Abstract
Groundwater is important for human survival and development, particularly in arid and semi-arid regions. This study aimed to analyze the hydrochemical characteristics, influencing factors, and the impact of human activities on groundwater in the semi-arid plains of western Jilin Province, northwest China. The study collected 88 and 151 phreatic and confined water samples, respectively, which were analyzed for 13 water quality indicators using statistical and graphical methods. In order to investigate the impact of anthropogenic activities on water quality and health risks, the improved combined weighted water quality index (ICWQI) based on the entropy weight, criteria importance though inter-criteria correlation (CRITIC), the coefficient of difference method, subjective weight based on quality grading criteria, and the water quality index (WQI) were proposed to evaluate the water quality of the study area. Meanwhile, the human health risk assessment (HHRA) model was used to assess the risks of nitrate to the health of humans in different ages and sex categories. The results indicated that the groundwater in the study area was weakly alkaline and the main hydrochemical types in the phreatic and confined water were HCO3−·Ca–Mg and HCO3−–Na. Rock weathering was the dominant process responsible for the generation of groundwater ions, the ions in groundwater primarily originate from the dissolution of halite, gypsum, and feldspar, while dolomitization promotes an increase in Mg2+. Human activities lead to an increase in NO3− in groundwater and have an impact on water quality and human health risks. The ICWQI method was found to yield more precise and rational assessments of water quality. Groundwater quality is primarily affected by nitrate ions. The areas in which groundwater nitrate posed a higher risk to human health were found to be mainly in the saline-alkali lands of Qian’an, Tongyu, and Zhenlai. Fertilizers, pesticides, and livestock farming activities contribute to the pollution of surface water. This surface contamination then infiltrates abandoned confined wells, leading to contamination of the confined aquifers. This study can improve the understanding of groundwater hydrochemical characteristics and the impact of human activities on groundwater in the study area. This study can also contribute to the study of groundwater in semi-arid regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is vital for human survival and economic development. Groundwater represents a major source of water and is important for human social development, the livelihoods of residents, and ecological balance and diversity (Li et al. 2020; Ramakrishnaiah et al. 2009), particularly in arid and semi-arid regions (Li et al. 2015; Wang et al. 2020). Therefore, the groundwater quality can directly affect the health of residents. Groundwater chemistry undergoes complex changes due to constant water-rock interactions (Subramani et al. 2010). However, groundwater pollution due to anthropogenic activity has become an increasingly important factor affecting groundwater chemistry in recent decades due to urbanization and industrialization, resulting in localized nitrate pollution (Zhai et al. 2019). Achieving sustainable development of groundwater resources has become a major challenge (Abbasnia et al. 2018).
Understanding water quality and hydrochemical characteristics is a fundamental requirement for the conservation and rational utilization of water resources. There has been an increased focus on the assessment and hydrochemical characteristics of groundwater in recent decades due to increasing concern over groundwater protection (Li et al. 2016b). This focus includes many studies conducted internationally and in China, including investigations of groundwater in the Yinchuan Plain, an arid area in northwest China (Chen et al. 2016; Wei et al. 2022; Zhang et al. 2020), the Loess Plateau in northern China (He et al. 2019; Li et al. 2019b), western Jilin Province in northeast China (Li et al. 2020, 2019a; Wang et al. 2022), Telangana in India (Adimalla and Li 2019), the Shagaya water well fields in Kuwait (Rashid et al. 2022), and North Sulawesi in Indonesia (Suherlina et al. 2022).
In previous water quality studies, a wide range of water quality assessment methods is used in groundwater assessment, including the fuzzy comprehensive assessment method (Jha et al. 2020), the technique for order of preference by similarity to ideal solution (TOPSIS) (Gorgij et al. 2019), the trapezoid grey relational degree method (Yan et al. 2016a), the Bayesian water quality assessment model (Tang et al. 2022), the artificial neural network method (Abba et al. 2022), and the water quality index (WQI) (Mladenovic-Ranisavljevic et al. 2018). The WQI is commonly used to assess water quality and was first proposed by Horton and Uddin (1965) and Brown et al. (1970). Nevertheless, the WQI suffers from a lack of objectivity in the determination of the weights of its component indicators (Bordalo et al. 2006; Li et al. 2010), leading to recent interest in improving weighting in the WQI. The methods developed for objective weighting of the WQI include the entropy weight method (Naik et al. 2022), CRITIC (Zhang et al. 2020), and integrated data envelopment analysis (DEA) (Oukil et al. 2022). The different weighting methods are characterized by different priorities and reference standards. Some studies have attempted to develop a more reasonable and objective index weighting method by combining weighting. Ding et al. (2022) combined the analytic hierarchy process (AHP) and entropy weight method within an improved comprehensive water quality identification index; Zhao et al. (2021) combined entropy weight and over-standard multiple and single-factor assessment methods and a fuzzy synthetic assessment method to evaluate the water quality of Chagan Lake. The combination of subjective and objective weighting is emerging as a novel trend to enhance the weighting of WQI indicators. For example, Yan et al. (2016b) used AHP, the entropy method, and coefficient of variation to combine subjective and objective weights within the establishment of an urban drinking water quality model. These studies have advanced the comprehensive weighting method and confirmed its feasibility. The water quality index (WQI) employs a multi-indicator evaluation approach, considering the differences among various individual and overall indicators in water quality assessment. By appropriately assigning weights, it effectively captures and incorporates the unique characteristics and importance of each indicator. This allows for a comprehensive and accurate water quality assessment in specific research contexts, aligning perfectly with the objectives of our study. However, the previous WQI method calculates indicator weights from an overall perspective, disregarding differences in individual indicator weights. This deficiency can result in deviations in water quality assessment outcomes from the actual conditions.
To address the shortcomings of previous studies, the present study developed a new compound index with subjective and objective weights, based on the WQI, which is referred to as the “ICWQI” in the present paper. In order to make the weight more objective, the ICWQI integrates two objective weighting methods which refer to different standards, namely, the entropy weight and CRITIC methods. Furthermore, this study innovatively employs graded quantitative criteria (qi) as subjective weights based on objective weighting to account for both overall and individual indicator variability. By combining subjective and objective weights, the resulting combined weights are more objective and accurate.
Previous research has demonstrated variations in hydrochemical compositions among different regional types. However, these studies have primarily focused on administrative districts, agricultural zones, or semi-arid basins, neglecting investigations in semi-arid low-lying plains. The western part of Jilin Province in northeast China is a typical semi-arid low-lying plains, characterized by a large area of saline-alkali land, lakes, and marshes resulting from strong evaporation, differing from other research areas in terms of its unique hydrological and ecological features. The low precipitation and high evaporation in the area have contributed to the dominant role of groundwater in limiting human activities and economic development. Recent studies on the western region of Jilin Province have mainly focused on ecosystem service capacity (Li et al. 2016a), the requirements of the ecological environment (Zhang et al. 2016a), rainfall and groundwater prediction (Lu et al. 2015; Yang et al. 2009), groundwater exploitation schemes (An et al. 2015), and soil salinization (Li et al. 2022). Studies on hydrochemistry have mainly focused on the migration, distribution, and assessment of the risk of harmful groundwater elements, such as fluorine, arsenic, and cadmium, to human health (Adeyeye et al. 2021; Cao et al. 2009; Jianmin et al. 2015; Xu et al. 2020, 2021; Zhang et al. 2003), as well as on the evolution of hydrology and geochemistry (Li et al. 2019a). Recent groundwater quality assessments have mainly focused on Chagan Lake (Zhang et al. 2016b; Zhao et al. 2021), Songyuan City (Yan et al. 2021), and Songnen Plain (Chen et al. 2021). However, there is insufficient knowledge of the hydrochemical characteristics, water quality, and risks posed to human health by nitrates of groundwater over the entire western plain of Jilin Province.
This study presents the first systematic analysis of the hydrochemical characteristics and formation mechanisms in the semi-arid low plain region. It investigates the impact of human activities on groundwater from the perspectives of water quality and health risks, while proposing an improved combined weighting water quality index method (ICWQI). This study is of significant importance in understanding the hydrochemical characteristics of groundwater in semi-arid regions and comprehending the impact of human activities on groundwater in such areas. It also emphasizes the significance of groundwater management and protection in these regions.
Study area
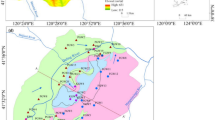
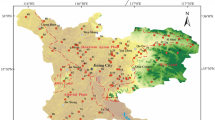
The study area of the present study was the western low plain area of Jilin Province and the southern part of Songnen Plain (Fig. 1 a). The study area has a flat terrain and is bordered by the Taoer River alluvial fan in the northwest, the tableland in the east, and the Nen River valley plain in the northeast. The study area falls within the Baicheng and Songyuan administrative regions and covers an area of 30,324.18 km2. The climate of the study area is a temperate continental monsoon, with low precipitation and high evaporation. The average annual temperature and precipitation in the study area range between 3 and 6 °C and 400 and 500 mm, respectively. Pleistocene subsidence in the Songnen Plain resulted in the formation of several different subsidence areas in the study area, which subsequently developed into many lakes and marshes (Lin et al. 2005).
The main rivers in the study area are the Songhua, Taoer, and Huolin rivers, which have a centripetal distribution. Groundwater flows along the topography from the west, south, and east to the center and north, and flows into the Taoer and Nen rivers in the north (Li et al. 2020). The dominant land use types in the study area are cropland, barren land, and grassland, accounting for 45%, 27%, and 13% of the total area, respectively, and are widely distributed in various areas of the western low plains (Fig. 1 b). As shown in Fig. 1 c, fine sand and loess aquifers dominate the study area, and groundwater recharge mainly occurs through rainfall infiltration, with lateral runoff recharge having a smaller contribution, whereas evaporation and artificial exploitation are the main forms of groundwater output (Li et al. 2020). Sand and gravel layers constitute the main lithology of the confined aquifers, which are characterized by buried depths of 40–60 m, thicknesses of 10–30 m, and impermeable mudstone floors.
Materials and methods
Sample collection and analysis
Water samples were collected in the study area between November 5 and 27, 2020. In total, 88 and 151 submersible and confined water samples, respectively, were collected, including 86 and 65 Quaternary and Neogene water samples, respectively (Fig. 1). Groundwater sampling was conducted in accordance with the Technical Specifications for Groundwater Environmental Monitoring (HJ/T164-2004). During sampling, source groundwater was first pumped for 5 min to avoid stagnant water in the pipes. Each water sample was filtered through a 0.45-μm filter membrane and sealed in a 100-mL polyethylene sampling bottle. Each sample bottle was rinsed 2 to 3 times with the sample water source before taking the sample. Each sampling point was sampled in triplicate. The water samples for measurement of cations were acidified with 10% HNO3 to a pH < 2. Water temperature and pH were measured in situ using a calibrated Hanna (HI99131) portable pH/temperature analyzer, whereas alkalinity was measured in situ by Gran titration. The collected water samples were sent for water chemistry analysis to the Pony Water Quality Testing Company within a week. Water quality testing methods were according to the Standard Inspection Method for Drinking Water (GB57550-2006). Groundwater cations (K+, Na+, Mg2+, and Ca2+) were measured by inductively coupled plasma atomic emission spectrometry (ICP-AES; optima 7000DV), whereas anions (Cl−, SO42−, NO3−, and F−) were measured by ion chromatography (ICS-600). HCO3−, chemical oxygen demand (COD), and total hardness (TH) were determined by titration. The results of groundwater quality analyses were within the allowable error range (± 5%) for water chemistry analysis.
Acquisition of land use types
The present study used remote sensing image data for the period June to September 2020. The dataset was obtained from the Data Center for Resources and Environmental Sciences, Chinese Academy of Sciences (RESDC) (http://www.resdc.cn). Cloud cover within the image data was < 2% and the band data followed a normal distribution with a meta-size of 30 × 30 m. ENVI5.3 software was used to conduct radiometric calibration and atmospheric correction for Landsat8 Operational Land Imager (OLI) remote sensing images, thereby eliminating the influences of the sun and atmosphere and improving the accuracy of land-use-type classification. The obtained raster image was divided into six land-use types using the ArcGis10.2 platform: (1) cropland; (2) forest; (3) grassland; (4) buildings; (5) water bodies; (6) barren land.
Water quality index method based on combination weighting
The weights of ICWQI were objectively selected based on the entropy weight and CRITIC methods. In addition, graded quantitative criteria values for the samples were introduced based on objective weighting to increase the weighting of anomalous indicators. The specific steps followed to derive the index are detailed below.
Creation of an initial water quality matrix
The initial water quality matrix was established for the water quality indicators of different samples in the study area:
where m and n are the numbers of water samples and water quality indices, respectively.
Data standardization
There was a need to standardize the different water quality indicators. This was achieved using the min-max normalization method:
where xij is the jth index value of the ith water sample and (xij) min and (xij)max represent the minimum and maximum values of the jth index of the ith water sample, respectively. After standardization, a Y matrix was established:
Combination weight calculation
Entropy weight method
The entropy weight method is a weight assessment method that considers the degree of confusion in the data. The present study applied the entropy weight method to calculate the weight of each water quality index:
Calculation of information entropy
In the formulation: \({p}_{ij}=\frac{{y}_{ij}}{\sum_{i=1}^{n}{y}_{ij}}\), \(k=1/\mathrm{lnm}\).
Weight determination
In the formulation: wj1∈[0,1], \(\sum_{j=1}^{n}{w}_{j1}=1\).
CRITIC weight
The CRITIC weight method considers data volatility and index conflict, and is calculated using the steps below.
Calculation of the standard deviation
Construction of the correlation coefficient matrix
Calculation of the weight of each indicator
Determination of the quantitative standards of grading
where Ci is the measured concentration of the Jth index; CpH is the measured pH concentration; and Sj is the standard limit of groundwater quality.
Combined objective weight
The weights wj1 and wj2 obtained by the entropy and CRITIC methods, respectively, were combined and weighted by the difference coefficient method:
where wj1 is the index weight obtained by the entropy weight method; wj2 is the index weight obtained by the CRITIC weight method; wO is the combined weight; α is the proportion of the CRITIC-calculated weight within the combined weighting. The difference coefficient method was used to reduce the influences of subjective factors:
where n is the number of assessment indices of the entropy weight method; w1, w2, …, wn is the weight of the assessment index determined by the entropy weight method.
Combined weight
The objective weights were multiplied by the sample grading criteria and then normalized to produce the final combined weights for the improved combined WQI method (ICWQI):
where wz is the combined weight.
Calculation of the improved combined water quality index
The improved combined weighted water quality index (ICWQI) was obtained by multiplying the quantitative criteria of each index classification by the combined weight and summing
Classification of groundwater quality
The calculated ICWQI was categorized into classes I to V as (I) excellent: ICWQI < 50; (II) good: 50 < ICWQI < 100; (III) medium 100 < ICWQI < 200; poor (IV) 200 < ICWQI < 300; (V) very poor ICWQI > 300.
Human health risk assessment
The present study used the internationally recognized HHRA assessment model proposed by the US Environmental Protection Agency (USEPA) to assess the risks of groundwater nitrates in the study area to human health.
Nitrates can enter the body through drinking water and skin contact. The intake resulting from oral administration (drinking water) was calculated as
where CDI is the concentration of nitrates obtained through ingestion (mg·kg−1·day−1); Cw is the concentration of nitrates in groundwater (mg·L−1); IR is the groundwater intake rate (L·day−1); EF is the exposure frequency (number of days in a year during which nitrate-containing groundwater is ingested) (day/year); ED is the duration of exposure (the number of years during which nitrate-containing groundwater was ingested) (year); BW is the average body weight (kg); AT is the average number of days (days).
The dose entering the body through skin contact was calculated as
where CDD is the average dose of nitrates entering the human body through skin contact (mg·kg−1·day−1); Ki is the skin permeability coefficient of pollutants (cm·h−1); SA is the skin contact area (cm2); AT is shower frequency (h·day−1); CF is a unit conversion factor; other parameters are as for Eq. (14).
The non-carcinogenic risk of ingesting nitrates can be expressed by the risk index (HQ):
where HQ is the non-carcinogenic risk index of nitrates; HQoral is the non-carcinogenic risk index of nitrates ingested orally; HQderm is the risk index of nitrates assimilated through skin contact; RfDoral is the reference dose of nitrates assimilated through skin contact; RfDderm is the reference dose of nitrates assimilated through skin contact.
Due to significant differences in computational parameters between children and adults, as well as between adult males and females, the present study aimed to calculate the potential health risks associated with exposure to nitrate in three distinct age groups, namely, children, adult males, and adult females, in the study area. The selected parameters were derived from relevant previous studies conducted in the western region of Jilin Province (Duan 2013). In order to better align with the actual conditions of the study area, the present study referred to the Chinese Population Exposure Parameter Manual for the choice of parameters to use in the assessment (Duan 2013). Table 1 summarizes the parameters used in the risk assessment.
Results
General hydrochemistry
The overall hydrochemistry of the groundwater in the study area was analyzed based on hydrochemical data of groundwater samples. Table 2 summarizes the hydrochemical profiles of collected water samples. The mean pH values of both the phreatic and confined aquifers exceeded 7, with a ranking of water samples according to pH of Neogene water samples (NW) > Quaternary water samples (QW) > unconfined aquifer water samples (UW). These results indicated that the groundwater in the study area was generally alkaline, whereas aquifers near the surface tended to be more acidic. Na+ and HCO3− were the main cation and anion, respectively, in confined and phreatic aquifers. The ranking of cations according to average groundwater content was Na+ > Ca2+ > Mg2+ > K+, whereas the ranking of anions was HCO3- > Cl− > SO42− > NO3−. The different aquifers showed the same ordering of anions and cations, indicating similar groundwater hydrochemical compositions of different aquifers.
The concentration of NO3− can reflect the impact of human activities on groundwater. The groundwater concentration of NO3− of the phreatic aquifers ranged between 0.01 and 298 mg L−1, while that for the Quaternary aquifer was 0.01–64 mg L−1, and the values for the Neogene aquifer were 0.01–3. 37 mg L−1. The result indicated that the phreatic aquifers were more susceptible to anthropogenic contamination. Also, groundwater NO3− concentration showed the highest coefficient of variation (CV) among all ions. The largest CV of NO3− concentration was observed in the water samples of the Quaternary confined aquifer (QW), indicating NO3− exhibits a pattern of localized contamination.. Total hardness (TH) and total dissolved solids (TDS) are two important indicators of groundwater quality, and groundwater with elevated TH and TDS values is not suitable for drinking. The average TDS concentrations of the water samples of the unconfined aquifer (UW), Quaternary aquifer (QW), and Neogene aquifer were 991.04 mg L−1, 642.29 mg L−1, and 517.63 mg L−1, respectively, whereas the average TH concentrations were 464.63 mg L−1, 297.08 mg L−1, and 211. 22 mg L−1, respectively (Table 2). The quality of groundwater in the study area generally met the quality standard, with only the TH of the phreatic water samples slightly exceeding the allowable quality standard of groundwater in China (SGQC2017) of 50 mg L−1. Most samples showed TDS values that were within the allowable quality standard (SGQC2017) of 1000 mg L−1, with only some water samples of the phreatic and Neogene confined water aquifers exceeding this standard. HCO3− and Na+ contributed the most to TDS in these water samples.
Hydrochemical types
The Piper diagram (Piper 1944) is a tool commonly used for analyzing hydrochemical water types. As shown in Fig. 2, the water samples of the three aquifers showed similar positions on the Piper diagram, indicating similarity in hydrochemistry between the groundwater of the three aquifers and a close hydraulic connection. The Piper diagram indicated the major water types of the groundwater samples to be the HCO3−–Na and HCO3− Ca–Mg types, with anions and cations dominated by HCO3− and Na+, respectively. In the phreatic aquifer, some of the water samples exhibit a SO4Cl−Ca·Mg water chemistry, which can be primarily attributed to the presence of Ca2+ ions in the overlying Loess-like subsandy soil. These ions filter into the groundwater through the leaching of precipitation and undergo an ion exchange reaction with Na+ ions, resulting in the formation of CaCl2−-type water.
Discussions
Factors controlling the chemistry of groundwater
Rock weathering, evaporation, and atmospheric precipitation are the three major mechanisms regulating the chemical composition of groundwater (Gibbs 1970). The present study used the Gibbs plot (Gibbs 1970) to analyze the factors regulating the hydrochemical compositions in the study area. As shown in the Gibbs plot in Fig. 3, the water samples of the phreatic and confined aquifers in the study area were mainly distributed in the rock weathering zone, indicating that rock weathering was the main factor regulating the hydrochemistry of groundwater in the study area. Some phreatic water samples falling into the evaporation-dominant zone indicate that the chemical composition of the groundwater is influenced by evaporation processes. In addition, as shown in Fig. 3 a, some of the water samples of the three aquifers were distributed in the middle right zone due to the Na+:(Na+ + Ca2+) ratio exceeding 0.5. This result indicated that groundwater in the study area may be affected by cation exchange, resulting in a high content of Na+. The water samples in the three aquifers in the study area showed similar distributions in the Gibbs plot, indicating that the same dominant factors regulate the hydrochemistry of phreatic water and confined water and that they have a close hydraulic connection. When compared to confined aquifers, the submerged aquifer is prone to significant evaporation resulting in elevated total dissolved solids (TDS) concentrations. This not only results in soil salinization but also has the potential to affect the water quality in the study area.
Sources of major ions
Mineral dissolution
The proportional coefficient diagram is an important tool for the analysis of sources of ion sources in groundwater. As shown in Fig. 4, the water samples of phreatic and confined aquifers in the study area were mainly concentrated in the lower right corner of the y = x line, indicating a high Na+ content in the study area, which can be attributed to albite dissolution and cation exchange.
As shown in Fig. 4 b, the water samples of unconfined and confined aquifers in the study area were mainly distributed above the y = x line, indicating that cation exchange may potentially have a significant influence on the chemical composition of groundwater, resulting in a decline in Ca2+ and Mg2+ and an increase in Na+. In addition, as observed from Fig. 5 c, the concentration of HCO3– is significantly higher than that of Ca2+, indicating that apart from carbonate minerals, HCO3– has additional sources. As shown in Fig. 4 d, some of the water samples are situated on the y = x line, indicating that gypsum dissolution is a significant source of Ca2+. Certain non-confined groundwater samples and Quaternary water samples lie above the reference line, implying that SO42− have additional sources. Due to the limited distribution of sulfide minerals in the study area, it is inferred that these SO42− may originate from anthropogenic activities. However, a majority of the water samples are located below the y = x line, suggesting that the primary source of Ca2+ is not gypsum dissolution but rather the dissolution of carbonates or silicates. Gaillardet et al. (1999) proposed ionic endmember diagrams based on the Ca2+:Na+, Mg2+:Na+, and HCO3-:Na+ ratios to determine the types of water-rock interaction affecting groundwater (silicate, carbonate, and evaporation). As shown in Fig. 4 e and f, groundwater in the study area was mainly located in the silicate weathering control zone, indicating that the hydrochemistry of the water samples in the study zone was mainly affected by silicate weathering. Some of the groundwater samples are influenced by evaporation processes. Due to the low abundance of carbonates and calcite saturation in the study area, the impact of carbonates on the water samples is minimal. Considering that the predominant silicate mineral in the study area is feldspar, the dissolution of feldspar can generate significant amounts of Ca2+, Na+, and HCO3−. Building upon the previous analysis, it can be inferred that Na+, Ca2+, and HCO3− in the study area primarily originate from the dissolution of feldspar. The relationship between Na++K+–Cl− and SO42−+ HCO3− –Ca2+–Mg2+ has been illustrated in Fig. 4 g, the groundwater phreatic and unconfined aquifer water samples in the ion diagram in the study area were distributed near the y = x line. The results showed that the Quaternary confined aquifer was most affected by albite dissolution, followed by the Neogene aquifer, and phreatic aquifer. The dissolution of feldspar can generate kaolinite, which has an affinity for ions and can facilitate cation exchange processes.As shown in Fig. 4 h, most of the phreatic and confined water samples in the study area were located in quadrant III, indicating that the hydrochemistry of the groundwater in the study area is mainly dominated by positive cation exchange. Figure 4 i shows the saturation state of concentrated minerals in the groundwater samples. Calcite and dolomite are saturated, while halite and gypsum are in an unsaturated state. Therefore, in the study area, the primary processes occurring are the dissolution of halite (NaCl) and feldspar, which contribute to the increase in Na+, Ca2+, and HCO3– concentrations and promote cation exchange reactions. Previous studies (Blasco et al. 2019) have indicated that the dissolution of halite enhances the dissolution of gypsum, the increased calcium ion concentration promotes the precipitation of calcite, and ultimately leads to dedolomitization and an increase in Mg2+.
Anthropogenic activities
Anthropogenic activities have had a significant impact on the water quality of the study area, with the discharge of domestic, industrial, and agricultural wastes and wastewater increasing the groundwater concentrations of Cl−, SO42−, and NO3−. The Cl− and SO42− in the groundwater samples were found to be mainly the result of evaporite dissolution and industrial activities, whereas groundwater NO3− mainly originated from domestic sewage and agricultural activities. The proportional coefficients of Cl−, NO3−, and Na+ were used to characterize the influence of anthropogenic activities on water quality. As shown in Fig. 5 a, some phreatic and Quaternary confined water samples was affected by agricultural activities, whereas agricultural activities had less of an impact on the Neogene groundwater. Figure 5 b demonstrates that the phreatic and the Quaternary confined water were affected by agricultural activities and domestic sewage, whereas the Neogene groundwater was less impacted by pollution. Previous studies have indicated that groundwater in the western plain region is influenced by the livestock manure and domestic wastewater causing higher content of TDS, NO3−, Cl−, SO42− in groundwater (Li et al. 2020). Human activities have impacted the water quality of groundwater in the study area, posing health risks to the residents.
Water quality assessment
To investigate the water quality in the study area, the ICWQI (the improved combined water quality index) method is used to assess the water quality and pollution. The combined weighting results are shown in Table 3. In order to illustrate the rationality of the improved method, this study combined the single factor index method and the traditional combined weight water quality index method to compare with the improved method.The “Groundwater Quality Standards for China (SGQC) (GB/T14848-2017)” are used as the standard for evaluating water quality in the study area, and for indicators (Ca2+, Mg2+, K+, HCO3−) not covered in the standard, constraints are applied based on the drinking water standards published by the World Health Organization (WHO). The water quality evaluation results derived from the three different methods are summarized in Table 4. Among the various ions, nitrate has the highest weight, implying that anthropogenic activities have a significant impact on water quality.
The results consistently indicate that the water quality of confined aquifers is superior to that of unconfined aquifers. The single-factor evaluation method only considers the influence of a single factor on water quality, leading to results that evaluate only poor water quality, namely, class IV (poor) and class V (very poor). The traditional combined weighted water quality index method incorporates both subjective and objective weights, aiming to enhance the overall reasonableness of the weight allocation. However, this method fails to consider the differences in the importance of different indicators in a single sample. This discrepancy may result in an assessment that is excessively optimistic and fails to align with the actual conditions.
To address these limitations, the proposed ICWQI method combines objective weights obtained through an improved weighting method with subjective weights derived from graded quantitative criteria values. The combined weights take into account both the overall and individual variability of the indicators, resulting in a more objective and accurate water quality evaluation. The water quality rating distribution of the ICWQI method is similar to that of the traditional combination of weighted WQI method but has both increased objectivity and sensitivity to individual sample indicator concentration. Thus, the ICWQI method provides results that are more aligned with the actual conditions.
Hence, the ICWQI method was employed in this study to further analyze the water quality in the study area based on the evaluation results. Figure 6 shows the distribution of groundwater water quality grades of the three aquifers in the study area. The groundwater quality grade in the study area ranges from class III to class V, with a small number of samples from the confined aquifer reaching class II. These samples are mainly found near the Tao’er River basin, where the groundwater flows faster and is renewed more frequently, resulting in better water quality. Conversely, areas with poorer water quality are primarily concentrated in the central plains and near saline zones, where groundwater accumulates in scattered lakes and is subject to strong evaporation. Meanwhile, agricultural land and livestock activities are relatively concentrated. The ionic components in the water become concentrated in the aquifer, leading to a deterioration in the water quality.
Notably, the water quality of the confined aquifer was found to be significantly better than that of the phreatic aquifer. However, the water quality of confined aquifers is significantly affected by pollution, leading to poor water quality conditions. Previous studies have indicated that in the western plain of Jilin, pollution in confined aquifers primarily originates from wastewater generated by human activities and contaminated recharge from shallow aquifers. Surface wastewater infiltrates into the confined aquifers through abandoned confined wells, resulting in contamination of the confined water. Additionally, excessive groundwater extraction due to human activities causes a decline in water levels, allowing infiltrating groundwater from unconfined aquifers to contaminate the confined aquifers.
Assessment of the risk of nitrate pollution to human health
The contamination of groundwater inevitably increases human health risks. The results of the present study showed that groundwater nitrates were greatly affected by anthropogenic activities and tended to accumulate in some areas. The concentration of groundwater nitrates in some regions of the study area exceeded the drinking water standard of the World Health Organisation (WHO) of 50 mg·L−1. The present study calculated the nitrate risk quotients according to Eqs. (15) to (19).The risk quotients for children, adult women, and adult men ranged between 0.00034 and 11.26, 0.00025 and 7.57, and 0.00019 and 5.77, respectively. The rank of age and sex categories according to their risks posed by groundwater nitrate was children > adult women > adult men. The mean values of the nitrate risk quotients for children, adult women, and adult men were 0.27, 0.18, and 0.14, respectively. On average, the nitrate risk quotient in the study area falls below the threshold. However, it is worth noting that some water samples in the study area exceeded the non-carcinogenic risk threshold by a significant margin.
Of the water samples, 14 phreatic water samples showed risk quotients that exceeded the non-carcinogenic risk thresholds for adult women and adult men by factors of 1.5 and 2, respectively. The hazard quotients for children, women, and men ranged between 1.23 and 11.26, 1.01 and 7.57, and 1.01 and 5.77, respectively. The hazard quotient for children exceeded those for adult women and adult men by factors of 1.5 and 2, respectively. Among the Quaternary confined water samples, the hazard quotient of three water samples exceeded the risk threshold for children, ranging between 1.28 and 2.42, whereas only one water sample exceeded the risk thresholds for adult women and adult men, at values of 1.63 and 1.24, respectively. All water samples of the Neogene aquifer showed hazard quotients < 1, indicating no non-carcinogenic risk. The non-carcinogenic risk posed by ingestion exceeded that of skin contact by a factor of 100. This result indicated groundwater nitrates in the study area pose a higher risk to the health of children. However, ingestion is the main source of the increased non-carcinogenic risk of nitrate.
As shown in Fig. 7, water samples of the phreatic aquifer in the study area showed a relatively large average nitrate risk quotient. These water samples were mainly distributed in densely populated areas with frequent industrial and agricultural activities in Qian’an and Tongyu. Lake marshes are widely distributed near these areas, and the poor water quality of these areas can be mainly attributed to agricultural activities and poor water flow, with high evaporation resulting in the concentration of nitrates. In comparison, the health risks associated with confined aquifers are significantly lower. The water samples from the confined aquifer with higher nitrate risk quotient were distributed in the saline-alkali land in northwest Zhenlai County and west and southwest Tongyu County. Due to the absence of widespread excessive nitrate levels in the confined aquifer, it can be inferred that the elevated nitrate content in individual confined aquifers is attributed to surface pollution infiltrating abandoned confined wells. These results showed that human activities pose significant health risks to groundwater, particularly to phreatic aquifers. The areas near lakes with intensive livestock farming exhibit the most apparent nitrate pollution. Surface contamination can potentially contaminate phreatic aquifers through abandoned confined wells. Therefore, implementing important measures such as controlling the use of pesticides and fertilizers, along with proper management of abandoned wells, is crucial for preserving the water quality of the local phreatic groundwater.
Spatial distribution of human health risks associated with groundwater nitrates of nitrates in the western plain of Jilin Province: a Health risks of phreatic groundwater to children; b health risks of phreatic groundwater to adult women; c health risks of phreatic groundwater to adult men; d health risks of Quaternary confined groundwater to children; e health risks of Quaternary confined groundwater to adult women; f health risks of Quaternary confined groundwater to adult men
Conclusions
This study conducted a systematic analysis of the hydrochemical characteristics, influencing factors, and the impact of human activities on groundwater in the semi-arid plains of western Jilin Province, northwest China. The conclusions are as follows:
-
(1)
Based on the statistical and graphical analysis of 13 water-quality indicators, it was found that the groundwater in the study area was weakly alkaline and dominated by the HCO3−·Ca–Mg and HCO3−–Na hydrochemical types in phreatic and confined water, respectively.
-
(2)
Rock weathering was the major mechanism controlling groundwater chemistry; the dissolution of halite, gypsum, and feldspar is the primary source of ions in the study area.
-
(3)
To evaluate the water quality of the study area, an improved combined weighted water quality index (ICWQI) was used. In comparison with other methods, the improved approach demonstrates better alignment with the actual circumstances. The research results reveal that nitrate exerts the greatest influence on water quality, indicating the significant impact of human activities on groundwater. Both phreatic and confined aquifers have been subjected to varying degrees of contamination, with pollution in confined aquifers primarily attributed to lateral groundwater flow and the infiltration of surface pollutants through abandoned confined wells..
-
(4)
The assessment using HHRA reveals that human activities have a significant impact on human health risks, particularly in phreatic groundwater. The results indicated that groundwater nitrates posed a higher risk to human health in the saline-alkali lands of Qian’an, Tongyu, and Zhenlai, with 15.9% of water samples exceeding the nitrate standard for children. Long-term exposure to or consumption of this groundwater thus poses a health risk to residents in the study area, particularly children. The lack of management in abandoned confined wells contributes to an increased health risk associated with elevated nitrate levels in certain confined water samples. Therefore, it is crucial to reduce the use of pesticides and fertilizers, as well as effectively manage abandoned confined wells, to ensure the protection of local groundwater resources.
Data availability
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abba SI, Abdulkadir RA, Sammen SS, Pham QB, Lawan AA, Esmaili P, Malik A, Al-Ansari N (2022) Integrating feature extraction approaches with hybrid emotional neural networks for water quality index modeling. Appl Soft Comput 114:1568–4946
Abbasnia A, Radfard M, Mahvi AH, Nabizadeh R, Yousefi M, Soleimani H, Alimohammadi M (2018) Groundwater quality assessment for irrigation purposes based on irrigation water quality index and its zoning with GIS in the villages of Chabahar, Sistan and Baluchistan, Iran. Data in Brief 19:623–631
Adeyeye OA, Xiao CL, Zhang ZH, Yawe AS, Liang XJ (2021) Groundwater fluoride chemistry and health risk assessment of multi-aquifers in Jilin Qianan, Northeastern China. Ecotoxicol Environ Saf 211:111926
Adimalla N, Li PY (2019) Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region, Telangana State, India. Hum Ecol Risk Assess 25:81–103
An Y, Lu W, Cheng W (2015) Surrogate model application to the identification of optimal groundwater exploitation scheme based on regression kriging method-a case study of Western Jilin Province. Int J Environ Res Public Health 12:8897–8918
Blasco M, Auque LF, Gimeno MJ (2019) Geochemical evolution of thermal waters in carbonate - evaporitic systems: the triggering effect of halite dissolution in the dedolomitisation and albitisation processes. J Hydrol 570:623–636
Bordalo AA, Teixeira R, Wiebe WJ (2006) A water quality index applied to an international shared river basin: the case of the douro river. Environ Manage 38:910–920
Brown RM, Mcclelland NI, Deininger RA, Tozer RG (1970) A water quality index—do we dare? Water Sewage Works 117:339–343
Cao H-c, Luan Z-q, Wang J-d, Zhang X-l (2009) Potential ecological risk of cadmium, lead and arsenic in agricultural black soil in Jilin Province, China. Stoch Env Res Risk Assess 23:57–64
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, Northwest China. Expos Health 8:349–359
Chen Y, Zhang Y, He J, Zhang J, Lang Q, Liu H, Wu C (2021) Assessment of groundwater quality and pollution in the Songnen Plain of Jilin Province, Northeast China. Water 13:2414
Ding F, Zhang WJ, Chen LY, Sun ZG, Li WP, Li CY, Jiang MC (2022) Water quality assessment using optimized CWQII in Taihu Lake. Environ Res 214:113713
Duan X (2013) Exposure factors handbook of Chinese population. China Environmental Press, Beijing
Gaillardet J, Dupre B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Gorgij AD, Wu JH, Moghadam AA (2019) Groundwater quality ranking using the improved entropy TOPSIS method: a case study in Azarshahr plain aquifer, east Azerbaijan, Iran. Hum Ecol Risk Assess 25:176–190
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess 25:32–51
Horton RK, Uddin J (1965) An index number system for rating water quality. J-Water Pollut Control Federation 37:300–305
Jha MK, Shekhar A, Jenifer MA (2020) Assessing groundwater quality for drinking water supply using hybrid fuzzy-GIS-based water quality index. Water Res 179:115867
Jianmin B, Yu W, Juan Z (2015) Arsenic and fluorine in groundwater in western Jilin Province, China: occurrence and health risk assessment. Nat Hazards 77:1903–1914
Li PY, Qian H, Wu JH (2010) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. E-J Chem 7:S209–S216
Li PY, Qian H, Howard KWF, Wu JH (2015) Building a new and sustainable “Silk Road economic belt.” Environ Earth Sci 74:7267–7270
Li F, Zhang SW, Yang JC, Bu K, Wang Q, Tang JM, Chang LP (2016a) The effects of population density changes on ecosystem services value: a case study in Western Jilin, China. Ecol Ind 61:328–337
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016b) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expos Health 8:331–348
Li MQ, Liang XJ, Xiao CL, Cao YQ, Hu SY (2019a) Hydrochemical evolution of groundwater in a typical semi-arid groundwater storage basin using a zoning model. Water 11:1334
Li PY, He XD, Li Y, Xiang G (2019b) Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese Loess Plateau: a case study of Tongchuan, Northwest China. Expos Health 11:95–107
Li M, Xiao C, Liang X, Cao Y, Hu S (2020) Hydrogeochemical evolution under a changing environment: a case study in Jilin, China. Water Supply 20:1653–1663
Li X, Li Y, Wang B, Sun Y, Cui G, Liang Z (2022) Analysis of spatial-temporal variation of the saline-sodic soil in the west of Jilin Province from 1989 to 2019 and influencing factors. Catena 217:106492
Lin NF, Bounlom V, Tang J, Bian JM (2005) Study on the relation between the formation of saline-alkali soil and the Neotectonic movement. Global Geol 03:282–288+311 (in Chinese)
Lu W, Chu H, Zhang Z (2015) Application of generalized regression neural network and support vector regression for monthly rainfall forecasting in western Jilin Province, China. J Water Supply Res Technol AQUA 64:95–104
Mladenovic-Ranisavljevic II, Takic L, Nikolic D (2018) Water quality assessment based on combined multi-criteria decision-making method with index method. Water Resour Manag 32:2261–2276
Naik MR, Mahanty B, Sahoo SK, Jha VN, Sahoo NK (2022) Assessment of groundwater geochemistry using multivariate water quality index and potential health risk in industrial belt of central Odisha, India. Environ Pollut 303:119161
Oukil A, Soltani AA, Zeroual S, Boutaghane H, Abdalla O, Bermad A, Hasbaia M, Boulassel M-R (2022) A DEA cross-efficiency inclusive methodology for assessing water quality: a composite water quality index. J Hydrol 612:128123
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–928
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. E-J Chem 6:523–530
Rashid T, Sabarathinam C, Al-Qallaf H, Bhandary H, Al-Jumaa M, Shishter A, Al-Salman B (2022) Evolution of hydrogeochemistry in groundwater production fields of Kuwait - inferences from long-term data. Chemosphere 307:135734
Subramani T, Rajmohan N, Elango L (2010) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ Monit Assess 162:123–137
Suherlina L, Newson J, Kamah Y, Brehme M (2022) The dynamic evolution of the Lahendong geothermal system in North-Sulawesi, Indonesia. Geothermics 105:102510
Tang M, Zeng H, Wang K (2022) Bayesian water quality evaluation model based on generalized triangular fuzzy number and its application. Environ Processes 9:6
Wang D, Wu J, Wang Y, Ji Y (2020) Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: analysis, assessment, and management. Expos Health 12:307–322
Wang XK, Xiao CL, Liang XJ, Li MQ (2022) Groundwater quality assessment in the northern part of Changchun City, Northeast China, using PIG and two improved PIG methods. Int J EnviroN Res Public Health 19:9603
Wei M, Wu J, Li W, Zhang Q, Su F, Wang Y (2022) Groundwater geochemistry and its impacts on groundwater arsenic enrichment, variation, and health risks in Yongning County, Yinchuan Plain of Northwest China. Expos Health 14:219–238
Xu P, Bian JM, Wu JJ, Li YH, Ding F (2020) Distribution of fluoride in groundwater around Chagan Lake and its risk assessment under the influence of human activities. Water Supply 20:2441–2454
Xu P, Bian JM, Wu JJ, Li YH, Li JL, Zeng X, Lin Z (2021) Simulation study on the migration of F- in soil around Chagan Lake, China. Environ Sci Pollut Res 28:45155–45167
Yan F, Qiao DY, Qian B, Ma L, Xing XG, Zhang Y, Wang XG (2016a) Improvement of CCME WQI using grey relational method. J Hydrol 543:316–323
Yan JH, Chen JS, Zhang WQ (2021) Study on the groundwater quality and its influencing factor in Songyuan City, Northeast China, using integrated hydrogeochemical method. Sci Total Environ 773:144958
Yan WW, Li JL, Bai XH (2016b) Comprehensive assessment and visualized monitoring of urban drinking water quality. Chemom Intell Lab Syst 155:26–35
Yang ZP, Lu WX, Long YQ, Li P (2009) Application and comparison of two prediction models for groundwater levels: a case study in Western Jilin Province, China. J Arid Environ 73:487–492
Zhai Y, Zheng F, Zhao X, Xia X, Teng Y (2019) Identification of hydrochemical genesis and screening of typical groundwater pollutants impacting human health: a case study in Northeast China. Environ Pollut 252:1202–1215
Zhang B, Hong M, Zhao YS, Lin XY, Zhang XL, Dong J (2003) Distribution and risk assessment of fluoride in drinking water in the west plain region of Jilin province, China. Environ Geochem Health 25:421–431
Zhang L, Hou GL, Zhang GX, Liu ZL, Sun GZ, Li MN (2016a) Calculation of wetlands ecological water requirement in China’s Western Jilin Province based on regionalization and gradation techniques. Appl Ecol Environ Res 14:463–478
Zhang L, Zhang GX, Li RR (2016b) Water quality analysis and prediction using hybrid time series and neural network models. J Agric Sci Technol 18:975–983
Zhang QY, Xu PP, Qian H (2020) Groundwater quality assessment using improved water quality index (WQI) and human health risk (HHR) evaluation in a semi-arid region of Northwest China. Expos Health 12:487–500
Zhao WB, Xiao CL, Chai YX, Feng XY, Liang XJ, Fang Z (2021) Application of a new improved weighting method, ESO method combined with fuzzy synthetic method, in water quality evaluation of Chagan Lake. Water 13:1424
Acknowledgements
We thank the anonymous reviewers and editors who contributed valuable comments, which were useful in improving the quality of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China, Research on the Impact of In-situ Oil Shale Exploitation on Groundwater Environment [project number 41572216], the China Geological Survey project, Regional water resources survey methods and groundwater ecological threshold survey research [project number DD20190340], Geological Exploration Fund of Jilin Province, and Geothermal Resources Survey in the Middle and West of Jilin Province [project number 2018–13].
Author information
Authors and Affiliations
Contributions
Linzuo Zhang: conceptualization, writing—original draft, data curation, and formal analysis. Xiujuan Liang: conceptualization, writing—review, supervision, and funding acquisition. Changlai Xiao: methodology, editing, and formal analysis. Weifei Yang: data curation, and reviewed and revised the manuscript. Jiang Zhang: software and validation. Xinkang Wang: methodology, editing, and formal analysis.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Liang, X., Xiao, C. et al. Hydrochemical characteristics and the impact of human activities on groundwater in a semi-arid plain: a case study of western Jilin Province, Northeast China. Environ Sci Pollut Res 30, 110204–110219 (2023). https://doi.org/10.1007/s11356-023-29603-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29603-5