Abstract
Lignocellulosic biomasses are considered the most suitable feedstocks for sustainable bioenergy generation in the future owing to their versatility, availability, and low cost. Pretreatment is considered an essential step for removing lignin and solubilizing hemicellulose to increase the sugar yield of cellulase hydrolysis of pretreated biomass because pretreatment may disrupt the crystalline structure of cellulose. To investigate the effect of ultrasound on ionic liquid-hydrochloric acid pretreatment with rice straw, this study adopted an hydrochloric acid–catalyzed process in 1-butyl-3-methylimidazolium chloride and 1-allyl-3-methylimidazolium chloride aqueous solutions under ultrasound irradiation to treat the rice straw at a medium-low temperature. The effects of temperature, acid concentration, and time on the production of reducing sugar were studied; moreover, the benefits of ultrasound during pretreatment were also explored according to the changes of enzymatic saccharification, components, morphology, crystallinity, and chemical structure. The results showed that temperature, acid concentration, and time have significant effects on enzymatic hydrolysis, and the production of reducing sugar, cellulose conversion, and delignification was increased by 20.13–28.96%, 31.69–35.23%, and 18.06–19.33%, respectively. On the other hand, the results of Scanning Electron Microscopy (SEM), X-ray diffraction (XRD), and Fourier Transform Infrared Spectroscopy (FTIR) analysis also demonstrate that ultrasound can promote the destruction of morphology, chemical structure, and crystallinity of rice straw in the hydrochloric acid–catalyzed process in ionic liquid aqueous solutions. (1) Temperature, acidity, and processing time had significant effects on the sugar yield of rice straw pretreated with US-IL-HCl. (2) Compared with treatment without ultrasound, the sugar yield, cellulose conversion rate, and delignification increased in ultrasonic-assisted pretreatment samples. (3) Ultrasound-assisted pretreatment can further improve the damage of the surface morphology and chemical structure of rice straw, reduce the crystallinity of cellulose, and improve the enzymatic hydrolysis effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background
China, as a big agricultural country, produces a large amount of straw waste. According to the data released by the implementation plan for the comprehensive utilization of crop straw in the “12th Five-Year,” the theoretical production of straw was 840 million tons in 2010, and the total available amount was about 700 million tons. The three major categories of straws were rice straw, wheat straw, and corn straw with approximate yields of 211 million tons, 154 million tons, and 273 million tons, respectively [1]. In China, direct incineration is a common straw waste treatment used to save space during collection and disposal and facilitate farming. However, the combustion process releases 70% CO2, 7% CO, 0.66% CH4, and 2.09% N2O, as well as large amounts of PM2.5 and greenhouse gases, causing serious pollution to the air environment [2]. Since the government has introduced a series of policies to prohibit the direct incineration of straw in recent years, the proportion of direct combustion has been effectively controlled. However, to solve this problem fundamentally, it is necessary to find a treatment that integrates economic, social, and environmental benefits and better utilizes straw waste. In the “13th Five-Year Plan for Biomass Energy,” the annual utilization of bio-liquid fuels will reach 6 million tons by 2020, of which bioethanol accounts for 4 million tons [3]. Therefore, the preparation of bioethanol using straw waste as a raw material is a popular technology for biomass energy utilization.
As a natural lignocellulose material, straw has a dense and stable structure, which hinders its contact with microorganisms and enzymes, and inhibits the decomposition and utilization of polysaccharide, thereby reducing the treatment efficiency and greatly increasing the production cost of ethanol. In order to break the natural barriers of lignocellulose, straw pretreatment is necessary. Ionic liquid can be used as a green solvent to dissolve cellulose. The dissolution-precipitation process can destroy both the dense structure of lignocellulose and the crystal structure of cellulose, remove lignin, increase the contact area with enzymes and microorganisms, and improve the availability of lignocellulose [4]. In particular, 1-allyl-3-methylimidazolium chloride AMIMCl and 1-butyl-3-methylimidazolium chloride BMIMCl show superior cellulose solubility characteristics. The viscosity, boiling, and melting points of AMIMCl are 685 mPa S, 50 °C, and 18 °C, as well as 147 mPa S, 125 °C, and 72 °C for BMIMCl. Previous research showed that rice straw pretreatment by AMIMCl and BMIMCl can increase cellulose conversion to 25% and 27%, respectively [5, 6]. (Enzymes dosage: 50 FPU/g rice straw cellulose and 40 CBU/g glucosidase.) Moisture affects most ionic liquids because it inhibits cellulose dissolution and reduces treatment effect [7]. However, moisture is inevitable during lignocellulose treatment because ionic liquids absorb moisture from the environment, and lignocellulose also contains some water. Studies have shown that the addition of a certain amount of hydrochloric acid can effectively improve the pretreatment effect of aqueous ionic liquid solutions on cellulose [8,9,10]. This new pretreatment method can effectively solve the problem caused by moisture, and also allows the water content to increase to 20~30% without significantly reducing the treatment effect. Increased moisture content can reduce the viscosity of ionic liquids, promote material transport, increase biomass loading, shorten the time spent on ionic liquid recovery, and eventually save costs [11].
As a mechanical acoustic wave, ultrasound produces effects through ultrasonic propagation in the medium, which plays a good role in stirring the medium. Ultrasound can emulsify two immiscible liquids, accelerate the dissolution of solute, and speed up the chemical reactions [12]. It has been reported that the use of ultrasonic-assisted ionic liquids can accelerate the pretreatment process and have a stronger effect on destroying crystalline cellulose and lignin [13, 14]. However, pretreatment of lignocellulose with an aqueous solution of ultrasonic-assisted ionic liquid-hydrochloric acid has not been reported so far. In aqueous ionic liquids, water molecules are more prone to ions in the ionic liquid, which in turn destroys the interaction between the anions and cations, resulting in reduced viscosity in the system [11]. It is generally believed that the reduced viscosity of a medium reduces the cavitation valve of the ultrasonic wave and promotes the mass transfer of the ultrasonic wave [15]. Therefore, rice straw waste was pretreated with an aqueous solution of ultrasonic-assisted ionic liquid under the catalysis of hydrochloric acid at medium and low temperatures in the current study. The change of sugars produced from enzymatic hydrolysis of rice straw and the change in microstructure were studied.
2 Materials and methods
2.1 Materials and reagents
The lignocellulosic material used in this experiment is rice straw, which was provided by the Institute of Urban Environment, Chinese Academy of Sciences, China. The rice straws were crushed, then screened through 40 mesh and dried at 60 °C for 24 h before being kept sealed. AMIMCl (≥ 99%) and BMIMCl (≥ 99%) were purchased from Shanghai Cheng-jie Chemical Co. Ltd., China. Thirty-six percent hydrochloric acid was purchased from Guangzhou Chemical Reagent Factory, China.
2.2 Biomass pretreatment and recycling
First, 0.27 g of rice straw (dry weight 0.25 g) was placed in a 20 mL bottle containing 5 g ionic liquid with a biomass loading of 5 wt%. A certain amount of HCl solution and deionized water were then added to prepare a mixed suspension with water content of 20% (w/w), hydrochloric acid concentration of 0–3.6% (w/w), and solid-liquid ratio of 1:20, before the suspension was placed in an ultrasonic cleaner water bath with 840 W (Ningbo Xinzhi Biotechnology Co., Ltd.) with the temperature controlled within the range of 30–70 (± 3) °C (acoustic energy density 0.0267 W cm−3) and frequency of 40 kHz. The ultrasonic reaction was carried out at a constant temperature for 30–240 min. After the reaction was completed and cooled, a certain amount of deionized water was added and stirred for 15 min before transferring the reaction mix to a 50-mL centrifuge tube. Deionized water was added to make a final volume of 50 mL, and centrifuged at 8000 g for 10 min to remove the supernatant. Then repeated the above operation 5 times until the washing liquid was colorless. After vacuum filtration, the filter residue was dried at 60 °C for 24 h and then weighed before being sealed and stored.
2.3 Enzymatic saccharification and composition analysis of lignocellulose samples
Then, it was mixed with enzymes including cellulase complex enzyme (Novozyme NS220086) 50 FPU g−1 and β-glucosidase (Novozyme NS221118) 40 CBU g−1, which is followed by a 150 r min−1 water bath shaker under 50 °C. Samples were taken at 0 h and 48 h, respectively. The total reducing sugar content in the reaction solution was determined by the DNS method [16]. Thus, the cellulose conversion was calculated using Formula (1) [17]
In the formula, c represents the concentration of reducing sugar (mg mL−1) produced by enzymatic hydrolysis at 48 h, V represents the total volume of the reaction solution (mL), η represents the content of cellulose and hemicellulose components (%), and m represents rice straw amount added to the reaction (mg). The equation assumes the hydrolysis factor is same for all the sugars present in the hydrolysis medium.
The cellulose, hemicellulose, and lignin contents of the samples were determined by the nitric acid-ethanol method, the sodium chlorite method and the 72% concentrated sulfuric acid method, respectively [18]. The lignin removal after pretreatment was calculated using Eq. (2) [19].
In the formula, LO and L represent the lignin content in rice straw before and after pretreatment, respectively, while YT is the sample recovery rate after pretreatment (%).
2.4 Characterization and analysis of lignocellulose samples
2.4.1 Ultima X-ray diffractometer (Japanese science)
X-ray diffractograms of untreated and pretreated samples were obtained on an Ultima X-ray diffractometer. The samples were scanned over a range of 5–50° (2θ), with step size and time of 0.02° and 1 s, respectively, and at 40 kV, 20 mA, and an ambient temperature. The crystallinity (CrI) of the sample was calculated using Segal’s Formula (3) [20].
In the formula, I002 is the (002) lattice plane diffraction intensity, and Iam is the diffraction intensity of the amorphous region between the lattice planes (001) and (002).
2.4.2 Nicolet 6700 Fourier infrared spectrometer (American ThermoFisher Scientific)
The functional groups of the pretreated and untreated samples were determined using a Nicolet 6700 Fourier infrared spectrometer within the wavenumber range of 600–4000 cm−1 with 20 scans at a 4 cm−1 resolution. The samples were mixed with potassium bromide (KBr) at a weight ratio of approximately 1:400 to form a pellet. Each FT-IR spectrum was recorded with a blank (KBr) pellet as the background.
2.4.3 Field emission scanning electron microscope JSM-7001F (Japan Electronics Co., Ltd.)
The morphologies of untreated and pretreated samples were examined on a scanning electron microscope. After freeze-drying, the samples were carefully mounted on the powder sample stubs by using a double-sided carbon tape and a 30-nm thick conductive gold coating was applied to the surface. Images were acquired at a 5-kV acceleration voltage.
3 Results and discussion
3.1 Effect of pretreatment temperature on sugar yield of rice straw
Ultrasonic-assisted pretreatment in an ionic liquid-hydrochloric acid (1.2 wt%) (US-IL-HCl) solution containing 20% water was utilized to pretreat rice straw at 30–70 °C for 120 min. The results of enzymatic hydrolysis and saccharification were shown in Fig. 1. The results showed that temperature has a significant effect on the pretreatment effect. With the increase of temperature, the yield of enzymatic hydrolysis increased. The yields of reducing sugar in ionic liquid-hydrochloric acid solutions US-BMIMCl-HCl and US-AMIMCl-HCl were 8.423 mg mL−1 and 9.717 mg mL−1 at 70 °C, respectively. The reducing sugar yield in US-BMIMCl-HCl and US-AMIMCl-HCl had significantly increased by 93.45% and 74.20% at 30 °C, 44.77% and 24.13% at 50 °C, respectively. Higher temperatures can reduce the viscosity of ionic liquids and accelerate the swelling and dissolution of cellulose because hydrogen bonds between cellulose are effectively disrupted at higher temperatures [21]. BMIMCl has a higher melting point than AMIMCl, and the mass transfer capacity of BMIMCl is lower than that of AMIMCl at low and medium temperatures [21], so enzymatic hydrolysis results show that the pretreatment capacity of US-BMIMCl-HCl is lower than that of US-AMIMCl-HCl. However, temperature has a greater effect on reducing sugar yield obtained in US-BMIMCl-HCl.
3.2 Effect of acid concentration on sugar yield of rice straw
Ultrasonic-assisted pretreatment was applied to pretreated rice straw in the ionic liquid-hydrochloric acid (0–3.6 wt.%) (US-IL-HCl) solution containing 20% water at 70 °C for 120 min. The results of enzymatic saccharification were shown in Fig. 2. As the hydrochloric acid concentration increased, the yield of sugar also increased, reaching the highest level at 2.4 wt.%. Compared to rice straw pretreated with ionic liquid without hydrochloric acid, the sugar yields of the rice straw pretreated with US-BMIMCl-HCl and US-AMIMCl-HCl were increased by 149.10% and 113.34%, respectively. The addition of a certain amount of hydrochloric acid can increase the concentration of hydrogen ions and chloride ions in the system. Chloride ion is a strong electronegative ion that can compete with hydroxyl radicals in cellulose for protons, and the increase of hydrogen ion concentration helps attack the intercellular cellulose glycosides [22, 23]. Therefore, the addition of a certain amount of hydrochloric acid can promote the dissolution and destruction of cellulose by US-BMIMCl and US-AMIMCl and further facilitate enzymatic hydrolysis.
3.3 Effect of processing time on sugar yield of rice straw
Ultrasonic-assisted pretreatment was used to pretreat rice straw in an ionic liquid-hydrochloric acid (2.4 wt.%) (US-IL-HCl) solution containing 20% water at 70 °C for 30–240 min. The results of enzymatic saccharification were shown in Fig. 3. The sugar yield remained unchanged after 60 min in US-BMIMCl-HCl, and reached its highest level at 180 min in US-AMIMCl-HCl (Fig. 3). Previous studies have reported elsewhere that the optimal pretreatment time of ionic liquid hydrochloric acid solution (IL-HCl) at a high temperature (≥ 120 °C) is 30–45 min [9, 10]. In the current study, the optimum pretreatment time was achieved at 60–180 min by lowering the pretreatment temperature and introducing assisted ultrasound. Pretreatment time is one of the important factors that directly affect the treatment effect. Although prolonging the reaction time can promote the destruction of lignocellulose structure, it also leads to excessive decomposition of sugar into furfural, hydroxymethylfurfural, and other enzymatic inhibitors [24].
3.4 Treatment effects of different pretreatment methods
In order to confirm the promoting effect of ultrasound in IL-HCl, the effects of different pretreatments were compared in the current experiment. As shown in Fig. 4, the sugar yield from digestion of untreated rice straw was only 3.83 mg mL−1, while the sugar yield of rice straw with ultrasonic-assisted pretreatment with 2.4 wt.% hydrochloric acid solution (US-HCl) increased by 36.80%. The sugar yield of rice straw with pretreatment using ultrasonic-assisted pure ionic liquid (anhydrous) (US-IL) increased by 73.39% and 95.06% in US-BMICMCl and US-AMIMCl, respectively. These two cases indicate that the combination of ultrasonic pretreatment with either hydrochloric acid solution or ionic liquid can improve the hydrolysis of sugar in rice straw, but the improvement effect is less than those of IL-HCl and US-IL-HCl. Figure 4 also showed that although the water content in IL-HCl is 20%, the sugar yield still increases by 113.06% and 164.63% for rice straw pretreated in IL-HCl and US-IL-HCl solution, respectively, compared to the sugar yield from untreated rice straw. In order to further improve the pretreatment effect, ultrasonic-assisted pretreatment in IL-HCl was used. The results of enzymatic hydrolysis showed that ultrasonic-assisted pretreatment can further increase the total yield of reducing sugar. The sugar yield of rice straws pretreated by US-BMIMCl-HCl and US-AMIMCl-HCl increased by 28.96% and 20.13%, respectively. Zhang et al. pretreated sorghum stalk by sole ultrasound. The optimum condition of ultrasonic pretreatment was 5 h, 50 °C, under which the sugar yield of 33.69% was obtained. It increased significantly, compared with the sugar yield of raw material (16.80%) [25]. Dong et al. pretreatment of corn stalk by ultrasonic-assisted (400 W) ionic liquid including 1-butyl-3-methylimidazolium chloride [BMIM]Cl, 1-H-3-methylimidazolium chloride [HMIM]Cl, and 1-(1-propylsulfonic)-3-imidazolium chloride [HSO3-pMIM]Cl at 70 °C for 2 h. Enzymatic hydrolysis of untreated, IL-US-treated, and IL-US/H2O-treated corn stalk for 12 h. Significantly high yield of reducing sugar was observed in the samples treated by [HMIM]Cl-US and [HSO3-pMIM]Cl-US, possibly because most of lignin was removed and the crystalline structure was decreased when corn stalk was pretreated by ionic liquid. After 12 h, a yield of 42% and 39% of reducing sugar was obtained when the corn stalk was treated by [HMIM]Cl-US and [HSO3-pMIM]Cl-US, respectively [26]. From our study and literature, ultrasound is an environmental-friendly method and shows great potential for the pretreatment of lignocellulosic biomass.
The enzymatic hydrolysis of pretreated rice straw in different pretreatment methods: US-HCl (2.4 wt.% HCl under ultrasound irradiation), US-IL (pure IL under ultrasound irradiation), IL-HCl (ionic liquid-2.4 wt.% HCl), US-IL-HCl (ionic liquid-2.4 wt.% HCl under ultrasound irradiation), and BMIMCl (70 °C, 60 min), AMIMCl (70 °C, 180 min)
3.5 Composition analysis
The lignocellulose contents of rice straw before and after pretreatment were shown in Table 1. The cellulose content of the rice straw increased from 38.10 to 52.54~56.09% after pretreatment because the soluble impurity was removed during the pretreatment and washing process. Comparing the component changes of rice straw pretreated by IL-HCl with or without ultrasonic-assisted treatment, it can be found that the contents of hemicellulose and lignin decreased in the ultrasonic-assisted treatment. The decrease of hemicellulose is mainly due to the cleavage of the β-ether bond between it and lignin. The cleavage rate of the β-ether bond and the number of cleaved bonds in the rice straw pretreated in IL-HCl depend mainly on solution acidity, the number of available protons, and temperature [9]. In this experiment, the hemicellulose content (18.18% and 18.86%) of rice straw pretreated in IL-HCl at a maximum temperature of 70 °C was not significantly decreased when compared with that of untreated rice straw (19.95%). However, with ultrasonic-assisted pretreatment in US-BMIMCl-HCl and US-AMIMCl-HCl, the hemicellulose content of rice straw was reduced 37.52% and 34.52%, respectively, when compared with samples treated with no ultrasound. This may be because the optimal conditions for β-ether bond cleavage are not sufficient at 70 °C when lacking ultrasound, but the conditions of transient high temperature and high pressure caused by the cavitation effect of ultrasonic waves promote the β-ether bond cleavage [12]. In addition, the lignin removal of BMIMC-HCl, AMIMCl-HCl, US-BMIMCl-HCl, and US-AMIMCl-HCl were 46.24%, 45.08%, 55.18%, and 53.22%, respectively. The lignin removal of US-BMIMCl-HCl and US-AMIMCl-HCl increased by 19.33% and 18.06%, respectively, when compared to those without ultrasound, indicating that ultrasonic-assisted treatment can further improve lignin removal. This is due to the separation of the disrupted lignin by ultrasound, which reduces the amount of lignin attached to the sample [27]. The cellulose conversion was used to compare the treatment effects on composition change and sugar yield between IL-HCl and US-IL-HCl. The calculated results show that US-BMIMCl-HCl and US-AMIMCl-HCl improved 35.23% and 31.69%, respectively, compared with cases without ultrasound. Therefore, pretreatment involving ultrasound can improve the sugar production rate of pretreated rice straw. Sorn et al. investigated the pretreatment of rice straw by using 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) as an ionic liquid (IL) and 1-butyl-3-methylimidazolium hydrogen sulfate ([Bmim]HSO4) as an acidic-IL (acidic-IL) under microwave irradiation. From their composition analysis, the untreated rice straw consists of cellulose (30.88 ± 0.39%), hemicellulose (15.73 ± 0.31%), and lignin (20.00 ± 0.44%). Compared to our results, the slight difference was because the rice straw was from different countries (Thailand and China). After pretreatment of rice straw with [Bmim]Cl only, [Bmim]HSO4 only, microwave-[Bmim]Cl, and microwave-[Bmim]HSO4, the lignin concentrations of the samples decreased to 11.79 ± 0.60%, 16.62 ± 0.39%, 8.59 ± 0.25%, and 15.86 ± 0.63%, respectively. With [Bmim]HSO4-only and microwave-[Bmim]HSO4 pretreatments, hemicelluloses decreased slightly to 12.94 ± 0.55% and 13.76 ± 0.45%, respectively. Most of the hemicellulose was destroyed by the [Bmim] HSO4-only and microwave-[Bmim]HSO4 treatment, and the cellulose concentration increased to 38.58 ± 0.48% and 36.98 ± 0.64%, respectively, whereas after treatment with [Bmim]Cl only and microwave-[Bmim]Cl, the hemicellulose concentrations of the samples did not differ significantly from that of the untreated sample. These results show that the increase in cellulose content was caused by the partial removal of hemicellulose and lignin [28].
3.6 Lignocellulose characterization
The surface morphology, chemical functional groups, and crystallinity of cellulose can also be used as bases for examining the pretreatment effect. Scanning electron microscopy (SEM) results of rice straw before and after pretreatment (magnification 2000 times) were shown in Fig. 5. Figure 5a shows the untreated rice straw, and the surface structure of the rice straw is found to be uniform and intact. The surface structure of the rice straw after pretreatment with IL-HCl or US-IL-HCl became disordered, irregular, and porous. Comparing the pictures of rice straw with or without ultrasonic-assisted treatment, such as in (b) and (c) and (d) and (e), it was shown that the cellulose from the rice straw not treated with ultrasound is again precipitated and deposited on the surface, showing bright protrusions. The fiber bundles on the surface of rice straw under ultrasonic-assisted treatment showed spalling and fracturing. This explains the reason for the improved efficiency with ultrasonic-assisted pretreatment of rice straw. The change of microstructure improves the accessibility of the enzyme to the material, destroys the natural barrier of lignocellulose, and improves the yield of enzymatic hydrolysis.
The crystallinity (CrI) of the rice straw before and after the pretreatment was analyzed by X-ray diffraction (shown in Fig. 6) and calculated according to Formula (3). The results were shown in Table 2. The CrI of untreated rice straw was 48.57%, and the crystallinity increased to 52.12% and 51.06% after treatment in US-BMIMCl-HCl and US-AMIMCl-HCl, respectively. The CrI value calculated by XRD analysis is the ratio of the crystalline region to the amorphous region in the sample [29]. The increase of CrI value is mainly due to the removal of amorphous substances such as lignin and hemicellulose and the collapse and flaking of amorphous regions, resulting in an increase in the relative content of crystalline cellulose. With the aid of ultrasound, the crystallinity of rice straw treated by US-BMIMCl-HCl and US-AMIMCl-HCl decreased to 47.77% and 46.19%, respectively. At this point, although the content of hemicellulose and lignin decreased more when treated with ultrasound than that of the rice straws without ultrasonic-assisted treatment, the crystallinity also decreased, indicating that the ultrasonic-assisted treatment can have a certain destructive effect on the crystalline cellulose. The decrease in the crystallinity of the cellulose helps to reduce the steric hindrance between it and the hydrolase and increases the reaction rate.
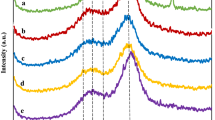
The chemical structure change of the sample can be observed by Fourier transform infrared spectroscopy (FTIR), and the results were shown in Fig. 7. The following characteristic peaks in cellulose and hemicellulose appear: 2920 cm−1 (C-H and CH2 stretching peak), 1372 cm−1 (bending vibration peak of C-H in cellulose and hemicellulose), 1160 cm−1 (C-O-C antisymmetric telescopic vibration peak), 1105 cm−1 (change in O-H when cellulose and hemicellulose are combined), 1063 cm−1 (stretching vibration peak of C-O in cellulose and hemicellulose), and 897 cm−1 (bending vibration peak of C-H in cellulose). The characteristic peaks in lignin are 1514 cm−1 (vibration peak of aromatic skeleton C-C), 1320 cm−1 and 1246 cm−1 (vibrational peak of C-O in Syringa base) [30].
In addition, the change in the characteristic peak of 1424 cm−1 (bending vibration peak of C-H in lignin and carbohydrate) usually represents the destruction of the amorphous zone [31]. From the FTIR results, it was found that the characteristic peaks of cellulose, hemicellulose, and lignin in rice straw were significantly changed after pretreatment (compare a and b–e). It is worth noting that by comparing the IR spectra of rice straws pretreated by IL-HCl and US-IL-HCl (i.e., comparing b and c and d and e) along with ultrasound, the characteristic peaks (1372 cm−1, 1160 cm−1, 1105 cm−1, 1063 cm−1) of cellulose and hemicellulose broadened. The peaks indicate that ultrasound-assisted pretreatment can further destroy the structure of cellulose and hemicellulose and change the absorption intensity of the binding bond.
In addition, the change of 897 cm−1 intensity can reflect the structural change of crystalline cellulose. It can also be found that the peak becomes less sharp and the peak width increases under ultrasonic-assisted treatment, which indicates that the characteristic functional groups of crystalline cellulose are damaged and absorbed. The intensity of lignin peaks at 1514 cm−1, 1320 cm−1, and 1246 cm−1 decreased after ultrasound-assisted treatment, which is consistent with the change of lignin content shown in Table 1.
The ratios of LOI (crystallinity index or collateral index) to the absorption intensities of 1424 cm−1 and 897 cm−1 and the ratio of the absorption intensity of 1372 cm−1 to 2920 cm−1 TCI (total crystallinity index) are usually used to judge of cellulose crystallinity. A higher index is usually related to a higher crystallinity and structural index [30]. The LOI and TCI indices of rice straw before and after pretreatment were shown in Table 2. The LOI values of untreated, BMICl-HCl, US-BMIMCl-HCl, AMIMCl-HCl, AMIMCl-HCl, and US-AMIMCl-HCl treated rice straw were 1.5955, 1.8159, 1.5123, 1.8029, and 1.4167, respectively. The CrI values are consistent. Moreover, 1424 cm−1 and 897 cm−1 are characteristic peaks of cellulose in amorphous and crystalline regions, respectively. When the amorphous zone is severely damaged, the absorption intensity of 1424 cm−1 decreases (as shown in Fig. 6), so the LOI value of the IL-HCl treated rice straw was significantly higher than those of the untreated straw (from 1.5955 to 1.8159 and 1.8029, respectively). With ultrasound assistance, further destruction of crystalline cellulose resulted in a significant decrease in the absorption intensity of 897 cm−1, therefore, the LOI value was lower than that with IL-HCl treatment. The TCI value decreased from 1.2399 to 0.8639~1.0562 as the treatment intensity increased, which is consistent with the trend of cellulose conversion rate.
4 Conclusions
Based on the results obtained herein, the rice straw pretreatment with US-IL-HCl led to the release of large quantities of fermentable sugar than other pretreatment methods. The increased cellulose conversions were mainly due to promote the destruction of morphology, chemical structure, and crystallinity of rice straw by ultrasound-assisted pretreatment. The reduced CrI and LOI revealed the positive effects of ultrasound on the breakdown of the hydrogen bond network of crystalline cellulose after pretreatment and increases the enzymatic saccharification performances. Contrary to conventional methods, ultrasound can bring several advantages such as environmentally friendly (no toxic chemicals are used or produced) and low cost. Furthermore, ultrasound operates in normal atmospheric conditions and easy to be installed and operated. However, ultrasound density is an important factor and should be studied in the future. It is a critical parameter for industrial use.
References
National Development and Reform Commission, Ministry of Agriculture, Ministry of Finance, The notice on issuing the implementation plan of comprehensive utilization of crop straw in 12th Five-year. http://www.mof.gov.cn/zhengwuxinxi/zhengcefabu/201112/t20111221_617842.htm
Li XG, Wang SX, Duan L, Hao J, Li C, Chen YS, Yang L (2007) Particulate and trace gas emissions from open burning of wheat straw and corn stover in China. Environ Sci Technol 41:6052–6058
National Energy Administration, Biomass 13th Five-year plan. http://www.gov.cn/xinwen/2016-12/05/content_5143612.htm
Sun N, Rahman M, Qin Y, Maxim ML, Rodriguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11:646–655
Chang K-L, Chen X-M, Wang X-Q, Han Y-J, Potprommanee L, Liu J-y, Liao Y-L, Ning X-a, Sun S-y, Huang Q (2017) Impact of surfactant type for ionic liquid pretreatment on enhancing delignification of rice straw. Bioresour Technol 227:388–392
Chang KL, Chen XM, Han YJ, Wang XQ, Potprommanee L, Ning XA, Liu JY, Sun J, Peng YP, Sun SY, Lin YC (2016) Synergistic effects of surfactant-assisted ionic liquid pretreatment rice straw. Bioresour Technol 214:371–375
Swatloski RP, Spear SK, Hobrey JD, Rogersj RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975
Qing Q, Hu R, He YC, Zhang Y, Wang LQ (2014) Investigation of a novel acid-catalyzed ionic liquid pretreatment method to improve biomass enzymatic hydrolysis conversion. Appl Microbiol Biotechnol 98:5275–5286
Zhang ZY, O’Hara IM, Doherty WUS (2012) Pretreatment of sugarcane bagasse by acid catalysed process in aqueous ionic liquid solutions. Bioresour Technol 120:149–156
Wang G, Zhang SP, Xu WJ, Qi W, Yan YJ, Xu QL (2015) Efficient saccharification by pretreatment of bagasse pith with ionic liquid and acid solutions simultaneously. Energy Convers Manag 89:120–1260
Pang ZQ, Dong CH, Pan XJ (2016) Enhanced deconstruction and dissolution of lignocellulosic biomass in ionic liquid at high water content by lithium chloride. Cellulose. 23:323–338
Luo J, Fang Z, Smith RL (2014) Ultrasound-enhanced conversion of biomass to biofuels. Prog Energy Combust Sci 41:56–93
Yang CY, Fang TJ (2015) Kinetics for enzymatic hydrolysis of rice hulls by the ultrasonic pretreatment with a bio-based basic ionic liquid. Biochem Eng J 100:23–29
Ninomiya K, Kohori A, Tatsumi M, Osawa K, Endo T, Kakuchi R, Ogino C, Shimizu N, Takahashi K (2015) Ionic liquid/ultrasound pretreatment and in situ enzymatic saccharification of bagasse using biocompatible cholinium ionic liquid. Bioresour Technol 176:169–174
Karimi M, Jenkins B, Stroeve P (2014) Ultrasound irradiation in the production of ethanol from biomass. Renew Sust Energ Rev 40:400–421
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Chang KL, Han YJ, Wang XQ, Chen XM, Qiu XS, Leu SY, Liu JY, Peng YP, Liao YL, Potprommanee L (2017) The effect of surfactant-assisted ultrasound-ionic liquid pretreatment on the structure and fermentable sugar production of a water hyacinth. Bioresour Technol 237:27–30
Shi SL. Pulp to analysis and detection. Beijing: China Light Industry Press; 2006. 1–380 p. (in Chinese)
Xu F, Chen L, Wang A, Yan Z (2016) Influence of surfactant-free ionic liquid microemulsions pretreatment on the composition, structure and enzymatic hydrolysis of the water hyacinth. Bioresour Technol 208:19–23
Segal L, Creely JJ, Martin JA, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Research J 29:786–794
Badgujar KC, Bhanage BM (2015) Factors governing dissolution process of lignocellulosic biomass in ionic liquid: current status, overview and challenges. Bioresour Technol 178:2–18
Zhao H, Jones CL, Baker GA, Xia S, Olubajo O, Person VN (2009) Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J Biotechnol 139:47–54
Li C, Wang Q, Zhao ZK (2008) Acid in ionic liquid: an efficient system for hydrolysis of lignocellulose. Green Chem 10:177–182
Zhou N, Zhang YM, Gong XW, Wang Q, Ma Y (2012) Ionic liquids-based hydrolysis of Chlorella biomass fermentable sugars. Bioresour Technol 118:512–517
Zhang Q, Zhao M, Xu QQ, Ren HG, Yin JZ (2019) Enhanced enzymatic hydrolysis of sorghum stalk by supercritical carbon dioxide and ultrasonic pretreatment. Appl Biochem Biotechnol 188:101–111
Dong SJ, Zhang BX, Gao YF, Hu XM (2015) An Efficient process for pretreatment of lignocelluloses in functional ionic liquids. Int J Polym Sci 2015:978983 6 pages
Montalbo-Lomboy M, Grewell D (2015) Rapid dissolution of switchgrass in 1-butyl-3-methylimidazolium chloride by ultrasonication. Ultrason Sonochem 22:588–599
Sorn V, Chang KL, Phitsuwan P, Ratanakhanokchai K, Dong CD (2019) Effect of microwave-assisted ionic liquid/acidic ionic liquid pretreatment on the morphology, structure, and enhanced delignification of rice straw. Bioresour Technol 293:121929
Li L, Yang DR, Liu DT, Yang F (2014) Influence of combined pretreatment of quadrol and anhydrous ionic liquid microemulsion on the physicochemical property of masson pine. J Appl Polym Sci 131:1–9
Ninomiya K, Kamide K, Takahashi K, Shimizu N (2012) Enhanced enzymatic saccharification of kenaf power after ultrasonic pretreatment in ionic liquid at room temperature. Bioresour Technol 103:259–265
Li LY, Niu K, Liu CG, Bai F (2013) Effect of ionic liquid pretreatment on lignocellulosic biomass from oilseeds. CIESC J 64:104–110
Funding
This research was funded by the Ministry of Science and Technology of Taiwan (106-2218-E-110-009-MY2 and 108-2221-E-110-051), the National Natural Science Foundation of China (No. 51608129, 51978175) and TEEP@Asia Plus 2018-2019, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Liu, J., Wang, Y. et al. Effect of ultrasound on ionic liquid-hydrochloric acid pretreatment with rice straw. Biomass Conv. Bioref. 11, 1749–1757 (2021). https://doi.org/10.1007/s13399-019-00595-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00595-y