Abstract
Accurate and reliable point of care tests (POCTs) for hemoglobin estimation is essential for early diagnosis and management of anemia. This study was aimed to assess the reliability and validity of two versions of digital hemoglobinometer (HemoCue 201+ and HemoCue 301) compared to the gold standard Sysmex autoanalyzer for hemoglobin estimation. Pregnant women attending antenatal clinics of Primary Health Centre and a Sub Divisional Hospital in Haryana, India, were recruited. After obtaining consent, capillary blood samples were collected and tested for hemoglobin levels with digital hemoglobinometers (HemoCue 201+ and HemoCue 301). Among same pregnant women venous blood was collected and hemoglobin levels were estimated using autoanalyzer. Validity and reliability of POCTs compared to Sysmex autoanalyzer were reported. Of the 102 pregnant women included in the study, 44 (43%) were primigravida, with mean (SD) age of 23.3 (3.4) years. The mean (SD) of difference in hemoglobin levels using HemoCue 201+ was − 0.53 (1.01) and using HemoCue 301 was − 0.25 (0.85) g/dL as compared to auto-analyzer. Lin’s concordance coefficient was 0.80 for HemoCue 201+ and 0.85 for HemoCue 301. Weighted Cohen’s Kappa indicated moderate degree of agreement with the gold standard. Sensitivity (HemoCue 201+: 93%; HemoCue 301: 90%) and specificity (HemoCue 201: 76% HemoCue 301: 80%) for detecting anemia was similar for both the POCT devices. The digital hemoglobinometers used in the study had moderate degree of agreement and concordance with the autoanalyzer for hemoglobin estimation. HemoCue 301 had higher validity as compared to HemoCue 201+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia, a severe public health problem in India, accounting for 1.1% Gross Domestic Product loss and is the leading cause of years lived with disability among both the genders in the country [1, 2]. National Family Health Survey reports that half of pregnant women are anemic in India and around 1% of them are severely anemic [3]. The adverse health consequences of anemia among pregnant women and the fetus are well established and include poor quality of life of pregnant women, increased postpartum bleeding, maternal mortality, intrauterine growth retardation, pre term delivery, low birth weight, fetal distress, cognitive defects, still birth and neonatal mortality [4,5,6].
Accurate and rapid diagnosis of anemia is crucial for appropriate management of anemia. World Health Organization (WHO) recommends full blood count for the diagnosis of anemia in routine antenatal care [7]. In resource limited settings (Low and Middle Income Countries) the laboratory options for full blood count is limited especially in peripheral health institutions. Hence point of care testing (POCT) needs to be employed in these settings for diagnosis and management of anemia. Point-of-care testing (POCT) is when the laboratory tests are performed close to the patient i.e. at or near the site of provision of clinical care using easy operable analyzers [8]. The commonly available POCT methods for detection of anemia are Indirect cyanmethaemoglobin method, Copper sulphate specific gravity method, Sahli’s method, WHO’s Hemoglobin Color Scale, digital hemoglobinometers (HemoCue 201, TrueHb, HemoCue 301 and DiaSpect).
Cyanmethemoglobin method still remains the gold standard for estimation of hemoglobin. However, the operational factors such as time requirement, operator dependent procedure and use of toxic reagents limits the usage of Cyanmethemoglobin method as POCT [9]. Hence, accurate, operator friendly POCT equipment which provide results in short time interval is the need of the hour in India. Digital hemoglobinometers are commonly being used as POCT for screening of anemia in the field and blood donation facilities and provide results instantly, enabling immediate management at the point of care [3, 10, 11].
The results of available validation studies with digital hemoglobinometer have been equivocal with some studies reporting good correlation and agreement while other reporting less than satisfactory results [12,13,14,15]. Most of the available studies have reported operational difficulties with use of HemoCue 201+, such as sensitivity to temperature, moisture and humidity of the reagent coated microcuvettes, inaccurate results due trapping of air bubbles during loading of the cuvette, cuvettes malfunctioning within days to weeks of opening the cuvette boxes and long time lag in reading the results by the device [16, 17].
The new POCT device (HemoCue 301) claims to have overcome these challenges as it operates under wider temperature range (10–40 °C) with reagent free microcuvettes which is not affected by moisture and does not require any specific storage conditions [18]. However, there are limited studies available globally or from India reporting the validity of this new POCT device (HemoCue 301).
The objective of this study was to assess validity and reliability of digital hemoglobinometers, HemoCue 201+ and 301 (using capillary blood sample) as compared with Sysmex auto-analyzer (based on Sodium Lauryl Sulphate method) (using venous blood) for hemoglobin estimation among the pregnant women attending Antenatal care clinics in North India.
Methods
Study Setting and Study Participants
The study was conducted in antenatal clinics of a Primary Health Centre (PHC) and a Sub Divisional Hospital (SDH) in Haryana state, India. Antenatal clinic was held once week in PHC, and thrice in a week in SDH. The average annual Antenatal clinic load was 500 pregnant women in PHC and 8000 pregnant women in SDH. The mean (range) temperature and relative humidity (Rh) in the study area during data collection period was 31 °C (range 26–36 °C) and 76% (range 53–100%) respectively.
Study Duration
The study was conducted in the month of August 2018.
Description of Devices
HemoCue 201+ (HemoCue AB, Ängelholm, Sweden): [19] is a portable battery-operated system that works on the principle of modified azidemethemoglobin determination. The microcuvette contains dried reagents (sodium deoxycholate to hemolyze red blood cells, sodium nitrite for conversion of hemoglobin to methaemoglobin and sodium azide for conversion of methemoglobin to azidemethemoglobin). The absorbance is then read at two wavelengths of 570 nm and 880 nm for turbidity compensation in the blood sample and hemoglobin level is estimated. HemoCue 301 (HemoCue AB, Ängelholm, Sweden): [20] The HemoCue Hb 301 is also a battery-operated system and estimates hemoglobin by measuring the absorbance of whole blood at the Hb/HbO2 isobestic points at the wave length of 506 nm and 880 nm for compensation of turbidity. Both HemoCue devices require only 10 µL amount of blood and the turnover time per sample is significantly less when compared to the automated analyzer.
Hematology auto-analyzer (Sysmex XS 1000i, Kobe, Japan) is a flow cytometry works that on the principle of non-cyanide, Sodium Lauryl Sulphate (SLS) method for hemoglobin estimation.
In this study, capillary blood samples were collected from finger-tip for estimation of hemoglobin in digital hemoglobinometer and venous blood sample from ante cubital fossa for Sysmex auto analyzer.
Sample Size and Sampling Method
Considering bias (Standard Deviation of difference) as 0.87 (0.27) alpha error of 5%, power 80% and maximum allowed difference 1.54, the minimum required sample size was 102 with 10% non-response rate [15, 21]. Fifty-one pregnant women were recruited from each of the two selected sites. The pregnant women were recruited using convenient sampling and all eligible pregnant women were enrolled consecutively.
Methodology
Pregnant women irrespective of trimester or parity registered for antenatal care (ANC) in either of the selected sites were included. No other exclusion criteria like trimester or parity were followed in this study. Pregnant women attending the ANC were assessed for eligibility and written informed consent was obtained. A trained nurse collected the data on socio-demographic and obstetric details using a pre-tested semi-structured interview schedule. Capillary blood samples were collected by a Senior Resident doctor and venous blood was collected by a trained laboratory technician.
Collection of Blood Samples
The microcuvettes of HemoCue 201+ and HemoCue 301 were filled from second and third drop of capillary blood (from middle or ring finger) for hemoglobin estimation. Both the microcuvettes were filled for all pregnant women and the order of cuvettes was alternated so as to overcome any bias that may arise due to difference in sequence drop of blood. Manufacturer’s guidelines on use of microcuvettes were followed.
Similarly, under strict aseptic precautions 2 ml of venous blood were collected from all pregnant women. All the venous samples were analyzed in the laboratory of SDH on the same day, within 6 h of blood sample collection.
Quality Control
Internal quality control (IQC) was run on daily basis for both digital hemoglobinometers and Sysmex autoanalyzer. All three levels of IQC samples (normal, low and high) were checked and plotted in Levy Jennings (LJ) plot. All values were found to be within the 2 standard deviation range in LJ plot.
The hemoglobin status of the pregnant women was shared with the medical officer/nurse of the health centers. Those who were identified with anemia by auto analyzer, were referred for treatment of anemia under routine antenatal care.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Ethics Committee of All India Institute of Medical Sciences, New Delhi (Institute Ethics Committee approval number: IEC-380/06.07.2018) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed Consent
Written informed consent was obtained from all pregnant women.
Statistical Analysis
Data were entered in Epicollect5 mobile application and analyzed using Stata 12 software (Stata Corp. 2011, College Station, TX: Stata Corp LP). Mean (SD) of hemoglobin estimated in HemoCue 201+, 301 and Sysmex auto analyzer were compared. Paired analysis was done between hemoglobin values of HemoCue 201+ versus Sysmex auto-analyzer and HemoCue 301 versus Sysmex auto-analyzer. The mean difference (SD) between the HemoCue and autoanalyzer and limits of agreement were calculated based on Bland Atman plot. Lin’s concordance coefficient and intra class correlation coefficient were calculated with 95% confidence interval. ROC curve was plotted for both digital hemoglobinometers, to compare the area under the curve for diagnosis of anemia using Z statistics. Weighted Kappa was calculated to assess the agreement of digital hemoglobinometer for grades of anemia compared to Sysmex auto analyzer. Sensitivity, specificity, positive and negative predictive values were calculated to assess the validity of the equipment. p value of < 0.05 was considered as cut off for ascertaining statistical significance.
Results
A total 102 pregnant women (51 each from PHC and SDH) were included in the study. The mean (SD) age of the pregnant women was 23.3 (3.4) years. Of the 102 pregnant women, 44 (43%) were primigravida.
The mean (95% CI) of hemoglobin (g/dL) values using HemoCue 201+ was 10.2 (9.9–10.5), HemoCue 301 was 10.5 (10.2–10.8) and Sysmex was 10.7 (10.4–11.0). There was statistically significant difference between the HemoCue values compared with Sysmex (HemoCue 201 vs. Sysmex p < 0.001; HemoCue 301 vs. Sysmex p = 0.003; paired t test). The prevalence of anemia (< 11g/dL) determined by HemoCue 201+ was 63% (95% CI 53–72%), HemoCue 301 was 59% (95% CI 49–68%) and Sysmex autoanalyzer was 56% (95% CI 46–66%). However, there was no statistically significant difference in prevalence of anemia between HemoCue and Sysmex (HemoCue 201+ vs. Sysmex p = 0.318; HemoCue 301 vs. Sysmex p = 0.671). In the subgroup analysis based on the sequence of blood drop used, similar results were observed in prevalence of anemia between both HemoCue and Sysmex (p > 0.05). HemoCue 201+ reported higher prevalence of anemia for 3rd drop (73%) compared to 2nd drop (53%), (p = 0.041). No difference in prevalence of anemia between second and third blood drops was detected with HemoCue 301. There was no significant difference between mean (SD) hemoglobin values determined by both HemoCue and Sysmex in regard to the sequence of blood drop (Table 1). The sensitivity was 93%, specificity was 76%, positive predictive value was 82% and negative predictive value was 90% for HemoCue 201+ for detection of anemia at the cutoff of 11 g/dL compared to the gold standard Sysmex. HemoCue 301 had 90% sensitivity, 80% specificity, 85% positive predictive value and 86% negative predictive value.
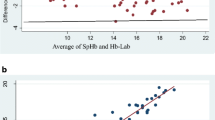
Bland–Altman plot was plotted comparing the hemoglobin values estimated by digital hemoglobinometers against Sysmex autoanalyzer. HemoCue 301 had less bias (HemoCue 301: − 0.25 vs. HemoCue 201: − 0.43) and narrow limits of agreement ([− 1.93 to 1.42] vs. [− 2.52 to 1.45]) compared to HemoCue 201+. Similar pattern of agreement was also observed in the subgroup analysis of digital hemoglobinometers based on sequence of blood drop (Fig. 1 and Table 2). Figure 2 illustrates the Pearson correlation coefficient and Lin’s concordance coefficient for digital hemoglobinometers compared with Sysmex. HemoCue 301 was found to have relatively better correlation (Lin’s concordance coefficient = 0.83; p < 0.001) than HemoCue 201+ (Lin’s CCC = 0.74; p < 0.001).
The intra class correlation coefficient for HemoCue 201+ and 301 were 0.75 (95% CI 0.55–0.85) and 0.91 (95% CI 0.75–0.87) respectively. The area under the curve (AUC) for HemoCue 201+ and 301 were 0.94 and 0.91 and there was no significant difference between the AUCs (p = 0.393) for the two devices.
The Cohen’s Kappa coefficient for HemoCue 201+ in diagnosing anemia (Hb < 11g/dL) compared to gold standard Sysmex was 0.697 indicating moderate degree of agreement. The kappa value for HemoCue 301 was 0.70 (moderate degree of agreement). The weighted kappa for HemoCue 201+ (weighted k = 0.65 [95% CI 0.50–0.75]) and 301 (weighted k = 0.67 [95% CI 0.51–0.83]) indicates moderate agreement. The number of pregnant women with no anemia, mild and moderate anemia determined by Sysmex were 45 (44%), 28 (27%) and 29 (28%) respectively. None of the pregnant women were diagnosed with severe anemia in Sysmex, whereas HemoCue 201+ (n = 5 [5%]) and 301 (n = 1 [1%]) reported severe anemia among the pregnant women.
Discussion
Digital hemoglobinometers used in this study had high sensitivity, acceptable specificity, moderate agreement and moderate concordance with the gold standard Sysmex autoanalyzer in estimating hemoglobin. Among the two different versions of the digital hemoglobinometer, HemoCue 301 was found to have relatively better validity and reliability compared to HemoCue 201+. Overall prevalence of anemia estimated by HemoCue 201+ (63%) and HemoCue 301 (59%) was higher than that estimated with Sysmex autoanalyzer (56%), however these estimates were not different statistically. The digital hemoglobinometers underestimated hemoglobin levels by 0.25 (95% CI 0.09–0.42) g/dL in HemoCue 301 and by 0.53 (95% CI 0.33–0.73) g/dL in HemoCue 201+.
HemoCue 301 reported mean (SD) of hemoglobin values closer to Sysmex (compared to HemoCue 201+), yet there was statistically significant difference. Earlier studies comparing digital hemoglobinometer with auto-analyzers have reported varied results with both underestimation and overestimation of the hemoglobin levels [15, 22]. A study conducted in Cambodia has reported similar results compared to our study [23]. But a study conducted among young children in USA and blood donors in Netherlands reported high mean (SD) values with HemoCue 301 compared to autoanalyzer [15, 24]. The difference in the age group, physiological conditions of the study participants, geographic regions and instrumental errors in the devices might have contributed for such differences. The possible reason for observed underestimation of Hb level by digital hemoglobinometers in this study might also be due to the Fåhræus effect which results in lower concentration of red blood cells in the capillaries compared to the venous vessels.
The minor underestimation of hemoglobin levels is not likely to be of much clinical significance, because it will not affect the overall management of the anemia. The observed bias of 0.25–0.53 g/dL in the study is lower than WHO acceptable level of bias of 1 g/dL for point of care of tests [25]. In case of surveys, due to clustering of measurements around cut off values, overall estimates of anemia might vary to significant extent. The deviation in anemia prevalence and mean hemoglobin estimation can be overcome by use of correction factors as has been proposed by several earlier studies also [26].
The prevalence of anemia was relatively higher, though not statistically significant. HemoCue 201+ has been widely used in National surveys of India for estimation of burden of anemia. HemoCue 201+ requires temperature in the range of 15–30 °C for the optimal functioning and stability of the reagents and is prone to error outside these temperature ranges. Digital hemoglobinometers requiring such narrow temperatures ranges may not be a good option in tropical countries like India. HemoCue 301 is a recently introduced reagent free digital hemoglobinometer and can function under wider range of temperature (0–40 °C). The price of HemoCue 301 equipment is higher as compared to HemoCue 201+ (INR 5000 difference per equipment), but the reagent free microcuvettes of 301 are less expensive (INR 1–3 difference per microcuvette), thus overall cost comes out to be similar [18]. Based on these observations, HemoCue 301 might be a better POCT, than HemoCue 201+.
The strengths of the study included minimal inter-observer, and inter-instrument error. The venous blood sample was collected within few minutes of capillary blood collection thus minimizing any within person variations. Adequate quality control of the equipment was in place and monitored daily. The internal quality control of the all three equipment (digital hemoglobinometer as well as the autoanalysers) was done daily for all three levels of control and found to be within 2(SD) in Levy Jennings plot. The pragmatic approach of comparing capillary (digital hemoglobinometer) and venous (auto-analyzer) was adopted in the study to closely reflect the real-life use of these devices. The study limitations included generalisability being limited to a specific group of population (pregnant women), whose physiological conditions may be different from general population. We had no severe anemia cases; and therefore, the findings of the study may not be valid for pregnant women with severe anemia. The study was conducted in a healthcare facility setting by trained laboratory personnel. Thus, the results may change in community setting where frontline workers use this instrument.
Conclusion
Digital hemoglobinometers were found to have moderate agreement in estimation of hemoglobin as compared to hematology autoanalyzer. HemoCue 301 was found to have better concordance and agreement than HemoCue 201+. Further studies across various age groups, in community setting and use by frontline workers are required to strengthen the evidence base on validation of digital hemoglobinometer.
References
Indian Council of Medical Research, Public Health Foundation of India, Institute for Health Metrics and Evaluation (2017) Health of the Nation’s States, India
Stein AJ, Qaim M (2007) The human and economic cost of hidden hunger. Food Nutr Bull 28:125–134
Ministry of Health and Family Welfare, Government of India. International Institute of Population Sciences (IIPS). National Family Health Survey 4. Fact sheets. [cited 2018 Jul 6]. http://rchiips.org/nfhs/factsheet_NFHS-4.shtml
Daru J, Zamora J, Fernández-Félix BM, Vogel J, Oladapo OT, Morisaki N et al (2018) Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob Heal 6(5):e548–e554
Breymann C, Honegger C, Hösli I, Surbek D (2017) Diagnosis and treatment of iron-deficiency anaemia in pregnancy and postpartum. Arch Gynecol Obstet 296(6):1229–1234
Brabin BJ, Hakimi M, Pelletier D (2001) An analysis of anemia and pregnancy-related maternal mortality. J Nutr 131(2):604S–615S
World Health Organization (2018) WHO recommendation on the method for diagnosing anaemia in pregnancy | RHL. [cited 2018 Aug 13]. https://extranet.who.int/rhl/topics/preconception-pregnancy-childbirth-and-postpartum-care/antenatal-care/who-recommendation-method-diagnosing-anaemia-pregnancy
Nichols JH (2007) Point of care testing. Clin Lab Med 27(4):893–908
Davis BH, Jungerius B (2010) International Council for Standardization in Haematology technical report 1-2009: new reference material for haemiglobincyanide for use in standardization of blood haemoglobin measurements. Int J Lab Hematol 32(2):139–141
Nkrumah B, Nguah SB, Sarpong N, Dekker D, Idriss A, May J et al (2011) Hemoglobin estimation by the HemoCue® portable hemoglobin photometer in a resource poor setting. BMC Clin Pathol 11(1):5
Litton E, Xiao J, Ho KM (2013) Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ 15(347):f4822
Bäck S, Magnusson CGM, Norlund LK, von Schenck HH, Menschik ME, Lindberg PES (2004) Multiple-site analytic evaluation of a new portable analyzer, Hemocue Hb 201+, for point-of-care testing. Point Care 3(2):60–65
Schapkaitz E, Mahlangu JN, Letsoalo M (2012) Point-of-care estimation of haemoglobin concentration in neonates and infants. S Afr J Child Health 6(1):10–11
Daves M, Cemin R, Zagler EM, Joos A, Platzgummer S, Hueber R et al (2016) Evaluation of capillary haemoglobin determination for anaemia screening in blood donation settings. Blood Transfus 14(5):387–390
Hinnouho G-M, Barffour MA, Wessells KR, Brown KH, Kounnavong S, Chanhthavong B et al (2018) Comparison of haemoglobin assessments by HemoCue and two automated haematology analysers in young Laotian children. J Clin Pathol 71(6):532–538
Henderson MA, Irwin MG (1995) High humidity affects HemoCue microcuvette function. Anaesth Intensive Care 23(3):407
Nguyen HT (2002) High humidity affects HemoCue cuvette function and HemoCue haemoglobin estimation in tropical Australia. J Paediatr Child Health 38(4):427–428
Morris LD, Osei-Bimpong A, McKeown D, Roper D, Lewis SM (2007) Evaluation of the utility of the HemoCue 301 haemoglobinometer for blood donor screening. Vox Sang 93(1):64–69
HemoCue Hb 201+ Operating Manual Manuel d’utilisation
HemoCue. Point-of-care anemia screening—HemoCue® Hb 301 System—HemoCue. [cited 2018 Jul 17]. https://www.hemocue.in/en-in/solutions/hematology/hemocue-hb-301-system
MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium). Sample size calculation: Bland–Altman plot. [cited 2018 Aug 20]. https://www.medcalc.org/manual/sampling_blandaltman.php
Levy TS, Mendez-Gomez-Humaran I, Ruan MDCM, Tapia BM, Hernandez SV, Avila MH (2017) Validation of Masimo Pronto 7 and HemoCue 201 for hemoglobin determination in children from 1 to 5 years of age. PLoS ONE 12(2):1–9
Rappaport AI, Karakochuk CD, Whitfield KC, Kheang KM, Green TJ (2017) A method comparison study between two hemoglobinometer models (Hemocue Hb 301 and Hb 201 +) to measure hemoglobin concentrations and estimate anemia prevalence among women in Preah Vihear, Cambodia. Int J Lab Hematol 39:95–100
Baart AM, de Kort WLAM, van den Hurk K, Pasker-de Jong PCM (2016) Hemoglobin assessment: precision and practicability evaluated in the Netherlands—the HAPPEN study. Transfusion 56:1984–1993
World Health Organization, Regional Office for the Eastern Mediterranean (2000) Selection of basic laboratory equipment for laboratories with limited resources. [cited 2018 Dec 8]. http://apps.who.int/iris/handle/10665/119641
Bansal PG, Toteja GS, Bhatia N, Gupta S, Kaur M, Adhikari T et al (2016) Comparison of haemoglobin estimates using direct indirect cyanmethaemoglobin methods. Indian J Med Res 144(4):566–571
Acknowledgements
We acknowledge the staff nurses, Auxiliary Nurse Midwifes and the laboratory technicians of Primary Health Centre, Dayalpur and Sub Divisional Hospital (SDH), Ballabgargh, Faridabad, Haryana for their support in conducting the study.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
KY contributed in designing the study, interpretation of results and final approval of the study. SK contributed in designing the study, providing critical comments on the manuscript and final approval of the study. RG collected the data and drafted the manuscript. FA contributed in designing the study and collection of data. OMJ contributed in designing the study. HV collected data. RK, SM and PK contributed in final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yadav, K., Kant, S., Ramaswamy, G. et al. Validation of Point of Care Hemoglobin Estimation Among Pregnant Women Using Digital Hemoglobinometers (HemoCue 301 and HemoCue 201+) as Compared with Auto-Analyzer. Indian J Hematol Blood Transfus 36, 342–348 (2020). https://doi.org/10.1007/s12288-019-01196-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-019-01196-5