. [5] reported a role for inflammation in the pathophysiology of AIS in humans. Similar studies have been reviewed by others [6, 7], and are consistent with reports in animal experiments [8]. Although various inflammatory markers, including IL-10, have been studied to understand the progression and prognosis of AIS [9, 10], to our knowledge no information is available regarding the role of IL-2 in AIS patients. Therefore, the present study was undertaken to determine the levels of the proinflammatory and anti-inflammatory cytokines IL-2 and IL-10, respectively, in sera of AIS patients at specified intervals to determine if markers might aid in prognosis.
Materials and methods
Subjects
Seventeen patients (11 males, 6 females), aged 22–76 years, admitted to Central India Institute of Medical Sciences, Nagpur, India, within 24 h of the onset of symptoms of AIS were included in the present study. Diagnosis was based on the WHO definition of stroke: rapidly developing signs of focal (or global) disturbance of cerebral function lasting >24 h (unless interrupted by surgery or death) with no apparent non-vascular cause, history, neurological examination, and computerized tomography (CT). Patients with transient ischemic attack, hemorrhage, malignancies, renal or hepatic diseases, or other types of brain injury were excluded from the study. Neurological deficit was assessed per the National Institute of Health Stroke Scale (NIHSS) score during the hospitalization period and functional recovery was assessed using the modified ranking scale (mRS) at the time of discharge. Twenty age- and sex-matched healthy subjects (control group) who did not have recent infection (i.e., within 1 month), other serious illness, or head trauma were included as controls. All patients were admitted to the intensive care unit (ICU), where the ambient temperature was maintained at 20–25°C. All patients received anti-platelet agents (aspirin 150 mg, clopidregel 75 mg once a day); three patients were thrombolysed using intravenous recombinant tissue-plasminogen activator; one patient was treated with decompressive hemicraniectomy and duroplasty for malignant middle cerebral artery syndrome; eight patients with symptoms of raised intracranial pressure received anti-edema measures (such as mannitol 20%, 0.25–0.5 g/kg over 20 min, not exceeding a total of 2 g/kg of body weight in 24 h; mean NIHSS 17.3); and one patient was treated with oral glycerol in addition to mannitol, IV fluids, and other supportive measures for the treatment of concurrent illnesses such as hypertension and diabetes mellitus. Out of the 17 patients, 13 patients survived and were discharged; these patients were classified as the improved group [Mean admission NIHSS 12.92 (range 7–18), mean discharge NIHSS 9.5 (range 3–16)]. Among the survivors, none exhibited significant clinical worsening (>4 point increase in NIHSS). Four patients expired during their hospital stays; these patients were classified as the expired group. The protocol of this study was reviewed and approved by the Institutional Ethics Committee of Central India Institute of Medical Sciences.
Blood Sampling
Venous blood was collected at 0 hrs (the time of admission) and 24, 48, 72, and 144 h after admission. Blood was allowed to clot, and, after centrifugation (1,000 g, 10 min), the serum was separated and stored at −20°C until use in experiments.
IL-2 and IL-10 determination
IL-2 was analyzed in triplicate by the sandwich ELISA method supplied by Bender Med System, Austria. Flat-bottom microtiter plates were coated with 100 µl of coating antibody solution and incubated at 2–8°C overnight. The wells were then washed once with wash buffer, and then 250 µl of blocking buffer was added to each well before incubation at 37°C for 2 h. This was followed by washing and the addition of 100 µl of serum from AIS patients collected at different time intervals or the control group along with 50 µl of biotin conjugate. The plates were then incubated at room temperature on a shaker for 2 h. After incubation, wells were washed thrice with wash buffer, followed by the addition of 100 µl diluted streptavidin-HRP and incubation at room temperature for 1 h. After this incubation, the wells were washed thrice and 100 µl of substrate was added to each well. After 10 min, a stop solution was added, and absorbance was measured at 450 nm. For IL-10 determination, the same procedure was followed as described above but instead of direct serum samples, samples were diluted 1:1 in assay buffer. Prior to AIS patient sampling, the assay was standardized using different dilutions of standard. The assay sensitivities reported by the manufacturer were 0.2 pg/ml for IL-2 and 0.15 pg/ml for IL-10.
Statistical analysis
Mean cytokine (IL-2 and IL-10) concentrations of control and AIS patients from different time intervals were compared using one-way ANOVA followed by Dunnett’s test. A P value of <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software.
Results
Table 1 shows the clinical characteristics of the AIS patients at the time of admission. Figure 1 shows IL-2 and IL-10 levels in serum samples of AIS patients collected at different time intervals (0, 24, 48, 72, and 144 h). We found a significant increase in IL-2 from AIS patient serum samples compared with the control group at 0 h (0.97, P < 0.01) and 24 h (1.011, P < 0.01). This gradually decreased in subsequent samples (48, 72, and 144 h samples, 0.324, P < 0.05) from improved patients. However, a significant decrease in IL-10 concentration was noted in serum samples collected from AIS patients at 24 h (0.180, P < 0.05), followed by a significant increase at 72 h (0.97, P < 0.01 vs. control) and 144 h (1.38, P < 0.01).
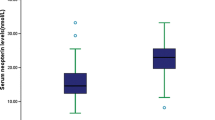
Cytokine concentrations were compared in AIS patients who improved with treatment and those who expired during treatment. Figure 2 shows IL-2 levels in serum samples from improved and expired AIS patients collected at different time intervals. Serum samples from expired AIS patients exhibited an initial increase in IL-2 at the time of admission, similar to those of improved AIS patients (Fig. 1). This was markedly increased in subsequent samples, especially at 144 h (0.877, P < 0.01), in contrast to improved patients, whose IL-2 levels decreased with treatment.
IL-10 levels were also compared in expired and improved AIS patients. Serum IL-10 concentrations were very low in the initial samples (0 and 24 h) of expired AIS patients, similar to those of improved AIS cases. However, IL-10 was markedly increased in subsequent samples from all improved AIS patients, whereas IL-10 levels were low in expired AIS patients (Fig. 3).
Discussion
Inflammation plays an important role in the pathogenesis of AIS. Many studies have demonstrated that various cytokines are unregulated in the brain after AIS and are expressed not only by cells of the immune system but also by the resident brain cells, including glia and neurons [11, 12]. The most widely studied cytokines related to inflammation in stroke are interleukin-1 (IL-1), TNF-α, interleukin-6, transforming-growth factor-β (TGF-β), and interleukin-10 (IL-10) [13]. To our knowledge, no information is available on IL-2 in AIS patients. IL-2 is generally viewed as a proinflammatory cytokine, but its role in AIS is not clear. IL-10 is an anti-inflammatory cytokine that inhibits proinflammatory cytokines and suppresses cytokine receptor expression and receptor activation [14]. It is synthesized in the central nervous system (CNS) and is unregulated in experimental stroke [15]. Here, we conducted a prospective study to compare IL-2 levels with those of IL-10 in sera from improved and expired AIS patients collected at different time intervals.
In the improved group, we observed increased IL-2 serum levels in the hyperacute stage of disease (0 and 24 h), which subsequently returned to normal (48, 72, and 144 h). In contrast, serum IL-10 was low in the acute stage of AIS, but increased at 72 and 144 h in AIS patients who improved with treatment. Interestingly, we found a significant inverse correlation between serum levels of these two cytokines, which seem to display opposite activities. However, the sera of AIS patients who expired exhibited initial increases in IL-2 at 0 and 24 h, but IL-2 concentrations continued to increase at subsequent times, especially at 144 h (0.877, P = 0.006). IL-10 levels in expired patients were low initially (0 and 24 h), similar to those of improved patients, but did not increase at subsequent times.
IL-10 is a major anti-inflammatory cytokine produced by several inflammatory cells, especially macrophages, Th2 (anti-inflammatory) T cells, and monocytes. Previously, Van Exel et al. [16] showed that subjects having low levels of IL-10 have an increased risk of stroke. Various studies have also reported anti-inflammatory and neuroprotective roles for IL-10 in AIS [17]. Using a rat model, Spera et al. [18] demonstrated that IL-10 levels were reduced after brain injury. Our study is in agreement with other studies indicating that increased IL-10 levels in stroke patients could be a good indicator of recovery.
However, increased levels of IL-2 in expired AIS patients and lower levels in improved AIS patients suggest that this cytokine can also be a predictor of stroke outcome. It is possible that IL-2 could act as a mediator of edema following the cerebral ischemia, as suggested by others [19]. Ellison et al. [20] showed that IL-2 interrupts the blood–brain barrier, producing cerebral edema when injected into normal rat brain.
Our experimental observations are similar to those observed by earlier workers that the infarct volume is greater in AIS patients who expired (169 × 10−6 ± 116 × 10−6 m3) compared with those who improved (49 × 10−6 ± 51 × 10−6 m3) [21, 22]. The larger volume of infarcted tissue may explain the difference in cytokine levels, however at this stage it is difficult to determine if the difference between the cytokine concentrations in the two groups is a cause or an effect of the difference in the volume of infarcted tissue. Secondly, it is also not clear; about the source of cytokines measured in this study either arrived peripherally or from CNS.
In the present study, we compared the levels of pro- and anti-inflammatory cytokines in serum from AIS patients at different time intervals. We observed that IL-2 levels decreased and IL-10 levels significantly increased in the sera of improved patients 72 and 144 h after admission. To our knowledge, this is the first report of a relationship between these two cytokines in the pathogenesis of AIS, as well as the serum levels of these pro- and anti-inflammatory cytokines in expired patients. Despite the small number of expired patients in this study, the observed results are useful for future studies.
In conclusion, our study demonstrates that IL-2 and IL-10 cytokines correlate well with the outcome of the AIS and may play an important role in prognosis. These cytokine markers have the potential for predicting AIS patient outcome and, therefore, may be useful in studying various forms of therapeutic intervention.
References
Dalal PM, Madhumita B. Stroke epidemic in India: hypertension-stroke control programme is urgently needed. J Assoc Physicians India. 2007;55:689–91.
Dalal PM. Burden of stroke: Indian perspective. J Assoc Physicians India. 2004;52:695–6.
Banerjee TK, Das SK. Epidemiology of stroke in India. Neurol Asia. 2006;11:1–4.
Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:0–04.
Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, et al. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci. 1994;22:135–9.
Chamoro A. Role of inflammation in stroke and atherothrombosis. Cerebrovasc Dis. 2004;17:1–5.
Pantoni L, Sarti C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia. Experimental bases and therapeutic perspectives. Arterioscler Thromb Vasc Biol. 1998;18:503–13.
Del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112.
Waje-Andreassen U, Krakenes J, Ulvestad E, Thomassen L, Myhr KM, Aarseth J, et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–5.
Mazzotta G, Sarchielli P, Caso V, Paciaroni M, Floridi A, Floridi A, et al. Different cytokine levels in thrombolysis patients as predictors for clinical outcome. Eur J Neurol. 2004;11:377–81.
Suzuki S, Tanaka K, Nogawa S, Nagata E, Ito D, Dembo T, et al. Temporal profile and cellular localization of interleukin-6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1256–62.
Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–8.
Sairanen T, Carpén O, Karjalainen-Lindsberg ML, Paetau A, Turpeinen U, Kaste M, et al. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke. 2001;32:1750–8.
Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investing Drugs. 2003;4:522–9.
Zhai QH, Futrell N, Chen FJ. Gene expression of IL-10 in relationship to TNF-alpha, IL-1beta and IL-2 in the rat brain following middle cerebral artery occlusion. Journal of Neurological Sciences. 1997;152:119–24.
Van Exel E, Gussekloo J, de Craen AJ, Bootsma-van der Wiel A, Frölich M, Westendorp RG. Inflammation and stroke: the Leiden 85-Plus Study. Stroke. 2002;33:1135–8.
Alison EB. The forgotten lymphocyte: immunity and stroke. Circulation. 2006;113:2035–6.
Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–92.
Rosenstein M, Ettinghausen SE, Rosenberg SA. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986;137:1735–42.
Ellison MD, Krieg RJ, Povlishock JT. Differential central nervous system responses following single and multiple recombinant interleukin-2 infusions. J Neuroimmunol. 1990;28:249–60.
Chamorro A, Vila N, Ascaso C, Saiz A, Montalvo J, Alonso P, et al. Early prediction of stroke severity: role of the erythrocyte sedimentation rate. Stroke. 1995;26:573–6.
Pullicino PM, Alexandrov AV, Shelton JA, Alexandrova NA, Smurawska LT, Norris JW. Mass effect and death from severe acute stroke. Neurology. 1997;49:1090–5.
Acknowledgment
We thank Mr. Prashant Deoras for assistance in statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: A. Bauhofer.
Rights and permissions
About this article
Cite this article
Nayak, A.R., Kashyap, R.S., Purohit, H.J. et al. Evaluation of the inflammatory response in sera from acute ischemic stroke patients by measurement of IL-2 and IL-10. Inflamm. Res. 58, 687–691 (2009). https://doi.org/10.1007/s00011-009-0036-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-009-0036-4