Abstract
Spatial analysis and stochastic risk analysis methodologies are proposed for the characterisation of major cations (Mg, Ca, K, Na and Zn) in deep sediments and surface waters of the Keban Reservoir, Turkey. Curves denoted as “iso-cation curves” were drawn using the spatial analysis of the major cations. Spatial diagrams and risk assessment allowed significant conclusions about the nature of the variations in cation concentration in the reservoir. Major cations are measured at 20 different locations within the reservoir, and their comparisons are presented through both spatial distribution and risk analysis models for each ion. Their collective behaviour was also analysed by comparing spatial diagrams of surface water and deep sediment concentrations. Finally, the iso-cation curves and achievement of the occurrence and/or non-occurrence cation probabilities in any research area can be generalised to other the environment sciences. This calculation can be extended easily to cover all pollutants at micro- and macroscales.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concept of geochemical cycling, or the movement of elements through various parts of the earth, including the atmosphere, is essential to a proper understanding of the overall role of these elements, especially their environmental significance (Swaine 2000).
Concentrations of major cations in the environment are important to the survival of living creatures. On Earth, Mg is the eighth most abundant element and constitutes 2 % of the Earth’s crust. Due to its high reactivity, Mg is not found in nature as a metal; instead, it is found in the form of various salts or ionised in water. Mg is needed both in various biological systems and in industrial processes and metallurgy. A higher content of Mg is characteristic of basic and ultra-basic magmatic and metamorphic rocks (e.g. basalt) and their weathering products (Merian et al. 2004a). Mg-rich rock-forming minerals include biotitic and other dark-coloured silicate minerals, as well as serpentine. Ca is enriched in limestone and dolomite in the form of CaCO3 and in lime-free basic silicate rocks, such as basalt. Soils formed in dry regions generally contain larger amounts of calcium than soils in humid regions (Merian et al. 2004a). K makes up 2.6 % of the Earth’s crust by weight. This element is enriched in acid magmatic rocks, such as granite, and in clay minerals. In uplifted marine sediments, the Na content may be high, and in igneous rocks, the ratio of K by weight is 0.92. Magmatic rocks contain, on average, 28 g Na kg−1, whereas sedimentary rocks accumulate lower Na concentrations (Merian et al. 2004b). Zn in soil is easily absorbed by mineral and organic substances; it accumulates primarily in the soil surface layer and is fairly uniformly distributed between the following fractions: dissolved in soil water, exchangeable bound to organic ligands, occluded in secondary clay minerals and metal oxides/hydroxides and present in primary minerals (Viets 1962).
Although various chemical elements are found in rocks, their solutions are also abundant in running water. They may therefore accumulate in deep sediments in reservoirs, though the surface water mineral content may be rather different. The difference between the deep sediment and surface layer mineral content provides extra information about the existence of such elements and their distribution in the sediment under lakes.
In this study, the risk levels and distributions of major cations in a lake are estimated using stochastic risk analysis and spatial methodologies (Kim et al. 2016; Xue et al. 2015; Mor et al. 2006). Keban Reservoir is chosen as a pilot area for the proposed method due to its geological and geographical diversity, and applications of the methodologies are carried out on its reservoir lake. A risk assessment formulation is presented for the concentrations obtained from cation field measurements. Risk levels for different cations are presented through Weibull, lognormal and exponential models, and the theoretical probability distribution function (pdf) and cumulative distribution function (cdf) of each cation measurement location are obtained. In addition, the spatial and transport characterisations of the cations are established using two-dimensional iso-curves created using the Kriging methodology (You et al. 2016; Külahcı and Şen 2009; Matheron 1963).
Experimental
Study area and water sampling
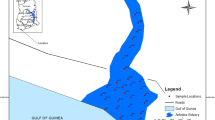
The Keban Dam Lake is the second largest freshwater body in Turkey. The study area (Uluova Region of Keban Reservoir) is approximately 150 km2. It is situated at latitude 38°5′N and longitude 38°4′E, with an elevation of 1134 m above mean sea level. Surface water and deep sediment samples were taken at 20 stations in April and May 2011 in the geographic locations shown in Fig. 1. In particular, residential areas on the southern side of the lake contribute to the lake and the stations are arranged as in Fig. 1.
According to the observation values between 1937 and 1987, the average annual atmospheric temperature is 13.4 °C. Monthly average temperatures vary between −0.1 and 27.1 °C. The seasonal temperature of the lake changes between 2 and 35 °C.
The bottom of the lake is covered with clay, and the annual amount is estimated to be approximately 40,000 tonnes of silt and clay. The total hardness (TH), electrical conductivity (EC) and pH values of water are also calculated. The experimental procedures are performed three times for each station, and the standard deviations are calculated for about 10 %. Accordingly, TH, EC and pH values for all stations are measured as 191.73 mgCaCO3/L, 343.91 μmho/cm and 8, respectively. pH measurements are performed with Orion (230) digital pH meter, and the electrical conductivities are measured with Jenway (4070) digital conductivity meter. The total hardness determinations are carried out using the titration system by titrimetric methods.
Surface water samples were taken at 25 cm depth and stored in 2-L polyethylene bottles for subsequent preparation and analysis. The bottles were rinsed at least three times with double-distilled water and 1:1 HNO3/H2O prior to sample collection (Özmen et al. 2004). Water samples were then passed through Whatman glass microfibre filters (GF/C) and acidified with (0.2 v/v) ultra-pure nitric acid (E. Merk, Darmstadt, Germany) to pH < 2 to minimise the absorption of metals onto the walls of the containers. Samples were stored at approximately 4 °C. Twenty sediment samples were collected using a stainless steel dredge. Sampling depth varied between 30 and 35 m. The sediment samples were placed in polyethylene bags and stored at 4 °C during their transport to the laboratory. They were then dried in an oven at 50 °C for 48 h. Approximately 200 mg of each sample was digested with HNO3, HF and H2O2 in a Teflon bomb. Among the major elements measured in the water and sediment samples were Zn (Shimadzu AA-660 Model AAS), Ca, Mg, Na and K (Eppendorf Model AES). The concentrations of each element in both water and sediment samples are shown in Table 1. The measurements are performed three times with the standard deviation of about 10 % according to the reference materials proposed by NIST (Clesceri et al. 1989).
Theoretical methodology
Risk analysis
The simple risk, R, can be defined as the probability that the concentration of a major cation, X, will exceed a chosen major cation threshold, Q, at least once during a certain time duration, T, or over an area, A. In this study, the area will be defined by the number of sampling points, n, for a given major cation. If the sequence of future likely occurrences of X is X 1, X 2,…, X n , then the joint probability of non-occurrence, N, is defined according to Şen (1976, 1991, 1999) as:
Hence, the simple risk, R, as a complementary event, is defined as:
In the risk assessment of any design project, it is necessary to first establish the frequency of design major cation occurrence, i.e. the return period, T, after which it is possible to determine the magnitude of the major cation on the basis of the most suitable pdf. The return period is defined as the average length of time over which Q will be exceeded once. The random variable T r, which specifies the time or distance between any two successive exceedances of the selected major cation threshold, is referred to as the waiting time. Its distribution, in the case of independent discrete observations at intervals of 1.Δt, is given by Feller (1967) as
or
where j is the discrete duration of non-exceedance. Hence, the return period, which is the expected value of the waiting time, is found by
where p = P(x > Q), i.e. the probability of exceedance of the selected major cation threshold value. The return period cannot be less than one. The theoretical distribution of the return period is given by Eq. (3), the solution of which is presented in Table 2, which shows T r as a function of \( 1 + \ln \,[P(T_{\text{r}} \ge j)]/\ln \,(1 - 1/T) \).
Table 2 shows that over a long period or distance, 25 % of the intervals between instances of major cation concentrations exceeding 100 are less than approximately 30 time (distance) units, while an equal number are expected to be in excess of approximately 139 time (distance) units. In other words, to ensure 75 % safety that the desired threshold level will not be exceeded by a major cation within the next 30 time or distance units, the system must be designed for non-exceedance over 100 time (or distance) units.
The risk of overtopping a given major cation threshold can be obtained in terms of the return period using Eq. (2), which leads to
Conversely, the risk can also be computed in terms of the rank and number of samples using Eq. (7)
where m is the rank and n is the number of samples.
Probability and cumulative distribution functions for distribution characterisations of major cations
The major cations (Mg, Ca, K, Na and Zn) in the Keban Dam Lake are described here by three different pdfs and cdfs: the lognormal, Weibull and exponential distribution functions. The distribution functions were computed using Eqs. 8–13.
Lognormal distribution
The lognormal pdf for x > 0 is given by Eq. 8. In a lognormal distribution, the parameters denoted μ and σ are, respectively, the mean (for a given location) and the standard deviation (scale).
The cdf for the lognormal distribution is given by Eq. 9
Weibull distribution
For the x ≥ 0 Weibull pdf, the α shape parameter and the β scale parameter are given by Eq. 10
The Weibull cdf is also given by Eq. 11
Exponential distribution
The pdf of an exponential distribution is
The cdf is given by
Results and discussion
Summary statistics for each cation are presented in Table 3. There are significant differences between the surface water and deep sediment cation concentrations. The following conclusions can be deduced from this table.
-
1.
Comparison of the median and mean values indicates the skewness of the available data. If the median and mean values of any element are equal within ±5 % limits, then this element has a symmetric pdf; otherwise, the pdf is asymmetric. On this basis, although the surface water data for Mg++, Ca++, K+ and Na+ have symmetric distributions similar to the normal pdf, the deep sediment data have asymmetric pdfs. This discrepancy represents one of the most significant differences between the surface and deep sediment results.
-
2.
The skewness coefficient of Zn in surface waters is greater than 1, which implies that it does not abide with a normal distribution. On the other hand, Zn in bottom sediments has positive range as shown in Table 3. Accordingly, Zn distributions in the bottom sediments conform to the normal distribution.
-
3.
The deep sediment cation concentrations have comparatively greater values than the corresponding surface water concentrations, indicating that the cations studied here accumulate in the sediment in this reservoir.
-
4.
The standard deviation estimates the average deviation around the arithmetic mean value; in other words, it is a measure of the spread of the probability distribution. If the standard deviation is large, the distribution of the data points is widespread, and if the standard deviation is small, then the risk associated with this element is also small. A large standard deviation indicates that the concentration values are unstable. In general, a large standard deviation is not required in the hydro-chemical analysis of major cations because in such a case, hazardous materials are more difficult to control and may display many extreme values.
-
5.
For surface water, the sequence of the concentration values of the cations surveyed, in decreasing order, is Mg > Ca > Na > Zn > K. The Mg and Ca concentrations in surface water are greater than those of the other cations because the lake is rich in carbonate compounds. The order of the compounds in deep sediments is Ca > K > Mg > Zn > Na. Ca has the greatest concentration in the deep sediments, and, as mentioned in the introduction, it is the fifth most abundant element by mass in the Earth’s crust. This element is enriched in limestone and dolomite in the form of CaCO3 and in lime-free basic silicate rocks such as basalt (Merian et al. 2004a). The research area is rich in CaCO3. Ca is also more abundant in clay than other basic ions (Merian et al. 2004b). Na is not bound to clay minerals, as it has low bonding energy and so can be found both in the soil solution and in water. Na concentration is higher in saline soils and waters. There is fresh water in the research area; especially Na in the bottom sediments is ranked as the last in terms of the variables of interest. Distribution of the Zn is very irregular. It is therefore difficult to make meaningful interpretations about Zn. In this study, the main element affecting the distribution of Zn is clay in bottom sediments. Zn is easily absorbed by clay minerals (Viets 1962). On the other hand, K is also enriched in the clay minerals. K in the bottom sediments has a high concentration according to other elements. However, it has low concentration in the surface waters. The amount of K is transported from deep sediments towards the surface water, and one can say that it is of relatively small rates. Transport of the Mg in the water is comparatively more than other elements.
-
6.
The coefficient of variation (CV) is the standard deviation divided by the mean, and it is a dimensionless measure of variation. This index allows comparison between various compounds even when their original units are different. A higher CV indicates greater dispersion of the variable, and a small CV implies smaller residuals attached to the prediction values. The CV plays a similar role as other measures of spread in experimental data, including the standard deviation or root mean squared residuals (SAS 2007). Of the various cations listed in the table, the CV of the surface water concentration of Zn is the highest, though Zn has the lowest mean concentration. This result implies that the Zn concentration has the most irregular variability of any of the cations measured in the surface water, and its variability is even greater than that of any element in the deep sediment. In the surface water, the cations in the order of decreasing CV are Zn > Ca > Na > Mg > K. According to these findings, the measurements of Zn in surface waters have a wider range than those of the other cations. Depending on the proximity of the sampling location to various sources, the main source of Zn in the surface water may be a smelter, a power station discharge, fertiliser production, vegetation or motor tyre wear (Swaine 2000). The Zn reserves in the basin are designated by the Turkey General Directorate of Mineral Research and Exploration (MTA 2011).
-
7.
In deep sediments, the cations in the order of decreasing CV are Mg > K > Ca > Zn > Na. The basin of the Keban Dam contains magmatic and metamorphic rocks (e.g. basalt), both of which have a high Mg content. Mg-laden mineral, rock or soil particles may be transported into the lake floor by soil and wind erosion, increasing the Mg CV in deep sediments. Na has the smallest CV in the deep sediments. Similarly, Mg in deep sediments has a wider quantity range in comparison to other cations.
-
8.
Kurtosis is a measure of the sharpness of the pdf peak. Any symmetric pdf, such as the normal (Gaussian) pdf, has a kurtosis value equal to zero. An increase in the kurtosis value implies a sharper pdf (Golden Software 2009). In surface water, Ca and Zn have positive coefficients of kurtosis (CK), as do Mg and Na in deep sediment. Mg, K and Na in surface water and Ca, K and Zn in deep sediments have negative CK values with a flat pdf, i.e. small kurtosis values.
The Ca and Zn in the surface water and Ca, K and Na in the bottom sediments are outside of the range, (−1, +1), and therefore, the concentration values of these elements do not fit the normal distribution. This result indicates to their non-homogeneous distributions in the lake.
-
9.
The coefficient of skewness (CS) is a measure of asymmetry in the pdf. A positive skew indicates a longer tail to the right, while a negative skew indicates a longer tail to the left. A perfectly symmetric distribution, such as the normal distribution, has a skew equal to zero (Golden Software 2009). Among the surface water cation concentrations, that of K has a CS closest to zero. This finding implies that K is homogeneously distributed in surface waters. Ca, K, Na and Zn have positive CS, indicating that their concentration values tend to be higher than the arithmetic averages. However, Mg has a negative CS, and its concentration values tend to be less than the average. Among the concentrations measured in the deep sediments, Mg, K and Zn have positive CS, whereas Ca and Na have negative values. The most symmetric pdf is that of Zn; its CS is close to 0. This result implies that Zn concentrations within the lake and in the deep sediments are in equilibrium.
Table 4 shows the correlation coefficient matrix between the surface water sediments and deep sediments.
There is a weak correlation between surface water sediments and deep sediments. The one-way ANOVA test for these elements is shown in Table 5. The critical F value for the α = 0.05 rejection region, denoted as F crit in Table 5, corresponds to F .05 = 1.92. Thus, one can reject the null hypothesis that the ten means are equal if F > F .05 = 1.92 because the computed value of the test statistic, F = 38.09, is greater than the critical value.
The ANOVA analysis indicates that the means of the surface water concentrations are statistically different from those of the deep sediments. This finding is consistent with the correlation coefficient analysis shown in Table 4. That is, statistical analysis based on the given data indicates that there is no statistically significant correlation between the surface water concentrations and those in the deep sediments.
Spatial variation features: iso-cation curves
It is possible to represent the spatial distribution of each element separately using the Kriging methodology, originally suggested and developed by Krige (1951) and Matheron (1963), which is based on the concept of semivariogram and subsequent regionalisation of a given variable. This methodology maps regional features within the variation domain, which is the dam reservoir sample area in this study. The curve maps are prepared for deviations from the areal averages of each element, and one is thus able to identify deviations from the areal average concentration in addition to zonal patterns. Spatial distributions of the deviations are shown in Figs. 2, 3, 4, 5 and 6. In the following discussion, regions with greater or less than the zone value will be described as high or low regions, respectively.
The spatial analysis of Mg concentrations in surface waters reveals high zones at sample points 5, 9 and 10 and low zone at sample points 17 and 19, respectively (Fig. 2a). The high and low zones are adjacent. The existence of two zones of unusually high or low concentration is expected because water enters the reservoir from the centre, as in Fig. 1. Consequently, the flow is separated into two directions, east and west, and therefore, two vorticities form, causing regions of intensification (high zones) and dilution (low zones) in the Mg concentration. In the surface water domain, the high-concentration zone is on the west because the water outlet is in this direction. A high-concentration zone exists here due to the accumulation of stagnant water near the coastal area without any outlet.
Results for the deep sediment are shown in Fig. 2b. Due to the topography of the reservoir, Mg accumulates on the west coastal area, and there is also a minor high-concentration zone at the coastal area opposite to the entrance, which is due to low entrance velocity. In general, there is a low zone stretching from the south-west towards the outlet, which may imply an outlet on the east and groundwater recharge in the south-west low zonal area (Keban Dam Lake Limnological Study Report 1982).
In the surface water, the Ca concentration shows a dominant high zone around sample number 10, with a minor one at location 7 (Fig. 3a). This finding is attributable to the surface water entrance velocity, coupled with a current towards the outlet. There is a generally increasing trend in Ca concentrations in the surface water from the west towards the outlet in the east. The Ca deep sediment concentrations, shown in Fig. 3b, also display spatial variation from the areal mean, with the same high-concentration zone observed in the surface water also appearing in the deep sediment. Additional Ca accumulation is also apparent in the north-western coastal area. The locations with low Ca concentrations might result from groundwater leakage from the bottom.
K concentrations in the surface waters are distributed more uniformly than either Ca or Mg (Fig. 4a); the data for K show relatively small concentrations throughout the reservoir volume. There is a sequence of high-K zones near the middle of the reservoir area, oriented from west to east, with the highest concentrations in the centre of the reservoir area. As with Ca and Mg, a low zone for K is observed at the south-west location; it may be due to groundwater seepage, minor surface water inflow or both.
On the other hand, deep sediments have a different K accumulation pattern than the surface water. A very large low-concentration zone occurs near the south-western coastal area, suggesting steady groundwater flow or minor runoff contributions to this area (Fig. 4b).
The spatial variability of Na is shown in Fig. 5a for surface water concentrations; a belt of high concentration occurs on the east region of the lake and extends south-east. The rest of the reservoir shows low-Na concentrations in the surface water. The deep sediment data vary greatly from the surface water values, showing very high accumulation concentrations with three low zones around sample locations 4, 8 and 14 (Fig. 5b).
The surface water Zn concentration data, shown in Fig. 6a, have an entirely different spatial pattern from all of the previous surface water maps. Its highest concentration zone lies near the south-western coastal area, opposite from the high-concentration zones for all of the previous elements. This result suggests that groundwater seepage or inland surface inflow in this area does not convey Zn. Zn seems to enter the lake only from the main entrance water and, as expected based on flow velocity direction and dispersion, there are two high zones, with the second occurring at the western coastal area south of the outlet point.
The deep sediment map in Fig. 6b shows high-concentration zones at the coastal areas opposite from the inlet point and their sequence covers sample locations starting from 2 and continuing towards the east, including sites 3–10. Almost all of the sites near the inlet and outlet points show low concentrations of Zn in the deep sediment layer.
Risk assessments
The surface water and the deep sediment concentrations of the cations shown in Table 1 are sorted from smallest to largest and ranked. The risk (R) and return period (T) are then computed using Eqs. 6 and 7, and the risk graphs for the data are shown in Fig. 7a, b. A MATLAB® program was written to find the most appropriate pdfs for the sample concentrations in Table 1. The lognormal, Weibull and exponential pdfs were calculated, and the α, β, μ, σ and λ parameters were obtained as shown in Table 6a, b. Equations 8–13 were used to calculate these parameters.
a Fitted probability distribution functions for Mg, Ca, K, Na and Zn surface water cation measurements. The most appropriate pdfs for each cation were drawn using a MATLAB program written by the authors. b Fitted probability distribution functions for Mg, Ca, K, Na and Zn deep sediment cation measurements
Figure 7a, b indicate the best-fit pdfs for each of the cations. Mg, K, Na and Zn concentrations in the surface water of the lake fitted best to the Weibull pdf, and only Ca concentrations in the surface water fitted best to the lognormal pdf (Table 6a). Mg and Ca concentrations in the deep sediments of the lake fitted best to the exponential pdf, and K, Na, and Zn concentrations in the deep sediments fitted best to the lognormal pdf (Table 6b).
The change in β’s size is one of the most important aspects of the effect of β on the Weibull distribution. Weibull distributions with β > 1 have a failure rate that increases with time, also known as wear-out failure (Fig. 7; Külahcı 2011). The lognormal distribution is closely related to the normal distribution (Evans et al. 2000). Hence, the Ca concentrations in the surface water and the K, Na, and Zn concentrations in the deep sediments were dispersed more regularly than the other elements measured in the lake (Fig. 7a, b). The risk resulting from Mg and Ca concentrations in the deep sediments decreased exponentially (Fig. 7b).
The theoretical pdfs of the cations were determined using Eqs. 8, 10 and 12 and Table 6a, b. Figure 7a, b imply that high Mg, Ca, K, Na and Zn values occur rarely, whereas small values are abundant. The cumulative pdfs were obtained using Eqs. 9, 11 and 13, yielding Figs. 8a and 9a. These figures show the change in the probability of occurrence of concentrations less than any given threshold value on the horizontal axis. By definition, the probability of non-occurrence is a complementary value to the probability of occurrence, as apparent in Figs. 8b and 9b.
Conclusions
The distributional behaviours and the probabilities of occurrence and non-occurrence of major cations are very difficult to predict in a research area intended. Therefore, by taking advantage of spatial methodology in this study, iso-cation curves are suggested, which represent visually the distribution behaviours of the cations. In addition, the “return period” concept from the meteorology, climate and hydrology disciplines is adapted for the distribution behaviours of the cations and finally, the probabilities of presence or absence of the cations are calculated in an area. These calculations are very important to understand whether the cation concentrations cross a certain threshold level or not.
Iso-cation curves provide great benefits to understand how the distribution of cations comes into existence. Hence, cation transportation can be realised completely in the field of research. Such a situation is very significant for researchers in the fields of all environmental scientists and hydrologists as well as researchers in related fields. Likewise, estimation of the probability distribution of cations is of paramount importance for the prevention of environmental pollution.
The statistical parameters of the various elements are explained, and the patterns resulting from the spatial distributions of the elements are interpreted. The Kriging methodology of regionalisation is used to depict the spatial variation in cation concentration through a sequence of iso-cation maps, and stochastic risk analysis methodology applications to the data. Distribution models, specifically pdfs and cdfs, are used to assess the risk of any major cation concentration at a given location exceeding a threshold level.
The Kriging method is recommended in order to see the spatial distribution of cations, because this method provides significant contributions for interpretations.
Invariably and expectedly, the deep sediments have far greater cation concentrations than the surface waters, which are in a constant dynamic state due to water inflow and outflow. The high sediment concentrations are due to accumulation processes in the sediments. The cation concentrations in the bottom sediments tend to accumulate. One of the biggest causes of this phenomenon in the bottom of the lake is the coverages of clay, and the clay layers capture the cations.
The surface water element concentrations appear to result from dispersion and dilution processes, whereas the sediment concentrations of each element depend on the bottom topography, groundwater seepage or other minor land surface inflows into the reservoir.
Mg, K, Na and Zn cations in the surface water of the lake are fitted to the Weibull pdf, suggesting that the distribution of these cations in the lake has a randomness that increases with time. Ca concentrations in the surface water fit best to the lognormal pdf, as K, Na and Zn concentrations in the lake deep sediments. This result means that these cations have relatively regular distributions in the lake. Mg and Ca concentrations in the deep sediments decreased exponentially with time, suggesting faster transport of Mg and Ca than other cations.
Mg and particularly Ca in both surface water and sediments in the bottom of some stations have high concentrations. These regions are rich in terms of limestone and dolomite.
According to the statistical calculations, Zn in surface waters and Na in bottom sediments are scattered over a wide area. The coefficient of variation (CV) to address this situation is a useful statistical tool.
Finally, as a result of this work, iso-cation curves and achievement of the probabilities of occurrence and/or non-occurrence of cations in any research area can be generalised to all of the environment sciences. This calculation can be done not only for cations but also easily for all pollutants at micro- and macroscales.
References
Clesceri LS, Greenberg AE, Trussell RR (1989) Standart methods for the examination of water and wastewater. American Public Health Association, Washington, USA, Part 7000-7500
Evans M, Hastings N, Peacock B (2000) Statistical distributions, 3rd edn. Wiley, New York
Feller W (1967) An introduction to probability theory and its application, vol 1. Wiley, New York, p 509
Golden Software (2009) Grapher version 8.0.278. Golden, Colorado
Keban Dam Lake Limnological Study Report (1982) Ministry of Energy and Natural Resources, Operations and Maintenance Department, Ankara
Kim H-S, Chung C-K, Kim H-K (2016) Geo-spatial data integration for subsurface stratification of dam site with outlier analyses. Environ Earth Sci 75:168
Krige DG (1951) A statistical approach to some basic mine valuation problems on the Witwatersrand. J Chem Metall Min Soc S Afr 52:119–139
Külahcı F (2011) A risk analysis model for radioactive wastes. J Hazard Mater 191:349–355
Külahcı F, Şen Z (2009) Spatio-temporal modeling of 210Pb transportation in lake environments. J Hazard Mater 65:525–532
Matheron G (1963) Principles of geostatistics. Econ Geol 58:1246–1266
Merian E, Anke M, Ihnat M, Stoeppler M (2004a) Elements and their compounds in the environment, vol 1. Wiley, Weinheim
Merian E, Anke M, Ihnat M, Stoeppler M (2004b) Elements and their compounds in the environment, vol 2. Wiley, Weinheim
Mor S, Ravindra K, Dahiya RP et al (2006) Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Monit Assess 118:435–456
Özmen H, Külahcı F, Çukurovalı A, Doğru M (2004) Concentrations of heavy metal and radioactivity in surface water and sediment of Hazar Lake (Elazığ, Turkey). Chemosphere 55:401–408
SAS (2007) UCLA: Academic Technology Services, Statistical Consulting Group. http://www.ats.ucla.edu/stat/sas/notes2/
Şen Z (1976) Wet and dry periods of annual flow series. ASCE J Hydraul Div 102:1503–1514
Şen Z (1991) Probabilistic modelling of crossing in small samples and application of runs to hydrology. J Hydrol 124:345–362
Şen Z (1999) Simple risk calculations in dependent hydrological series. J Hydrol Sci 44:871–878
Swaine DJ (2000) Why trace elements are important. Fuel Process Technol 65:21–33
Viets FG (1962) Chemistry and availability of micronutrients in soil. J Agric Food Chem 10:174–178
Xue Y, Meng X, Wasowsk J et al (2015) Spatial analysis of surface deformation distribution detected by persistent scatterer interferometry in Lanzhou Region, China. Environ Earth Sci 75:80
You M, Huang Y, Lu J, Li C (2016) Fractionation characterizations and environmental implications of heavy metal in soil from coal mine in Huainan, China. Environ Earth Sci 75:78
Acknowledgments
This study is part of a research project supported by The Scientific and Technical Research Council of Turkey (TÜBİTAK) and The Council of Higher Education (YÖK), Turkey. The author would like to thank TÜBİTAK and YÖK for financial support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Külahcı, F. Proposals for risk assessment of major cations in surface water and deep sediment: iso-cation curves, probabilities of occurrence and non-occurrence of cations. Environ Earth Sci 75, 980 (2016). https://doi.org/10.1007/s12665-016-5788-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5788-x