Abstract
In Aotearoa New Zealand (NZ), ethnic inequities in health outcomes exist. Non-Māori experience better access to healthcare than Māori, including access to the quality use of medicines. Quality medicines use requires that medicines provide maximal therapeutic benefit with minimal harm. As older adults are more at risk of harm from medicines, and, because inequities are compounded with age, Māori older adults may be at more risk of medicines-related harm than younger and non-Māori populations. This narrative review examined ethnic variation in the quality use of medicines, including medicines utilisation and associated clinical outcomes, between Māori and non-Māori older adult populations in NZ. The review was structured around prevalence of medicine utilisation by medicine class and in particular disease states; high-risk medicines; polypharmacy; prevalence of potentially inappropriate prescribing (PIP); and association between PIP and clinical outcomes. 22 studies were included in the review. There is ethnic variation in the access to medicines in NZ, with Māori older adults often having reduced access to particular medicine types, or in particular disease states, compared with non-Māori older adults. Māori older adults are less likely than non-Māori to be prescribed medicines inappropriately, as defined by standardised tools; however, PIP is more strongly associated with adverse outcomes for Māori than non-Māori. This review identifies that inequities in quality medicines use exist and provides a starting point to develop pro-equity solutions. The aetiology of inequities in the quality use of medicines is multifactorial and our approaches to addressing the inequitable ethnic variation also need to be.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is ethnic variation in access to quality use of medicines for older adults in New Zealand. |
Māori older adults have reduced access to medicines compared with non-Māori older adults and are less likely to be prescribed medicines inappropriately, as defined by standardised tools. |
Compared with non-Māori, Māori older adults may be at increased risk of adverse clinical outcomes as a result of the inappropriate prescription of medicines. |
1 Background
The Treaty of Waitangi, one of Aotearoa New Zealand’s (NZ’s) founding documents, guarantees Māori, the Indigenous people of NZ, the right to equitable health outcomes [1]. National and regional health policies state the importance of the Treaty of Waitangi and health equity. Despite these legislative and political mandates, sustained disparities in health outcomes exist in NZ [1, 2]. Compared with Māori, non-Māori experience significantly lower rates of morbidity including chronic medical conditions, cancer and mental health conditions [3, 4]. Non-Māori also enjoy the privilege of higher life expectancy, with Māori men dying, on average, 7.4 years earlier than non-Māori men [4]. Māori represent 16% of the total population in NZ, but < 7% of the population aged 65 years and older [5].
Colonisation and racism are recognised to drive differential access to the wider determinants of health in NZ, including housing, employment, education and the judicial system, contributing to inequitable health outcomes [6, 7]. Colonisation and racism also impact directly on Māori wellbeing and health outcomes [8,9,10]. Non-Māori have better access to and quality of healthcare than Māori in both primary [1] and secondary care [11,12,13] and across the spectrum of clinical contexts including general practitioner consultations [14], revascularisation for ischaemic heart disease [15] and mental health [16]. Non-Māori are more likely than Māori to have earlier treatment, higher levels of appropriate intervention and better healthcare outcomes [11,12,13,14,15,16,17].
Inequities in access to the quality use of medicines also exist. Quality use of medicines requires rational medicines use, defined by the World Health Organisation as the use of “medications appropriate to [patients’] clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community” [19]. Medicines are a cornerstone of best practice prevention and treatment of most common chronic medical conditions including cardiovascular disease [20], chronic obstructive pulmonary disorder [21] and diabetes mellitus [22]. These are conditions in which Māori bear a disproportionate disease burden both in terms of prevalence and associated morbidities, including hospitalisation rates [3, 23]. However, at a population level, compared with non-Māori, Māori have reduced access to medicines that are beneficial to long-term health outcomes [24, 25]. Although we have information relating to medicines use in the general Māori population, there is less information regarding potential disparities in the Māori older adult population.
1.1 Ethnic Disparities in Older Age
There are several reasons to consider Māori older adults as a distinct sub-group when assessing medicines. Older adults experience higher rates of chronic co-morbidities, and are more likely to be on multiple medicines for their associated treatment [26]. The increasing complexity of medicines regimens and physiological changes associated with ageing amplifies the risk of medicines-related harm in older adults [27, 28]. Furthermore, inequities are experienced across the life course and are compounded by age [29]. There is the potential for Māori older adults to be at risk both due to differential access to optimal medicines and other health services earlier in life, and increased risk of medicines-related harms associated with ageing. It is reasonable to hypothesise that Māori older adults may experience inequities in the quality use of medicines compared with both non-Māori older adults and younger Māori adults, in NZ. We have not identified any other reviews that examine ethnic variation of quality medicines use in older adults across a range of clinical contexts in NZ or internationally.
This review aimed to examine ethnic variation in the quality use of medicines, including medicines utilisation and associated clinical outcomes, between Māori and non-Māori older adult populations in NZ, through a narrative review of the literature.
2 Narrative Review Process and Study Identification
2.1 Narrative Review Process
Searches were undertaken in three biomedical databases: Ovid Medline, Embase and SCOPUS, from inception dates until 21 April 2020. Search terms and strategies were adapted for the different syntax requirements of each database. No limits were placed on language, study type or publication type. Search terms related to ‘older adults’, ‘New Zealand’ and ‘medication’. The search strategy used in Ovid Medline is shown in Online Resource 1 (see electronic supplementary material [ESM]). Relevant reports that are publicly available from the New Zealand Health Quality and Safety Commission (HQSC) were also included. The HQSC publishes the Atlas of Healthcare Variation [30] with data relating to health service utilisation and outcomes, including topics focused on medicines utilisation such as polypharmacy and opioid prescribing. It allows commentary and consideration on different patterns of use based on age, ethnicity, gender and geographical locations. The information relating to medicines is sourced from national pharmaceutical dispensing data disaggregated by age and ethnicity.
Studies were included if outcomes were analysed by ethnicity (including Māori as a subgroup) and more than 50% of the study population were over 55 years of age. This age limit was used as Māori have an earlier onset of chronic co-morbidity and are generally able to access ‘older adult’ health services in NZ from this younger age. Intervention studies were excluded, as were those relating to cancer treatment or immunisation programmes. Studies were also excluded if they related to medicines administration in the hospital setting, as diverse, acute clinical presentations may impact on the findings. Studies that took place in long-term care settings alone were excluded as the very low Māori populations in such facilities often preclude ethnicity analysis. Case studies, editorials, methodology papers, conference abstracts and letters were also excluded.
Zotero (reference management system) was used to collate studies identified from the biomedical database searches. Duplicates were excluded, titles and abstracts reviewed, and non-relevant studies were excluded. Full texts for the remaining studies were then obtained and reviewed for inclusion, along with those obtained from HQSC. Data from relevant studies were extracted and presented as a narrative review. These steps were all undertaken independently by the lead author. A narrative approach was chosen due to the expected heterogeneity of studies and to allow for contextual discussions and considerations of the results to be presented throughout the text.
2.2 Characteristics of Included Studies
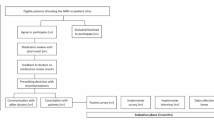
Biomedical database searches yielded 1970 unique studies once duplicates were removed, from which 19 studies were identified for inclusion. A further three relevant reports were identified from the HQSC on the topics of polypharmacy [31], opioids [32] and gout [33] (see screening and assessment process in Fig. 1). A total of 1,996,255 (range 225–537,387) people were included across 19 studies; three studies did not specify the population number. Māori representation in the study populations ranged from 4.8 to 39.8%. The majority of studies included population-level data with the remainder collecting regional-level data. Sixteen of the studies included only older adults with the remainder of the included studies either stratifying results by age or having a median study population age > 55 years. Medicines utilisation was the focus of 14 studies [31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Four studies reported on the prevalence of potentially inappropriate prescribing (PIP) using medicines appropriateness tools to measure this [45,46,47,48] and a further four studies investigated the association between PIP and clinical outcomes [49,50,51,52]. In 20 studies, ethnicity data was collected from information attached to the unique National Health Identifier (NHI). Prioritised ethnicity was reported whereby if participants identify with multiple ethnicities, they are only included under one ethnicity in the analysis. The ethnicity allocation order is prioritised in NZ according to the national prioritisation standard with Māori being the highest priority and European being the lowest [53].
3 Measuring Quality Medicines Use in Older Adults
There are several ways in which the ‘quality’ use of medicines is measured and the findings in this review will be structured around these, as follows:
-
The prevalence of use of particular medicines, which may include their appropriateness/place in specific disease conditions or clinical contexts [31].
-
‘Best practice’ treatment in particular disease states [31].
-
Medicines use associated with an increased risk of harm; also known as ‘high-risk’ medicines [31].
-
A count of the number of prescribed medicines. The risk of adverse outcomes increases with the number of medicines that older adults are prescribed [54].
-
‘Appropriate medicines use’. Medicines appropriateness tools, which involve a combination of the factors above, may be used to assess the quality of medicine use in older adults. These are often linked to the therapeutic use of medicines in particular clinical scenarios [56,57,58,59]. Associations between PIP (as defined by medicines appropriateness tools) and clinical outcomes can also be investigated.
3.1 Medicines Utilisation in Māori Older Adults
A number of studies investigated the prevalence of medicines utilisation in relation to medicine classes, clinical conditions and contexts, and high-risk medicines [31,32,33,34,35,36,37,38,39,40,41,42,43,44].
3.1.1 Prevalence of Medicine use by Medicine Class
Māori older adults have significantly higher rates of cardiovascular disease than non-Māori [4]. Medicines, including antihypertensives, statins and antiplatelets, form part of the best practice management to prevent and treat the associated morbidity [20] and our review suggests Māori have reduced access to these medicines compared with non-Māori. Statins are recommended first-line for lipid-lowering treatment in the context of cardiovascular disease [20]. A study investigating statin use in one geographical region in NZ found similar rates of statin prescribing in Māori and non-Māori aged ≥ 65 years, findings replicated in a larger national study of the same [44]. This suggests potential under-utilisation of these medicines in Māori who have higher levels of cardiovascular disease [36]. This could be affected by factors including under-prescription in Māori, lack of provision of appropriate education for Māori to increase medicine uptake, increased adverse medicine effects in Māori and greater barriers to access medicines from pharmacies.
Similarly, the use of antiplatelets at a population level increased more over time for NZ European than Māori, potentially increasing treatment disparities [44]. This study also demonstrated a greater reduction in warfarin use and uptake in dabigatran use for NZ European, suggesting that they may have had better access to newer therapies than Māori. Warfarin and dabigatran are oral anticoagulants used to treat and prevent venous thromboembolism. Warfarin has historically been the main agent used in NZ and elsewhere; however, newer agents such as dabigatran, with less intense monitoring requirements, have been introduced over the last decade in NZ and tend to be the first-line choice in first-time users [59].
The utilisation of bisphosphonate therapy, used first-line for the medical management of osteoporosis, has also been investigated [44]. Bisphosphonate dispensing rates reduced for all ethnic groups over this period, but to a greater extent for NZ European than Māori. There are several possible explanations for this variation, including under-utilisation in NZ European or increased rationalisation of bisphosphonate use in NZ European (bisphosphonate use should be reviewed after 3–5 years of treatment with cessation of therapy recommended in those at lower risk of fracture [60]). The study data only included primary care dispensing data and would not have captured the use of zoledronate in secondary care. This medicine is administered intravenously in the outpatient and inpatient hospital setting with no direct cost to the patient and was increasing in popularity during the study period. Again, this difference could reflect the reduced ability of Māori to access newer treatment options.
A variety of medicine classes are classified as psychotropic medicines, including antidepressants, antipsychotics, hypnotics and anti-seizure medicines. In general, Māori older adults appear to be prescribed psychotropic medicines at lower rates than non-Māori [39]. A study investigating medicines use in Māori and non-Māori octogenarians found lower rates of central nervous system medicines in Māori [51]. In another study focusing on psychotropic utilisation in older adults, Māori had lower rates of antidepressants, anxiolytics and hypnotics [37]. These results are replicated in the HQSC data with Māori older adults receiving benzodiazepines and zopiclone at less than half the rate of NZ Europeans (5.6% and 12.0%, respectively) [31]. Benzodiazepines and zopiclone are associated with an increased risk of medicines-related harm in older adults [57], and the difference in prescription rates between Māori and NZ European likely reflects over-prescription in NZ European. Māori experience higher rates of depression and anxiety than non-Māori [16], yet had lower rates of antidepressant prescription [37]. Ethnic disparities in access to antidepressants in older age were also noted in a study from the United States, which found that those belonging to ethnic minorities were less likely to receive treatment [61]. To understand treatment access more fully, the inclusion of non-pharmacological management, which may include rongoā Māori (traditional Māori system of healing and treatment) such as karakia (prayer), is needed. Non-pharmacological management of mild depression is more effective than medicines [62], although it has been noted that the mainstream delivery of these services may not meet the needs of Māori [63].
Antipsychotics form a subset of psychotropic medicines. They can be used to treat psychiatric disorders and, although rarely indicated, behavioural and psychological symptoms of dementia. Hence, indications and patterns of antipsychotic use may vary with age. The direction of ethnic variation in antipsychotic utilisation varies between different studies, with the most recent study (based on dispensing data from 2017) suggesting lower utilisation of antipsychotics for Māori older adults [31]. A study in which Māori had higher antipsychotic dispensing rates than NZ European used earlier data (2005–2013) [37] and may signal changes in the utilisation of antipsychotics over time. Māori have higher rates of, and more severe, psychiatric disorders than non-Māori [16], which may be more prevalent in younger cohorts. The prevalence of a formal dementia diagnosis is lower in Māori than in NZ European [64]. Variation in utilisation may reflect a change in therapeutic indication with age. Antipsychotics are rarely indicated in the treatment of dementia and, therefore, lower utilisation in Māori suggests more appropriate prescribing in Māori; however, further study is needed to better understand the clinical reasoning behind the medicine use and whether or not the differences relate to place of residence (i.e. community-dwelling compared with institutional care). None of these studies differentiate between typical and atypical antipsychotic use in the analysis, which has been shown to be influenced by ethnicity in a previous systematic review in the general adult population [65]. It is of significance as typical antipsychotics are associated with a greater incidence of adverse effects [65].
3.1.2 Best Practice Medicines Utilisation in Different Disease States
When data is analysed without accounting for differential morbidity rates, as above, it is difficult to interpret whether findings represent equitable access given the burden of disease. Investigating medicines use within certain disease states can give a better picture of equity in medicines use. This section includes a wide variety of studies where medicine use in certain disease conditions is explored.
A study looking at the dispensing of secondary prevention medicines in cardiovascular disease showed Māori were less likely than NZ Europeans to be maintained on ‘best practice’ triple therapy (anticoagulant/antiplatelet, antihypertensive and lipid-lowering treatment) [35]. This effect was predominantly seen in younger age groups included in the study. One study investigated the risk of cardiovascular events in those with atrial fibrillation who had undergone cardiovascular risk screening at least 1 year prior to the study [38]. It showed that in those not receiving anticoagulants, Māori were more likely to experience a stroke within 1 year of assessment compared with NZ Europeans. In those not receiving lipid-lowering treatment and antihypertensives, Māori were twice as likely to experience a major adverse cardiovascular disease event (non-fatal myocardial infarction, stroke, heart failure) [38]. This study suggests that the consequences of the omission of indicated medicines may be of greater clinical significance for Māori than non-Māori. However, to properly assess the consequences of omission, comparison with outcomes in those who did receive indicated medicines would be required, as there is the potential that other failures of care (such as access to primary care services) are associated with the findings in this study. Authors comment that the tools used to assess thromboembolic risk, and select appropriate treatment, are usually developed in European populations and may not accurately assess risk and did not accurately predict risk in their NZ study population [38]. In addition to variation in medicines access, findings may also reflect reduced access to primary and secondary care services that support prevention and treatment strategies related to these events.
For those with a diagnosis of gout, urate-lowering medicines reduce acute exacerbations and disease progression. The use of these medicines in older adults with gout diagnoses appears similar for Māori and NZ European older adults [33]. Initial analysis of the data seems to show that inequities in treatment access, which exist at a younger age [33], may not continue into older age. However, in those aged ≥ 65 years, gout prevalence is much higher in Māori compared with non-Māori, non-Pacific men (37.3% vs 17.2%, respectively). Of those aged ≥ 65 years with gout, 33% of Māori and 41% of non-Māori, non-Pacific people did not receive urate-lowering treatment [33]. Given the higher prevalence of gout in Māori older adults, but similar treatment levels between ethnicities, Māori older adults may have an increased absolute risk of missing out on preventative gout treatment compared with non-Māori, non-Pacific people. This analysis is also only relevant to those with gout diagnoses and it is thought that the methods used to assess gout incidence underestimate it by approximately 20% and to a greater extent for Māori [66], again signalling that unmet treatment needs are higher for Māori than non-Māori. Māori aged ≥ 65 years are hospitalised for a primary diagnosis of gout at a rate approximately five times that of NZ European. It is unclear whether those hospitalised were on urate-lowering treatment or not. Higher hospitalisation rates for Māori could be due to non-treatment in those with more severe disease or barriers to accessing earlier intervention in primary care. These findings may also indicate disparities in appropriate treatment. In other words, although Māori may be on urate-lowering therapy, the regimen confers less therapeutic benefit than for NZ Europeans, which could, in turn, be influenced by medicine choice, adequate dose titration, the stage of disease at which medicines are started and a lack of therapeutic relationships with health providers to support medicines use.
In a study that investigated predictors of mortality in those diagnosed with dementia (all types), Māori were less likely than NZ Europeans to receive acetylcholinesterase inhibitors (38% compared with 52%, p value 0.039), which delay the progression of dementia [43]. In this same study, Māori had lower rates of antipsychotic prescription than NZ Europeans, yet the mortality rate in Māori receiving antipsychotics was three times higher than NZ Europeans (3.62 compared with 1.19) [43]. The results did not reach statistical significance, likely due to the lower number of Māori participants and consequent reduced number of death events [43]. The difference in mortality may have been accounted for by lower rates of co-morbidity in NZ Europeans (although rates of co-morbidity were adjusted for), the stage of disease at which antipsychotics may have been used, or the severity of behavioural and psychological symptoms (more severe symptoms correlate with increased mortality rates) [43]. Ethnic disparities in acetylcholinesterase inhibitor use have previously been reported in a literature review, which found African Americans with Alzheimer’s dementia were up to 30% less likely to be prescribed these medicines than Whites [67].
One study looked at the prevalence of Parkinson’s disease, whereby prevalence rates were calculated using the dispensing of Parkinson’s treatments as a proxy [41]. Lower rates of Parkinson’s disease were noted in Māori compared with non-Māori although, given the method of calculating prevalence, the authors could not rule out the possibility that undertreatment with Parkinson’s medicines for Māori led to the apparent disparities in disease prevalence.
In a small (n = 225) study investigating medication exposure in older adults with end-stage renal disease, there was no significant difference in the number of medicines, or medicine classes, prescribed between Māori and non-Māori [40].
An 8-year follow-up study investigating long-acting bronchodilator use in first-time users in NZ in those with chronic obstructive pulmonary disease showed no ethnic variation in medicines use [42]. However, as entry into the study required the prescription of a long-acting bronchodilator, ethnic variation in initial access to these medicines is not accounted for.
3.1.3 High-Risk Medicines
Some medicines are classed as ‘high-risk’ medicines as they are associated with more risk of adverse outcomes. These include anticoagulants, insulin, and opioids [68]. The risks posed by these medicines often increase when used in older age groups [55]. Two studies looked at the incidence of high-risk prescribing; one study investigated opioids [32], the other investigated a combination of three medicines (non-steroidal anti-inflammatories, diuretics and angiotensin converting enzyme inhibitors/angiotensin receptor blockers) referred to as a ‘triple whammy’ [31]. When used together, the ‘triple whammy’ increases the risk of acute renal failure, particularly in those that already have renal insufficiency, the risk of which increases with age [31]. Māori aged 65–74 years are more likely to have a medicine regimen that includes a triple whammy than NZ Europeans at rates of 4% (95% confidence interval [CI] 3.7–4.2) compared with 3% (95% CI 2.9–3.1), respectively [31]. Rates of triple whammy prescription significantly reduce with age across all ethnicities but more substantively in Māori, so for the oldest age band (≥ 85 years) the incidence is similar between both ethnic groups. Strong opioids (which include morphine, fentanyl, methadone and oxycodone) increase the risk of adverse outcomes in older adults [69] and are of limited analgesic benefit in non-malignant pain [70]. There is ethnic variation in strong opioid use in NZ with Māori aged 65–79 years more likely to be prescribed opioids than NZ Europeans of the same age. This variation reverses in those aged ≥ 80 years [32]. In this report, strong opioid use was strongly associated with public hospital utilisation (inpatient and outpatient) in the week prior to dispensing. The variation seen in opioid use may reflect variation in access to secondary care for Māori and non-Māori as they age and is a demonstration of how access to health services impacts on medicines access [32].
3.1.4 Number of Prescribed Medicines and Polypharmacy
The measurement of polypharmacy is used as an indicator of prescribing quality. The definition of polypharmacy is not universally agreed upon; however, it is frequently defined as the prescription of five or more regular medicines [31, 71]. Polypharmacy is often analysed as a binary outcome (i.e. polypharmacy is either present or it is not), although the risk of medicines-related harm increases with every medicine prescribed [72]. The prevalence of polypharmacy in older adults is similar between Māori (36.5%) and NZ Europeans (33.8%) [31]. When this prevalence is further interrogated, we see that there are variations across the age bands within these groups (Table 1).
Māori experience polypharmacy at a younger age. Compared with NZ European, Māori aged 65–74 years and 75–84 years are more likely to experience polypharmacy; however, the direction of variation is reversed in those aged 85 years or older [31]. This pattern reflects the earlier onset of chronic conditions in Māori [73]. The prevalence of polypharmacy increases with age for both Māori and NZ Europeans, although the difference is much less marked for Māori with an increase (from the ages 65–74 years to ≥ 85 years) of 12.6% compared with 34.6%, respectively. It may also suggest Māori have more similar levels of chronic disease throughout older age compared to NZ European, although this suggestion differs from findings of a national study of multimorbidity which utilised pharmaceutical data as one of the measures of disease prevalence [73]. Although pharmaceutical data are often used as a proxy for medical diagnoses and the prevalence of comorbidity, there are limitations in using this method, with a potential lack of alignment with primary care diagnosis data. However, this method is used as NZ does not have national primary care morbidity data from which comorbidity prevalence can be calculated [73]. The prescription of multiple medicines is often appropriate to treat chronic co-morbidity and when using polypharmacy (and raw number counts) as a measure of medicines quality, appropriateness of prescribing in relation to medicine type, clinical context or patient characteristics are not able to be taken into consideration.
3.2 The Prevalence of Potentially Inappropriate Prescribing (PIP) in Māori Older Adults
There are several tools used to assess PIP [55,56,57,58]. These tools can be used in practice to guide the decision-making process [56, 57], and can be used in research to describe the ‘quality’ of medicines use [74] and to assess the impact of interventions [75]. The tools include criteria relating to the prescription of potentially inappropriate medicines (PIMs)—medicines whose potential for harm outweighs the potential therapeutic benefit [56,57,58,59]. Some tools also include criteria relating to potential prescribing omissions (PPOs)—the non-prescription of medicines that are indicated given certain diagnoses or clinical parameters [57].
A research group undertook two studies to assess the rate of PIMs in community dwellers aged ≥ 65 years receiving NZ publicly funded long-term community care services [45, 47]. The PIM rate was assessed using the 2015 Beers criteria [76] and utilised information from a comprehensive geriatric assessment using the standardised ‘international Resident Assessment Instrument—Home Care’ (interRAI‐HC) tool [77]. An interRAI-HC assessment is required for all that are being assessed for entry into publicly funded services. The first study showed that PIM prevalence was higher for Māori than NZ Europeans (9.5% compared with 7%, respectively; statistical significance not calculated) [47]. When the same researchers investigated similar data and adjusted for confounding factors, they found Māori were less likely to be prescribed PIMs than NZ Europeans [45]. The adjusted odds ratio (OR) for Māori to receive one or two PIMs was 0.76 (95% CI 0.59–0.98; p = 0.035) with NZ European as the comparator. The adjusted OR for Māori to receive three or more PIMs was 0.50 (95% CI 0.38–0.65; p < 0.001). The group then undertook a third study that investigated PIMs and clinical outcome association in the subset of those with dementia [46]. The prevalence of PIMs in all those with dementia was 66.9% with Māori again being less likely than NZ Europeans to receive a PIM (adjusted OR 0.68, 95% CI 0.54–0.87) [46]. Narayan and Nishtala investigated the prevalence of Beers criteria PIMs at a population level and again, Māori were significantly less likely to be prescribed PIMs than NZ Europeans (OR 0.85, 95% CI 0.82–0.87) [48].
In all four of these studies, increasing age was associated with a reduction in PIM use, suggesting that as people progress through the later stages of life, the prevalence of PIMs reduces [45,46,47,48]. Māori have an earlier onset of chronic co-morbidity and associated polypharmacy, and the impact this may have on PIM rates is not reflected when analyses between ethnicities are conducted across the same older adult age bands and only start at the age of 65 years.
3.3 The Association Between PIP and Clinical Outcomes in Māori Older Adults
There are a number of studies that have investigated the association between PIP and clinical outcomes [77, 78].
A longitudinal cohort study of Māori and non-Māori octogenarians investigated the impact of baseline medicines appropriateness on 12-month hospitalisation and mortality rates [51]. The STOPP/START criteria [57] were used to assess medicines appropriateness, and there was no significant ethnic variation in the occurrence of PIP overall, although Māori were more likely to have PPOs than non-Māori (p = 0.013). The prescription of PIMs did not affect hospitalisation rates. However, for Māori, the occurrence of PPOs was associated with an increased risk of hospitalisation (24.3% without PPO hospitalised compared with 51.7% with PPO hospitalised; p = 0.001). This association did not occur for non-Māori, suggesting Māori may be at more clinical risk from medicines omissions. The occurrence of PIMs and PPOs did not affect mortality in either ethnic group.
This research group also investigated the association between the Drug Burden Index (DBI) and clinical outcomes at 12, 24 and 36 months [52]. The DBI is a measure of medicines with anticholinergic and/or sedative properties [55]. A higher index number indicates an increased burden and lower quality prescribing. Non-Māori had higher DBIs at baseline than Māori. In Māori, DBI was associated with increased mortality at 36 months (adjusted hazard ratio 1.89, 95% CI 1.11–3.20; p = 0.02). In non-Māori, DBI was associated with higher mortality rates at 12 months (adjusted hazard ratio 2.26, 95% CI 1.09–4.70; p = 0.03) but not at other time points. DBI was not associated with increased risk of falls, hospitalisations or change in functional status over the study period in either ethnic group.
A study assessing the association between DBI and hip fractures found that higher DBI was associated with an increased incidence of hip fractures for both Māori and non-Māori older adults, with no ethnic variation in the association [50].
Nishtala et al. examined DBI in older adults at a population level utilising national databases [49]. Multivariate analysis showed that higher DBI was positively associated with GP visits and mortality. For Māori, DBI exposure was associated with reduced GP access compared with NZ Europeans (incidence risk ratio 0.972, 95% CI 0.963–0.980) and almost double the risk of 12-month mortality (hazard ratio 1.798, 95% CI 1.689–1.916). Due to differences in data collection methods between studies, there was less co-morbidity data available in the two studies above. Nishtala et al. [49] used a ‘chronic disease score’ to show there was no variation in chronic co-morbidity between Māori and non-Māori. However, the method uses pharmacy dispensing data as a proxy for disease status rather than diagnoses data sets or clinical assessments. This method has several limitations including the inability to account for untreated disease, which is likely to be higher for Māori. This study found that polypharmacy and DBI were both independent risk factors for all clinical outcomes in the study, supporting the idea that multiple methods are required to adequately assess quality medicines use [49].
4 Summary and Significance of the Findings
This review highlights that ethnic differences exist in medicines access in NZ, with Māori older adults often having reduced access to particular medicine types, or in particular disease states. Although Māori older adults may be less likely to be prescribed medicines inappropriately, as defined by standardised tools, inappropriate prescribing is more strongly associated with adverse outcomes for Māori than non-Māori. It is possible that quality medicines use is a marker for access to health services more generally.
This current review is the first of its kind to examine ethnic variation in the quality use of medicines in older adults across a range of clinical contexts. Although we could not find any reviews of medicines utilisation in older adult populations, ethnic variation in medicines treatment has been reported previously internationally. It follows the same patterns in the NZ population, with ethnic minorities being significantly less likely to receive medicine treatment [80]. There is also limited international literature showing the risk of inappropriate prescribing in older Indigenous populations with an Australian study showing that suboptimal prescribing put older Aboriginal Australians at high risk of medicines-related harm [81].
5 Limitations
Only one author reviewed the abstracts and papers for inclusion, increasing the potential for relevant papers to have been missed, although this approach is quite common when conducting a narrative review. Another limitation is the quality of ethnicity data, which has been well documented as an issue in NZ, although it has improved over time [82].
Although the medicines utilisation and medicines appropriateness data are useful for high-level monitoring and identification of trends, they are limited by the lack of data relating to disease burden and differential access to health-related resources that occur across the life course. This review includes studies with varying methods of data collection and analysis, varying clinical contexts and different populations and time periods. This presents challenges with being able to compare the data across studies; however, it has value in highlighting various findings relating to Māori older adults specifically, as well as acknowledging where gaps in the data lie.
Across all studies, Māori appear to have similar or lower rates of PIMs. Yet we know Māori continue to have higher rates of hospitalisations for conditions that can be improved with appropriate medicines use [3]. This suggests that tools may be of more use for screening rather than accurately assessing appropriate medicine use in an individual. These tools are developed outside of NZ by overseas experts, informed by clinical research relating to international, predominately European ethnicity, populations. Subsequent validation has also largely taken place outside of NZ. This review suggests these tools may not be as useful in assessing quality medicines use for Māori older adults.
Quality medicines use can be thought to incorporate the scientific and clinical basis for medicines use, patient choice and the general good (“…a mixture of issues, including societal and family-related consequences of prescribing”) [83]. All of the methods used to judge quality medicines use in this review centre on the therapeutic use of medicines without acknowledging the role of patient choice or wider societal impacts of medicine use. Patient inclusion, perspectives and choice are important aspects of quality considerations when delivering and evaluating Māori healthcare [1], as is making the most appropriate use of the health dollar. A limitation of this review was that economic factors were not addressed. None of the studies included economic analysis in their outcome measures. When assessing the quality of the health system, and the services within it, understanding cost implications to the individual, communities and overall system is important. These costs relate to medicines, medicines-related harm, the cost of non-treatment in terms of morbidity and mortality and wider societal ‘burdens’ of ill-health. Further study is needed to assess the economic impact of ethnic variation in the quality use of medicines.
Using medicine dispensing data as a proxy for appropriate prescribing fails to take into account other factors that may prevent prescribed medicines from being dispensed to individuals. In NZ almost all medicines used for the management of chronic conditions are subsidised by the government, with the patient being required to pay a co-payment ($5 per item at the time of writing). Māori are twice as likely not to obtain their medicines due to cost than non-Māori [84]. Māori also report higher rates of culturally unsafe pharmacy care, perceiving that this negatively impacts on their health outcomes [85].
Several included studies reported that PIP was more strongly associated with worse outcomes for Māori than non-Māori. Medicines-related outcomes relate to more than just prescribing. There are ethnic variations in a number of factors that influence medicines-related harm including medicines adherence [86], health literacy [87] and access to culturally safe health services, with Māori having lower rates of quality access [88]. In noting these, the authors wish to state their belief that it is the responsibility of health professionals, organisations and systems to negotiate medicines adherence and communicate in a health literate manner, rather than these being associated with individual or culturally-specific factors.
There are complexities in comparing medicines use between different ethnicities when differing age structures exist between the different ethnic groups. These difficulties have been articulated previously in whole-of-population medicines access research in NZ [89]. In NZ, Māori have a younger population than non-Māori (median age of 23.9 years compared with 38 years) [90], influenced by higher levels of chronic co-morbidity at younger ages for Māori, and subsequent reduced life expectancy. Given medicine use increases with age, one would expect higher prescription numbers in groups with older populations. Because of varying rates of comorbidity in different ethnic groups, comparison across the same age bands does not allow a fair assessment of levels of appropriate medicines use. The lower proportion of Māori compared with non-Māori in older age bands also means equal explanatory power is absent. This often precludes ethnic subgroup analysis, unless the studies use large data sets, or have been designed to include similar numbers of Māori and non-Māori older adults [51, 52, 89]. The impact of this can be seen in the large number of papers that were excluded in the full paper review (n = 53) because they either did not report Māori participation, or ethnicity was not used as a factor in the analysis. Māori have significantly lower life expectancy than non-Māori, and therefore survivor bias will feature in the reported figures, whereby Māori do not make it to the age of 65 years to the same extent as non-Māori. Even within the included studies, ethnicity analysis was not always undertaken across all outcome measures. The Māori population is ageing at a faster rate than that of non-Māori [92] and appropriate access to medicines will be increasingly important.
Some studies investigated effects in the total older adult group (≥ 65 years); however, it is important to understand that those aged ≥65 years are not one homogenous group. In several included studies, medicines utilisation and appropriateness changed significantly through the older adult age bands. Using the age of 65 years as the threshold for inclusion risks a lack of reporting for Māori who experience chronic comorbidity at a younger age. When results are analysed in 10-year age bands we see that there is more nuance in prescribing trends throughout the later years of life. For Māori, these ‘later years’ occur at a much earlier age than for non-Māori. There is variation between ethnicities and between age bands requiring further data interrogation, making it difficult to assess appropriateness when interpreting the discrete outcome measures used in these studies.
6 Implications for Practice and Future Direction
In clinical practice, the quality use of medicines in older adults involves skilled assessment by trained clinicians in partnership with patients. The complex decision-making processes need to take into account clinical, cultural and social contexts, multiple competing priorities, patient preferences and unique circumstances. Further investigation is needed to understand how culturally safe health services can be developed to ensure the quality use of medicines in Māori older adults and how the quality of these services can be appropriately assessed.
Understanding ethnic variation in the quality use of medicines for older adults in NZ allows us to identify whether inequities exist and what the particular issues are. Economic and patient-level impacts, such as quality of life and health service experiences, also need to contribute to this understanding in order to develop positive solutions. This review is a starting point in a process that can ultimately lead to pro-equity solutions to address any disparities in quality medicines use that do exist. The quality use of medicines is multi-factorial and our approaches to addressing inequity need to be multi-factorial. This review is of relevance to clinicians, researchers, funders and policy makers.
References
Tribunal W. Hauora-report on stage one of the health services and outcomes Kaupapa inquiry. Wai 2575. Wellington: Waitangi Tribunal; 2019.
Health and Disability System Review. Health and disability system review—final report—Pūrongo Whakamutunga. Wellington: HDSR; 2020. https://systemreview.health.govt.nz/assets/Uploads/hdsr/health-disability-system-review-final-report.pdf (Online).
Ministry of Health. Wai 2575 Māori Health Trends Report. Wellington: Ministry of Health; 2019. https://www.health.govt.nz/publication/wai-2575-maori-health-trends-report. Accessed: 29 Apr 2020 (Online).
Ministry of Health. Tatau Kahakura: Māori Health Chart Book 2015. 3rd ed. Wellington: Ministry of Health; 2015.
Te Pou o te Whakaaro Nui. DHB population profiles, 2019–2029: Statistics New Zealand projections 2018 update. Auckland: Te Pou o te Whakaaro Nui; 2019. https://www.tepou.co.nz/resources/dhb-population-profiles-2019-2029/901
Ministry of Health and University of Otago. Decades of disparity III: Ethnic and socioeconomic inequalities in mortality, New Zealand 1981–1999. Wellington: Ministry of Health; 2006.
Hobbs M, Ahuriri-Driscoll A, Marek L, Campbell M, Tomintz M, Kingham S. Reducing health inequity for Māori people in New Zealand. Lancet. 2019;394(10209):1613–4. https://doi.org/10.1016/S0140-6736(19)30044-3.
Harris R, Tobias M, Jeffreys M, Waldegrave K, Karlsen S, Nazroo J. Racism and health: the relationship between experience of racial discrimination and health in New Zealand. Soc Sci Med. 2006;63(6):1428–41.
Harris RB, Stanley J, Cormack DM. Racism and health in New Zealand: Prevalence over time and associations between recent experience of racism and health and wellbeing measures using national survey data. PLoS ONE. 2018. https://doi.org/10.1371/journal.pone.0196476.
Harris R, et al. The pervasive effects of racism: Experiences of racial discrimination in New Zealand over time and associations with multiple health domains. Soc Sci Med. 2012;74(3):408–15. https://doi.org/10.1016/j.socscimed.2011.11.004.
Rahiri J-L, Lauti M, Harwood M, MacCormick AD, Hill AG. Ethnic disparities in rates of publicly funded bariatric surgery in New Zealand (2009–2014). ANZ J Surg. 2018;88(5):E366–9. https://doi.org/10.1111/ans.14220.
Rumball-Smith J, Sarfati D, Hider P, Blakely T. Ethnic disparities in the quality of hospital care in New Zealand, as measured by 30-day rate of unplanned readmission/death. Int J Qual Health Care. 2013;25(3):248–54. https://doi.org/10.1093/intqhc/mzt012.
Rumball-Smith JML. Not in my hospital? Ethnic disparities in quality of hospital care in New Zealand: a narrative review of the evidence. N Z Med J. 2009;122(1297):17.
Mccreanor T, Nairn R. Tauiwi general practitioners’ explanations of Māori health: colonial relations in primary healthcare in Aotearoa/New Zealand? J Health Psychol. 2002;7(5):509–18. https://doi.org/10.1177/1359105302007005670.
Tukuitonga CF, Bindman AB. Ethnic and gender differences in the use of coronary artery revascularisation procedures in New Zealand. N Z Med J. 2002;115(1152):179–82.
Baxter J. Mental health: psychiatric disorder and suicide. In: Hauora: Māori Standards of Health IV: a study of the years 2000–2005, 4th edn. Wellington: Te Rōpū Rangahau Hauora a Eru Pōmare; 2007.
Tin ST, et al. Ethnic disparities in breast cancer survival in New Zealand: which factors contribute? BMC Cancer. 2018;18(1):58. https://doi.org/10.1186/s12885-017-3797-0.
Hill S, et al. Survival disparities in Indigenous and non-Indigenous New Zealanders with colon cancer: the role of patient comorbidity, treatment and health service factors. J Epidemiol Community Health. 2010;64(2):117–23. https://doi.org/10.1136/jech.2008.083816.
World Health Organisation. The rational use of drugs. report of the conference of experts. Geneva: World Health Organisation; 1985.
Ministry of Health. Cardiovascular disease risk assessment and management for primary care. Wellington: Ministry of Health; 2018.
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of Chronic Obstructive Pulmonary Disease: 2020 Report. Global Initiative for Chronic Obstructive Lung Disease; 2020. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf (Online).
bpacNZ. Optimising pharmacological management of HbA1c levels in patients with type 2 diabetes: from metformin to insulin. bpacNZ. 2019. https://bpac.org.nz/2019/docs/hba1c.pdf (Online).
Robson B, Harris R, Eru Pōmare Maori Health Research Centre. Hauora: Māori standards of health IV : a study of the years 2000–2005. Wellington: Te Rōpū Rangahau Hauora a Eru Pōmare; 2007.
Metcalfe S, Laking G, Arnold J. Variation in the use of medicines by ethnicity during 2006/07 in New Zealand: a preliminary analysis. N Z Med J. 2013;126(1384).
Metcalfe S, et al. Te Wero tonu—the challenge continues: Māori access to medicines 2006/07–2012/13 update. N Z Med J. 2018;131(1485).
Payne RA. The epidemiology of polypharmacy. Clin Med. 2016;16(5):465–9. https://doi.org/10.7861/clinmedicine.16-5-465.
Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. https://doi.org/10.1046/j.1365-2125.2003.02007.x.
Nair P, Chalmers L, Peterson G, Bereznicki B, Castelino R, Bereznicki L. Hospitalization in older patients due to adverse drug reactions—the need for a prediction tool. Clin Interv Ageing. 2016;11:497–505.
Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–9. https://doi.org/10.1016/S0140-6736(08)61690-6.
Health Quality and Safety Commission. Atlas of Healthcare Variation. Health Quality and Safety Commission of New Zealand; 2019. https://www.hqsc.govt.nz/our-programmes/health-quality-evaluation/projects/atlas-of-healthcare-variation/. Accessed 5 Jun 2020.
Health Quality and Safety Committee. Polypharmacy in people aged 65 and over. 2019. Accessed 15 Mar 2019. https://www.hqsc.govt.nz/our-programmes/health-quality-evaluation/projects/atlas-of-healthcare-variation/polypharmacy/ (online).
Health Quality and Safety Commission. Atlas of healthcare variation: opioids. Health Quality and Safety Commission of New Zealand; 2019. https://www.hqsc.govt.nz/our-programmes/health-quality-evaluation/projects/atlas-of-healthcare-variation/opioids/.
Health Quality and Safety Commission. Atlas of healthcare variation: Gout; 2020. https://www.hqsc.govt.nz/our-programmes/health-quality-evaluation/projects/atlas-of-healthcare-variation/gout/.
Wilkinson S, Mulder RT. Antidepressant prescribing in New Zealand between 2008 and 2015. N Z Med J. 2018;131(1485):52–9.
Kerr AJ, et al. Effect of age, gender, ethnicity, socioeconomic status and region on dispensing of CVD secondary prevention medication in New Zealand: The Atlas of Health Care Variation CVD cohort (VIEW-1). N Z Med J. 2014;127(1400):39–69.
Norris P, et al. Equity in statin use in New Zealand. J Prim Health Care. 2014;6(1):17–22.
Ndukwe HC, Wang T, Tordoff JM, Croucher MJ, Nishtala PS. Geographic variation in psychotropic drug utilisation among older people in New Zealand. Aust J Ageing. 2016;35(4):242–8. https://doi.org/10.1111/ajag.12298.
Poppe KK, et al. Identification, risk assessment, and management of patients with atrial fibrillation in a large primary care cohort. Int J Cardiol. 2018;254:119–24. https://doi.org/10.1016/j.ijcard.2017.11.045.
Norris P, et al. Medicalisation or under-treatment? Psychotropic medication use by elderly people in New Zealand. Health Sociol Rev. 2011;20(2):202–18. https://doi.org/10.5172/hesr.2011.20.2.202.
Samaranayaka S, Walker RJ, Samaranayaka A, Derrett S, Schollum JWB. Medication exposure and health outcomes in older patients with end-stage kidney disease: a prospective study undertaken in New Zealand. Drugs Aging. 2018;35(11):1005–15. https://doi.org/10.1007/s40266-018-0582-y.
Pitcher TL, et al. Parkinson’s disease across ethnicities: a nationwide study in New Zealand. Mov Disord. 2018;33(9):1440–8. https://doi.org/10.1002/mds.27389.
Parkin L, Barson D, Zeng J, Horsburgh S, Sharples K, Dummer J. Patterns of use of long-acting bronchodilators in patients with COPD: a nationwide follow-up study of new users in New Zealand. Respirology. 2018;23(6):583–92. https://doi.org/10.1111/resp.13235.
Cullum S, et al. Predictors of mortality in Maori, Pacific Island, and European patients diagnosed with dementia at a New Zealand Memory Service. Int J Geriatr Psychiatry. 2020;35(5):516–24. https://doi.org/10.1002/gps.5266.
Narayan SW, Tordoff JM, Nishtala PS. Temporal trends in the utilisation of preventive medicines by older people: a 9-year population-based study. Arch Gerontol Geriatr. 2016;62:103–11. https://doi.org/10.1016/j.archger.2015.10.007.
Bala SS, Jamieson HA, Nishtala PS. Factors associated with inappropriate prescribing among older adults with complex care needs who have undergone the interRAI assessment. Curr Med Res Opin. 2019;35(5):917–23. https://doi.org/10.1080/03007995.2018.1543185.
Bala SS, Jamieson HA, Nishtala PS. Determinants of prescribing potentially inappropriate medications in a nationwide cohort of community dwellers with dementia receiving a comprehensive geriatric assessment. Int J Geriatr Psychiatry. 2019;34(1):153–61. https://doi.org/10.1002/gps.5004.
Bala SS, Narayan SW, Nishtala PS. Potentially inappropriate medications in community-dwelling older adults undertaken as a comprehensive geriatric risk assessment. Eur J Clin Pharmacol. 2018;74(5):645–53. https://doi.org/10.1007/s00228-018-2412-x.
Narayan SW, Nishtala PS. Prevalence of potentially inappropriate medicine use in older New Zealanders: a population-level study using the updated 2012 Beers criteria. J Eval Clin Pract. 2015;21(4):633–41. https://doi.org/10.1111/jep.12355.
Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf. 2014;23(7):753–8.
Jamieson HA, et al. Drug burden index and its association with hip fracture among older adults: a national population-based study. J Gerontol A Biol Sci Med Sci. 2019;74(7):1127–33. https://doi.org/10.1093/gerona/gly176.
Ryan C, et al. Quality of prescribing predicts hospitalisation in octogenarians: life and living in advanced age: a cohort study in New Zealand (LiLACS NZ). BMC Geriatr. 2019;19(1):357. https://doi.org/10.1186/s12877-019-1305-x.
Cardwell K, et al. The Association between Drug Burden Index (DBI) and health-related outcomes: a longitudinal study of the ‘oldest old’ (LiLACS NZ). Drugs Aging. 2020;37(3):205–13. https://doi.org/10.1007/s40266-019-00735-z.
Ministry of Health NZ. HISO 10001:2017 Ethnicity Data Protocols. Ministry of Health; 2017.
Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. J Am Assoc Nurse Pract. 2005;17(4):123–32.
Hilmer SN, et al. A drug burden index to define the functional burden of medications in older people. Am Arch Intern Med. 2007;167:781–7.
American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–94. https://doi.org/10.1111/jgs.15767.
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8. https://doi.org/10.1093/ageing/afu145.
Hanlon JT, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045–51. https://doi.org/10.1016/0895-4356(92)90144-C.
January CT, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019. https://doi.org/10.1161/CIR.0000000000000665.
Adler RA, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for bone and mineral research. J Bone Miner Res. 2016;31(1):16–35. https://doi.org/10.1002/jbmr.2708.
Simpson SM, Krishnan LL, Kunik ME, Ruiz P. Racial disparities in diagnosis and treatment of depression: a literature review. Psychiatr Q. 2007;78(1):3–14. https://doi.org/10.1007/s11126-006-9022-y.
Malhi GS, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49(12):1087–206.
Kopua DM, Kopua MA, Bracken PJ. Mahi a Atua: a Maori approach to mental health. Transcult Psychiatry. 2019. https://doi.org/10.1177/1363461519851606.
Deloitte. Dementia economic impact report 2016. Alzheimers; 2016. https://www.alzheimers.org.nz/getmedia/79f7fd09-93fe-43b0-a837-771027bb23c0/Economic-Impacts-of-Dementia-2017.pdf
Puyat JH, et al. Racial and ethnic disparities in the use of antipsychotic medication: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2013;48(12):1861–72. https://doi.org/10.1007/s00127-013-0753-4.
Winnard D, et al. Gout, diabetes and cardiovascular disease in the Aotearoa New Zealand adult population: co-prevalence and implications for clinical practice. N Z Med J. 2013;126(1368).
Lines L, Sherif N, Wiener J. Racial and ethnic disparities among individuals with Alzheimer’s disease in the United States: a literature review. North Carolina: RTI Press; 2014. https://doi.org/10.3768/rtipress.2014.RR.0024.1412.
Health Quality and Safety Commission. Launch of open for better care campaign focus on medication safety. Health Quality and Safety Commission; 2014. https://www.hqsc.govt.nz/assets/Open-for-better-care/Medication/NEMR/Launch-FAQ-Oct-2014.pdf. Accessed 5 Feb 2020.
Papaleontiou M, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353–69. https://doi.org/10.1111/j.1532-5415.2010.02920.x.
Chou R, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–30. https://doi.org/10.1016/j.jpain.2008.10.008.
Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014;(10).
Tangiisuran B, Gozzoli M, Davies J, Rajkumar C. Adverse drug reactions in older people. Rev Clin Gerontol. 2010;20(3):246–59. https://doi.org/10.1017/S0959259810000171.
Stanley J, Semper K, Millar E, Sarfati D. Epidemiology of multimorbidity in New Zealand: a cross-sectional study using national-level hospital and pharmaceutical data. BMJ Open. 2018;8(5):e021689. https://doi.org/10.1136/bmjopen-2018-021689.
Ailabouni NJ, Nishtala PS, Tordoff JM. Examining potentially inappropriate prescribing in residential care using the STOPP/START criteria. Eur Geriatric Med. 2016;7(1):40–6. https://doi.org/10.1016/j.eurger.2015.11.004.
Gallagher P, O’Connor MN, Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6).
American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46. https://doi.org/10.1111/jgs.13702.
Schluter PJ, et al. Comprehensive clinical assessment of home-based older persons within New Zealand: an epidemiological profile of a national cross-section. Aust N Z J Public Health. 2016;40(4):349–55. https://doi.org/10.1111/1753-6405.12525.
Lee D, Martini N, Moyes S, Hayman K, Zolezzi M, Kerse N. Potentially inappropriate medication use: the Beers’ Criteria used among older adults with depressive symptoms. J Prim Health Care. 2013;5(3):182–90.
Gnjidic D, Couteur DGL, Abernethy DR, Hilmer SN. Drug Burden Index and Beers Criteria: impact on functional outcomes in older people living in self-care retirement villages. J Clin Pharmacol. 2012;52(2):258–65. https://doi.org/10.1177/0091270010395591.
Hall-Lipsy EA, Chisholm-Burns MA. Pharmacotherapeutic disparities: Racial, ethnic, and sex variations in medication treatment. Am J Health Syst Pharm. 2010;67(6):462–8. https://doi.org/10.2146/ajhp090161.
Page A, et al. Potentially suboptimal prescribing of medicines for older Aboriginal Australians in remote areas. Med J Aust. 2019;211(3):119–25. https://doi.org/10.5694/mja2.50226.
Cormack D, Kukutai T. Ethnic group classification in Aotearoa New Zealand. In: Heterogeneity/granularity in ethnicity classidications outside the United States (HGEC Project). Edinburgh: The Robert Wood Johnson Foundation; 2016.
Spinewine A, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–84. https://doi.org/10.1016/S0140-6736(07)61091-5.
Jatrana S, Richardson K, Norris P, Crampton P. Is cost-related non-collection of prescriptions associated with a reduction in health? Findings from a large-scale longitudinal study of New Zealand adults. BMJ Open. 2015;5(11):1–9. https://doi.org/10.1136/bmjopen-2015-007781.
Bassett-Clarke D, Krass I, Bajorek B. Ethnic differences of medicines-taking in older adults: a cross cultural study in New Zealand. Int J Pharm Pract. 2012;20(2):90–8. https://doi.org/10.1111/j.2042-7174.2011.00169.x.
Horsburgh S, et al. Allopurinol use in a New Zealand population: prevalence and adherence. Rheumatol Int. 2014;34(7):963–70.
Ministry of Health NZ. Kōrero Mārama: Health Literacy and Māori. Results from the 2006 Adult Literacy and Life Skills Survey. Wellington: Ministry of Health; 2010.
Jansen P, Bacal K, Crengle S. He Ritenga Whakaaro: Māori experiences of health services, vol. 200. Auckland: Mauri Ora Associates; 2008.
Auckland UniServices Ltd. Variation in medicines use by ethnicity: a comparison between 2006/7 and 2012/13. Auckland: The University of Auckland; 2018.
Statistics New Zealand. 2013 Census ethnic group profiles. Māori; 2013. http://archive.stats.govt.nz/Census/2013-census/profile-and-summary-reports/ethnic-profiles.aspx?request_value=24705&tabname=Age,sex,andethnicities. Accessed 24 May 2018.
Dyall L, et al. Engagement and recruitment of Māori and non-Māori people of advanced age to LiLACS NZ. Aust N Z J Public Health. 2013;37(2):124–31. https://doi.org/10.1111/1753-6405.12029.
Ministry of Health NZ. Older people’s health data and stats. Ministry of Health NZ, Jun. 18; 2018. https://www.health.govt.nz/nz-health-statistics/health-statistics-and-data-sets/older-peoples-health-data-and-stats. Accessed 15 Mar 2019.
Ministry of Health. Pharmacy action plan 2016 to 2020. Ministry of Health, Wellington; 2016. https://www.health.govt.nz/system/files/documents/publications/pharmacy-action-plan-2016-to-2020.pdf. Accessed 25 Mar 2019.
Spinewine A, Fialová D, Byrne S. The role of the pharmacist in optimizing pharmacotherapy in older people. Drugs Aging. 2012;29(6):495–510.
Te Karu L, Bryant L, Harwood M, Arroll B. Achieving health equity in Aotearoa New Zealand: the contribution of medicines optimisation. J Prim Health Care. 2018;10(1):5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
JH was funded by a Health Research Council of NZ, Clinical Research Training Fellowship (HRC: 17/134). The funders had no role or influence over study design; the collection, analysis and interpretation of data; in the writing of the report; or the decision to submit the article for publication.
Conflict of Interest
The authors declare they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author Contributions
JH developed the methodology, performed the literature search and data analysis, drafted, edited and finalised the manuscript. NM, RJ, CH and MJC provided input to the structure of the report and interpretation and critical review of the findings. All authors provided comprehensive, critical reviews of the manuscript and approved the final draft of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hikaka, J., Jones, R., Hughes, C. et al. Ethnic Variations in the Quality Use of Medicines in Older Adults: Māori and Non-Māori in Aotearoa New Zealand. Drugs Aging 38, 205–217 (2021). https://doi.org/10.1007/s40266-020-00828-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-020-00828-0