Abstract
Uric acid crystals, the causative agent of gout, have recently gained widespread attention due to their role as a natural endogenous adjuvant. Uric acid crystals, first sensed extracellularly by membrane lipid alterations, are internalized and subsequently activate the NLRP3 inflammasome. Currently, various aspects of this particular novel pathway are poorly defined. This short review will focus on some recent discoveries regarding this simple crystalline structure and address areas requiring further investigation. The fact that uric acid crystals activate innate host defense mechanisms, triggering robust inflammation and immune activation, may lead to engineering potent adjuvants for future vaccines. Furthermore, the elucidation of uric acid’s mechanism of inflammation may lay the foundation for other solid inflammatory structures such as silica, asbestos, and alum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major goal of immunological research is to prevent disease through the development of effective vaccines. In principle, vaccines contain two essential parts: a small portion of the infectious agent, usually microbial proteins, and an adjuvant, a substance that amplifies the immune response against the co-administered pathogenic agent. The efficacy of vaccines is highly dependent upon adjuvants. Despite tremendous strides in immunological research, the development of appropriate adjuvants for vaccination purposes has been limited to laboratory studies using mixtures of killed mycobacterial components and mineral oil (Freund’s adjuvants) or for human applications using aluminum salt precipitates (Glenny et al. 1926). The modern extensions of these practices are still by and large empirical in nature; immunologists do not fully understand how these adjuvants work. Thus much effort within this field has been initiated to uncover the relevant mechanisms as outcomes from these efforts will provide a theoretical basis for the rational development of future prophylactic strategies against infection and neoplasm.
A difficulty with inducing prophylaxis is that an appropriate and robust response against pathogens must be initiated without indiscriminately injuring host tissues. The immune system contains an innate set of mechanisms to accomplish this crucial discrimination. One of the pivotal events in immune recognition is the interaction between professional antigen-presenting cells (APCs) and CD4 T cells, which essentially determines subsequent immune activation events. At this step, self–nonself distinction is accomplished by the development of two activation signals. For productive immune activation, two signals must be present on APCs, for example dendritic cells. The first signal consists of processing proteins from the infectious agent into short epitope peptides and loading these peptides onto major histocompatibility complexes. The second signal consists of costimulatory molecules, cytokine release, etc. In natural infection, this “second signal” is activated by microbial components, otherwise known as pathogen-associated molecular patterns (PAMPs), which engage Toll-like receptors (TLRs) (Janeway 1989; Janeway 1992). There is also a second class of substances, first proposed by P. Matzinger, that activate the second signal (Matzinger 1994; Matzinger 1998). These substances, damage-associated molecular patterns, are released from injured host cells to perpetuate immune responses in place of PAMPs. In both scenarios one can see that the role of the adjuvant in a vaccine is to provoke the second signal.
An example of such an endogenous signal is uric acid or, more precisely, monosodium urate (MSU) crystals. They are also the causative agent of gout (Garrod 1848). Naturally, it has become a topic of immunological research due to its adjuvanticity and CTL activity (Shi et al. 2003). Attention to this simple structure has been further elevated due to the requirement of the NLRP3 inflammasome in its recognition (Martinon et al. 2006). Coincidentally, alum, a human adjuvant, is also known to activate the NLRP3 inflammasome and induce upregulation of costimulatory molecules and other activation markers on professional APCs (Eisenbarth et al. 2008; Marrack et al. 2009; Ulanova et al. 2001). Interestingly, alum has also been shown to elicit its adjuvant properties by inducing MSU release (Kool et al. 2008). However, one conceptual barrier remains as to how an intracellular protein complex can recognize an extracellular structure. This short review will focus on two major advances in the adjuvanticity of uric acid crystals.
Uric Acid Crystals as a Rheumatological Concern
For years, research in MSU-associated arthritis was purely a rheumatological study. Gouty arthritis was first linked to MSU crystal deposition in the 1960s; Faires and McCarty reproduced gout symptoms by injecting the crystal into human and dog joints (Faires and McCarty 1962). Since the discovery, there have been ongoing investigations into the inflammatory mechanism of MSU.
The surface of a MSU crystal is deprotonated and “sticky”. The electrostatic charge of solid structures allows for the binding of serum proteins to the crystal surface via ionic interactions or hydrogen bonding (personal communication with Tonglei Li, University of Kentucky). Indeed, the binding of serum proteins has been observed. In particular, complement adsorption to MSUs surface dominated early research into the mechanism of MSU-mediated inflammation. Both the classical and alternative complement pathways have been implicated in MSUs mode of action (Doherty et al. 1983; Giclas et al. 1979; Hasselbacher 1979a, b; Naff and Byers 1973). The complement cascade consists of a series of proteins that ultimately activates C3 or C5 convertase. Complement in association with MSU was first investigated on the hypothesis that complement bound to MSU contributed to the membranolytic events, premature cell rupture, and cell death associated with the inflammatory nature of MSU crystals (Wallingford and McCarty 1971). As a result, lysosomal damage and content release mechanisms were further investigated in leukocytes (Mandel 1976). Taken together, these factors likely contribute to the inflammatory properties of MSU.
It was previously documented that antibodies are also found adsorbed to crystalline MSU. These antibodies were first thought to play a role in MSUs inflammatory potential through the complement cascade (Hasselbacher 1979a, b). However, evidence following this path of research has since diminished. In 1992, Adaddi’s group proposed a new hypothesis that serum from uric acid-immunized animals aids in its precipitation (Kam et al. 1992). They subsequently showed that uric acid crystallization is aided by serum from uric acid-immunized rabbits (Kam et al. 1994). This is important because at normal serum concentrations, uric acid fails to crystallize in vitro (Fiddis et al. 1983; Iwata et al. 1989; Loeb 1972). Our group recently identified uric acid-binding antibodies as the soluble factor that mediates crystal precipitation in vivo (Kanevets et al. 2009). The presence of these antibodies increased basal inflammation and enhanced CTL activity in antigen-dependent assays. This finding may explain MSUs ability to function as an endogenous adjuvant and immune activator.
MSU Crystals in Immunology
Monosodium urate crystals soon entered the realm of immunological research when they were discovered to act as a danger signal to alert the immune system to injured or dying cells (Shi et al. 2003). Shi et al. further proposed that MSU crystals could be used for vaccination purposes as an adjuvant for cytolytic T cells. While earlier studies shed light onto the mechanisms of the robust symptoms of gouty inflammation, the mechanism of MSU-associated adjuvanticity may be different; in contrast to the systemic inflammation of gout, adjuvanticity is usually a local and transient event.
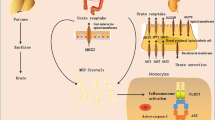
In 2006, Tschopp’s group reported that MSU crystals activate the NLRP3 inflammasome (Martinon et al. 2006). The NLRP3 inflammasome is an intracellular protein complex composed of NLRP3, ASC, and caspase-1 (Martinon et al. 2002). Activation of NLRP3 leads to activation of caspase-1, which cleaves pro-IL-1β and pro-IL-18 to their active secreted products. IL-1β and IL-18 are pro-inflammatory cytokines and are believed to play a significant role in MSU-mediated inflammation. Several other solid-structure stimulants have also been implicated in the activation of the NLRP3 inflammasome, among others silica, asbestos, and alum (Dostert et al. 2008; Eisenbarth et al. 2008). These findings did not address how the solid structures directly activate an intracellular “sensing” mechanism. Subsequently, Hornung et al. published an interesting result: APCs internalize the solid structure into a phagosome that subsequently undergoes acidification and maturation into a lysosome (Hornung et al. 2008). Due to the internalized solid structure, the lysosome destabilizes, ruptures, and releases its contents, particularly cathepsin B, into the cytoplasm. Cathepsin B signals for K+ efflux and ROS production for the activation of the NLRP3 inflammasome (Dostert et al. 2008; Petrilli et al. 2007). Recently it was shown that ROS production can cleave thioredoxin (TRX)-interacting protein (TXNIP) from TRX to allow its interaction with NLRP3 (Zhou et al. 2010). The interaction of TXNIP with NRLP3 allows for activation of the inflammasome complex. Activation of the inflammasome allows for IL-1β and IL-18 release. They are believed, at least in vivo, to enhance activities characteristic of Th2 responses: antibody production, T-cell expansion, and T-cell polarization (Brewer et al. 1999; Brewer 2006).
Certain questions remain. Among them is how MSU or other solid structures are detected prior to their entry into the cell. Phagolysosomal destabilization leading to cathepsin B release is one possible method for the downstream NLRP3-dependent sensing (Hornung et al. 2008). This same mechanism was proposed for alum, the most widely used adjuvant. Alum stimulates a Th2 response preferentially, while MSU has a preferential Th1 type response. A simplistic reenactment fails to explain the distinct outcomes. Furthermore, how K+ efflux and ROS production initiates NLRP3 is unknown and how NLRP3 affects later immune responses, for instance, adjuvanticity, is speculative at best.
Using atomic force microscopy as a tool for real-time single-cell force analysis, we recently proposed that MSU sensing occurs prior to crystal internalization via a lipid-sorting mechanism (Ng et al. 2008). Cholesterol-rich domains in the lipid membrane, also known as lipid rafts, have a natural affinity to MSU crystals, possibly due to hydrogen bonding interactions. These domains are home to various ITAM-associated transmembrane proteins such as FcR gamma and DAP12. The aggregation of cholesterol-rich regions to MSU crystals increases the spatial density of such ITAM motifs such that the basal phosphorylation of these ITAMs spontaneously activates Syk signaling in an extracellular protein receptor-independent manner. Syk signaling results in PI3K signaling, cytoskeletal rearrangement, and phagocytosis of the crystal (Berton et al. 2005). How Syk signaling contributes to NLRP3 inflammation and adjuvanticity is currently unknown. However, clues from other systems present themselves. It is now known that Syk signaling in fungal sensing is coupled to NLRP3 activation by controlling, via CARD9, pro-IL-1β production and ROS/K+ efflux (Gross et al. 2009). Our finding implies that lipid membrane remodeling may be a primitive form of structure recognition with the ability to sense a wide variety of solid structures. Additionally, this finding suggests that lipid sorting may be a broader sensor than TLRs and that it may precede the evolutionary development of surface protein receptors as a form of external sensing.
Lipids are certainly an understudied and underappreciated aspect in immunological studies. However, recent evidence has placed enhanced emphasis on the importance of lipid moieties in phagocytosis, protein localization, and T-cell activation (Yeung et al. 2006; Yeung et al. 2008; Lillemeier et al. 2010). Large-scale investigations are necessary to extend the implications and importance of lipids in immunological settings. Of particular importance is the role of lipids in the adjuvant action of aluminum adjuvants.
Is Inflammasome Activity Tied to Adjuvanticity?
A recent article by Ghiringhelli et al. demonstrates a role of NLRP3 and IL-1β in adaptive antitumor responses (Ghiringhelli et al. 2009). Although the authors did not investigate T-cell cytotoxicity against tumor cell lines, they show that dying tumor cells releasing ATP activate NLRP3. Subsequent IL-1β release aids in antigen-specific T-cell activation based on IFN-γ readouts. However, it was not determined whether T-cell activation was a specific event and whether the activation resulted in a prolonged memory response against further tumor inoculation. Therefore the roles that IL-1β and NLRP3 play in the development of memory, or adjuvanticity in general, remain to be elucidated.
Thus a dogmatic perception about inflammation is that it is coupled to adjuvanticity and that neither can exist without the other. This is certainly the impression from the initial discovery of NLRP3 sensing MSU (Martinon et al. 2006). Perhaps the reason for this link is the vague description of adjuvanticity. In our opinion, the all-encompassing definition of adjuvanticity is the eventual activation and proliferation of immune cells and subsequent development of memory, which is the ultimate goal of vaccine development. On the other hand, inflammation is defined as the host’s attempt to remove harmful stimuli, but the process itself is self-destructive. Therefore, how inflammation is connected to adjuvanticity should be a subject of study rather than a conceptual synonym. At least in NLRP3-independent alum investigations, many groups report that the absence of pro-inflammatory cytokines such as IL-1β does not result in attenuated adjuvanticity (Franchi and Nunez 2008; Marrack et al. 2009), and in our experience, secondary activation signals are intact under NLRP3 deficiency (unpublished data). Furthermore, upregulation of costimulatory markers remains present under IL-1R deficiency (unpublished data), a setting devoid of inflammation during MSU-induced peritonitis (Chen et al. 2006). These results certainly suggest that inflammation and adjuvanticity are two independent events. Furthermore, these findings raise the possibility of developing vaccines that have adjuvant activity without the inflammatory properties. Thus, to investigate the mechanism of adjuvants in general, one must look beyond pro-inflammatory cytokines and consider other activation signals because they likely play a prominent role in adjuvanticity.
Conclusion
In the past four decades, many rheumatological advances have contributed to the current understanding of MSU-mediated gouty inflammation. MSUs role in NLRP3 sensing, which allows for IL-1β release leading to inflammation, has spurred intense interest in this field. These events are certainly informative about gouty inflammation. However, an open mind should be kept regarding adjuvanticity because it may involve identical mechanisms as NLRP3-dependent pro-inflammatory cytokines. Our recent proposal of lipid-dependent sorting mechanisms as a sensor for solid structures for immune activation is a novel attempt to address this issue. Sensing the crystal extracellularly may play a role in the intracellular signaling events associated with adjuvanticity. We currently lack advanced tools for large-scale investigations and for extending this research to other applications. However, because of the explanatory power of lipid-based theories, we believe that the addition of lipid biology to immunological research may lead to the accelerated development of new vaccines.
References
Berton G, Mocsai A, Lowell CA (2005) Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol 26:208–214
Brewer JM (2006) (How) do aluminium adjuvants work? Immunol Lett 102:10–15
Brewer JM, Conacher M, Hunter CA et al (1999) Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol 163:6448–6454
Chen CJ, Shi Y, Hearn A et al (2006) MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest 116:2262–2271
Doherty M, Whicher JT, Dieppe PA (1983) Activation of the alternative pathway of complement by monosodium urate monohydrate crystals and other inflammatory particles. Ann Rheum Dis 42:285–291
Dostert C, Petrilli V, Van Bruggen R et al (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320:674–677
Eisenbarth SC, Colegio OR, O’Connor W et al (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453:1122–1126
Faires J, McCarty D (1962) Acute synovitis in normal joints of man and dog produced by injection of microcrystalline sodium urate, calcium oxalate and corticosteroid esters. Arthritis Rheum 5:295–296
Fiddis RW, Vlachos N, Calvert PD (1983) Studies of urate crystallisation in relation to gout. Ann Rheum Dis 42(Suppl 1):12–15
Franchi L, Nunez G (2008) The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol 38:2085–2089
Garrod A (1848) Observations on certain pathological conditions of the blood and urine of gout, rheumatism and Bright’s disease. Med Chir Trans 31:83–97
Ghiringhelli F, Apetoh L, Tesniere A et al (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15:1170–1178
Giclas PC, Ginsberg MH, Cooper NR (1979) Immunoglobulin G independent activation of the classical complement pathway by monosodium urate crystals. J Clin Invest 63:759–764
Glenny A, Waddington H, Wallace U (1926) The antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol 29:38–45
Gross O, Poeck H, Bscheider M et al (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459:433–436
Hasselbacher P (1979a) Binding of IgG and complement protein by monosodium urate monohydrate and other crystals. J Lab Clin Med 94:532–541
Hasselbacher P (1979b) C3 activation by monosodium urate monohydrate and other crystalline material. Arthritis Rheum 22:571–578
Hornung V, Bauernfeind F, Halle A et al (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9:847–856
Iwata H, Nishio S, Yokoyama M et al (1989) Solubility of uric acid and supersaturation of monosodium urate: why is uric acid so highly soluble in urine? J Urol 142:1095–1098
Janeway CA Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54(Pt 1):1–13
Janeway CA Jr (1992) The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 13:11–16
Kam M, Perl-Treves D, Caspi D et al (1992) Antibodies against crystals. FASEB J 6:2608–2613
Kam M, Perl-Treves D, Sfez R et al (1994) Specificity in the recognition of crystals by antibodies. J Mol Recognit 7:257–264
Kanevets U, Sharma K, Dresser K et al (2009) A role of IgM antibodies in monosodium urate crystal formation and associated adjuvanticity. J Immunol 182:1912–1918
Kool M, Soullie T, van Nimwegen M et al (2008) Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 205:869–882
Lillemeier BF, Mortelmaier MA, Forstner MB et al (2010) TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol 11:90–96
Loeb JN (1972) The influence of temperature on the solubility of monosodium urate. Arthritis Rheum 15:189–192
Mandel NS (1976) The structural basis of crystal-induced membranolysis. Arthritis Rheum 19(suppl 3):439–445
Marrack P, McKee AS, Munks MW (2009) Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol 9:287–293
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426
Martinon F, Petrilli V, Mayor A et al (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 44:237–241
Matzinger P (1994) Tolerance, danger, and the extended family. Annu Rev Immunol 12:991–1045
Matzinger P (1998) An innate sense of danger. Semin Immunol 10:399–415
Naff GB, Byers PH (1973) Complement as a mediator of inflammation in acute gouty arthritis. I. Studies on the reaction between human serum complement and sodium urate crystals. J Lab Clin Med 81:747–760
Ng G, Sharma K, Ward SM et al (2008) Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 29:807–818
Petrilli V, Papin S, Dostert C et al (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14:1583–1589
Shi Y, Evans JE, Rock KL (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425:516–521
Ulanova M, Tarkowski A, Hahn-Zoric M et al (2001) The common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun 69:1151–1159
Wallingford WR, McCarty DJ (1971) Differential membranolytic effects of microcrystalline sodium urate and calcium pyrophosphate dihydrate. J Exp Med 133:100–112
Yeung T, Terebiznik M, Yu L et al (2006) Receptor activation alters inner surface potential during phagocytosis. Science 313:347–351
Yeung T, Gilbert GE, Shi J et al (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319:210–213
Zhou R, Tardivel A, Thorens B et al (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11:136–140
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ng, G., Chau, E.M.T. & Shi, Y. Recent Developments in Immune Activation by Uric Acid Crystals. Arch. Immunol. Ther. Exp. 58, 273–277 (2010). https://doi.org/10.1007/s00005-010-0082-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-010-0082-1