Abstract
There is little information on the levels of chemical elements in pet food considering the dietary requirements as well as risk assessment of toxicity. This study aimed to determine the essential and toxic elements in dry and canned foods for dogs and cats and estimate their daily intake. We compared the levels of the chemical elements between the dry and wet (canned) food to the levels recommended by the Association of American Feed Control Officials (AAFCO) and the European Pet Food Industry Federation (FEDIAF) and the maximum tolerable level proposed by European Commission (EC). In addition, the estimated daily intake (EDI) for each one of the elements through food was calculated. Seventy-six dry food samples (dogs n = 62 and cats n = 14) from 43 brands and 12 canned foods (dogs n = 6 and cats n = 6) from 5 brands, were purchased from Brazilian supermarkets. Mean levels of all essential elements reached the minimum level recommended by AACFO. Selenium levels were very close to the maximum limit proposed by AAFCO. Besides, the iron concentrations in canned (moist) food were statistically higher than in dry food and its EDI for cats (54 mg/day × kg body weight) exceeded the maximum limit recommended by FEDIAF. Regarding the toxic metals, the concentrations of mercury and cadmium, in dry and canned food, were considerably higher than the maximum tolerable level proposed by EC. Overall, the results show that levels of essential elements are in agreement with the nutrient requirement. On the other hand, mercury and cadmium in pet food are an issue of concern.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Surveys have shown that pet ownership has increased in the United States with about 70% of people having at least one pet (APPA 2013). In Brazil, data from 2013 indicate that 52.2 million dogs are owned as pets with 44.3% of the homes having at least one dog; for cats, the numbers are estimated to be 22.1 million cats distributed across 17.7% of the homes (at least one cat per home) (IBGE 2013).
Due to the relatively high demand for pet services and products, a variety of pet food has been progressively introduced in the market. Dry, canned, semi-moist, and frozen-chilled foods are currently available options that have been developed based on the pets’ breed, size, health condition, and age (Duran et al. 2010; Costa et al. 2013). The main difference between the three basic forms of commercial pet foods (dry, semi-moist, and canned) is the water content. Dry foods usually contain < 11% water, semi-moist foods between 25 and 35% and canned (moist) food between 60 and 87% water (Zicker 2008).
Pet foods are formulated to address the specific nutritional requirements, as recommended by the Association of American Feed Control Officials (AAFCO 2003). Continuous ingestion of either insufficient or excessive amount of essential elements leads to structural disorders that will depend on the element, level and time of the dietary deficiency, age, sex and the animal species (Priego-Capote and Luque de Castro 2004). Furthermore, deficiency of essential nutrients can lead to diseases such as anemia, while its overconsumption may lead to accumulation in the body, causing intoxication (González-Martín et al. 2006). In the same way, pets that are fed with diets containing high amounts of harmful chemical elements are at risk of intoxication. In both scenarios, monitoring the risk is highly necessary to ensure homeostasis and avoid both short- and long-term pet intoxication. For instance, Furr et al. (1976) found that arsenic, lead, bromine, cadmium, chromium, mercury were present at high concentrations in cat food containing fish proteins. On the other hand, Duran et al. (2010) found that the concentrations of copper, iron, nickel, lead, manganese, chromium and, cadmium in pet food from Turkey were within the levels recommended by the local authorities. Nonetheless, evaluations on the chemical elements concentrations in pet food and their implication considering their dietary requirements as well as the risk of toxicity are still scarce in the literature. Therefore, this study aimed to determine essential and toxic elements in dry and canned foods for dogs and cats. Moreover, the data obtained were compared with the levels recommended by regulatory agencies and the daily intake for each one of the elements was estimated.
2 Materials and methods
2.1 Reagents, accessories and instrumentation
All reagents used were of analytical grade and were purchased from Fluka (St. Louis, United States). The nitric acid (14 M HNO3, Synth, São Paulo, Brazil) was previously purified in quartz sub boiler (Kürner, Rosenheim, Germany). A clean lab and laminar flow hood (Filter flux, Piracicaba, Brazil) capable of producing a class 100 was used to prepare the solutions. High-purity deionized water (resistivity 18.2 MΩ/cm) obtained from a Milli-Q water purification system (Millipore, Milli-Q RiOs, Bedford, MA, United States) was used throughout. Plastic bottles and glassware were cleaned by soaking in 10% (v/v) HNO3 for 24 h, rinsed 5 times with Milli-Q water and dried in a class 100 laminar flow hood before use. A multi-element stock solution containing 10 mg/l was obtained from PerkinElmer (Norwalk, CT, United States). Rhodium was used as internal standard at 10 μg/l (PerkinElmer, Norwalk, CT, United States). The determination of the chemical elements was performed using an inductively coupled plasma mass spectrometer (ICP-MS, ELAN DRC II, PerkinElmer, United States). The ICP-MS was operated with a sampler and skimmer platinum cones.
2.2 Sampling and analysis
The most consumed pet food in Brazil is the dry type. The canned type is consumed to a lesser extent compared to the dry type. Therefore, 76 samples of dry food for adult pets (dogs n = 62 and cats n = 14) comprising 43 different brands and 12 canned foods for adult pets (n = 6 for dogs and n = 6 for cats) comprising 5 different brands, were purchased from supermarkets of the states of Minas Gerais and São Paulo (Southeast Brazil, the most populous region). Considering the dry foods for dogs, approximately 36.5% was from beef, 60.3% from chicken and, 3.2% from fish. The dry foods for cats presented beef (38%), chicken (44%) and fish (18%) as the primary source of protein. In canned food, for dogs, the main sources of protein were beef (67%), sheep (16.5%) and fish (16.5%) and for cats were fish (83.5%) and beef/liver (16.5%).
After quartering, fifty grams of each sample (in triplicate) were individually milled using an electric ultra-centrifugal mill (Retsch ZM200 with DR100 auto-sampler, Haan, Germany) at 6000 rpm, homogenized and, sieved (0.25 mm). The samples were weighted (0.1 g) and 5 ml HNO3 20% v/v + 2 ml H2O2 30% v/v were added. The digestion was conducted in a closed vessel microwave system (Milestone ETHOS 1600, Sorisole, Italy), according to Nardi et al. (2009). The volume was made up to 25 ml and the samples were analyzed by ICP-MS. The limits of detection (LOD) were estimated from blank analyses (10 replicates) and determined as 3 times the standard deviation (SD) of blanks signals divided by the slope (S) of the calibration curve from each element (LOD = 3 × SD/S). The found LODs (in ng/g) were: cobalt (Co) = 0.3, strontium (Sr) = 0.42, rubidium (Rb) = 0.72, vanadium (V) = 0.19, magnesium (Mg) = 5.6, calcium (Ca) = 373, molybdenum (Mo) = 0.4, phosphorus (P) = 43.8, uranium (U) = 0.04, aluminum (Al) = 0.60, antimony (Sb) = 0.80, manganese (Mn) = 0.40, selenium (Se) = 0.75, zinc (Zn) = 0.28, copper (Cu) = 0.90, iron (Fe) = 2.5, arsenic (As) = 0.70, lead (Pb) = 0.20, cadmium (Cd) = 0.60, nickel (Ni) = 0.50, mercury (Hg) = 0.50 and, chromium (Cr) = 0.40.
2.3 Quality control for analysis
The method accuracy was evaluated by analyzing the standard reference materials (SRM) 8415 (whole egg powder), SRM 1568a (rice flour) and, SRM 1577 (bovine liver) from the National Institute of Standard and Technology (NIST, United States). The SRMs were analyzed after every 10 ordinary samples. The recoveries ranged from 95 to 105% and 1.3 to 4.3, respectively.
2.4 Estimated daily intakes
The estimated daily intakes (EDI) of the elements were calculated in the present study as
where EDI is the estimated daily intake (μg/kg × day) of the elements per kg of body weight, [E] is the mean concentration (μg/kg) of the chemical element found in pet foods, M is the mass of the pet food consumed daily (kg/day) and W is the weight (kg) of pet animals. The correspondent food consumption (in grams) and the average weight of dogs and cats were based on the Canadian report (GBC 1996).
2.5 Statistics
Mean levels of the chemical elements in canned and dry foods were compared by using t-Test. The level of significance was set at 0.05. Statistical analyses were done using the GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, United States).
3 Results and discussion
Essential elements in pet diets have to be maintained within narrow limits to protect the functional and structural integrity of the tissues and to ensure adequate growth, health, and development. The recommendations of AAFCO and FEDIAF are the most commonly used in Brazil for essential elements. However, the maximum tolerable levels of toxic elements were proposed by the European Commission (EC 2012). The maximum concentrations recommended by FEDIAF and AAFCO are the maximum level of a nutrient that has not been associated with adverse effects in healthy dogs and cats. Levels exceeding the nutritional maximum may still be safe. Considering the undesirable substances, the maximum levels preconized by the European Commission are applied for products used for animal feed. If this limit is exceeded, the source must be identified to decrease or eliminate the contamination.
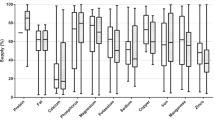
The concentrations of essential elements in dog and cat food are presented in Table 1, along with the recommended concentrations established by the AAFCO (2003) and FEDIAF (2011). Considering these recommended limits, these agencies proposed different values for the different age periods. The present study included only feed for adult animals; therefore, we compared the results with the recommended limits corresponding to that age period.
In general, mean levels of essential elements in dog and cat foods are above the minimum recommended by AAFCO (2003). For most of the essential elements, there is no maximum limit, which makes it difficult to assess the risk. The concentrations of iron (dog = 910 and cat = 896 mg/kg), zinc (dog = 235 mg/kg), copper (dog = 25.7 ± 15.4 mg/kg) and selenium (dog = 1.5 ± 0.19 and cat = 1.2 ± 0.43 mg/kg) were statistically higher (p < 0.05) in canned foods compared to dry foods (Table 1).
Iron concentration in dry food showed agreement with those provided by Costa et al. (2013) which evaluated dry food for dogs purchased from Brazilian supermarkets (mean = 337 mg/kg). Their values were higher than the mean found by Duran et al. (2010) in wet pet food from New Zealand (71 ± 3.4 mg/kg) and the minimum recommended by FEDIAF (2011). In the present study, we observed some samples with iron above the maximum limit recommended by FEDIAF (2011) for dogs and cats (2032 mg/kg and 1913 mg/kg, respectively, in canned foods, Table 1).
The EDI is a prediction of the daily intake based on the amount of the chemical element determined in pet food samples and the best available food consumption data for a specific population. However, there are no limits defined for EDI for pet foods. Considering cats that only eat canned food, the EDI for iron was estimated to be about 54 mg/day × kg body weight (Table 3), close to a single dose concentration of this metal (60 mg/kg body weight) administered to swine orally that produced toxicity (Dean et al., 1996). Similarly, Rallis et al. (1989) observed hepatocellular necrosis and death in sheep daily supplemented with 40 mg/kg body weight of iron for 22 weeks.
The mean levels of zinc in dog and cat dry food (133 mg/kg and 167 mg/kg, respectively) and in cat canned food (158 mg/kg) are within the same range of those found by Kelly et al. (2013) in commercially dry dog food (79–330 mg/kg) from the United States (Table 1).
Selenium mean concentrations in dry food (0.56 mg/kg for dog and 0.74 mg/kg for cat) and canned food (1.5 mg/kg for dog and 1.2 mg/kg for cat) (Table 1) were higher than the maximum limit proposed by FEDIAF (2011) and higher than those found by Kelly et al. (2013) in dry dog food (0.22–0.79 mg/kg). However, Todd et al. (2012) observed that cats fed with high selenium levels (2 mg/kg) did not show symptoms of toxic effects, only increased urinary excretion of selenium. Goehring et al. (1984) studied pigs and observed decreased growth and feed intake when the doses of selenium were 4 and 8 mg/kg feed, respectively. On the other hand, Grotto et al. (2018) showed that after 85 days of selenium supplementation (2 and 6 mg/l in drinking water) there was an augment of the systolic blood pressure in Wistar rats. Therefore, the high levels of selenium found in pet foods are of concern and should continuously be monitored.
Dog and cat canned food samples had mean values of 45.6 and 41.3 mg/kg of copper, respectively (Table 1). These levels, about twice the maximum limit (28 mg/kg) recommended by FEDIAF (2011), are not considered toxic as the EDI for copper was lower than the dose which adverse effects are observed (WHO 1998). Despite its essentiality, copper can be toxic at high concentrations, and chronic toxicity may be associated with liver disorders (Rifkin and Miller 2014). Therefore, copper is an issue of concern in pet foods and need to be continuously monitored.
Magnesium and calcium concentrations are within the limits recommended by FEDIAF (2011). For phosphorus, the level in dogs’ dry food was within but very close to the upper limit recommended by AAFCO (2003). Molybdenum, vanadium, and cobalt concentrations are in agreement with the levels reported in the study of Kelly et al. (2013), which also analyzed dog food from Brazil.
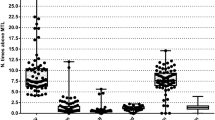
Risk assessment of the levels of the toxic elements arsenic, lead, cadmium, and mercury in food is critical as they can produce deleterious effects such as DNA damage, lipid peroxidation, depletion of protein containing sulfhydryl groups, and others. These harmful actions may cause liver toxicity, neurotoxicity, nephrotoxicity and genotoxicity (Valko et al. 2005; Jomova and Valko 2011). The concentrations of toxic elements in dog and cat foods and the maximum tolerable level proposed by European Commission (2012) are given in Table 2. Arsenic and lead concentrations were below the limit (EC 2012). Lead was found in levels twice higher in dry food than in canned food. The EDI for arsenic (0.63 mg/day kg body weight) (Table 3) was higher for dogs that eat dry food (Neiger and Osweiler 1992; Byron et al. 1967). The EDI for lead was below the dose at which adverse effects (reduction of feed intake, weight loss, and mortality) were observed in dogs. Cadmium concentration in dry and canned foods was higher than the maximum tolerable level (EC 2012) and lower than the dose (30 mg/kg) at which Loeser and Lorke (1977) observed no toxicity effects in rats. Although the cadmium levels were lower than that found in the study of Loeser and Lorke, our findings show that cadmium in pet food may pose the animals at risk as nowadays new toxic effects are known about the presence of cadmium in the organism, even at very low levels (Lovásová et al. 2013).
Mercury is potentially related to the content of fish in pet foods as this element can accumulate in the marine environment and biota (Morel et al. 1998; Rahman et al. 2012). The mercury concentration was higher than the maximum tolerable level (EC 2012) for pets eating dry and canned food. However, the EDI is below the dose for effects such as ataxia, loss of equilibrium and motor incoordination in cats (74 mg/day × kg body weight) (Charbonneau et al. 1976).
No maximum tolerable limit was found in the literature for aluminum, strontium, and uranium in pet foods. Considering the maximum tolerable level in the swine’s food for aluminum (1000 mg/kg) and strontium (2000 mg/kg) and rodent’s food for uranium (100 mg/kg), the levels found in our samples are lower (NRC 2005). On the other hand, considering the highest EDI observed for aluminum (4.5 mg/day × kg body weight, for cats eating dry food) and uranium (11.5 µg/day × kg body weight for dogs eating dry food) (Table 2), the doses are above those ones for toxic effects (Katz et al. 1984; Pettersen et al. 1990; Maynard et al. 1953).
The mean levels of chromium in dog’s dry food and nickel in cat dry food (5.1 and 1.0 mg/kg, respectively) and chromium in canned food (6.1 and 5.1 mg/kg, for dog and cat, respectively) (Table 2) were higher than those reported by Tuzen and Soylak (2007) (0.19–0.52 mg/kg) and Ikem and Egiebor (2005) (0.0–0.30 mg/kg) in canned foods for dogs or cats.
The concentration of antimony in dry food were 11.8 and 17.9 µg/kg in dog and cat, respectively (Table 2). For canned food, we found lower levels (5.4 µg/kg in dog and cat). Severe weight loss, diarrhea, vomiting, muscle weakness and difficulty in moving hind limbs were effects observed in dogs who ingested 6,600 µg/day × kg body weight of antimony (Fleming 1982). Our findings were below to this dose. The highest antimony EDI observed were for pets that eat dry foods (0.44 and 1.1 µg/day × kg body weight for dogs and cats, respectively (Table 3).
For chromium, nickel, and antimony no maximum tolerable level for food of dogs and cats exists. However, when considering the maximum tolerable intake level suggested by National Research Council for nickel (for cattle = 100 mg/kg), antimony (for rodents = 70–150 mg/kg) and chromium (for swine = 100 mg/kg) (NRC 2005) our results are lower. The highest and lowest EDI for nickel in this study were 0.07 and 0.03 mg/day × kg body weight, respectively (Table 3). These concentrations are below the NOAEL (no observed adverse effect level) (25 mg/day × kg body weight) observed by Ambrose et al. (1976).
4 Conclusion
Our findings show that almost all the essential elements in pet food are in agreement to nutrient requirement and are within the safe maximum limit and minimum recommended by AACFO and FEDIAF. However, iron, selenium, and copper concentrations in canned food were statistically higher than in dry food and above the maximum safe limit. The levels of mercury and cadmium are predominant in canned foods and were higher than the maximum tolerable level (EC 2012). Therefore, there is a concern about the concentrations of chemical elements in pet food indicating that constant measures are needed to ensure the pet food safety.
References
AAFCO, Association of American Feed Control Officials (2003) Non-pet food: label design & format guide. AAFCO, Oxford
Ambrose AM, Larson PS, Borzelleca JF, Hennigar GR Jr (1976) Long term toxicologic assessment of nickel in rats and dogs. J Food Sci Technol 13:181–187
APPA, American Pet Products Association (2013). http://www.americanpetproducts.org/pubs_survey.asp. Accessed June 2018
Byron WR, Bierbower GW, Bouwer JB, Hansen WH (1967) Pathologic changes in rats and dogs from two-year feeding of sodium arsenite or sodium arsenate. Toxicol Appl Pharmacol 10:132–147
Charbonneau SM, Munro IC, Nera EA, Armstrong FA, Willes RF, Bryce F, Nelson RF (1976) Chronic toxicity of methylmercury in the adult cat. Interim Rep Toxicol 5:337–349
Costa SSL, Pereira ACL, Passos EA, Alves JPH, Garcia CAB, Araujo RGO (2013) Multivariate optimization of an analytical method for the analysis of dog and cat foods by ICP OES. Talanta 108:157–164
Dean BS, Oehme FW, Krenzelok EP, Griffith GR, Hines RH (1996) Iron complexation with oral deferoxamine in a swine model. Vet Hum Toxicol 38:96–98
Duran A, Tuzen M, Soylak M (2010) Trace element concentrations of some pet foods commercially available in Turkey. Food Chem Toxicol 48:2833–2837
EC, European Commission (2012) Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Off J L140:1–10 (30.5.2002, as last amended by Commission Regulation (EU) Official Journal 1)
FEDIAF, European Pet Food Industry Federation (2011) Nutritional Guidelines for complete and complementary pet food for cats and dogs. http://www.fediaf.org/self-regulation/nutrition.html. Accessed Jun 2018
Fleming AJ (1982) The toxicity of antimony trioxide. Sponsored by E.I. Du Pont de Nemours and Co., Wilmington DE. OTS215027
Furr AK, Bache CA, Gutenmann WH, Pakkala IS, Lisk DJ (1976) Element and chlorinated hydrocarbon content of commercial pet foods. Cornell Vet 66:513–527
GBC, Government of British Columbia (1996) Animal weights and their food and water requirements, Resource document. ministry of environment, lands and parks. http://www.env.gov.bc.ca/wat/wq/reference/foodandwater.html#table2. Accessed Jun 2018
Goehring TB, Palmer IS, Olson OE, Libal GW, Wahlstrom RC (1984) Toxic effects of selenium on growing swine fed corn-soybean meal diets. J Anim Sci 59:733–737
González-Martín I, Alvarez-García N, González-Pérez C, Villaescusa-García V (2006) Determination of inorganic elements in animal feeds by NIRS technology and a fibre-optic probe. Talanta 69:711–715
Grotto D, Carneiro MF, de Castro MM, Garcia SC, Barbosa-Junior F (2018) Long-term excessive selenium supplementation induces hypertension in rats. Biol Trace Elem Res 182:70–77
IBGE, Instituto Brasileiro de Geografia e Estatística (2013) Pesquisa Nacional de Saúde, 2013: acesso e utilização dos serviços de saúde, acidentes e violências: Brasil, grandes regiões e unidades da federação. http://www.ibge.gov.br. Accessed Jun 2018
Ikem A, Egiebor NO (2005) Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America). J Food Compos Anal 18(8):771–787
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Katz AC, Frank DW, Sauerhoff MW, Zwicker GM, Freudenthal RI (1984) A 6-month dietary toxicity study of acidic sodium aluminium phosphate in beagle dogs. Food Chem Toxicol 22:7–9
Kelly DG, White SD, Weir RD (2013) Elemental composition of dog foods using nitric acid and simulated gastric digestions. Food Chem Toxicol 55:568–577
Loeser E, Lorke D (1977) Semichronic oral toxicity of cadmium. 2. Studies on dogs. Toxicology 7:225–232
Lovásová E, Rácz O, Cimboláková I, Nováková J, Dombrovský P, Ništiar F (2013) Effects of chronic low-dose cadmium exposure on selected biochemical and antioxidant parameters in rats. J Toxicol Environ Health A 76:1033–1038
Maynard EA, Down WL, Hodge HC (1953) Oral toxicity of uranium compounds. In: Voegtlin C, Hodge HC (eds) Pharmacology and toxicology of uranium compounds. McGraw-Hill, New York, pp 1221–1369
Morel FM, Kraepiel AM, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Ann Rev Ecol Syst 29(1):543–566
Nardi EP, Evangelista FS, Tormen L, SaintPierre TD, Curtius AJ, de Souza SS, Barbosa F Jr (2009) The use of inductively coupled plasma mass spectrometry (ICP-MS) for the determination of toxic and essential elements in different types of food samples. Food Chem 112:727–732
Neiger RD, Osweiler GD (1992) Arsenic concentrations in tissues and body fluids of dogs on chronic low-level dietary sodium arsenite. J Vet Diagn Investig 4:334–337
NRC, National Research Council (2005) Mineral tolerance of animals: second revised. Committee on Minerals and Toxic Substances in Diets and Water for Animals, Washington, DC (8 1/2 × 11)
Pettersen JC, Hackett DS, Zwicker GM, Sprague GL (1990) Twenty-six week toxicity study with KASAL® (basic sodium aluminum phosphate) in beagle dogs. Environ Geochem Health 12:121–123
Priego-Capote F, Luque de Castro MD (2004) Dynamic ultrasound-assisted leaching of essential macro and micronutrient metal elements from animal feeds prior to flame atomic absorption spectrometry. Anal Bioanal Chem 378:1376–1381
Rahman MA, Hasegawa H, Lim RP (2012) Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ Res 116:118–135
Rallis T, Spais AG, Papasteriadis A, Agiannidis A, Leondidis S (1989) Iron toxicity in sheep. J Trace Elem Electrol Health Dis 3:131–137
Rifkin J, Miller MD (2014) Copper-associated hepatitis in a Pembroke Welsh corgi. Can Vet J 55(6):573–576
Todd SE, Thomas DG, Bosch G, Hendriks WH (2012) Selenium status in adult cats and dogs fed high levels of dietary inorganic and organic selenium. J Anim Sci 90:2549–2555
Tuzen M, Soylak M (2007) Evaluation of trace element contents in canned foods marketed from Turkey. Food Chem 102:1089–1095
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
WHO, World Health Organization (1998) Copper: environmental health criteria 200. World Health Organization, Geneva
Zicker CS (2008) Evaluating pet foods: how confident are you when you recommend a commercial pet food? Top Companion Anim Med 23:121–126
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Paulelli, A.C.C., Martins, A.C., de Paula, E.S. et al. Risk assessment of 22 chemical elements in dry and canned pet foods. J Consum Prot Food Saf 13, 359–365 (2018). https://doi.org/10.1007/s00003-018-1178-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-018-1178-5