Abstract
Myasthenia gravis (MG) is an autoimmune disease caused by an immunological response against the acetylcholine receptor (AChR) at the neuromuscular junction. Anti-AChR antibodies induce degradation of the receptor, activation of complement cascade and destruction of the post-synaptic membrane, resulting in a functional reduction of AChR availability. The pathophysiological role of autoantibodies (auto-Abs) and T helper lymphocytes has been studied in the experimental autoimmune MG (EAMG) models. EAMG models have been employed to investigate the factors involved in the development of MG and to suggest new therapies aimed to preventing or modulating the ongoing disease. EAMG can be induced in susceptible mouse and rat strains, which develop clinical symptoms such as muscular weakness and fatigability, mimicking the human disease. Two major types of EAMG can be induced, passive and active EAMG. Passive transfer MG models, involving the injection of auto-Abs, are helpful for studying the role of complement molecules and their regulatory proteins, which can prevent neuromuscular junction degradation. Active models, induced by immunization, are employed for the analysis of antigen-specific immune responses and their modulation in order to improve disease progression. In this review, we will concentrate on the main pathogenic mechanisms of MG, focusing on recent findings on EAMG experimental models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Myasthenia Gravis
Acquired myasthenia gravis (MG) is a B cell-mediated T cell-dependent autoimmune disease, characterized by impairment of the neuromuscular junction (NMJ) transmission caused by specific autoantibodies (auto-Abs) against the acetylcholine receptors (AChR) on the post-synaptic membrane of skeletal muscle cells (Engel et al. 1977; Lindstrom et al. 1976; Meriggioli and Sanders 2009). The majority of patients with generalized MG (85%) and 50% with ocular MG develop antibodies against AChR, usually belonging to IgG1 and IgG3 isotypes (Rodgaard et al. 1987); these auto-Abs can be detected by the standard radioimmunoassay method (Lindstrom et al. 1976; Vincent 1994). In approximately 40% of MG patients without anti-AChR antibodies (AChR negative MG), antibodies directed to a muscle-specific tyrosine kinase can be detected (Hoch et al. 2001; Lindstrom 2008). The development of anti-AChR auto-Abs is apparently due to breakdown of self-tolerance in the thymus (Melms et al. 2006; Newsom-Davis et al. 1981), with induction or activation of AChR-specific CD4+ T helper cells and production of pro-inflammatory cytokines, leading to the synthesis of high-affinity antibodies (Hoedemaekers et al. 1997b; Vincent 2002).
Anti-AChR Abs are the most frequent cause of NMJ impairment. Neuromuscular transmission can also be impaired by antibodies against the voltage-gated calcium channels on the presynaptic membrane as occurs in the Lambert-Eaton myasthenic syndrome (Mareska and Gutmann 2004), or in rare occasions by drug-induced breakdown tolerance to the AChR, as observed in penicillamine induced MG (Hill et al. 1999; Penn et al. 1998).
MG generally satisfies the clinical criteria for an antibody-mediated autoimmune disease (Drachman 2003):
-
1.
Autoantibodies are present in patients with the disease. Elevated amounts of anti-AChR Abs are found in 85% of sera from myasthenic patients, but not in sera from individuals without MG, including those with other neurologic or autoimmune diseases (Lindstrom et al. 1976; Vincent 1991);
-
2.
Antibodies interact with the target antigen. IgGs are found localized on segments of the post-synaptic membrane and fragments of degenerating junctional folds in MG patients, whereas no immune complexes are evident in non-myasthenic controls (Engel et al. 1977). Moreover, the majority of AChR-specific antibodies are directed against an extracellular area of the receptor, defined as main immunogenic region (MIR), localized between residues 67 and 76 of the α-subunit of the receptor (Luo et al. 2009; Tzartos et al. 1988);
-
3.
Passive transfer of antigen-specific antibodies in animals reproduces features of the disease. Daily injections into mice of an immunoglobulin fraction of MG patients’ sera reduce amplitudes of miniature end-plate potentials and responses on repetitive nerve stimulation, and decrease the number of AChR at the NMJ (Toyka et al. 1975);
-
4.
Immunization with the specific antigen produces a disease model. Patrick and Lindstrom (1973) first demonstrated in rabbits that repeated injections of AChR, purified from the electric organ of Electrophorus electricus, result in the production of high levels of anti-AChR antibodies; the authors also observed flaccid paralysis in animals and typical MG electromyographs. The immunization with AChR from the electric organ of Electrophorus electricus or Torpedo californica induced experimental autoimmune MG (EAMG) in rats and guinea pigs (Lennon et al. 1975), in association with an autoantibody response to the AChR. Clinical signs of the disease could be also observed in female Lewis rats, injected intradermally with a recombinant protein spanning the extracellular domain (human AChR α-subunit, aa 1–210) (Lennon et al. 1991);
-
5.
Reduction of antibody levels ameliorates the disease. Plasma exchange is a successful therapeutic procedure for the treatment of compromised myasthenic patients. The removal of circulating IgGs, including pathogenic anti-AChR antibodies, is associated with a rapid improvement of clinical symptoms in MG patients (Antozzi et al. 1991; Dau 1981).
Impairment of the neuromuscular transmission by AChR-specific auto-Abs results from three different mechanisms: (a) complement-induced damage of the NMJ, (b) increased degradation of AChR, and (c) direct binding of auto-Abs to the ACh binding site.
The binding of auto-Abs (of the IgG1 and IgG3 subtypes) to the antigen at the NMJ activates the complement cascade, a process triggered by the interaction between the Fc fragment of anti-AChR Abs and the C1 component. The complex activation leads to formation of the membrane attack complex (MAC) and consequently to the focal lysis of the muscle cell at the NMJ (Engel et al. 1977). The destruction of the post-synaptic membrane results in a morphological alteration, in which the typical deep junctional folds are replaced with a relatively flat surface and the density of functional AChRs is reduced (Conti-Fine et al. 2006). An associated phenomenon, due to the loss of the AChR at the NMJ, is a marked reduction in the number of voltage-gated sodium channels in MG patients and passively transferred EAMG rats (Ruff and Lennon 1998), and of AChR-associated proteins utrophin (Slater et al. 1997), and rapsyn (Losen et al. 2005; Martinez–Martinez et al. 2007); for a review see (Gomez et al. 2010). The reduction of functional muscle AChRs might also result from antigenic modulation caused by anti-AChR Abs. Indeed, it has been demonstrated in vitro that the addition of myasthenic IgGs, or the divalent fragment F(ab′)2, to skeletal muscle cultures increases threefold the rate of AChR degradation (Drachman et al. 1978). Hence, the formation of immune-complexes induces an accelerated internalization of AChR by endocytosis, which is not compensated by ex novo synthesis, and increases AChR degradation by lysosomal mechanisms reducing its availability on the post-synaptic membrane (Engel and Fumagalli 1982). Moreover, a subset of the polyclonal anti-AChR repertoire might interact with ACh-binding sites on the receptor and induce a direct impairment of AChR function. Administrations of monoclonal antibodies (mAbs) directed to the α-bungarotoxin binding sites are able to produce an acute paralysis in injected chickens (Gomez and Richman 1983); this evidence suggests that the presence of antibody-binding sites might be a crucial factor influencing the severity of the disease, more than antibody concentration in serum.
Experimental Autoimmune MG
EAMG represents an excellent model to investigate the pathogenic mechanisms underlying the human disease and develop new immunotherapies for its treatment.
The first report on an experimental model of MG dates back to almost 30 years ago, when Patrick and Lindstrom (1973) demonstrated that rabbits immunized with AChR, purified from the Electrophorus electricus electric organ, developed MG-like symptoms. After that pivotal discovery, many animal studies confirmed that an autoimmune response was occurring in MG patients against muscle AChR, and that anti-AChR Abs were responsible for the structural and functional damage of the NMJ.
Myasthenia gravis and its animal models share several features, in particular muscle weakness and fatigability, decremental response after repetitive nerve stimulation, temporary improvement of muscle strength following treatment with anti-cholinesterase drugs and increased curare sensitivity (Christadoss et al. 2000). Moreover, MG and EAMG appear similar in several immunopathological features, such as presence anti-AChR antibodies in serum, deposition of IgGs and C3 complement components at the NMJ, MHC class II-restricted presentation of AChR epitopes and involvement of T helper cells in B-cell antibody production (Christadoss et al. 2000) (Table 1).
The human disease differs from experimental MG for only a few features. Factors inducing MG are unknown and thymic alterations are common in myasthenic patients, suggesting the potential role of the thymus in the pathogenesis of the disease (Meinl et al. 1991). In contrast, animals develop EAMG after AChR-immunization and the auto-sensitization process seems to occur only in draining lymph nodes (Christadoss et al. 2000), apparently without affecting the thymus, as in MG patients (Table 1).
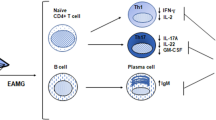
Although EAMG can be induced in a wide variety of animals, among which also monkeys (Tarrab-Hazdai et al. 1975; Toro-Goyco et al. 1986) and rabbits (Eldefrawi 1978; Patrick and Lindstrom 1973), about 65% of experimental models are established in rats and 35% in mice, mainly due to a higher incidence of clinical EAMG signs in the former (Link and Xiao 2001). The lower murine susceptibility to EAMG may be due to a high safety factor of the NMJ, characterized by the release of a large amount of acetylcholine quanta from the nerves (Wood and Slater 2001). In susceptible rats, EAMG is routinely induced by active immunization with torpedo AChR (Link and Xiao 2001), but also a short fragment of the rat (self) AChR (aa 97–116 of the α-subunit) is capable to break immunological tolerance (Baggi et al. 2004). EAMG can also be induced by passive transfer of anti-AChR antibodies (Lindstrom et al. 1976; Tzartos et al. 1987), the simplest protocol for studying the pathogenic effects of auto-Abs in vivo. The accepted general distinction between actively induced and passively transferred models of EAMG implies that the passive transfer models allow the study of the effector phase of the human disease (IgG deposit, complement activation, NMJ destruction), while the active models also include breakdown of AChR tolerance and the immune cell activation mechanisms that might occur in human MG. The next paragraphs will describe these models in detail (Fig. 1).
Schematic representation of EAMG induction factors and treatments. Three different mechanisms to induce experimental MG are shown in the upper left box: active immunization with injection of AChR; passive transfer with administration of anti-AChR antibodies purified from myasthenic animals or MG patients; adoptive EAMG induced with transplantation of PBLs derived from MG patients. In the lower left box, two potential mechanisms to suppress EAMG are represented: cellular therapy, via either DCs or Treg cells, characterized by inhibition of T cell proliferation and pro-inflammatory cytokine production, or pharmacological treatment. Findings in the pathogenetic mechanisms and effective treatments can hopefully be translated from the animal model to the human disease (right box)
Active EAMG in Rats
Various inbred rat strains have been tested for the induction of active EAMG via immunization with Torpedo californica AChR (TAChR). While Wistar Furth and Copenhagen rats seem to be resistant to disease induction, maintaining normal weight and muscle strength, Fischer and Wistar Munich rats appear the most susceptible strains showing severe EAMG symptoms and rapid progression of the disease (Biesecker and Koffler 1988). Nevertheless, Lewis rats, which exhibit intermediate susceptibility, represent the rat model most commonly used to induce EAMG, as clinical manifestations in this strain are similar to those of human MG (Biesecker and Koffler 1988).
EAMG in Lewis rats is commonly induced by a single immunization with purified AChR in complete Freund’s adjuvant (CFA), triggering the production of antibodies to foreign AChR, which are able to cross-react with the self-AChR (Lindstrom 1980; Link and Xiao 2001). The disease can also be induced by immunization with a synthetic peptide, corresponding to the region 97–116 of rat AChR α-subunit (R97-116), emulsified in CFA, followed by a second immunization boost of R97-116 in incomplete Freund’s adjuvant (IFA) 30 days after the first immunization. In this induction protocol, the production of auto-Abs and the activation of R97-116-specific T cells derive from the breakdown of self-tolerance (Baggi et al. 2004).
The course of EAMG is evaluated monitoring the loss of body weight and muscular strength of immunized animals. Myasthenic symptoms are assessed after exercise (repetitive paw grips on the cage grid) for 30 s, and are characterized by tremor, hunched posture, muscle weakness and fatigability.
When EAMG is induced in the Lewis rat by injection of Torpedo AChR (40 μg/rat), emulsified in CFA supplemented with additional Mycobacterium tuberculosis H37Ra (0.5 mg/rat) (Aricha et al. 2008), two different disease phases can be clinically distinguished. A transient acute phase of muscle weakness can also occur in EAMG models induced with AChR/CFA supplemented with with Bordetella pertussis; if the additional adjuvant is not used, the acute phase is not observed (Lindstrom 1980). The first one (the acute transient phase) begins approximately 7 days post immunization and is characterized by the synthesis of anti-AChR Abs that induce complement depositions on muscle membrane. Chemotactic signals are released, leading to extensive phagocytic invasion at the NMJ, with destruction of the post-synaptic membrane. The cellular invasion firstly results in a rapid decrease of muscle AChR content, which is followed (after 2–3 days) by an abnormal increase in AChR content, probably due to the formation of extra-junctional AChR (Lindstrom 1980). The second phase (the progressive chronic phase), begins approximately 28 days post immunization (Link and Xiao 2001) and is characterized by production of a larger amount of antibodies and complement deposition at the post-synaptic membrane, which now appears as a nearly flat surface lacking its typical junctional folds. In this phase, there are no phagocytic cells, and there is a drastic reduction in skeletal muscle AChR content reduced to about one-third compared with healthy controls. Importantly, the progressive chronic phase reflects the clinical course of the human disease.
Active EAMG in Mice
Mice would represent the ideal model for the development of the experimental disease due to the availability of transgenic, knockout, and mutant mice as tools to investigate the biological mechanisms at the basis of MG pathogenesis (Berman and Patrick 1980b; Christadoss et al. 2000). Moreover, an almost infinite and a never-ending array of monoclonal antibodies specific for cell markers (cell surface antigens, chemokines, cytokines, grow factors) is available for murine research. Indeed, EAMG has been intensively studied in mice with defined immunological aberrations to better understand genetic factors involved in the disease pathogenesis and investigate their potential modulation and regulation (Table 2).
C57Bl/6, SJL and AKR mice were classified as highly susceptible strains due to the development of myasthenic symptoms, induced by TAChR immunization, in 50–70% of animals; on the contrary, BALB/c and SWR strains appeared poorly susceptible showing low incidence of muscular weakness and flaccid paralysis (Berman and Patrick 1980a).
EAMG in the mouse is commonly induced by two or three immunization boosts with purified AChR in CFA/IFA (Christadoss et al. 2000), does not show a transient acute phase and myasthenic symptoms typically appear 7–14 days after the last injection. Due to the several immunization boosts required to induce this model, it is relatively difficult to define the appropriate window for a preventive/therapeutic treatment in mice EAMG; moreover, clinical features are less severe compared with those observed in the Lewis rat model.
Passive Transfer MG
Experimental MG can be also induced by passive transfer of auto-Abs via two distinct mechanisms. The first one regards daily injections into healthy recipient animals with serum IgG fraction isolated from MG patients (Toyka et al. 1975) or with anti-AChR antibodies purified from AChR-immunized donor animals with chronic EAMG (Lindstrom et al. 1976). Otherwise, the disease can be passively induced via administration of monoclonal antibodies, generally of IgG1 and IgG2a subclasses, directed to the AChR α-subunit and derived from AChR-immunized animals (Tzartos et al. 1987) or from cell line culture supernatants (Piddlesden et al. 1996). Recipient animals show the typical EAMG symptoms 24 h after antibodies transfer, developing the acute phase characterized by phagocytic invasion. Passive transfer MG has also been shown in rhesus monkeys injected with a human monoclonal anti-AChR antibody (IgG1 subtype) isolated from MG thymus (van der Neut Kolfschoten et al. 2007).
Adoptive Transfer EAMG
Finally, EAMG can be induced via transplantation of human tissues or cells in severe combined immunodeficiency (SCID) mice, lacking mature B and T cells and tolerating xenografts (Schönbeck et al. 1992; Wang et al. 1999). This chimeric human-mouse model represents an important tool to investigate the mechanisms involved in the pathogenesis of MG. Published studies show that SCID mice engrafted with thymus tissue fragments derived from MG patients, produce human anti-mouse AChR antibodies 1–2 weeks after transplantation. Moreover, these studies demonstrate that a myasthenic thymus contains all the cellular components required for producing auto-Abs and maintaining or increasing their synthesis for at least 11 weeks after transplantation (Schönbeck et al. 1992). Besides, other published data refer that SCID mice injected with peripheral blood lymphocytes (PBL), derived from MG patients, show the typical signs of the human disease, characterized by circulating anti-AChR antibodies and human IgG deposits at the NMJ. The same myasthenic manifestations are observed in SCID mice AChR-immunized and simultaneously injected with PBL isolated from healthy controls (Martino et al. 1993). Finally, studies on SCID mice treated with PBL from MG patients show the role of CD4+ and CD8+ T cells in the development of MG proving that only CD4+ T cells are necessary for the pathogenesis of the disease (Wang et al. 1999).
EAMG Models as a Tool to Investigate Therapeutic Approaches
The main aims of experimental MG are to understand the pathological mechanisms of the disease and to investigate potential new therapies (Fig. 1). The currently used immunosuppressive antigen-unspecific drugs such as, corticosteroids, mitomycin C, cyclosporine A, azathioprine, linomide and cyclophosphamide, were indeed initially tested in EAMG models, as recently reviewed by Sanders and Evoli (2010) and Gomez et al. (2010); in some occasions, the experimental model was also used to investigate the mechanisms of actions associated with specific drugs used in the treatment of the human disease (Janssen et al. 2008).
Induction of Peripheral Tolerance
Current conventional therapies for MG are not effective in a proportion of patients and immunosuppressive drugs induce numerous side effects; moreover, these drugs mainly suppress lymphocyte activation and proliferation without having much effect on longlived plasma cells that are terminally differentiated cells, which continue producing pathogenic antibodies (Arce et al. 2002; Gomez et al. 2011). Hence, new therapies are necessary to suppress antigen-specific immune cells and reduce the undesired effects usually observed following the inhibition of the whole immune system in MG patients. In order to develop new therapeutic approaches, we must first understand and define the pathogenesis of the disease.
The most supported pathogenetic hypothesis for MG induction is the loss of self-tolerance in the thymus, which induces the production of AChR-specific auto-reactive CD4+ T cell and consequently anti-AChR auto-Abs. The progression of EAMG seems to be associated to an altered balance in the T cell subsets: Th17 cells (and the secreted IL-17) were found significantly increased in EAMG animals. IL-17 is a pleiotropic pro-inflammatory cytokine that enhances T-cell priming and stimulates the production of multiple pro-inflammatory mediators, including IL-1, IL-6, TNF-α and chemokines (Mu et al. 2009). Normally, the immune response is kept under control by a peripheral immune-surveillance system, which eliminates self-reactive T cells that escaped from the selection processes in the thymus. This immune-surveillance is maintained in a steady state by the balance between different CD4+ T cell subsets: breaking that balance leads to failure of immune-surveillance.
The nasal administration to myasthenic rats of human recombinant fragments of the AChR α-subunit, including the whole extracellular domain of AChR (Hα1-210), induces tolerance to the AChR. The treatment prevents the development of EAMG and suppresses the progression of the disease, inhibiting antigen-specific T-cell proliferative responses and reducing the levels of anti-AChR antibodies (Barchan et al. 1999). Moreover, EAMG rats, orally treated during the acute and chronic phase with a human recombinant extracellular domain of the AChR α-subunit (Hα1-205), are similarly tolerized. This treatment results in a shift from Th1 to Th2 response and from IgG2 to IgG1 Abs isotypes, and improvement of the disease (Im et al. 1999). Similar evidence is observed after oral administration of Tα146-162 synthetic peptide, corresponding to the immunodominant epitope of TAChR α-subunit, to mice immunized with TAChR. This treatment modulates the ongoing disease in a dose-dependent manner, reducing T cell proliferative response to both TAChR and Tα146-162 peptide, the production of pathogenic antibodies and the loss of muscle AChR content (Baggi et al. 1999).
Immunomodulatory therapeutic strategies addressing the induction of peripheral T-cell tolerance to AChR should promote the re-establishment of a non-pathological balance among the T helper Th1/Th2/Th17/Treg subsets. The importance of Th1 (CD4+IFNγ+) and Th17 (CD4+IL17+) cells in EAMG induction has been demonstrated (Mu et al. 2009), while the role of the Th2 subset in EAMG is controversial. Deficiency of IL-5 or IL-10 (Th2-type cytokines) makes mice susceptible to EAMG induction; deficiency of IL-4 (also a Th2-type cytokine) does not significantly affect EAMG pathogenesis, and this cytokine is not required for EAMG induction (Balasa et al. 1998).
The pathogenic role of complement-fixing anti-AChR IgGs has been confirmed both in mouse and rat EAMG. However, it should be noted that in rats both Th1 and Th2 cells induce secretion of complement-fixing IgG subclasses. Hence, the restoration of the correct balance between Th1/Th2 subsets in the rat EAMG model might have a different effect compared to the mouse EAMG, because the rat IgG1 also activates complement, in contrast to the mouse IgG1. In human, Th1-induced IgG1, IgG2, and IgG3 subclasses bind and activate complement whereas Th2-induced IgG4 antibodies do not. The role of anti-AChR Th1 and Th2 responses in the pathogenesis of human MG is not clear yet (Milani et al. 2006).
Another therapeutic strategy designed to suppress the antigen-specific response causing the disease involves cellular components participating in the control of peripheral tolerance to the AChR. Dendritic cells (DC) are specialized antigen-presenting cells that recognize and process foreign antigens in the periphery and migrate to lymphoid organs where they expose the processed peptides to naïve T cells. Depending on their maturation and differentiation state, DC acquires tolerogenic rather than immunogenic activity (Banchereau and Steinman 1998). In the absence of inflammation, immature DC seem to control peripheral tolerance by promoting regulatory T-cell differentiation; in contrast, inflammatory conditions provoke morphological and functional changes leading to mature DC able to induce the activation of effector T cells (Roncarolo et al. 2001). Many therapeutic strategies try to modulate DC maturation and differentiation with anti-inflammatory agents rather than growth factors. DC, isolated from spleens of healthy rats and conditioned in vitro with transforming growth factor 1-beta (TGF-β1), can be arrested at their immature differentiation stage. The administration to AChR-immunized rats of TGF-β1-DC reduces the severity of EAMG symptoms (Yarilin et al. 2002). Moreover, healthy animals injected with bone marrow DC, isolated from healthy donors and in vitro pulsed with AChR, and subsequently immunized with the same AChR antigen, do not show clinical signs of EAMG. This observation confirms the role of immature DC in the control of peripheral tolerance (Xiao et al. 2003).
AChR-immunized rats injected intraperitoneally with DC derived from spleens of myasthenic animals and in vitro exposed to IL-10, show amelioration of EAMG clinical symptoms, due to DC ability in modulating T and B cell responses (Duan et al. 2004; Xiao et al. 2006). Curiously, the same effect is not achieved when DC are injected subcutaneously. The failure of this second easier injection route represents a limit of IL-10-DC treatment as a potential immunotherapy for human MG (Xiao et al. 2006).
In addition, treatment with granulocyte–macrophage colony-stimulating factor (GM-CSF) can suppress the development of EAMG manifestations when administered to mice before AChR-immunization. The efficacy of this treatment is due to the activation of specific DC subpopulations and expansion of the regulatory T-cell compartment (Sheng et al. 2006). Finally, recent data demonstrate that the administration of bone marrow DC, RelB-silenced and pulsed with Tα146-162, is able to suppress EAMG progression in mice, by inducing a positive shift in favour of Th2/regulatory T cell responses (Yang et al. 2010).
A third alternative therapeutic approach acts directly on the CD4+CD25+ regulatory T-cell (Treg) compartment. Treg cells arise in the thymus, represent 5–10% of CD4+ T cells in the periphery and constitutively express CD25 molecule (IL-2 receptor α-chain). They exert an essential role in maintenance of peripheral tolerance by suppressing proliferation and cytokine production of CD4+ effector T cells (Sakaguchi 2004). A defect in Treg cell subset is often observed in myasthenic patients: the number of Treg cells is reduced in the peripheral blood (Fattorossi et al. 2005), while their suppressive function, but not their number (Matsui et al. 2010), is altered in the thymus (Balandina et al. 2005). Therefore, the restoration or expansion of the Treg cell compartment can represent an important therapeutic tool for the disease. Induced Tregs can be prepared by ex vivo purification of CD4+ T cells from spleens of healthy rats and in vitro stimulation with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β and IL-2. Published studies show that induced CD4+CD25+ cells, with functional features identical to naturally occurring Treg cells, can suppress clinical signs of EAMG in AChR-immunized rats (Aricha et al. 2008). Other studies show that also naturally occurring Treg cells, purified from spleens of healthy rats, can modulate EAMG progression when administered to AChR-immunized rats (Nessi et al. 2010). The administration of naturally occurring CD4+CD25+ cells is effective in reducing T-cell proliferation in response to the immunizing antigen, decreasing pathogenic Abs and increasing muscle AChR content, when given according to a preventive schedule (starting 7 days post immunization). In contrast, the therapeutic injection of Treg cells (starting 30 days post immunization, at overt clinical symptoms) does not reduce the severity of EAMG (Nessi et al. 2010). Moreover, published data demonstrate that the administration of IL-2/anti-IL-2 mAb complexes inhibits the development of EAMG, mediating the expansion of CD4+CD25+Foxp3+ T cells and the conversion of CD4+CD25− T cells in the periphery (Nessi et al. 2010). The suppression of the disease seems mainly due to the shift of Th1/Th2 ratio in favour of a Th2 phenotype and to the increased production of TGF-β (Liu et al. 2010).
A further candidate to study new potential cell therapies for human MG is represented by bone marrow stromal cells (BMSC), which can modulate the functions of T and B cells, NK and DC. In particular, BMSC inhibit lymphocytes responses to different stimuli by secretion of immunosuppressive factors (Kong et al. 2009a, b). Indeed, stromal cells, derived from healthy rats, induce a strong reduction of disease severity when injected in EAMG rats at the appearance of clinical signs. Such treatment results in the suppression of both T and B cell responses to the immunizing antigen and in modulation of cytokine production, decreasing Th1 and Th17 subsets and increasing Th2 and Treg subpopulations, due to BMSC secretion of immunosuppressive factors, like indoleamine 2,3-dioxygenase as well as TGF-β (Kong et al. 2009a, b).
Pharmacological Immunotherapy
Current pharmacological therapies for the treatment of MG include steroids and immunosuppressants, as long-term drugs, and immunomodulating agents, as short-term therapy (Mantegazza et al. 2011). Azathioprine and cyclophosphamide provided successful results in the treatment of experimental MG in rabbits (Abramsky et al. 1976) and rats (Pestronk et al. 1983), respectively. The synthetic immunomodulant drug, Linomide, has been tested for its effect on EAMG and other experimental autoimmune diseases, showing promising results for the future (Karussis et al. 1994). Other emerging drugs, such as mycophenolate mofetil (MMF) (Janssen et al. 2008), pixantrone (BBR2778; PIX) (Ubiali et al. 2008), and bortezomib (Gomez et al. 2011), show excellent efficacy in suppressing EAMG. MMF is a synthesized pro-drug of mycophenolic acid that inhibits the immune system by preferentially depleting guanosine and deoxyguanosine on both T and B-lymphocyte lines; Bortezomib is a proteasome-inhibitor that depletes auto-Abs-producing plasma cells; PIX is an anti-neoplastic drug, characterized by a reduced cardiotoxicity compared with the structurally-related compound Mitoxantrone. These drugs have been administrated to AChR-immunized rats via different treatment schedules, either preventive (before clinical onset) or therapeutic protocol (at overt clinical symptoms), and the showed effects were on antigen-specific T-cell proliferative responses, on the pathogenic Abs and increasing muscle AChR content. These results offer a new possibility for the treatment of human MG.
Modulation of Complement-Mediated NMJ Destruction
The role of complement components in MG and its experimental models has been intensively studied in the past. The terminal and lytic complement components (C9) are located at the motor end-plate in acquired autoimmune MG: the NMJ is characterized by an inverse relationship between the structural integrity of the junctional folds and the abundance of C9 (Sahashi et al. 1980). Complement breakdown products might play several roles in EAMG development: for instance, C3a and C5a could promote inflammation by recruiting and activating phagocytic cells. Published data demonstrate that anti-C5 antibody treatment ameliorates EAMG weakness and disease course (Zhou et al. 2007); however, C5a-knockout mice are resistant to EAMG induction, showing no direct C5 involvement in EAMG pathogenesis (Qi et al. 2008), other complement factors like C3b and C4b lead to muscle membrane lysis (Morgan et al. 2006). Depleting the complement cascade via treatment with cobra venom factor decreases the formation of anti-AChR Abs/ACh-receptor complexes and ameliorates the acute phase of EAMG in rats (Lennon et al. 1978). Moreover, the effects of some complement components have been analyzed in transgenic models. For instance, following AChR-immunization, C5-deficient mice do not show the same increased incidence of clinical symptoms, significant loss of muscle AChR and disease-induced death as in C5-sufficient control animals (Christadoss 1988).
The role of the MAC has also been investigated in acute passively transferred EAMG in Wistar rats, in which the administration of anti-C6 Fab leads to the inhibition of MAC formation and suppression of clinical and electrophysiological signs of experimental MG (Biesecker and Gomez 1989).
A further study aimed to investigate the role of complement has been performed in C6−/− rats passively transferred with monoclonal anti-AChR antibodies mAb35, an IgG1 immunoglobulin that specifically binds the MIR. Here, C6-deficient rats are resistant to EAMG development and neither C9 nor MAC are observed at the NMJ. Confirming these results, the reconstitution of C6 in EAMG C6−/− rats by injection of human C6 is able to induce the disease with severity and symptoms comparable to that observed in wild-type animals (Chamberlain-Banoub et al. 2006). All these evidences demonstrate the importance of complement activation, in particular MAC formation, in NMJ destruction and generally in the pathogenesis of EAMG. Therefore, depletion of components necessary to MAC assembly could represent an essential element for the treatment of human MG.
An alternative to depleting approaches is represented by blocking the NMJ destruction by pharmacological inhibition of the complement activation pathway. Accordingly, the administration of rEV576, a specific C5 complement component inhibitor identified in the saliva of the tick, is able to reduce the severity of passive transfer MG and the progression of acute experimental MG, reducing C9 deposits at the NMJ (Soltys et al. 2009).
Besides, daily intraperitoneal injections with a soluble recombinant form of human complement receptor 1 reduce weight loss and severity of clinical symptoms in myasthenic Lewis rats, in which EAMG has been induced by passive administration of mAb35 (Piddlesden et al. 1996). Following this line of experimental approach, several studies have been recently performed. Regulatory proteins that inhibit MAC formation (such as MIRL-CD59 which inhibits MAC assembly) (Kaminski et al. 2006) or control the activation of the complement cascade (such as the decay-accelerating factor, DAF or CD55, which inactivates C3 and C5 convertase enzymes) (Lin et al. 2002) can represent crucial players in EAMG development and their depletion might have a role in the pathogenesis of EAMG. Hence, the modulation of these regulatory proteins may be a target for preventing or attenuating the destruction of the NMJ. For instance, Daf1−/− mice passively transferred with McAb-3, an activator of complement belonging to IgG2b isotype, show an increased susceptibility to EAMG compared with normal animals, characterized by muscle weakness, fatigability and C3b deposits at the NMJ already 24 h post the disease induction (Lin et al. 2002). Daf1−/−, CD59a−/− and Daf1−/−CD59a−/− mice intraperitoneally injected with McAb-3 can develop passive transfer MG with different severity: both knockout mice for a single gene become mildly sick, while mice with depletion of both genes show a severe muscle inflammation and loss of AChR (Morgan et al. 2006). Similar findings can be observed after intravenous administration of McAb-3 in mice with depletion of the same genes. Daf1−/−CD59a−/− mice develop a very severe disease leading to death, and Daf1−/− mice appear weaker than CD59a−/− with evident deposits of C9 at the NMJ 48 h post EAMG induction (Kaminski et al. 2006).
These and other experimental approaches underline the fact that complement activation is an essential factor in the destruction process of the post-synaptic membrane. Indeed, IL-12−/− mice provided indirect but strong evidence on the role of complement in EAMG, since they do not produce complement activating IgG2b and IgG2c antibodies, and hence knockout mice are protected from EAMG after immunization with Torpedo AChR (Karachunski et al. 2000).
Increase of NMJ Resistance to Complement-Mediated Lysis
The susceptibility to experimental MG in rats correlates with the age of the animals. Aged rats show a mild disease, characterized by low antibody titer and high resistance of the post-synaptic membrane to the Abs-mediated attack (Hoedemaekers et al. 1997a). These events are accompanied by an increase of rapsyn concentration at the level of the NMJ in aged animals. An increased interaction between rapsyn and AChR may stabilize the receptor molecules, leading to minor AChR loss and muscle weakness in acute EAMG (Losen et al. 2005). Thus, rapsyn and its overexpression could represent a further therapeutical target. However, findings that are more recent show that the increased expression of rapsyn alone is not able to efficiently anchor the AChR to the post-synaptic membrane in chronic EAMG, once the destruction of the NMJ has already occurred (Martinez–Martinez et al. 2007).
Conclusions
Human diseases like MG involve different compartments of the organism: the immune system and the NMJ. The EAMG model allows investigation of both compartments, focusing on the pathogenic mechanisms and the clinical outcome. In vitro models able to fully represent complex pathologies where more tissues and systems are involved are not yet available. Therefore, animal models are necessary to deeply comprehend the pathogenic mechanisms and investigate new therapies. Animal models involving transgenic approaches may not be easily translated to human, considering the difficulties associated with gene therapy, but new drugs and chemicals can be easily tested on experimental models and later on translated to clinical trials.
Although several antigen-unspecific immunosuppressive strategies tested in EAMG were transferred to the clinics (Abramsky et al. 1976; Karussis et al. 1994), other antigen-specific therapeutic approaches, more recently tested and proved effective in EAMG, have not been transferred yet to the human disease. This may be due to two reasons. Firstly, EAMG has a less complex pathogenesis compared to the human disease; secondly, MG is a rare disease and generally new approaches are tested in different experimental models belonging to more common autoimmune disorders. Nevertheless, as we had the opportunity to demonstrate, EAMG has been a valuable tool to prove the efficacy of pixantrone in the experimental model (Ubiali et al. 2008), with results that encourage its investigation in the human disease. This approach is propaedeutic to the investigation of any new immunosuppressive or immunomodulating compound of potential interest.
Always bearing in mind the importance of Russel and Burch (1959) 3R rule for replacement of experimental animal procedures with alternative methods, reduction of the number of used animals and refinement of the animal conditions, we cannot forget that complex diseases such as autoimmune disorders need to be addressed with pre-clinical research in order to obtain therapies that are more efficient. Despite the evident differences between EAMG and MG, primarily the fact that the MG pathology cannot spontaneously arise in mice and rats, the experimental approach remains a very useful tool and an unavoidable method to discover new and more efficient therapies.
Abbreviations
- aa:
-
Amino acids
- AChR:
-
Acetylcholine receptor
- TAChR:
-
Torpedo californica AChR
- auto-Abs:
-
Autoantibodies
- BMSC:
-
Bone marrow stromal cells
- DC:
-
Dendritic cells
- MG:
-
Myasthenia gravis
- MIR:
-
Main immunogenic region
- EAMG:
-
Experimental autoimmune MG
- CFA:
-
Complete Freund’s adjuvant
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- IgG:
-
Immunoglobulin G type
- MAC:
-
Membrane attack complex
- NMJ:
-
Neuromuscular junction
- PBL:
-
Peripheral blood lymphocytes
- PIX:
-
Pixantrone (BBR2778)
- SCID:
-
Severe combined immunodeficiency
- TGF-β1:
-
Transforming growth factor 1-beta
- Treg:
-
Regulatory T-cell
References
Abramsky O, Tarrab-Hazdai R, Aharonov A et al (1976) Immunosuppression of experimental autoimmune myasthenia gravis by hydrocortisone and azathioprine. J Immunol 117:225–228
Antozzi C, Gemma M, Regi B et al (1991) A short plasma exchange protocol is effective in severe myasthenia gravis. J Neurol 238:103–107
Arce S, Cassese G, Hauser A et al (2002) The role of long-lived plasma cells in autoimmunity. Immunobiology 206:558–562
Aricha R, Feferman T, Fuchs S et al (2008) Ex vivo generated regulatory T cells modulate experimental autoimmune myasthenia gravis. J Immunol 180:2132–2139
Aricha R, Feferman T, Scott HS et al (2011) The susceptibility of Aire(−/−) mice to experimental myasthenia gravis involves alterations in regulatory T cells. J Autoimmun 36:16–24
Baggi F, Andreetta F, Caspani E et al (1999) Oral administration of an immunodominant T-cell epitope downregulates Th1/Th2 cytokines and prevents experimental myasthenia gravis. J Clin Invest 104:1287–1295
Baggi F, Annoni A, Ubiali F et al (2004) Breakdown of tolerance to a self-peptide of acetylcholine receptor alpha-subunit induces experimental myasthenia gravis in rats. J Immunol 172:2697–2703
Balandina A, Lecart S, Dartevelle P et al (2005) Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood 105:735–741
Balasa B, Deng C, Lee J et al (1997) Interferon gamma (IFN-gamma) is necessary for the genesis of acetylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J Exp Med 186:385–391
Balasa B, Deng C, Lee J et al (1998) The Th2 cytokine IL-4 is not required for the progression of antibody-dependent autoimmune myasthenia gravis. J Immunol 161:2856–2862
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Barchan D, Souroujon MC, Im SH et al (1999) Antigen-specific modulation of experimental myasthenia gravis: nasal tolerization with recombinant fragments of the human acetylcholine receptor alpha-subunit. Proc Natl Acad Sci USA 96:8086–8091
Bellone M, Ostlie N, Lei SJ et al (1991) The I-Abm12 mutation, which confers resistance to experimental myasthenia gravis, drastically affects the epitope repertoire of murine CD4+ cells sensitized to nicotinic acetylcholine receptor. J Immunol 147:1484–1491
Berman PW, Patrick J (1980a) Experimental myasthenia gravis. A murine system. J Exp Med 151:204–223
Berman PW, Patrick J (1980b) Linkage between the frequency of muscular weakness and loci that regulate immune responsiveness in murine experimental myasthenia gravis. J Exp Med 152:507–520
Biesecker G, Gomez CM (1989) Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J Immunol 142:2654–2659
Biesecker G, Koffler D (1988) Resistance to experimental autoimmune myasthenia gravis in genetically inbred rats. Association with decreased amounts of in situ acetylcholine receptor-antibody complexes. J Immunol 140:3406–3410
Chamberlain-Banoub J, Neal JW, Mizuno M et al (2006) Complement membrane attack is required for endplate damage and clinical disease in passive experimental myasthenia gravis in Lewis rats. Clin Exp Immunol 146:278–286
Christadoss P (1988) C5 gene influences the development of murine myasthenia gravis. J Immunol 140:2589–2592
Christadoss P, Poussin M, Deng C (2000) Animal models of myasthenia gravis. Clin Immunol 94:75–87
Conti-Fine BM, Milani M, Kaminski HJ (2006) Myasthenia gravis: past, present, and future. J Clin Invest 116:2843–2854
Dau PC (1981) Response to plasmapheresis and immunosuppressive drug therapy in sixty myasthenia gravis patients. Ann NY Acad Sci 377:700–708
Deng C, Goluszko E, Tuzun E et al (2002) Resistance to experimental autoimmune myasthenia gravis in IL-6-deficient mice is associated with reduced germinal center formation and C3 production. J Immunol 169:1077–1083
Drachman DB (2003) Autonomic “myasthenia”: the case for an autoimmune pathogenesis. J Clin Invest 111:797–799
Drachman DB, Angus CW, Adams RN et al (1978) Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med 298:1116–1122
Duan RS, Adikari SB, Huang YM et al (2004) Protective potential of experimental autoimmune myasthenia gravis in Lewis rats by IL-10-modified dendritic cells. Neurobiol Dis 16:461–467
Eldefrawi ME (1978) Experimental autoimmune myasthenia gravis: the rabbit as an animal model. Fed Proc 37:2823–2827
Engel AG, Fumagalli G (1982) Mechanisms of acetylcholine receptor loss from the neuromuscular junction. Ciba Found Symp (90):197–224
Engel AG, Lambert EH, Howard FM (1977) Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc 52:267–280
Fattorossi A, Battaglia A, Buzzonetti A et al (2005) Circulating and thymic CD4 CD25 T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunology 116:134–141
Goluszko E, Deng C, Poussin MA et al (2002) Tumor necrosis factor receptor p55 and p75 deficiency protects mice from developing experimental autoimmune myasthenia gravis. J Neuroimmunol 122:85–93
Gomez CM, Richman DP (1983) Anti-acetylcholine receptor antibodies directed against the alpha-bungarotoxin binding site induce a unique form of experimental myasthenia. Proc Natl Acad Sci USA 80:4089–4093
Gomez AM, Van Den Broeck J, Vrolix K et al (2010) Antibody effector mechanisms in myasthenia gravis-pathogenesis at the neuromuscular junction. Autoimmunity 43:353–370
Gomez AM, Vrolix K, Martinez–Martinez P et al (2011) Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol 186:2503–2513
Hill M, Moss P, Wordsworth P et al (1999) T cell responses to D-penicillamine in drug-induced myasthenia gravis: recognition of modified DR1:peptide complexes. J Neuroimmunol 97:146–153
Hoch W, McConville J, Helms S et al (2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 7:365–368
Hoedemaekers AC, Verschuuren JJ, Spaans F et al (1997a) Age-related susceptibility to experimental autoimmune myasthenia gravis: immunological and electrophysiological aspects. Muscle Nerve 20:1091–1101
Hoedemaekers AC, van Breda Vriesman PJ, De Baets MH (1997b) Myasthenia gravis as a prototype autoimmune receptor disease. Immunol Res 16:341–354
Im SH, Barchan D, Fuchs S et al (1999) Suppression of ongoing experimental myasthenia by oral treatment with an acetylcholine receptor recombinant fragment. J Clin Invest 104:1723–1730
Janssen SP, Phernambucq M, Martinez–Martinez P et al (2008) Immunosuppression of experimental autoimmune myasthenia gravis by mycophenolate mofetil. J Neuroimmunol 201–202:111–120
Kaminski HJ, Kusner LL, Richmonds C et al (2006) Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp Neurol 202:287–293
Karachunski PI, Ostlie NS, Okita DK et al (1999) Interleukin-4 deficiency facilitates development of experimental myasthenia gravis and precludes its prevention by nasal administration of CD4+ epitope sequences of the acetylcholine receptor. J Neuroimmunol 95:73–84
Karachunski PI, Ostlie NS, Monfardini C et al (2000) Absence of IFN-gamma or IL-12 has different effects on experimental myasthenia gravis in C57BL/6 mice. J Immunol 164:5236–5244
Karussis DM, Lehmann D, Brenner T et al (1994) Immunomodulation of experimental autoimmune myasthenia gravis with linomide. J Neuroimmunol 55:187–193
Kong QF, Sun B, Bai SS et al (2009a) Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol 207:83–91
Kong QF, Sun B, Wang GY et al (2009b) BM stromal cells ameliorate experimental autoimmune myasthenia gravis by altering the balance of Th cells through the secretion of IDO. Eur J Immunol 39:800–809
Lennon VA, Lindstrom JM, Seybold ME (1975) Experimental autoimmune myasthenia: a model of myasthenia gravis in rats and guinea pigs. J Exp Med 141:1365–1375
Lennon VA, Seybold ME, Lindstrom JM et al (1978) Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J Exp Med 147:973–983
Lennon VA, Lambert EH, Leiby KR et al (1991) Recombinant human acetylcholine receptor alpha-subunit induces chronic experimental autoimmune myasthenia gravis. J Immunol 146:2245–2248
Lin F, Kaminski HJ, Conti-Fine BM et al (2002) Markedly enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J Clin Invest 110:1269–1274
Lindstrom J (1980) Experimental autoimmune myasthenia gravis. J Neurol Neurosurg Psychiatry 43:568–576
Lindstrom J (2008) ‘Seronegative’ myasthenia gravis is no longer seronegative. Brain 131(Pt 7):1684–1685
Lindstrom JM, Seybold ME, Lennon VA et al (1976) Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 26:1054–1059
Link H, Xiao BG (2001) Rat models as tool to develop new immunotherapies. Immunol Rev 184:117–128
Liu R, Hao J, Dayao CS et al (2009) T-bet deficiency decreases susceptibility to experimental myasthenia gravis. Exp Neurol 220:366–373
Liu R, Zhou Q, La Cava A et al (2010) Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol 40:1577–1589
Losen M, Stassen MH, Martinez–Martinez P et al (2005) Increased expression of rapsyn in muscles prevents acetylcholine receptor loss in experimental autoimmune myasthenia gravis. Brain 128(Pt 10):2327–2337
Luo J, Taylor P, Losen M et al (2009) Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. J Neurosci 29:13898–13908
Mantegazza R, Bonanno S, Camera G et al (2011) Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr Dis Treat 7:151–160
Mareska M, Gutmann L (2004) Lambert-Eaton myasthenic syndrome. Semin Neurol 24:149–153
Martinez–Martinez P, Losen M, Duimel H et al (2007) Overexpression of rapsyn in rat muscle increases acetylcholine receptor levels in chronic experimental autoimmune myasthenia gravis. Am J Pathol 170:644–657
Martino G, DuPont BL, Wollmann RL et al (1993) The human-severe combined immunodeficiency myasthenic mouse model: a new approach for the study of myasthenia gravis. Ann Neurol 34:48–56
Matsui N, Nakane S, Saito F et al (2010) Undiminished regulatory T cells in the thymus of patients with myasthenia gravis. Neurology 74:816–820
Meinl E, Klinkert WE, Wekerle H (1991) The thymus in myasthenia gravis. Changes typical for the human disease are absent in experimental autoimmune myasthenia gravis of the Lewis rat. Am J Pathol 139:995–1008
Melms A, Luther C, Stoeckle C et al (2006) Thymus and myasthenia gravis: antigen processing in the human thymus and the consequences for the generation of autoreactive T cells. Acta Neurol Scand Suppl 183:12–13
Meriggioli MN, Sanders DB (2009) Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 8:475–490
Milani M, Ostlie N, Wu H et al (2006) CD4+ T and B cells cooperate in the immunoregulation of experimental autoimmune myasthenia gravis. J Neuroimmunol 179:152–162
Moiola L, Galbiati F, Martino G et al (1998) IL-12 is involved in the induction of experimental autoimmune myasthenia gravis, an antibody-mediated disease. Eur J Immunol 28:2487–2497
Morgan BP, Chamberlain-Banoub J, Neal JW et al (2006) The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol 146:294–302
Mu L, Sun B, Kong Q et al (2009) Disequilibrium of T helper type 1, 2 and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology 128(1 suppl):e826–e836
Nessi V, Nava S, Ruocco C et al (2010) Naturally occurring CD4+ CD25+ regulatory T cells prevent but do not improve experimental myasthenia gravis. J Immunol 185:5656–5667
Newsom-Davis J, Willcox N, Calder L (1981) Thymus cells in myasthenia gravis selectively enhance production of anti-acetylcholine-receptor antibody by autologous blood lymphocytes. N Engl J Med 305:1313–1318
Ostlie N, Milani M, Wang W et al (2003) Absence of IL-4 facilitates the development of chronic autoimmune myasthenia gravis in C57BL/6 mice. J Immunol 170:604–612
Patrick J, Lindstrom J (1973) Autoimmune response to acetylcholine receptor. Science 180:871–872
Penn AS, Low BW, Jaffe IA et al (1998) Drug-induced autoimmune myasthenia gravis. Ann NY Acad Sci 841:433–449
Pestronk A, Drachman DB, Teoh R et al (1983) Combined short-term immunotherapy for experimental autoimmune myasthenia gravis. Ann Neurol 14:235–241
Piddlesden SJ, Jiang S, Levin JL et al (1996) Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol 71:173–177
Poussin MA, Goluszko E, David CS et al (2001) HLA-DQ6 transgenic mice resistance to experimental autoimmune myasthenia gravis is linked to reduced acetylcholine receptor-specific IFN-gamma, IL-2 and IL-10 production. J Autoimmun 17:175–180
Qi H, Tuzun E, Allman W et al (2008) C5a is not involved in experimental autoimmune myasthenia gravis pathogenesis. J Neuroimmunol 196:101–106
Rodgaard A, Nielsen FC, Djurup R et al (1987) Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clin Exp Immunol 67:82–88
Roncarolo MG, Levings MK, Traversari C (2001) Differentiation of T regulatory cells by immature dendritic cells. J Exp Med 193:F5–F9
Ruff RL, Lennon VA (1998) End-plate voltage-gated sodium channels are lost in clinical and experimental myasthenia gravis. Ann Neurol 43:370–379
Russel WMS, Burch RL (1959) The principles of humane experimental technique. Special edition published by Universities Federation for Animal Welfare (UFAW), 1992 edn. Methuen & Co., London
Sahashi K, Engel AG, Lambert EH et al (1980) Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J Neuropathol Exp Neurol 39:160–172
Sakaguchi S (2004) Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22:531–562
Sanders DB, Evoli A (2010) Immunosuppressive therapies in myasthenia gravis. Autoimmunity 43:428–435
Schönbeck S, Padberg F, Hohlfeld R et al (1992) Transplantation of thymic autoimmune microenvironment to severe combined immunodeficiency mice. A new model of myasthenia gravis. J Clin Invest 90:245–250
Sheng JR, Li L, Ganesh BB et al (2006) Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol 177:5296–5306
Slater CR, Young C, Wood SJ et al (1997) Utrophin abundance is reduced at neuromuscular junctions of patients with both inherited and acquired acetylcholine receptor deficiencies. Brain 120(Pt 9):1513–1531
Soltys J, Kusner LL, Young A et al (2009) Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann Neurol 65:67–75
Tarrab-Hazdai R, Aharonov A, Silman I et al (1975) Experimental autoimmune myasthenia induced in monkeys by purified acetylcholine receptor. Nature 256:128–130
Toro-Goyco E, Cora EM, Kessler MJ et al (1986) Induction of experimental myasthenia gravis in rhesus monkeys: a model for the study of the human disease. PR Health Sci J 5:13–18
Toyka KV, Brachman DB, Pestronk A et al (1975) Myasthenia gravis: passive transfer from man to mouse. Science 190:397–399
Tzartos S, Hochschwender S, Vasquez P et al (1987) Passive transfer of experimental autoimmune myasthenia gravis by monoclonal antibodies to the main immunogenic region of the acetylcholine receptor. J Neuroimmunol 15:185–194
Tzartos SJ, Kokla A, Walgrave SL et al (1988) Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67–76 of the alpha subunit. Proc Natl Acad Sci USA 85:2899–2903
Ubiali F, Nava S, Nessi V et al (2008) Pixantrone (BBR2778) reduces the severity of experimental autoimmune myasthenia gravis in Lewis rats. J Immunol 180:2696–2703
van der Neut Kolfschoten M, Schuurman J, Losen M et al (2007) Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317:1554–1557
Vincent A (1991) Autoimmunity to acetylcholine receptors in myasthenia gravis. Biochem Soc Trans 19:180–183
Vincent A (1994) AChR from cell line TE671 cannot replace human muscle AChR in the conventional diagnostic immunoprecipitation RIA. J Neuroimmunol 53:115
Vincent A (2002) Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2:797–804
Wang ZY, Karachunski PI, Howard JF et al (1999) Myasthenia in SCID mice grafted with myasthenic patient lymphocytes: role of CD4+ and CD8+ cells. Neurology 52:484–497
Wang W, Ostlie NS, Conti-Fine BM et al (2004) The susceptibility to experimental myasthenia gravis of STAT6−/− and STAT4−/− BALB/c mice suggests a pathogenic role of Th1 cells. J Immunol 172:97–103
Wood SJ, Slater CR (2001) Safety factor at the neuromuscular junction. Prog Neurobiol 64:393–429
Xiao BG, Duan RS, Link H et al (2003) Induction of peripheral tolerance to experimental autoimmune myasthenia gravis by acetylcholine receptor-pulsed dendritic cells. Cell Immunol 223:63–69
Xiao BG, Duan RS, Zhu WH et al (2006) The limitation of IL-10-exposed dendritic cells in the treatment of experimental autoimmune myasthenia gravis and myasthenia gravis. Cell Immunol 241:95–101
Yang H, Goluszko E, David C et al (2002) Mapping myasthenia gravis-associated T cell epitopes on human acetylcholine receptors in HLA transgenic mice. J Clin Invest 109:1111–1120
Yang H, Zhang Y, Wu M et al (2010) Suppression of ongoing experimental autoimmune myasthenia gravis by transfer of RelB-silenced bone marrow dentritic cells is associated with a change from a T helper Th17/Th1 to a Th2 and FoxP3+ regulatory T-cell profile. Inflamm Res 59:197–205
Yarilin D, Duan R, Huang YM et al (2002) Dendritic cells exposed in vitro to TGF-beta1 ameliorate experimental autoimmune myasthenia gravis. Clin Exp Immunol 127:214–219
Zhang GX, Xiao BG, Bai XF et al (1999) Mice with IFN-gamma receptor deficiency are less susceptible to experimental autoimmune myasthenia gravis. J Immunol 162:3775–3781
Zhou Y, Gong B, Lin F et al (2007) Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J Immunol 179:8562–8567
Acknowledgments
We regret that the essential work of many investigators and colleagues could not be included in this review due to space constraints.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Baggi, F., Antozzi, C., Toscani, C. et al. Acetylcholine Receptor-Induced Experimental Myasthenia Gravis: What Have We Learned from Animal Models After Three Decades?. Arch. Immunol. Ther. Exp. 60, 19–30 (2012). https://doi.org/10.1007/s00005-011-0158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-011-0158-6