Abstract
Pollution of water bodies and sediments/soils by trace elements remains a global threat and a serious environmental hazard to biodiversity and human’s health. Globalization and industrialization resulted in the increase and availability of these substances in the environment posing unpredictable adverse effects to living organisms. To determine pollution status and risk contamination by trace elements, data available in the literature of the last 40 years on trace elements occurrence in three environmental matrices (water bodies, sediments/soils, and biota) from Continental Portugal were collected (about 90 studies). Data were compared to water and sediment quality guidelines to assess potential ecological risks. Most environmentally relevant hazardous elements include Zn, Cu, Cd, Pb, and As. Various studies found trace elements at levels higher than those considered safe by environmental guidelines. In surface waters, Al, Zn, Se, and Ag were found above aquatic life limits in about 60% of the reviewed papers, while Cu, Zn, and As exceed those values in more than 60% of mining waters. Hg and Cd in sediments from mining areas exceeded aquatic life limits and potential ecological risk showed extremely high risk for most of the elements. The data compiled in this review is very heterogenous, varying in terms of sampling schemes, trace elements analysed, and spatiotemporal settings. This heterogenicity leads to data differences that make meaningful comparisons difficult. Nevertheless, the compilation of scattered environmental spatial and temporal trace elements data, of either natural sources or human activity as well as the ultimate effect on biological systems, is of the upmost importance to broaden its knowledge, risk assessment, and implementation of mitigation measures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution is among the main challenges of today’s society and is a major source of living organism’s health problems (Guerranti et al. 2019, Mandal and Kaur, 2019, Samoli et al. 2019, Taati et al. 2020, Wang and Zhou, 2021). All environmental compartments are affected by environmental pollutants though aquatic environments are perhaps the most impacted ecosystems as they are frequently the ultimate reservoir for many contaminants (Acosta-Coley et al. 2019, Ferreira da Silva et al. 2020, Liu et al. 2020). Among environmental contaminants, trace elements gained special visibility due to excessive concentrations, non/low-degradability, persistence, bio/ accumulative nature, and harmful effects on living organisms (Acosta-Coley et al. 2019, Ferreira da Silva et al. 2020, Fuentes et al. 2020, Li et al. 2018, Liu et al. 2020, Taati et al. 2020). Trace elements include naturally occurring elements present throughout the earth’s crust and those originated from anthropogenic activities such as the industry (e.g., refineries, coal burning, petroleum combustion, nuclear power stations among others), agriculture, pharmaceutical, domestic effluents, and atmospheric sources and transport systems (e.g., wind, rain) (Fig. 1) (Fuentes et al. 2020, Liu et al. 2020, Mandal and Kaur, 2019). Most living organisms’ exposure results from human activities that lead to undesirable environmental contamination. However, exposure can also be a result of natural processes such as metal corrosion, atmospheric deposition, soil erosion of metal ions and leaching of heavy metals, sediment re-suspension, and metal evaporation from water resources to soil and ground water (Fig. 1) (Moiseenko et al. 2019, Zhang et al. 2014).
The presence of trace elements in the aquatic ecosystems is widely reported and both abiotic and biotic processes affect their distribution and circulation (Chon et al. 2012, Moiseenko et al. 2019, Zhang et al. 2014). Sediments play an important role in trace elements cycling (Chon et al. 2012, Martins et al. 2013). In fact, sediments can behave as both reservoir and non-point source of trace elements in the water column contributing to their persistence and recalcitrance in the aquatic environment (Chon et al. 2012, Ribeiro et al. 2018). Exposed organisms may absorb dissolved elements from surrounding water and food, and/or accumulate in various tissues in significant amounts causing harmful effects or entering the food web (Fuentes et al. 2020, Li et al. 2018). Trace element bioavailability is influenced by physical and chemical factors (e.g., temperature, phase association, adsorption, speciation at thermodynamic equilibrium, complexation kinetics, and solubility), climatic factors, and biogeochemical cycling. Therefore, an accurate ecological status of the aquatic environment should evaluate the occurrence and distribution of trace elements in different compartments such as water, sediments, and biota to have integrative data on the system.
Portugal is located along the Atlantic coast in southwestern Europe. It has more than 10 million inhabitants, and most of the Portuguese population is fixed in the coastal areas, where most industrial, agricultural, and port activities are implemented. As a result, estuaries, rivers, and coastal areas are impacted by intensive anthropogenic activities that result in the release of diverse pollutants including trace elements in these compartments. Several works stressed the ecological status regarding the occurrence of trace elements in coastal waters and estuaries, mainly Douro, Mondego, Tagus, and Sado (Alves et al. 2009, Antunes et al. 2018a, Couto et al. 2019, França et al. 2005, Ribeiro et al. 2018). Besides, mining is a relevant economic activity in Portugal; however, mining activities are responsible for trace elements pollution of the surrounding environment. In fact, several works reported the occurrence of high levels of trace elements nearby mining areas in compartments such as water, sediments, and soils and even biota (Carvalho et al. 2011, Favas et al. 2016, Ferreira da Silva et al. 2009). Therefore, trace element contamination emerged as an issue of major concern due to their high levels and consequently threat to biodiversity and humans. This review intends to (a) summarize the occurrence of trace elements in water, sediments, and aquatic biota in Portugal, (b) current gaps, (c) to assess potential risk by identifying the contaminants that represent a high concern due to their concentration and/or toxicological effects, and (d) propose solutions for environmental risk management. This manuscript provides the current state of the art regarding trace elements pollution status in Portugal. Compliance with these data is important not only for researchers, local regulatory authorities, and environmental protection measures (regional, national and global) but also contributes to the global worldwide knowledge about trace elements crucial to strengthen international cooperation, to assure a more sustainable global development and support scientific policymaking.
Data collection and characterization of the study area
This search was based on ScienceDirect and ISI web of Knowledge databases considering articles published between the 1980s and 2021 that comprise waters, sediments/soil, and biota.
The area focused on this review is mainland Portugal (geographic coordinates 42°9′8" and 36°57′39"N and 6°11′10" and 9"29′45"W) corresponding to less than 1/6 of the Iberian Peninsula.
Mainland Portugal has a coast of nearly 832-km, mostly low, level, and straight, apart from several harbors located in river indentations. The northern coast is characterized by rocky bays or rias, and the southern one is rich in lagoons and sandbanks. The Portuguese Instituto Nacional de Estatística (Europa, 1985) reports 92 000 ha of rivers, streams, estuaries and bays in the territory. The Portuguese average annual runoff from rainfall is 20 000 million m3. More 17 000 million m3 are obtained from rivers whose springs are located in Spanish territory, causing a total annual river discharge of 37 000 million m3 leaving the country. The entire drainage is the Atlantic Ocean, to which all the most important rivers flow in a predominantly east-west direction. There are 11 independent river systems in Portugal with the length of the main river within the country exceeding 60 km (Table 1, Fig. 2).
Map of the main Portuguese rivers (adapted from FAO, available at http://www.fao.org/3/T0798E08.htm)
Ecological risk assessment
To assess ecological risk, both water and sediments reported maximum values were compared to Freshwater long-term Water Quality Guidelines for the Protection of Aquatic Life (CCME 2017) and Sediment Quality Guidelines for the Protection of Aquatic Life (CCME 2015), respectively. Furthermore, the environmental risks of trace elements in sediments were assessed by the determination of the potential ecological risk index. This index was proposed by Hakanson (1980) and indicates the degree of biological risk and can be calculated as follows: (Devanesan et al. 2017, Hakanson 1980, He et al. 2021)
where \({E}_r^i\) is the potential ecological risk index of single trace element i in sediment samples. \({T}_r^i\) is the toxicity response factor for a trace element i: As, Cd, Cr, Cu, Ni, Pb, Zn, and Hg are 10, 30, 2, 5, 2, 5, 1, and 40, respectively (Hakanson, 1980). Ci is the measured concentration of trace element i and \({C}_n^i\) is the reference value of trace element i collected from the natural geochemical background (Ribeiro et al. 2018). Average concentrations for 90 naturally occurring elements in the Earth’s crust can be found in the literature (Wedepohl 1995). The terminologies used to describe the potential ecological risk are low risk (\({E}_r^i\) ≤ 40), moderate risk (40 < \({E}_r^i\) ≤ 80), high risk (80 < \({E}_r^i\) ≤ 160), very high risk (160 < \({E}_r^i\) ≤ 320), or extremely high risk (\({E}_r^i\) > 320).
Occurrence of trace elements in water bodies, sediments/soils, and biota of Portugal
Water bodies
Trace elements occurrence in water bodies was reported in estuaries and coastal areas, lagoons, rivers, and streams and also water bodies near mining areas (streams, rivers, ground water, and irrigation waters) (Table 2). Various trace elements have been examined, though Zn, Cu, Pb, and Cd are among the most investigated (Table 2). Both Douro River and estuary are among the most studied ecosystems and various studies were conducted in order to determine a wide panel of trace elements (Li, Be, Al, V, Cr, Co, Ni, Cu, Zn, Se, Mo, Ag, Cd, Sb, Ba, Tl, Pb, and U) in surface estuarine waters in Douro estuary (Couto et al. 2014, Ribeiro et al. 2018). In the work developed by Couto and co-workers, estuarine waters were collected in 11 sampling sites in four sampling campaigns in 2007/2008. Results showed sporadic high levels for most trace elements, suggesting punctual and local sources. Significant spatial differences were also found. Most of the elements tended to increase in the inner stations to the mouth of the river. Indeed, some trace elements associated with agriculture procedures (Zn, Cu, and Ni) were higher in the middle part of the estuary, suggesting a possible common source. A comprehensive study considering different matrices such as water, sediments, and biota was also done in 2013 in the Douro River estuary. In that study, the overall concentrations were sorted as follows: Al > Zn > Li > Se > Ba > V > Cu > Mo > Pb > Ni > U > Cr > Sb > Ag > Co > Cd > Tl ~ Be. Water mean Al, Cr, Cu, Zn, Se, Ag, and Pb concentrations were above acceptable values for aquatic organisms (Ribeiro et al. 2018). A similar study was performed in Ave estuary where the trend Al > Zn > Se > Mo > Li > Ba > V > Cu > Pb > Ni > Cr > U > Be > Co ≈ Sb > Ag ≈ Cd > Tl was found (Couto et al. 2019). Al, Zn, Se, Cu, Ag, Pb, and Cd mean values were also found above aquatic life limits.
The occurrence of mercury (Hg) has also been investigated in Portuguese waters, and various works reported Hg analysis (Iglesias et al. 2020, Lillebø 2011, Ramalhosa et al. 2006). The presence of this element has been investigated in Douro River estuary, Oporto coastal area, Ria de Aveiro, Sado River estuary, and Caveira stream water with values up to 0.08 μg L-1. In most of the published studies, Hg levels were not of environmental concern.
In a country frequently devastated by summer fires, a recent paper correlated the forest fires of October 2017 with changes in the water’s chemical watercourses in the Mondego hydrological basin (showing increases in Al, Fe, Mn, As, Ba, and Zn concentrations) and biological constituents, after the beginning of rainfall due to sediments, ashes, debris and products of combustion runoff (Sequeira et al. 2020a, Sequeira et al. 2020b).
The presence of trace elements in water bodies has also been investigated in mining areas (Table 2). For instance, both surface and/or groundwater contamination has been observed in the vicinity of many post-mining areas, namely with an increase of As, Cd, Cu, Pb, and Zn resulting from 129 years of pyrite and Cu exploitation, spread along the Grândola stream (Ferreira da Silva et al. 2015). U mining pose, particular challenges for remediation and future land use. There are two studies in U mining areas, one in Mangualde, extensively explored until 1993, with high production of poor ore, where toxic levels of Mn, Fe, Al, U, and Sr were found. The other was in Horta da Vilariça, with U concentrations in stream water toxic to aquatic plants and invertebrates in one sampling point of the 15 sampling points selected (Antunes et al. 2007, Cordeiro et al. 2016). One study states the risk of inhabitants of a nearby village (S. Francisco de Assis) located downstream the Barroca Grande tailings deposit and impoundments, probably exposed to some potential health risks through the intake of As, Cd, and also Pb via vegetable consumption, even though waters had low metal concentrations. Zn and Mn were present in significant concentrations though below the standard parametric values. The concentrations of other elements were all legally acceptable, with values up to 7.8 μg L−1 for As; 0.36 μg L−1 for Cd, and 23.1 μg L−1 for Pb (Candeias et al. 2014).
Remediation processes, applied in the period of 2005–2008, using confination, tailings and debris control, and phytoremediation of the Murçós complex, contributed to a soil and water decrease of metals and arsenic; nevertheless, these procedures were not sufficient to assure a rehabilitation of the area (Antunes et al. 2016). In fact, mining activities have a considerable effect in the vicinity of the mine during its exploration. The mining places that have been abandoned or improperly closed may however continue to provoke damages to soils, water courses, and even the atmosphere. Post-mining regions represent, therefore, an important environmental issue. This topic has been a European problem of political debate and scientific concern for some 50 years (Keenan and Holcombe, 2021, Wirth et al. 2012).
Sediments and soils
Sediments are among the most studied matrices concerning the presence of trace elements and their occurrence was reported in estuaries, rivers, lagoons, and streams (Table 3). Different sediment sampling strategies (location, depth, grain size/fraction, etc.) and sediment digestion procedures (different mixtures of acids (HF, HCl, HNO3), microwave vs conventional digestion, etc.) make inter-studies comparison of the published papers impossible. This section highlights some of the most relevant studies comparing, whenever possible, studies in the same geographical areas.
The Sado estuary is among the most studied ecosystems (Caeiro et al. 2005, Cortesäo and Vale, 1995). Caeiro et al. (2005) reported that from 78 sampling stations, analysed in May 2006, 3% were highly contaminated and registered a high potential for adverse biological effects, while 47% had moderate contamination by Cd, Cu, Pb, Cr, Hg, Al, Zn, and As. Nevertheless, another study, also conducted in 2006, showed that Hg was not a cause of environmental risk in this estuary (Lillebø 2011). Another study in 2009 referred that the most important anthropogenic metal sources in this estuary have been historically related to pyrite mines that discarded mining waste directly into the river without appropriate treatment (high levels of Cd and Zn), the industries on the north shore (pulp and paper mills, pesticides and fertilizers factories) and shipyards (high levels of Pb, Cu, Cr, and Ni), as well as intensive farming, mostly of rice, and fish farms around the estuary. Also, the weak residual current flow characteristic of this system enhanced the accumulation of sediment; therefore, locally introduced pollutants settled out rather than being transported away (Serafim et al. 2013).
Tagus (Tejo), one of the largest estuaries on the Atlantic coast of Europe, has also been studied in several works. For instance, Caçador et al. (1996) evaluated the presence of various trace elements in two salt marshes sediments and showed that profiles of Zn, Pb, and Cu concentrations in vegetated sediments differed from those recorded in non-vegetated areas and that at subsurface layers (with higher root density) Zn, Pb, and Cu were enriched. In a monitoring study conducted by Duarte et al. (2014) in 2011, 19 sampling points were selected along Tagus estuary and concluded that with the exception of Cr and Cu, the analyzed metals (Zn, Pb, Cd, Ni, Cu, and Cr) showed similar distributions and a homogenous distribution in the proximity of discharge areas. The presence of yttrium and rare-earth elements collected in 78 sampling stations was also investigated in this estuary (Brito et al. 2018). Distribution of yttrium and rare earth elements was correlated with sediment grain size and associated with wastewater treatment plants (WWTP) located in the north margin and the legacy of an abandoned industrial complex in the south margin of the estuary (Brito et al. 2018). A study of heavy metal concentrations in sediment, benthic invertebrates, and fish in three salt marsh areas subjected to different pollution loads carried out in 2003 (França et al. 2005) obtained lower values than those obtained in previous studies for the Tagus estuary in 1993 (Caçador et al. 1993). This change could be related to a reduction in pollutant input, since the Tagus River was heavily polluted during the 1980s and the 1990s, but some industries stopped activity and several WWTP begun to operate since then.
Douro, one of the rivers with the larger hydrographic basin on the Iberian Peninsula, has also been studied in several works, from the transboundary Douro River basin to its mouth. In a study where 107 samples were collected from stream sediments in 2004 (Reis et al. 2014) higher concentrations of Cu, Zn, and, in particular, Pb, in the most labile fractions, were found. The higher values were found where the total element contents were also higher, suggesting an important contribution of anthropogenic activities to the total contents of these elements in the sediments. Cr and Ni were the main metals from a lithological source. Several other studies were focused on estuarine sediments. Water, sediments, and plants were collected in May 2013 and the possible occurrence of several trace elements as Li, Be, Al, V, Cr, Co, Ni, Cu, Zn, Se, Mo, Ag, Cd, Sb, Ba, Tl, Pb, and U were checked; Al and Zn were the trace element found at the higher concentrations at both sediments and water. Pb, Cu, and Zn levels in sediments were critical in comparison to the established probable effect levels (Ribeiro et al. 2018). Prior studies have shown an evident signature of anthropogenic trace metal contamination (Zn, Cu, Pb, Cr, Cd, and Ni) with consequences for estuarine communities and salt marsh vegetation (Almeida et al. 2006, Mucha et al. 2004b). A more recent study carried out in 2019 in 6 sampling sites showed a potential for Cd, Hg, and Pb to accumulate in organisms, with consequences for the entire trophic chain (Iglesias et al. 2020).
Ave and its tributaries have also been the focus of several research works. In 1992, a study emphasized the contribution of leather tanning, metal plating, and textile industries as the main sources of toxic metal contamination (Cd, Cr, Cu, Pb, and Zn) in these streams (Gonçalves et al. 1992). Other work, performed in1999, included more sampling stations to accomplish a better description of the basin showing similar results in Ave river and its tributary, river Este. Cd showed to be the most problematic pollutant followed by Zn, Cu, and Cr, with a strong correlation to local industry (Soares et al. 1999). Data from 2013 collected in the lower basin near the mouth of Ave river showed strong contamination by anthropogenic activities for Al, Mn, Ba, and Zn and high enrichment factors (EF)for Se, Cd, Zn, Li, Cu, Ag, Pb, and U (Couto et al. 2019).
Sediments of the biggest artificial lake of the Iberian Peninsula in the Guadiana Basin, Alqueva, that drains the western part of the Iberian Pyrite Belt, were studied and Cd was shown to contribute to the highest pollution levels followed by Pb and As. Despite the trace element contamination of the Alqueva sediments, sequential extraction studies showed that most of them were found in the oxidizable and residual fractions indicating that they were sparingly bioavailable, with exception of Cd (acid-labile fraction) and Pb (reducible fraction) (Palma et al. 2015).
Portugal is a country with a long mining tradition and still has a strong mining activity. Land pollution due to mining activities is a major issue in many European countries and Portugal is not an exception. Therefore, the presence of trace elements in mining sediments (stream sediments and soils) were reported in several works (Table 3) and at 18 different mining sites across Portugal. Environmental potential problems were found in Castromil (Au mining areas abandoned since 1940), Ag–Pb–Zn-Terramonte mines (closed in 1973), Pb-Zn Coval da Mó mines (total shutdown in 1972), Alto da Várzea radium mine (closed in 1946), tin–tungsten Panasqueira mine (still active), the tin–tungsten sector located in the Central Iberian Zone of the Iberian Massif, in the Portuguese–Spanish border - Monfortinho, Caveira (closed in de 1980s), Lousal (closed in 1988), Aljustrel (Iberian pyrite belt mining sites still active), and S. Domingos (closed in 1966) (Alvarenga et al. 2012, Antunes et al. 2018a, Antunes et al. 2018b, Candeias et al. 2011, Candeias et al. 2014, Ferreira da Silva et al. 2009, Ferreira da Silva et al. 2015, Silva et al. 2005). A recent review on the soils affected by mining activities in the Portuguese sector of the Iberian Pyrite Belt describes some of the rehabilitation actions, from constructive techniques to dig and contain the contaminated tailings and waste materials to more unconventional processes (Mourinha et al. 2022).
Biota
Trace elements have also been found in algae, plants, bivalve mollusk (e.g., mussels), and fish (Table 4). For instance, the presence of various trace elements was investigated in Ave and Douro estuaries in algae and plants located near the estuaries (Couto et al. 2019, Ribeiro et al. 2018). Estuarine plants slowly accumulate trace elements, being identified as bioindicators of estuary pollution. Those studies showed the presence of trace elements in distinct families of native estuarine flora. In the Douro estuary, high levels of Al, Zn, and Ba were determined in plants and macroalgae. No correlation was observed between flora and waters and sediments concentrations at the same sampling locations. Such differences could be related to the various parameters that affect the sorption of trace metals including plant life cycle, metal availability among others (Antunes et al., 2018a, b, Bonanno et al. 2018). Additionally, most of these plants are annual or biannual while trace elements sediments concentrations reveal chronic exposure and may explain the variations found among species and the concentrations found in sediments. In Ave estuary flora, the trace element concentrations were similar to high levels found for Al, Zn Ba, and Cu. The highest trace element levels were found in specimens of Plantago sp. and in macrophytes such as Oenanthe crocata and Veronica anagallis-aquatica, showing that these species may be metal accumulators and can be used as phytoindicators of local pollution. The occurrence of trace elements was also analyzed in aquatic mosses (Fontinalis antipyretica) in Ave (and tributaries) and Cávado rivers and a correlation was found between the elements found in selected flora and sediments (Gonçalves et al. 1992, Gonçalves et al. 1994). Data demonstrated that this species was a bioaccumulator and highlighted its importance as a metal bioindicator. Cr and Zn concentrations up to 107 and 70 times the natural concentration, respectively, were found in the plants. As mosses do not have conductive tissues, toxic metals are completely taken up from the water in accordance with their relative dissolved quantities. The increase in Hg contamination was related to the decrease of the local microbenthic community (~8400 microorganisms corresponding to 31 macrobenthic taxa) in Ria de Aveiro (Nunes et al. 2008). Invertebrates and fish trace element contamination has also been described. For instance, mussels Mytilus species have been wildly studied not only because they are commercially important but also because they are considered sentinels of environmental pollution making them an excellent metal biomonitoring species (Coimbra et al. 1991, Figueiredo et al. 2022, Machado et al. 1999, Santos et al. 2014). Seasonal and spatial variations of trace elements were reported in M. galloprovincialis (Figueiredo et al. 2022) and Santos et al. (2014) reported levels of several trace elements including Hg, Pb, Cr, and Cd below maximum allowed values.
The presence of Hg was found in green alga (Ulva sp.), bivalves (Scrobicularia plana and Cerastoderma edule), worms (Hediste diversicolor) or crabs (Carcinus maenas) collected from the intertidal mudflats in Sado estuary (Lillebø 2011). Nevertheless, no correlation was found among sediments and water levels and biota. In the Tagus estuary, the presence of significant levels of heavy metals was found in worms (Nereis diversicolor), bivalves (S. plana), brown shrimp (C. crangon), shore crab (C. maenas), grey mullet (L. ramada), sole (Solea senegalensis), and sand gob (Pomatoschistus minutus). Various studies have been showing the adverse effects of trace elements on aquatic organisms. In fact, exposure to various trace elements has been shown to induce biochemical, genotoxicity effects, and decreases survival (Gárriz and Miranda, 2020, Velma and Tchounwou, 2010).
The presence of various trace elements in diatoms and various plant species associated with mine areas was also reported (Table 4). In Fílvida stream Coval da Mó, the presence of Pb, Zn, Cd, Co, Cu, Mn, and Ni in benthic diatoms communities was shown and geochemical results showed a very toxic environment. The mixture of metals and their high concentrations in stream sediments near the mine was too toxic to allow a stable diatom community development. Further downstream, the decrease in metal concentrations to lower levels (two orders of magnitude) permitted the growth of diatoms, many of which with deformations particularly in F. capucina var. rumpens.
Global assessment of trace elements in Portugal
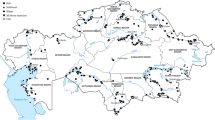
Our literature survey revealed that currently available data is heterogenous, varying in terms of study design, selected trace elements, and spatiotemporal locations. This heterogenicity leads to data differences that make meaningful comparisons difficult. Furthermore, comprehensive studies are scarce in what concerns spatiotemporal monitoring studies using different matrices (e.g., water, sediments, and biota) as they require multidisciplinary teams to obtain, integrate and correlate different data. Transboundary cooperative monitoring programs should also be promoted as many hydrographic basins are shared between Portugal and Spain. Additionally, the establishment of environmental geochemical background levels of trace elements is highly needed as they have strong regional characteristics and are crucial for accurate risk assessment. The overall spatial distribution of studies is represented in Fig. 3. The coastline and particularly in the north of Portugal had coverage for multiple environmental matrices types, whereas a notable number of interior districts had no sample coverage for any matrix type (Fig. 3). The spatial register of sample locations may indicate that there is enhanced research interest in those regions. Most papers published are focused on coastal areas and estuaries, mainly along the Atlantic coastline. Even in the main rivers, most studies analyze their mouths, where the biggest Portuguese cities are located. In fact, coastal transition ecosystems as estuaries and coastal lagoons are among the most productive in terms of biological importance and therefore of high environmental relevance. However, they are also amongst the most modified due to anthropogenic activities and vulnerable to contamination by diverse classes of pollutants including trace elements.
The total number of research regarding matrix type may also represent the scientific priority for environmental trace metals research. For example, there is a larger number of papers reporting trace metals in sediments/soil in contrast to water. This can indicate a shift away from this historically important exposure pathway. In fact, since the launch of the Water Framework Directive, significant efforts were made regarding the release of pollutants in addition to the implementation of WWTPs that contributed to the reduction of water trace element concentrations. Also, water trace elements reflet recent or occasional discharges while sediments and soils are major reservoirs and frequently act as sources of their presence in water bodies (Chon et al. 2012). Therefore, the majority of papers (59%) published in this period focus on sediments while a similar percentage focus either on waters or biota (~20%) (Fig. 4). There are only 8% of the papers with an integrated approach with determinations on water, sediments, and biota. The distribution of trace elements in each type of matrix (water, sediments/soils, and biota) as can be seen in Fig. 5.
Maximum values found and the percentage of papers with values above Aquatic Life limits are summarized in Table 5.
In waters, Zn, Cu, Pb, Cd, and As are among the trace elements most determined and various works reported trace element maximum levels higher than those established by CCME guidelines for aquatic life for Al, Zn, Se, Cu, Pb, Ag Cd, Cr, U, Ni, and As suggesting that adverse biological effects are expected to occur (Table 5, Fig. 5). In sediments, the pattern is similar, Pb, Cu, Zn, Cr, and Cd, and various works reported maximum levels surpassing the corresponding Interim sediment quality guidelines (ISQGs). Among them, As, Cd, Cr, Pb, and Hg rank among the priority metals that are of public health significance ranging from extremely high risk to high risk (Table 5, Fig. 5) (Tchounwou et al. 2012). Thus, adverse biological effects are likely to occur namely due to the presence of high levels of As, Cd, and Pb. The mean concentration of trace elements in surface water sediments is lower than that from mining areas (Table 5), nevertheless, both sediments/soils from surface waters and mining areas indicate that the degree of pollution by these trace elements is severe and deserves attention.
These metallic elements are considered systemic toxicants recognized as multiple organ damage inducers, even at reduced exposure concentrations. They are also classified as human carcinogens (known or probable) according to the US Environmental Protection Agency, and the International Agency for Research on Cancer. Pb, Cr, Zn, As, and Cu exceeded water quality guidelines in some studies (Abreu et al. 2008). A worse scenario is found for sediments with Zn, Cu, As, Pb, and Cr exceeding acceptable values in the majority of the studies (Table 3) (Abreu et al. 2008, Couto et al. 2019, Gonçalves et al. 1992, Ribeiro et al. 2018). Though Al is not among the most studied element, Al values also exceed acceptable values for both water and sediments in some studies.
Hg contamination is also a serious environmental health problem and its complexity in the environment has been systematically examined (Eagles-Smith et al. 2018). Even if Hg is not among the most analyzed elements, its occurrence was investigated in all matrices (Tables 1, 2, and 3). Hg is an element of natural occurrence found in trace amounts in air, water, and soil. Inorganic Hg appears naturally in surface water because of rocks and soil erosion and weathering processes. Hg in surface waters remains inorganic, but in certain environmental conditions, such as acidic pH, high organic matter and low dissolved oxygen, a fraction of it may be in a more toxic organic form, methylHg. MethylHg has a tendency to bioaccumulate, entering the food chain, therefore becoming a human health threat (Eagles-Smith et al. 2018). Hg is one of the most harmful environmental contaminants and therefore mentioned in the high-priority environmental pollutants directory within the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR Commission 2009), the European Union Water Framework Directive (EU-Directive 2000), and the United States Environmental Protection Agency (U.S. EPA). Hydrodynamic flow patterns have been related to Hg dispersion and pathways in coastal areas, causing harmful effects on biota (Iglesias et al. 2020). Several estuaries show problematic and deep-concerning levels (see boldface levels in Table 3).
Arsenic, usually detected at low concentrations, has a wide distribution in practically all environmental matrices in both inorganic (trivalent arsenite and pentavalent arsenate) and organic forms (methylated metabolites). Environmental As pollution occurs because of natural phenomena such as volcanic eruptions and soil erosion, but also by anthropogenic activities (Mandal 2017, Tchounwou et al. 2012). As water concentration is usually less than 10 μg L-1, although higher levels have been reported near natural mineral deposits or mining sites. Higher values were reported in two Portuguese mining sites, and at Segura, the As water content in December 2006 (1.190 mg L-1) was even higher than in October 2006 (0.636 mg L-1) and was related to the As released from Fe oxy-hydroxide (Antunes and Albuquerque, 2013, Antunes et al. 2018b). Natural levels of As in soil usually range from 1 to 40 μg g-1 (Tchounwou et al. 2012) and CCME report values of probable effect levels (PELs) (CCME 2006) of up to 41.6 μg g-1 in marine/estuarine sediments and a guideline value of 12 μg g-1 in soils. Higher values were reported in several estuarine or stream sediments (see boldface values Table 3) in Minho (Mil-Homens et al. 2013), transboundary Douro (Reis et al. 2014), Tagus (Vale et al. 2008), Ria Formosa (Sousa et al. 2019), and Guadiana (Delgado et al. 2011). In mining areas, values of 2000 μg g-1 or higher were found in Castromil (values up to 6909 μg g-1) (Silva et al. 2004), Aljustrel (maximum value of 3936 μg g-1) (Candeias et al. 2011) and S. Domingos (Alvarenga et al. 2012), where a value of 7955 μg g-1 was reported and As presented high mobilizable contents in all sampled soils, even though its effective bioavailable fraction represented less than 10% of the pseudo-total content.
Cd is broadly disseminated in the earth’s crust with a mean concentration of about 0.1 mg kg-1. Cd is commonly used in industry in activities such as the production of alloys, pigments, and batteries (Tchounwou et al. 2012). Cd compounds are considered human carcinogens by various regulatory agencies such as The International Agency for Research on Cancer (IARC) (IARC 1993, 2009) and the US National Toxicology Program; it has also been extensively investigated by United Nations Environment Programs and the International Commission on Occupational Health. US Poison and Disease Registry (ATSDR 1997) rated Cd as the sixth most toxic substance. World Health Organization (Lata and Mishra, 2019) placed cadmium in a priority position in the study of food contaminants. Plants act as a Cd carrier, in different salt chemical water-soluble forms, into the food chain, so Cd polluted vegetable consumption or living close to highly-industrialized places enhance toxicity potential (Lata and Mishra, 2019).
CCME reports values of 0.1 μg L-1 for long-term exposure in freshwaters and 4.2 μg g-1 of PEL in marine/estuarine soils and a guideline value of 1.4 μg g-1 in agricultural soils (CCME 2006). Values superior to 19.5 μg g-1 were reported in Cávado (Gonçalves et al. 1994) and in Mondego (Dias-Ferreira et al. 2016) estuarine sediments. Waters in mining areas with values above CCME guideline were reported in three of the mines studied (Antunes et al. 2007, Candeias et al. 2014, Favas et al. 2016) and in Ave estuary, where 80% of the samples collected exceeded this limit (Couto et al. 2019).
Cr is naturally present in the earth’s crust, with oxidation states (II) to (VI). Cr compounds are stable in the trivalent form and occur in nature in this state in minerals. Cr reaches different environmental matrices (air, water, and soil) from various natural and anthropogenic sources with the greatest contribution coming from industries (Tchounwou et al. 2012). Hexavalent Cr, a powerful oxidizing agent, is a toxic industrial pollutant classified as a human carcinogen by several regulatory and non-regulatory agencies such as ATSDR, IARC, and EPA (ATSDR 2012, EPA 1992, IARC 1990). CCME reports a guideline value for the protection of aquatic life of 1 μg L-1 for freshwater and ISQGs and PELs of 52.3 and 160 μg g-1. A very high value (1187 μg g-1) in the Selho river (Ave tributary) was reported and reflected the wastewater discharged from the leather tanning industries located in Guimarães city (Gonçalves et al. 1992). This Ave tributary presented very high metallic levels related to industrial activities and probably illegal industrial wastewater discharges without prior treatment.
Pb is present in trace amounts in the earth’s crust. Pb is the second most toxic metal after As because of its toxic effects on living organisms (ATSDR 2016). Although Pb occurs naturally in the environment, anthropogenic activities such as fossil fuel burning, mining, and manufacturing contribute to its release in elevated concentrations. Pb has many different industrial (batteries, ammunition, metal products), agricultural and domestic applications (Tchounwou et al. 2012). It is considered by the IARC as a probable human carcinogen. CCME ISQGs and PELs for Pb are 30.2 and 112 μg g-1 respectively; these values were exceeded in several places in the literature cited in this review (boldface in Table 3).
Remediation and risk management
Human activities such as agricultural production, urban expansion, industrial activities, and mining have been pointed out as the main contributors of trace elements input into the environment in the last years. Trace elements pollution remediation is difficult mostly because of their persistence and non-biodegradability in the environment. Additionally, as a sink and source, soils and sediments represent a repository of bioavailable heavy metals/trace elements and take part in the returning of contaminants into circulation in the aquatic environment depending on favorable situations. Therefore, sediment chemistry provides valuable information essential to assessing sediment quality in contaminated sites and potentially harmful effects (Sarkar et al. 2014). Hence, the improvement/implementation of tools for their successful and effective environmental removal and well as protection policies are needed to diminish their contamination potential and production, respectively.
There are several techniques that can be used, depending on the concentration and nature of the contaminant, the soil and site characteristics, the contaminant’s availability, and the existence of specific regulations. The remediation can be performed by the containment/isolation of the contaminated matrices or soils, by using constructive techniques/physical treatments; the immobilization/stabilization of the contaminants in the contaminated material (soils or tailings); or by extraction/removal of the contaminants from the soil (Liu et al. 2018, Song et al. 2021, Wang et al. 2021). The technique used is always site-specific, and, often, it combines different strategies. In or ex situ remediation techniques for contaminated sites targeting specifically the contaminants can be used (Liu et al. 2018). The techniques can be further classified as physical (e.g., soil washing, electrokinetic), chemical (e.g., chemical addition to the soil to react and immobilize the contaminants), or biological (e.g., plants and/or microorganisms to degrade, immobilize or extract the contaminant). In mining areas (Lousal, Aljustrel, and S.Domingos) constructive techniques were widely applied in the rehabilitation of soil (excavation, storage, and capping) (Mourinha et al. 2022). In Aljustrel, for instance, the dispersed slag deposits, mining residues, and contaminated soils were removed and confined, and the deposits were sealed with limestone and clay and covered with clean clay soil and vegetation. Channels were constructed on the perimeter to collect drainage waters, conducted to evaporation-concentration ponds, and processed in artificial wetlands to protect the hydrological environment (Mourinha et al. 2022).
In contrast to the conventional physical and chemical techniques for soil remediation, phytoremediation is a plant-based and cost-effective technology that has been pointed out as an alternative or complementary strategy to constructive techniques. The main phytoremediation mechanisms are based on phytostabilization (immobilization of pollutants in the rhizosphere by the action of roots, bacteria, and soil amendments); phytoextraction (plant aerial part uptake and accumulation); phytostimulation (degradation in the rhizosphere by microorganisms, stimulated by the plant’s exudates); phytodegradation (plant enzymes degradation within the plant tissues); phytovolatilization (conversion to volatile forms and atmospheric release), phytodesalinization (salt removal in saline soils with halophytes), and rhizofiltration (removal of contaminants from polluted aquatic environments).
In the cited data phytoremediation is the most used approach and various species have been used including native plants. In fact, phytoremediation was developed as a sustainable alternative to chemical and physical pollution remediation approaches but is less expensive and environmentally friendly. One study carried out in Ria Formosa showed that Spartina maritima and S. fruticosa acted as Ag, Cd, Mo, Cu, Pb, and Zn remediators, altering the sediment metal distribution in depth and accumulating them, mostly in roots (and in rhizomes for S. maritima). Metal translocation to aerial organs was found residual. S. maritima proved to be a more effective metal stabilizer than S. fruticosa (Moreira da Silva et al. 2015). Another study compared Scirpus maritimus and Juncus maritimus from Douro salt marshes in the bioaccumulation of Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn. Both plants affected the sediment composition and revealed the potential for Cd phytostabilization. S. maritimus could also concentrate Pb in its roots (Almeida et al. 2006). Various studies showed Brassica juncea, Medicago sativa, Echinophora platyloba, Chara aculeolata to be Pb hyperaccumulators (Zulfiqar et al. 2019). Durães et al. (2015) compared the capability to uptake, translocate and tolerate Cu, Zn, and Pb by macrophytes (Juncus effusus L., Scirpus holoschoenus L., Thypha latifolia L., and Juncus sp.) and land plants (Cistus ladanifer L., Erica andevalensis C.-R., Nerium oleander L., Isatis tinctoria L., Rosmarinus officinalis L., Cynodon dactylon L. and Hordeum murinum L.) from Aljustrel, Lousal and São Domingos mining sites and Morocco (Tighza and Zeida) and concluded that the aquatic plants showed a higher capacity for Zn bioaccumulation and translocation with the metal mobility sequence Zn>Cu>Pb. Another study highlighted the uranium concentrations in water–soil–plant matrices and the efficiency, taking into account a heterogeneous assemblage of terrestrial and aquatic native plant species to act as biomonitor and phytoremediator for environmental U-contamination in the Sevilha mine (Favas et al. 2016). In that study, a total of 53 plant species belonging to 22 families were collected from 24 sampling sites. The maximum potential of U accumulation was recorded in roots of Juncus squarrosus (450 mg kg−1), Carlina corymbosa (181 mg kg−1), Juncus bufonius (39.9 mg kg−1), Callitriche stagnalis (55.6 mg kg−1), Lemna minor (53.0 mg kg−1), and Riccia fluitans (50.6 mg kg−1) confirming the unique efficiency of roots in accumulating this element from soil or sediments (phytostabilization) (Favas et al. 2016). U accumulation by Scorpiurium deflexifolium, Fontinalis antipyretica, Nasturtium officinale (roots), Oenanthe crocata (rhizomes/ roots), and Rorippa sylvestris (aerial parts) was also demonstrated and showed a consistent higher trend of its concentration in the majority of the plants in comparison to water (Cordeiro et al. 2016). The perennial herb Rorippa sylvestris (creeping yellowcress) had significantly higher U concentration in the shoots compared to roots, with translocation factor (TF) 700 times higher than unity, acting as a possible phytoextractor. The process of natural attenuation of contamination by phytostabilization of U in the rhizosphere, with the contribution of the native plant community offered an cost-effective and technical benefit, and this study could contribute to improve U-contaminated areas using the studied plant species in future environmental projects (Cordeiro et al. 2016). However, phytoremediation as also some drawbacks (Farraji et al. 2016) The extensive treatment period makes it only suited for remote areas, and the trace elements accumulated in biomass may lead to secondary pollution. Also, this process does not degrade the trace elements, but decreases the compound’s ability to migrate to soil and water. It is also possible that if flora is consumed by wildlife, these pollutants could enter the food chain. However, various complementary processes and methods can be used to enhance phytoremediation efficiency and overcome its present disadvantages. Phytoremediation can also be operated at large scales and contribute to the conservation of soil and ecosystem structure, prevention of erosion and leaching of metal. Furthermore, phytoremediation in degraded areas can offer more habitats for wildlife.
Nevertheless, the overexploitation of natural resources leads to their depletion and negative ecological impact affecting not only all living organisms’ health but also economic growth. Therefore, governments and policymakers should implement measures for better management and sustainable production as well as strategies for the reduction and release of these contaminants to the environment, promoting sustainable use and consumption.
Conclusions
Trace elements are ubiquitous environmental pollutants in aquatic and terrestrial ecosystems and have been considered a severe environmental problem. The potential hazard of an environmental chemical is a function of various factors including its persistence, toxicity, and bio/accumulative potential. Due to these three main characteristics trace elements are considered hazardous. Most hazardous trace elements environmentally relevant include Cr, Zn, Cd, Pb, Hg, and As. The trophic transfer of these elements in aquatic and terrestrial food chains/webs has important implications for both wildlife and human health. Reviewed data on surface waters showed that Al, Zn, Se, and Ag were above aquatic life limits in 60% of the published works. Cu, Zn, and As exceed aquatic life limits in more than 60% of mining waters. Hg and Cd in sediments from mining areas exceeded aquatic life limits and potential ecological risk showed extremely high risk for most of the elements. According to a potential ecological risk assessment, an extremely high risk was detected for Hg, Cu, As, Cd, and Pb. Therefore, it is crucial to continue to monitor the concentrations of trace elements in different environmental matrices for environmental protection works, management and mitigation measures. Furthermore, the establishment of environmental background concentrations of trace elements should be documented in the different environmental matrices and specific research area for later use as reference and to allow an accurate environmental risk assessment.
References
Abreu MM, Matias MJ, Magalhães MCF, Basto MJ (2008) Impacts on water, soil and plants from the abandoned Miguel Vacas copper mine, Portugal. J Geochem Explor 96:161–170
Acosta-Coley I, Mendez-Cuadro D, Rodriguez-Cavallo E, de la Rosa J, Olivero-Verbel J (2019) Trace elements in microplastics in Cartagena: a hotspot for plastic pollution at the Caribbean. Marine Pollut Bull 139:402–411
Almeida CMR, Mucha AP, Vasconcelos MTSD (2006) Comparison of the role of the sea club-rush Scirpus maritimus and the sea rush Juncus maritimus in terms of concentration, speciation and bioaccumulation of metals in the estuarine sediment. Environ Pollut 142:151–159
Alvarenga P, Palma P, de Varennes A, Cunha-Queda AC (2012) A contribution towards the risk assessment of soils from the São Domingos Mine (Portugal): chemical, microbial and ecotoxicological indicators. Environ Pollut 161:50–56
Alves CM, Boaventura RRAR, Soares HMVM (2009) Evaluation of heavy metals pollution loadings in the sediments of the Ave River Basin (Portugal). Soil Sediment Contam: Intl J 18:603–618
Antunes IMHR, Albuquerque MTD (2013) Using indicator kriging for the evaluation of arsenic potential contamination in an abandoned mining area (Portugal). Sci Total Environ 442:545–552
Antunes IMHR, Gomes MEP, Neiva AMR, Carvalho PCS, Santos ACT (2016) Potential risk assessment in stream sediments, soils and waters after remediation in an abandoned W>Sn mine (NE Portugal). Ecotoxicol Environ Safety 133:135–145
Antunes IMHR, Albuquerque MTD, Roque N (2018a) Spatial environmental risk evaluation of potential toxic elements in stream sediments. Environ Geochem Health 40:2573–2585
Antunes IMHR, Neiva AMR, Albuquerque MTD, Carvalho PCS, Santos ACT, Cunha PP (2018b) Potential toxic elements in stream sediments, soils and waters in an abandoned radium mine (central Portugal). Environ Geochem Health 40:521–542
Antunes M, Santos A, Valente T, Albuquerque T (2020) Spatial Mobility of U and Th in a U-enriched Area (Central Portugal). Appl Sci 10
Antunes SC, de Figueiredo DR, Marques SM, Castro BB, Pereira R, Gonçalves F (2007) Evaluation of water column and sediment toxicity from an abandoned uranium mine using a battery of bioassays. Sci Total Environ 374:252–259
ATSDR (1997): Toxicological Profile for Cadmium (Update). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service
ATSDR (2012): Toxicological Profile for Cromium Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service
ATSDR (2016): Addendum to the toxicological profile for arsenic Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service
Blasco J, Gomes T, García-Barrera T, Rodríguez-Romero A, González-Rey M, Morán-Roldán F, Tromibini C, Miotk M, Gómez-Ariza JL, Bebianno M (2010) Trace metal concentrations in sediments from the southwest of the Iberian Peninsula. Scientia Marina 74:99–106
Bonanno G, Orlando-Bonaca M (2018) Trace elements in Mediterranean seagrasses and macroalgae. A review. Sci Total Environ 618:1152–1159
Bonanno G, Vymazal J, Cirelli GL (2018) Translocation, accumulation and bioindication of trace elements in wetland plants. Sci Total Environ 631-632:252–261
Brito P, Prego R, Mil-Homens M, Caçador I, Caetano M (2018) Sources and distribution of yttrium and rare earth elements in surface sediments from Tagus estuary, Portugal. Sci Total Environ 621:317–325
Cabrita MT (2014) Phytoplankton community indicators of changes associated with dredging in the Tagus estuary (Portugal). Environ Pollut 191:17–24
Caçador I, Vale C, Catarino F (1993): Effects of plants on the accumulation of Zn, Pb, Cu and Cd in sediments of the Tagus estuary salt marshes, Portugal. In: Vernet JP (Editor), Studies in Environmental Science. Elsevier, pp. 355-364
Caçador I, Vale C, Catarino F (1996) Accumulation of Zn, Pb, Cu, Cr and Ni in Sediments Between Roots of the Tagus Estuary Salt Marshes, Portugal. Estuarine, Coastal and Shelf Science 42:393–403
Caçador I, Vale C, Catarino F (2000) Seasonal variation of Zn, Pb, Cu and Cd concentrations in the root–sediment system of Spartina maritima and Halimione portulacoides from Tagus estuary salt marshes. Marine Environ Res 49:279–290
Caeiro S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A, Medeiros G, Painho M (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Ind 5:151–169
Canário J, Branco V, Vale C (2007) Seasonal variation of monomethylmercury concentrations in surface sediments of the Tagus Estuary (Portugal). Environ Pollut 148:380–383
Canário J, Vale C, Poissant L, Nogueira M, Pilote M, Branco V (2010) Mercury in sediments and vegetation in a moderately contaminated salt marsh (Tagus Estuary, Portugal). J Environ Sci 22:1151–1157
Candeias C, da Silva EF, Salgueiro AR, Pereira HG, Reis AP, Patinha C, Matos JX, Ávila PH (2011) Assessment of soil contamination by potentially toxic elements in the aljustrel mining area in order to implement soil reclamation strategies. Land Degradation Dev 22:565–585
Candeias C, Melo R, Ávila PF, Ferreira da Silva E, Salgueiro AR, Teixeira JP (2014) Heavy metal pollution in mine–soil–plant system in S. Francisco de Assis – Panasqueira mine (Portugal). Appl Geochem 44:12–26
Carraça S, Ferreira A, Coimbra J (1990) Sr transfer factors between different levels in the trophic chain in two dams of Douro river (Portugal). Water Res 24:1497–1508
Carvalho P, Neiva A, Silva M (2011): Assessment to the potential mobility and toxicity of metals and metalloids in soils contaminated by old Sb–Au and As–Au mines (NW Portugal). Environ Earth Sci 65
Carvalho P, Neiva A, Silva M, Antunes M (2014): Metal and metalloid leaching from tailings into streamwater and sediments in the old Ag–Pb–Zn Terramonte mine, northern Portugal. Environ Earth Sci71
CCME (2015): Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. In: Council C (Hrsg.)
CCME (2017): Water Quality Guidelines for the Protection of Aquatic Life. In: Environment CCoMot (Hrsg.)
CCME (2006): Arsenic Canadian Sediment Quality Guidelines for the Protection of Aquatic Life, https://ccme.ca/en/res/arsenic-canadian-sediment-quality-guidelines-for-the-protection-of-aquatic-life-en.pdf. Accessed 21 Nov 2021
CCME (2019): Water Quality Guidelines for the Protection of Aquatic Life. http://stts.ccme.ca/en/index.html
Cesário R, Monteiro C, Nogueira M, O’Driscoll N, Caetano M, Hintelmann H, Mota A, Canário J (2016): Mercury and Methylmercury Dynamics in Sediments on a Protected Area of Tagus Estuary (Portugal). Water, Air, & Soil Pollution 227
Cesário R, Brito P, Caetano M, Prego R (June, 2018): Rare earth elements in the Mira river estuary sediments XIX SEMINARIO IBÉRICO DE QUÍMICA MARINA, Vigo, Spain
Chon H-S, Ohandja D-G, Voulvoulis N (2012) The role of sediments as a source of metals in river catchments. Chemosphere 88:1250–1256
Coimbra J, Carraça S, Ferreira A (1991) Metals in Mytilus edulis from the northern coast of Portugal. Marine Pollut Bull 22:249–253
Cordeiro C, Favas PJC, Pratas J, Sarkar SK, Venkatachalam P (2016) Uranium accumulation in aquatic macrophytes in an uraniferous region: Relevance to natural attenuation. Chemosphere 156:76–87
Cortesäo C, Vale C (1995) Metals in sediments of the Sado estuary, Portugal. Marine Pollut Bull 30:34–37
Costa C, Jesus-Rydin C (2001) Site investigation on heavy metals contaminated ground in Estarreja — Portugal. Eng Geol 60:39–47
Couto C, Pinto I, Madureira TV, Rocha MJ, Tiritan ME, Lopes JA, Agostinho AA (2014) Lower Douro river basin (Portugal) water quality - focus on trace element changes and anthropogenic sources of contamination. Global Nest J 16:252–269
Couto C, Ribeiro C, Ribeiro AR, Maia A, Santos M, Tiritan ME, Pinto E, Almeida AA (2019) Spatiotemporal Distribution and Sources of Trace Elements in Ave River (Portugal) Lower Basin: Estuarine Water, Sediments and Indigenous Flora. Intl J Environ Res 13:303–318
Delgado J, Nieto JM, Boski T (2010) Analysis of the spatial variation of heavy metals in the Guadiana Estuary sediments (SW Iberian Peninsula) based on GIS-mapping techniques. Estuarine Coast Shelf Sci 88:71–83
Delgado J, Barba-Brioso C, Nieto JM, Boski T (2011) Speciation and ecological risk of toxic elements in estuarine sediments affected by multiple anthropogenic contributions (Guadiana saltmarshes, SW Iberian Peninsula): I. Surficial sediments. Sci Total Environ 409:3666–3679
Devanesan E, Suresh Gandhi M, Selvapandiyan M, Senthilkumar G, Ravisankar R (2017) Heavy metal and potential ecological risk assessment in sedimentscollected from Poombuhar to Karaikal Coast of Tamilnadu using energy dispersive X-ray fluorescence (EDXRF) technique. Beni-Suef Univ J Basic Appl Sci 6:285–292
Dias-Ferreira C, Pato RL, Varejão JB, Tavares AO, Ferreira AJD (2016) Heavy metal and PCB spatial distribution pattern in sediments within an urban catchment—contribution of historical pollution sources. J Soils Sediments 16:2594–2605
Duarte B, Silva G, Costa JL, Medeiros JP, Azeda C, Sá E, Metelo I, Costa MJ, Caçador I (2014) Heavy metal distribution and partitioning in the vicinity of the discharge areas of Lisbon drainage basins (Tagus Estuary, Portugal). J Sea Res 93:101–111
Durães N, Bobos I, Ferreira da Silva E, Dekayir A (2015) Copper, zinc and lead biogeochemistry in aquatic and land plants from the Iberian Pyrite Belt (Portugal) and north of Morocco mining areas. Environ Sci Pollut Res Int 22:2087–2105
Eagles-Smith CA, Silbergeld EK, Basu N, Bustamante P, Diaz-Barriga F, Hopkins WA, Kidd KA, Nyland JF (2018) Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 47:170–197
EPA (1992): Environmental Criteria and Assessment Office.
EU-Directive (2000) Directive of the European Parliament and of the Council 2000/60/EC, establishing a framework for community action in the field of water policy. Official J Eur Commun L 327:1–72
Farraji H, Zaman NQ, Tajuddin RM, Faraji H (2016): Advantages and disadvantages of phytoremediation : A concise review
Favas PJC, Pratas J, Mitra S, Sarkar SK, Venkatachalam P (2016) Biogeochemistry of uranium in the soil-plant and water-plant systems in an old uranium mine. Sci Total Environ 568:350–368
Fernandes C, Fontaínhas-Fernandes A, Cabral D, Salgado MA (2008) Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz–Paramos lagoon, Portugal. Environ Monit Assess 136:267–275
Ferreira da Silva E, Almeida SFP, Nunes ML, Luís AT, Borg F, Hedlund M, de Sá CM, Patinha C, Teixeira P (2009) Heavy metal pollution downstream the abandoned Coval da Mó mine (Portugal) and associated effects on epilithic diatom communities. Sci Total Environ 407:5620–5636
Ferreira da Silva E, Durães N, Reis P, Patinha C, Matos J, Costa MR (2015) An integrative assessment of environmental degradation of Caveira abandoned mine area (Southern Portugal). J Geochem Explor 159:33–47
Ferreira da Silva M, Fontes MPF, Pacheco AA, Lima HN, Santos JZL (2020) Risk assessment of trace elements pollution of Manaus urban rivers. Sci Total Environ 709:134471
Figueiredo C, Oliveira R, Lopes C, Brito P, Caetano M, Raimundo J (2022) Rare earth elements biomonitoring using the mussel Mytilus galloprovincialis in the Portuguese coast: Seasonal variations. Marine Pollut Bull 175:113335
França S, Vinagre C, Caçador I, Cabral HN (2005) Heavy metal concentrations in sediment, benthic invertebrates and fish in three salt marsh areas subjected to different pollution loads in the Tagus Estuary (Portugal). Mar Pollut Bull 50:998–1003
Freire Ávila P, Santos Oliveira JM, Ferreira da Silva E, Cardoso Fonseca E (2005) Geochemical signatures and mechanisms of trace elements dispersion in the area of the Vale das Gatas mine (Northern Portugal). J Geochem Explor 85:17–29
Fuentes I, Márquez-Ferrando R, Pleguezuelos JM, Sanpera C, Santos X (2020) Long-term trace element assessment after a mine spill: pollution persistence and bioaccumulation in the trophic web. Environ Pollut 267:115406
Gárriz Á, Miranda LA (2020) Effects of metals on sperm quality, fertilization and hatching rates, and embryo and larval survival of pejerrey fish (Odontesthes bonariensis). Ecotoxicology 29:1072–1082
Gonçalves EPR, Boaventura RAR, Mouvet C (1992) Sediments and aquatic mosses as pollution indicators for heavy metals in the Ave river basin (Portugal). Sci Total Environ 114:7–24
Gonçalves EPR, Soares HMVM, Boaventura RAR, Machado AASC, Esteves da Silva JCG (1994) Seasonal variations of heavy metals in sediments and aquatic mosses from the Cávado river basin (Portugal). Sci Total Environ 142:143–156
Gredilla A, Stoichev T, Fdez-Ortiz de Vallejuelo S, Rodriguez-Iruretagoiena A, de Morais P, Arana G, de Diego A, Madariaga JM (2015) Spatial distribution of some trace and major elements in sediments of the Cávado estuary (Esposende, Portugal). Mar Pollut Bull 99:305–311
Guerranti C, Martellini T, Fortunati A, Scodellini R, Cincinelli A (2019) Environmental pollution from plasticiser compounds: do we know enough about atmospheric levels and their contribution to human exposure in Europe? Curr Opin Environ Sci Health 8:1–5
Hakanson L (1980) An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res 14:975–1001
He N, Liu L, Wei R, Sun K (2021) Heavy metal pollution and potential ecological risk assessment in a typical mariculture area in Western Guangdong. Intl J Environ Res Pub Health 18:11245
IARC (1990) Chromium, nickel and welding. IARC monographs on the evaluation of carcinogenic risks to humans 49:121–145
IARC (1993) Cadmium and cadmium compounds. IARC monographs on the evaluation of carcinogenic risks to humans 58:119–237
IARC (2009) Cadmium and cadmium compounds. IARC monographs on the evaluation of carcinogenic risks to humans 100C:121–145
Iglesias I, Almeida CMR, Teixeira C, Mucha AP, Magalhães A, Bio A, Bastos L (2020) Linking contaminant distribution to hydrodynamic patterns in an urban estuary: the Douro estuary test case. Sci Total Environ 707:135792
Keenan J, Holcombe S (2021) Mining as a temporary land use: a global stocktake of post-mining transitions and repurposing. The Extractive Industries and Society 8:100924
Lata S, Mishra T (2019): Cadmium bioremediation: a Review. International Journal of Pharmaceutical Sciences and Research
Leal MCF, Vasconcelos MT, Sousa-pinto I, Cabral JPS (1997) Biomonitoring with benthic macroalgae and direct assay of heavy metals in seawater of the Oporto coast (northwest Portugal). Marine Pollut Bull 34:1006–1015
Li L, Wu J, Lu J, Min X, Xu J, Yang L (2018) Distribution, pollution, bioaccumulation, and ecological risks of trace elements in soils of the northeastern Qinghai-Tibet Plateau. Ecotoxicol Environ Safety 166:345–353
Lillebø AI (2011): Assessment of Mercury in Water, Sediments and Biota of a Southern European Estuary (Sado Estuary, Portugal). Water, air, and soil pollution v. 214, pp. 667-680-2011 v.214 no.1-4
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci Total Environ 633:206–219
Liu S, Liu Y, Yang D, Li C, Zhao Y, Ma H, Luo X, Lu S (2020) Trace elements in shellfish from Shenzhen, China: implication of coastal water pollution and human exposure. Environmental Pollution 263:114582
Machado LM, Bebianno MJ, Boski T, Moura D (1999): Trace metals on the Algarve coast, II: Bioaccumulation in mussels Mytilus galloprovincialis (Lamarck, 1819)
Mandal P (2017) An insight of environmental contamination of arsenic on animal health. Emerging Contaminants 3:17–22
Mandal R, Kaur S (2019) Impact of environmental pollution on trace elements in vegetables and associated potential risk to human health in industrial town Mandi-gobindgarh (India). Chemosphere 219:574–587
Martins MV, Mane M, Frontalini F, Santos JF, da Silva FS, Terroso D, Miranda P, Figueira R, Laut LL, Bernardes C, Filho JG, Coccioni R, Dias JM, Rocha F (2015) Early diagenesis and clay mineral adsorption as driving factors of metal pollution in sediments: the case of Aveiro Lagoon (Portugal). Environ Sci Pollut Res Int 22:10019–10033
Martins VA et al (2013) Assessment of the health quality of Ria de Aveiro (Portugal): heavy metals and benthic foraminifera. Mar Pollut Bull 70:18–33
Mil-Homens M, Stevens RL, Abrantes F, Cato I (2006) Heavy metal assessment for surface sediments from three areas of the Portuguese continental shelf. Continental Shelf Research 26:1184–1205
Mil-Homens M, Costa A, Lopes C, Duarte H, Rodrigues T, Santos C, Silva AA, Mateus S, Cavaleiro C, Abrantes F, Fonseca S, Serrano R, Trancoso M, Sousa R, Mateus M, Melo Z (2012): Multi-parameter characterization of surface sediments from the Minho estuary
Mil-Homens M, Costa AM, Fonseca S, Trancoso MA, Lopes C, Serrano R, Sousa R (2013) Characterization of heavy-metal contamination in surface sediments of the Minho River Estuary by way of factor analysis. Archives of Environmental Contamination and Toxicology 64:617–631
Mil-Homens M, Vale C, Raimundo J, Pereira P, Brito P, Caetano M (2014) Major factors influencing the elemental composition of surface estuarine sediments: the case of 15 estuaries in Portugal. Marine Pollution Bulletin 84:135–146
Moiseenko TI, Dinu MI, Gashkina NA, Kremleva TA (2019) Aquatic environment and anthropogenic factor effects on distribution of trace elements in surface waters of European Russia and Western Siberia. Environmental Research Letters 14:065010
Moreira da Silva M, Anibal J, Duarte D, Chicharo L (2015) Sarcocornia fruticosa and spartina maritima as heavy metals remediators in Southwestern European Salt Marsh (Ria Formosa, Portugal). Journal of environmental protection and ecology 16:1468–1477
Mourinha C, Palma P, Alexandre C, Cruz N, Rodrigues SM, Alvarenga P (2022) Potentially toxic elements’ contamination of soils affected by mining activities in the Portuguese Sector of the Iberian Pyrite Belt and optional remediation actions: a review. Environments 9:11
Mucha AP, Bordalo AA, Vasconcelos MT (2004a) Sediment quality in the Douro river estuary based on trace metal contents, macrobenthic community and elutriate sediment toxicity test (ESTT). J Environ Monit 6:585–592
Mucha AP, Vasconcelos MTSD, Bordalo AA (2004b) Vertical distribution of the macrobenthic community and its relationships to trace metals and natural sediment characteristics in the lower Douro estuary, Portugal. Estuarine, Coastal and Shelf Science 59:663–673
Neves MO, Matias M (2008) Assessment of groundwater quality and contamination problems ascribed to an abandoned uranium mine (Cunha Baixa region, Central Portugal). Environmental Geology 53:1799–1810
Nunes JR, Ramos-Miras J, Lopez-Piñeiro A, Loures LG, C.; , Coelho J, Loures A (2014): Concentrations of available heavy metals in Mediterranean agricultural soils and their relation with some soil selected properties: a case study in typical Mediterranean soils. . Sustainability 6, 9124-9138.
Nunes M, Coelho JP, Cardoso PG, Pereira ME, Duarte AC, Pardal MA (2008) The macrobenthic community along a mercury contamination in a temperate estuarine system (Ria de Aveiro, Portugal). The Science of the total environment 405:186–194
OSPAR Commission ( 2009): Evaluation of the OSPAR System of Ecological Quality Objectives for the North Sea. Biodiversity Series.
Palma P, Ledo L, Alvarenga P (2015) Assessment of trace element pollution and its environmental risk to freshwater sediments influenced by anthropogenic contributions: The case study of Alqueva reservoir (Guadiana Basin). CATENA 128:174–184
Pereira P, Vale C, Ferreira AM, Pereira E, Pardal MA, Marques JC (2005) Seasonal variation of surface sediments composition in Mondego River Estuary. J Environ Sci Health, Part A 40:317–329
Pereira P, Raimundo J, Vale C, Kadar E (2009) Metal concentrations in digestive gland and mantle of Sepia officinalis from two coastal lagoons of Portugal. Sci Total Environ 407:1080–1088
Ramalhosa E, Segade SR, Pereira E, Vale C, Duarte A (2006) Mercury cycling between the water column and surface sediments in a contaminated area. Water Res 40:2893–2900
Reis A, Parker A, Alencoão A (2014) Storage and origin of metals in active stream sediments from mountainous rivers: a case study in the River Douro basin (North Portugal). Appl Geochem 44:69–79
Reis AR, Parker A, Carter J, Ferreira MP (2005) Distribution of Selected Heavy Metals in Sediments of the Águeda River (Central Portugal). J Environ Sci Health, Part A 40:305–316
Reis PA, Antunes JC, Almeida CMR (2008) Metal levels in sediments from the Minho estuary salt marsh: a metal clean area? Environ Monit Assess 159:191
Ribeiro C, Couto C, Ribeiro AR, Maia AS, Santos M, Tiritan ME, Pinto E, Almeida AA (2018) Distribution and environmental assessment of trace elements contamination of water, sediments and flora from Douro River estuary, Portugal. Sci Total Environ 639:1381–1393
Said OB, Moreira da Silva M, Hannier F, Beyrem H, Chícharo L (2019) Using Sarcocornia fruticosa and Saccharomyces cerevisiae to remediate metal contaminated sediments of the Ria Formosa lagoon (SE Portugal). Ecohydrol Hydrobiol 19:588–597
Samoli E, Stergiopoulou A, Santana P, Rodopoulou S, Mitsakou C, Dimitroulopoulou C, Bauwelinck M, de Hoogh K, Costa C, Marí-Dell’Olmo M, Corman D, Vardoulakis S, Katsouyanni K (2019) Spatial variability in air pollution exposure in relation to socioeconomic indicators in nine European metropolitan areas: a study on environmental inequality. Environ Pollut 249:345–353
Santos I, Diniz MS, Carvalho ML, Santos JP (2014) Assessment of essential elements and heavy metals content on Mytilus galloprovincialis from river Tagus estuary. Biol Trace Elem Res 159:233–240
Sarkar SK, Favas PJC, Rakshit D, Satpathy KK (2014): Geochemical Speciation and Risk Assessment of Heavy Metals in Soils and Sediments. InTechOpen, Maria C. Hernandez Soriano (Eds.), London, UK, 723 - 757
Sequeira MD, Castilho AM, Dinis PA, Tavares AO (2020a): Impact assessment and geochemical background analysis of surface water quality of catchments affected by the 2017 Portugal Wildfires. Water 12
Sequeira MD, Castilho AM, Tavares AO, Dinis P (2020b) Assessment of superficial water quality of small catchment basins affected by Portuguese rural fires of 2017. Ecological Indicators 111:105961
Serafim A, Company R, Lopes B, Pereira C, Cravo A, Fonseca VF, França S, Bebianno MJ, Cabral HN (2013) Evaluation of sediment toxicity in different Portuguese estuaries: Ecological impact of metals and polycyclic aromatic hydrocarbons. Estuarine, Coastal and Shelf Science 130:30–41
Silva E, Zhang C, Ls P, Patinha C, Reis A (2004) Hazard assessment on arsenic and lead in soils of Castromil gold mining area, Portugal. Appl Geochem - Appl Geochem 19:887–898
Silva EF, Fonseca EC, Matos JX, Patinha C, Reis P, Santos Oliveira JM (2005) The effect of unconfined mine tailings on the geochemistry of soils, sediments and surface waters of the lousal area (Iberian Pyrite Belt, Southern Portugal). Land Degradation & Development 16:213–228
Silva HF, Silva NF, Oliveira CM, Matos MJ (2021): Heavy Metals Contamination of Urban Soils—A Decade Study in the City of Lisbon, Portugal. Soil Systems 5
Soares HMVM, Boaventura RAR, Machado AASC, Esteves da Silva JCG (1999) Sediments as monitors of heavy metal contamination in the Ave river basin (Portugal): multivariate analysis of data. Environ Pollut 105:311–323
Song N, Hursthouse A, McLellan I, Wang Z (2021) Treatment of environmental contamination using sepiolite: current approaches and future potential. Environ Geochem Health 43:2679–2697
Sousa CAM, Delgado J, Szalaj D, Boski T (2019) Holocene background concentrations and actual enrichment factors of metals in sediments from Ria Formosa, Portugal. Marine Pollut Bull 149:110533
Stoichev T, Coelho JP, De Diego A, Valenzuela MGL, Pereira ME, de Chanvalon AT, Amouroux D (2020) Multiple regression analysis to assess the contamination with metals and metalloids in surface sediments (Aveiro Lagoon, Portugal). Marine Pollut Bul 159:111470
Taati A, Salehi MH, Mohammadi J, Mohajer R, Díez S (2020) Pollution assessment and spatial distribution of trace elements in soils of Arak industrial area, Iran: Implications for human health. Environ Res 187:109577
Tchounwou PB, Yedjou CG, Patlolla AK (2012) Sutton DJ (2012): Heavy metal toxicity and the environment. Experientia Supplementum 101:133–164
Vale C, Canário J, Caetano M, Lavrado J, Brito P (2008) Estimation of the anthropogenic fraction of elements in surface sediments of the Tagus Estuary (Portugal). Marine Pollut Bull 56:1364–1367
Vasconcelos RP, Reis-Santos P, Maia A, Ruano M, Costa MJ, Cabral HN (2011) Trace metals (Cu, Zn, Cd and Pb) in juvenile fish from estuarine nurseries along the Portuguese coast. Scientia Marina 75:155–162
Velma V, Tchounwou PB (2010) Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish, carassius auratus. Mutation Research 698:43–51
Wang G, Zhang Q, Du W, Lin R, Li J, Ai F, Yin Y, Ji R, Wang X, Guo H (2021) In-situ immobilization of cadmium-polluted upland soil: A ten-year field study. Ecotoxicol Environ Safety 207:111275
Wang X, Zhou D (2021) Spatial agglomeration and driving factors of environmental pollution: a spatial analysis. J Clean Prod 279:123839
Wedepohl KH (1995) The composition of the continental crust. Geochimica et Cosmochimica Acta 59:1217–1232
Wirth P, Mali BČ, Fische W (2012): Post-mining regions in Central Europe - problems, potentials, possibilities. oekom, München
Zhang C, Yu Z-g, Zeng G-m, Jiang M, Yang Z-z, Cui F, Zhu M-y, Shen L-q, Hu L (2014) Effects of sediment geochemical properties on heavy metal bioavailability. Environ Intl 73:270–281
Zulfiqar U, Farooq M, Hussain S, Maqsood M, Hussain M, Ishfaq M, Ahmad M, Anjum MZ (2019) Lead toxicity in plants: Impacts and remediation. J Environ Manage 250:109557
Data availability
Applicable
Funding
This work was supported by CESPU project MYCOBIOENV-PFT-IINFACTS-2019.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Cristina Couto and Cláudia Ribeiro. The first draft of the manuscript was written by Cristina Couto and Cláudia Ribeiro and both authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Applicable
Consent for Publication
Applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Luke Mosley
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Couto, C.M.C.M., Ribeiro, C. Pollution status and risk assessment of trace elements in Portuguese water, soils, sediments, and associated biota: a trend analysis from the 80s to 2021. Environ Sci Pollut Res 29, 48057–48087 (2022). https://doi.org/10.1007/s11356-022-20699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20699-9