Abstract
Introduction
Iron-mediated oxidative damage has been implicated in the genesis of cerebral vasospasm in animal models of SAH. We sought to explore the relationship between levels of non-protein bound iron in cerebrospinal fluid and the development of brain injury in patients with aneurysmal SAH.
Methods
Patients admitted with aneurysmal subarachnoid hemorrhage to a Neurointensive care unit of an academic, tertiary medical center, with Hunt and Hess grades 2–4 requiring ventriculostomy insertion as part of their clinical management were included in this pilot study. Samples of cerebrospinal fluid (CSF) were obtained on days 1, 3, and 5. A fluorometric assay that relies on an oxidation sensitive probe was used to measure unbound iron, and levels of iron-handling proteins were measured by means of enzyme-linked immunosorbent assays. We prospectively collected and recorded demographic, clinical, and radiological data.
Results
A total of 12 patients were included in this analysis. Median Hunt and Hess score on admission was 3.5 (IQR: 1) and median modified Fisher scale score was 4 (IQR: 1). Seven of 12 patients (58 %) developed delayed cerebral ischemia (DCI). Day 5 non-transferrin bound iron (NTBI) (7.88 ± 1 vs. 3.58 ± 0.8, p = 0.02) and mean NTBI (7.39 ± 0.4 vs. 3.34 + 0.4 p = 0.03) were significantly higher in patients who developed DCI. Mean redox-active iron, as well as day 3 levels of redox-active iron correlated with development of angiographic vasospasm in logistic regression analysis (p = 0.02); while mean redox-active iron and lower levels of ceruloplasmin on days 3, 5, and peak concentration were correlated with development of deep cerebral infarcts.
Conclusions
Our preliminary data indicate a causal relationship between unbound iron and brain injury following SAH and suggest a possible protective role for ceruloplasmin in this setting, particularly in the prevention of cerebral ischemia. Further studies are needed to validate these findings and to probe their clinical significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent evidence suggests that oxidative injury due to hemoglobin (Hb) and iron overload is a major contributor to neuronal damage following intracerebral hemorrhage (ICH) [1], and an iron-chelating drug is being tested as a potential therapeutic intervention in ICH patients [2, 3]. In aneurysmal subarachnoid hemorrhage (SAH), erythrocytes lyse in the subarachnoid space and expose the brain to high concentrations of Hb [4]. The catabolism of heme, mediated by heme-oxygenase, leads to the release of iron, which easily overwhelms the homeostatic mechanisms normally present in the CNS [5].
In humans, iron can be found either tightly bound to carrier proteins (i.e. transferrin) or liberated/translocated (also referred to as “free iron”) [6]. The reduction of bound ferric iron from carrier proteins to its “free” ferrous form is likely accomplished by the acidic pH and cathecolamines found in the extracellular fluid following brain injury [7]. In its reduced (i.e. ferrous) form, iron is free to participate in the Fenton reaction leading to the formation of reactive oxygen species (ROS) and subsequent lipid peroxidation, which have been implicated in the etiology of vasospasm (VSP) and neuronal injury following experimental subarachnoid hemorrhage (SAH) [8].
Non-transferrin bound iron (NTBI) encompasses all forms of the metal that are not tightly associated with transferrin. Redox-active iron (REDOX-Fe) represents the component of NTBI that is both redox-active (ferrous) and chelatable [9]. No clinical studies have evaluated the role of non-protein bound iron in SAH and iron-mediated neurotoxicity following aneurysm rupture represents a promising therapeutic target that thus far remains unexplored. In this pilot study, we set out to examine the changes in REDOX-Fe and NTBI, as well as the concentration of the main iron-handling and regulating proteins in the cerebrospinal fluid (CSF) of patients over the first 5 days following SAH.
Methods
Subjects
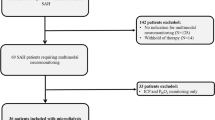
This study was reviewed and approved by the local institutional review board and consent for enrollment was obtained from a next of kin or a legal representative if patients were deemed unfit to provide informed consent. Consecutive eligible patients between the ages of 18 and 80 years admitted to the Cleveland Clinic Neurointensive care unit following aneurysmal SAH were included. Only patients with Hunt and Hess clinical grades between 2 and 4 at presentation, with a clear time of onset of symptoms, and requiring insertion of an external ventricular drain (EVD) were considered for participation in this pilot study. Clinical management was at the discretion of the neurocritical care and neurosurgical teams and consistent with published guidelines and current standards of care [10, 11]. Demographic and clinical data were prospectively collected and recorded.
Demographic, Clinical, and Radiographic Data
Age, gender, past medical history, baseline and 90-day modified Rankin scale (mRS), admission systolic (SBP) and mean arterial pressure (MAP), core body temperature, Glasgow coma scale (GCS), Hunt and Hess (HHS), modified Fisher scale (mFS), and aneurysm location and size (on its largest axis) were recorded.
Episodes of clinical deterioration secondary to delayed cerebral ischemia (DCI) defined as any focal neurological impairment or GCS reduction >2 points lasting at least 1 h and not attributable to any other condition [12] were documented, as was the initiation of hypertensive hypervolemic therapy (HHT) in response to DCI. Duration of EVD and eventual need for ventriculo-peritoneal shunt was also recorded and subsequent outpatient assessment was performed at 90 days from hospital admission.
Functional status was assessed by means of the mRS by certified investigators during an outpatient visit; for the purpose of the present study an mRS score of 0–2 was considered good functional outcome, while a score of 3 or higher, a poor one. Acute cerebral infarcts (CI) present on diffusion-weighted (DWI) sequences from magnetic resonance imaging (MRI) obtained as part of clinical care were recorded following a previously validated classification that has been shown to correlate with clinical outcomes [37] and that relies on the number and location of positive DWI lesions [13]. Cerebral angiograms were reviewed by two interventional neurologists for the presence of radiographic vasospasm, defined as a reduction of at least 50 % in vessel caliber in one or more vascular axis. Investigators recording clinical and radiographic data were blinded to results of CSF analysis.
CSF Samples
Samples were obtained at 24 ± 8, 72 ± 8, and 120 ± 8 h from onset of symptoms. Fluid was obtained from the burette attached to the EVD system, allowing only sampling of CSF drained during the preceding hour. A total of 6 ml were collected at each time point and immediately centrifuged at 2,000×g for 10 min. The supernatant was aliquoted in small polypropylene cryovials and stored at −80 °C. Protease inhibitor (Halt, Thermo scientific) was added a priori to the cryovials destined for protein determination [14].
Samples of CSF obtained from four patients who had lumbar drain insertion and fluid drainage to rule out the presence of normal pressure hydrocephalus and that resulted normal and free from pathological alterations after testing analysis were used as controls for the determination of iron and protein concentrations for comparison purposes.
Redox-Active Iron and Non-transferrin Bound Iron Determination
Both REDOX-Fe and NTBI were determined by a modification of protocol previously described for labile plasma iron [15]. Briefly, quadruplicates of 10 μl of CSF were transferred to clear-bottom, 384-well plates (ThermoFisher Scientific, USA). Iron-free HEPES-buffered saline (90 μl; HBS; HEPES 20 nM, NaCl 150 mM, pH 7.4) containing 40 μM ascorbate and 50 μM DHR (dihydrorhodamine 123, di-hydrochloride salt, Sigma-Aldrich, St Louis, MO, USA) was added to two of the wells. Ninety microliters of the same solution containing 50 μM of the iron chelator deferiprone (L1; Sigma-Aldrich, St Louis, MO) was added to the other two wells. Following reagent addition, the kinetics of fluorescence increase were followed at 37 °C in a Spectramax M2e microplate reader (Molecular Devices, Sunnyvale, CA) with a 485/538 nm excitation/emission filter pair, for 40 min, with readings every two minutes. The slopes (r) of DHR fluorescence intensity with time were calculated from measurements taken between 15 and 40 min and were recorded as FU/min (fluorescence units per minute). The duplicate values of r in the presence and absence of L1, r L1 and r, respectively, were averaged and the redox-active CSF iron (REDOX-Fe) concentration was determined from calibration curves relating the difference in slopes with and without L1 against Fe concentration: REDOX-Fe = ∆r/r st = (r – r L1 )/r st , where ∆r and r st denote the L1 sensitive component of r and the calibration factor relating ∆r to the Fe concentration, respectively. Calibration curves were prepared by serial dilutions of Fe:nitriloacetate, (1:7, mol:mol) to give final concentrations of 5, 2.5, 1.25, 0.625, 0.313, 0.156, and 0.078 μM Fe in HBS buffer containing 0.3 mg/ml bovine serum albumin.
For the calculation of NTBI, the process described above was repeated, but nitriloacetic acid (NTA; 0.5 μM; Sigma-Aldrich, St Louis, MO), a weak iron mobilizer was added to the well prior to the measurement of the kinetics of fluorescence. Cerebrospinal fluid REDOX-Fe and NTBI are expressed in micromolar (μM).
Iron-Handling Proteins Determination
Measurements of ferritin, transferrin, lactoferrin, ceruloplasmin, (Abcam ELISA kits; Cambridge, MA), and pro-hepcidin (MyBiosource, San Diego, CA) in CSF were performed using enzyme-linked immunosorbent assays and the concentrations expressed following the standards set by the International System of Units (SI Units).
Statistical Analysis
All values for CSF tests are expressed as mean + standard error of the mean. Comparison between SAH patients and control subjects and between patients who developed DCI and those who did not was made using the Wilcoxon Rank Sum test (assuming a non-parametric distribution of data) and intergroup comparisons among the 3 groups were determined by 1-way analysis of variance (ANOVA). Student’s T test was used to compare means of normally distributed data, bivariate analysis, and logistic regression to explore relationships between continuous variables and Fisher’s exact test for analysis of categorical datasets. For statistical purposes, the 90-day mRS was dichotomized to < 2 to define favorable functional outcome and > 2 for unfavorable outcome. A p value of < 0.05 was considered statistically significant. All the computations were done using JMP 10.0 and SAS 9.2 (SAS Institute, INC.)
Results
Demographic and Clinical Data
Fourteen patients with SAH were initially consented, but only 12 are included in the present analysis. One patient was made comfort care status within 24 h of admission and another had malfunction of the ventriculostomy system that lead to early discontinuation of the EVD system. Table 1 summarizes the demographic and clinical characteristics of our patients overall and by DCI status. The majority of patients were women (83 %) with a mean age of 57.5 + 6.3 years. The prevalence of history of hypertension and smoking was high in this cohort (83 %). Four of 12 patients were comatose on admission; the median HHS score at presentation was 3.5 (IQR 1), while the median mFS score on admission was 4 (IQR 1). Most aneurysms were located in the anterior circulation (75 %). Eleven patients (92 %) underwent aneurysm coiling, while only one patient was treated with craniotomy and clipping.
Unbound Iron and Iron-Handling Proteins Concentration
We were able to obtain CSF on day 1 in only 6 patients due to either initially low ventriculostomy output, admission > 24 h from onset of symptoms or emergent surgery or endovascular intervention precluding proper sample collection. For subsequent days (i.e., days 3 and 5), CSF was available for all 12 patients. Figure 1 depicts the change in unbound iron (i.e., NTBI and REDOX-Fe) concentration in CSF over the first 5 days following SAH. Values for NTBI and REDOX-Fe in control CSF were 0.12 + 0.05 and 0.07 + 0.02 μM (mean + SEM), respectively. A significant increase in both NTBI and REDOX-Fe levels was observed over time, with a peak level that was about tenfold that of control subjects (0.69 + 0.07 vs. 0.07 + 0.02 μM for REDOX-Fe and 1.07 + 0.03 vs. 0.1 + 0.03 μM for NTBI, p = 0.01), and a change in magnitude in concentration ranging between 120 and 170 % from days 1 to 5 (0.41 + 0.05 vs. 0.69 + 0.07 μM for REDOX-Fe, p = 0.008; and 0.91 + 0.28 vs. 1.07 + 0.16 μM for NTBI, p = 0.08)
Figure 2 depicts the changes in iron handling and regulating proteins over time. The mean ceruloplasmin (Cp) concentration in CSF samples from SAH patients on day 1 was almost 80 times higher than that of control subjects (134.9 + 27.2 vs. 1.7 + 0.7 mg/L; p = 0.04), but it decreased by the third day (55.3 + 9.4 mg/L) and remained virtually unchanged through day 5 (55.5 + 7.8 mg/L). The levels of REDOX-Fe were inversely related to the Cp concentration in a simple linear regression analysis (p = 0.03)
The mean CSF ferritin (Ft) on day 1 in SAH patients was 877.6 + 163.8 pmol/L, compared to 408.9 + 87.6 pmol/L in controls (p = 0.1). Ferritin steadily increased and quadrupled by day 5 reaching a concentration of 3,534.5 + 826.9 pmol/L (p = 0.01). Similarly, mean lactoferrin (Lf) levels were significantly higher in patients compared to controls (398 + 77 vs. 0 μg/L; p = 0.004), and Lf spiked on day 3 in SAH patients and exhibited a small decrement in subsequent sampling. Transferrin (Tf) was also significantly elevated on the first day (0.02 + 0.007 vs. 0.005 + 0.001 g/L; p = 0.01), but CSF levels trended down over time and by day 5 they were similar to that of control subjects (0.007 + 0.002 vs. 0.005 + 0.001 g/L; p = 0.8); whereas Pro-hepcidin (Ph) levels were initially similar to those of control subjects, but progressively increased over the first five days after SAH (151 + 29 Vs. 8.2 + 3.2 μg/L in controls; p = 0.01).
Clinical and Radiographic Outcomes
Delayed Cerebral Ischemia
A total of 7 patients (58 %) met the pre-specified criteria for DCI. Development of DCI was associated with larger aneurysm size (p = 0.02), and expectedly longer duration of HHT (p = 0.01) and prolonged mechanical ventilation (P = 0.04) (Table 1). As Table 2 illustrates, these patients also had a significantly higher CSF NTBI on day 5 and mean NTBI than those who did not develop DCI (1.39 + 0.17 vs. 0.62 + 0.12 μM; p = 0.02 and 1.27 + 0.17 vs. 0.62 + 0.08 μM; p = 0.03, respectively). In an univariate logistic regression analysis, day 5 NTBI, peak NTBI, mean NTBI, day 3 Ft, day 1 Ph, and day 3 Ph was significantly associated with the development of DCI, however, in a multivariate analysis only day 5 NTBI, mean NTBI, and peak NTBI remained significant (p = 0.001).
Angiographic Vasospasm
Five patients (41.6 %) developed angiographic evidence of vasospasm. Only day 3 REDOX-Fe and mean REDOX-fe correlated with the development of VSP on cerebral angiogram in a regression analysis (p = 0.02).
Cerebral Ischemia
Nine patients (75 %) underwent MRI scans and evidence of acute ischemia on DWI sequences was identified in 8 of them. Most strokes involved deep brain structures (50 %), and only one instance of exclusive cortical involvement was identified, while the rest were a combination of cortical and deep strokes. Day 1 REDOX-Fe, day 1 Cp, day 3 Lf ,and change in Cp concentration between days 3 and 5 correlated with development of cerebral ischemia in univariate logistic regression, however, only day 1 REDOX-Fe and change in Cp between days 3 and 5 remained significant in multivariate regression analysis (p = 0.02). Patients who developed deep cerebral infarcts had a significant lower level of Cp on day 3, compared to those who did not (34.1 + 11.9 vs. 81.4 + 14.2 mg/L; p = 0.03). The development of deep-seated strokes was associated with mean REDOX-Fe, day 3, day 5, and peak Cp, and day 5 and mean Tf in univariate logistic regression, but only mean REDOX-fe, day 3 Cp, day 5 Cp, peak Cp, and mean Tf remained significant in multivariate regression (p = 0.03).
Functional Outcomes (Dichotomized 90-Day mRS)
A total of 7 patients (58 %) had a mRS score of 2 or less at 90 + 18 days. Patients who developed DCI were more likely to have poor functional outcomes (i.e., 90-day mRS > 2) than patients who did not (57 % vs. 20 %; p 0.2), but this difference did not reach statistical significance (Table 1). Overall, patients in the favorable outcome group had lower mean REDOX-Fe concentrations in CSF (0.53 + 0.05 vs. 0.77 + 0.05 μM; p = 0.05). Subjects in the favorable outcome group also had lower levels of Lf (320 + 60 vs. 532 + 153 μg/L; p = 0.1) and Ft (1,804.3 + 478.6 vs. 3,051.4 + 541.5 pmol/L; p = 0.1), and higher mean Cp concentration in CSF (80.2 + 5.9 vs. 54.3 + 15.8 mg/L; p = 0.1), but none of these differences were statistically significant.
Discussion
In this small pilot study, we found that measures of unbound iron (NTBI and REDOX-Fe) in the CSF of patients with SAH were higher than in controls, and that their concentrations rose over the first 5 days following ictus. We also found that development of DCI, angiographic vasospasm, and presence of cerebral ischemia on DWI-MRI was associated with higher concentration of non-protein bound iron in CSF, while patients who developed ischemic strokes had lower CSF ceruloplasmin levels.
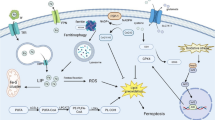
Following aneurysm rupture, phagocytosis, and breakdown of red blood cells (RBCs) occur and hemoglobin rapidly distributes over the entire brain and easily penetrates the deeper layers of the cortex [16–18]. Iron, a byproduct of heme degradation by heme-oxygenase 1, can react with H2O2 via the Fenton reaction to form ROS [19] that have been shown to mediate vasoconstriction, arterial structural damage, microcirculatory derangement, and neurotoxicity in experimental SAH [8, 17, 20]. While the mechanisms normally responsible for iron homeostasis in the CNS have not been fully elucidated [38], considering the amount of iron released after RBCs lysis, it is likely that many of these become quickly saturated. For instance, CSF has a low iron-binding capacity that is close to saturation under normal conditions [5] and the increase in the labile iron pool following brain injury has been shown to be too large to be sequestered by cellular ferritin [19].
Coexistence of ROS and iron deposits in periarterial spaces, the tunica adventicia of cerebral arteries and infiltrating neutrophils in experimental SAH supports the hypothesis that iron-mediated generation of ROS results in cerebral vasospasm [8]. Furthermore, iron may facilitate the formation of bilirubin oxidation products (BOXes) which have been postulated to play a role in arterial vasoconstriction [21]. Iron and ROS have also been linked to blood brain barrier dysfunction and brain edema formation through activation of matrix metalloproteinase-9 [22]. A progressive increase in brain non-heme iron following SAH leading to increased intracellular deposits has been implicated in oxidative DNA injury and activation of autophagic and apoptotic cell death pathways [16, 23], and prolonged ferritin elevation within neurons is known to lead to cell death [24]. In contrast, the use of iron-chelating agents can effectively prevent the development of cerebral vasospasm following experimental SAH [25, 26]. We decided to examine the role of unbound and redox-active iron, as opposed to total CSF iron, since in a previous investigation it did not correlate with the development of cerebral vasospasm [27], and because preliminary data from animal studies suggest that redox-active iron-mediated production of superoxide anion strongly correlated with development of vasospasm [8]. Our findings add to the accumulating evidence implicating iron-mediated toxicity as a major contributor to the pathophysiology of acute brain injury following SAH.
The prevention of vasospasm through ferritin-mediated iron detoxification has been postulated recently [27]. While it is primarily an intracellular protein, in pathological conditions Ft is secreted into the CSF where it may help clear iron released following red cell lysis. The Ft concentration in CSF of SAH patients in our study was very similar to that reported previously (3,534.5 + 826.9 vs. 3,509.8 + 563.9 pmol/L), however, we did not observe a significant difference between DCI and no DCI groups. This lack of correlation may be due to the small number of patients included in our study, but also to the use of different endpoints (DCI versus transcranial Doppler and Xenon CT scan) between our investigation and the one reported by Suzuki et al. [27]. The exact role that Ft plays in the metabolism of iron following SAH remains to be firmly established.
Earlier studies had implicated a role for Tf in the pathogenesis of cerebral vasospasm through a mechanism involving increased cytosolic free calcium in smooth muscle cells. Moreover, significantly elevated CSF levels have been recently described in patients who developed this condition [28, 29]. We could not replicate these findings and CSF Tf, while elevated in SAH patients, trended down over time and no significant difference by DCI status was observed. It is important to note that we measured Tf levels at an earlier time frame and that may at least partially account for this discrepancy. Also, our study was likely underpowered to demonstrate such an effect. Whether transferrin plays any significant role in the pathogenesis of brain injury following aneurysmal SAH remains to be elucidated.
Lactoferrin is a small iron-binding glycoprotein found in various secretory fluids, where it serves primarily an antimicrobial function [39]. It has also been found to have anti-inflammatory, anti-oxidant, anti-tumorigenic, and transcription effects [41], which seem to be modulated by its ability to donate and sequester iron. Moreover, CSF lactoferrin levels were found to be elevated in patients following both ischemic and hemorrhagic stroke [40]. In our study, Lf levels did not correlate with any of the outcomes studied, and therefore, seems unlikely to play a major role in the body’s response to SAH.
We also sought to explore whether hepcidin played a role in brain iron metabolism following SAH. Hepcidin is secreted primarily by hepatocytes in response to inflammatory stimuli, iron, and hypoxia and is responsible for the regulation of body iron balance. It has also been shown to be widely expressed in the murine brain [43]. It is initially synthesized as a prepropeptide, which is in turn processed into a 60- to 64-residue pro-hepcidin peptide, and then finally into the mature and biologically active 25-amino acid hepcidin. We chose to determine pro-hepcidin levels due to the ubiquitous availability of enzyme-linked immunoassays [42]. In our small pilot study pro-hepcidin did not correlate with any of the outcomes explored. It remains to be seen whether determination of hepcidin-25 levels with recently available immunoassays affects the development of DCI, vasospasm or cerebral infarcts following aneurysmal SAH.
Ceruloplasmin is a serum alpha-2 glycoprotein that belongs to the family of multi-copper oxidase enzymes [30]. It is an acute phase reactant with a number of attributed functions including, ferroxidase activity; [31] anti-oxidant functions and the prevention of the formation of free radicals; [32] inhibition of neutrophil myeloperoxidase; [33], and protective anti-inflammatory roles, among others [34]. Interestingly, patients with aceruloplasminemia, an autosomal recessive disorder characterized by mutations that abolish functional Cp and impair iron homeostasis, exhibit abundant iron deposition in microglia and neurons, selective neuronal loss and evidence of increased ROS generation in the CNS [35]. Moreover, brains of adult transgenic Cp-/- mice show evidence of increased lipid peroxidation and free radical-mediated injury, intracellular iron accumulation and increased susceptibility to free radical injury [36]. We found Cp levels to be significantly and inversely correlated with REDOX-Fe concentration in CSF. Also, lower CSF ceruloplasmin levels were associated with higher likelihood of ischemic strokes, particularly in deep locations. To our knowledge, a potential protective effect of Cp in SAH has not been explored before.
This study has some limitations. First, given the small number of patients included, these findings need to be considered preliminary and require further validation in a larger cohort of patients; furthermore, the sample size significantly limits the validity of multivariate analysis. Second, CSF on day 1 was only available in half of the patients in our analysis, considerably diminishing the number of samples available for testing. Third, control CSF was obtained from patients as part of the work up for normal pressure hydrocephalus and may not represent truly normal CSF. Furthermore, we do not have demographic data available for this cohort. Nonetheless, the levels of REDOX-active iron measured in control CSF were very similar to levels reported recently in another cohort of normal subjects [44]. Finally, We were unable to obtain serial MRI-DWI imaging in this cohort and instead reviewed MRIs obtained as part of clinical care, which could potentially have introduced biases.
In conclusion, our preliminary data indicate a causal relationship between unbound iron and brain injury following SAH and suggest a possible protective role for ceruloplasmin in this setting, particularly in the prevention of cerebral ischemia. Further studies are needed to validate these findings and to probe their clinical significance.
References
Xi G, Keep RF, Nakamura T, et al. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5:53–63.
Selim M, Yeatts S, Goldstein JN, Gomes J, et al. Safety and tolerability of deferoxamine mesylate in patients with acute intracerebral hemorrhage. Stroke. 2011;42:3067–74.
Yeatts SD, Palesch YY, Moy CS, Selim M. High dose deferoxamine in intracerebral hemorrhage (HI-DEF) trial: rationale, designs, and methods. Neurocrit Care. 2013;19:257–66.
Lee J-Y, Sagher O, Keep R, et al. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery. 2009;65:331–43.
Savman K, Nilsson UA, Blennow M, et al. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatr Res. 2001;49:208–12.
Nilsson UA, Bassen M. Savman, et al. A simple and rapid method for determination of “free” iron in biological fluids. Free Radical Res. 2002;36:677–84.
Bishop GM, Robinson SR. Quantitative analysis of cell death and ferritin expression in response to cortical iron: implications for hypoxia-ischemia and stroke. Brain Res. 2001;907:175–87.
Mori T, Nagata K, Town T, et al. Intracisternal increase of superoxide anion production in a canine subarachnoid hemorrhage model. Stroke. 2001;32:636–42.
Cabantchik ZI, Breuer W. LPI-Labile plasma in iron overload. Best Practice & Research in Clinical Haematology. 2005;18:277–87.
Diringer MN, Bleck TP, Hemphill JC III, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15:211–40.
Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012 (published online May 3, 2012).
Vergouwen MDI. – The participants in the International multi-disciplinary consensus conference on the critical care management of subarachnoid hemorrhage. Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocrit Care. 2011;15:308–11.
Rabinstein AA, Weigend S, Atkinson JL, Wijdicks EF. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36:992–7.
Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914–22.
Breuer W, Ghoti H, Shattat A, et al. Non-transferrin bound iron in Thalassemia: differential detection of redox active forms in children and older patients. Am J Hematol. 2012;87:55–61.
Lee JY, Keep RF, Hua Y et al: The role of iron in brain following subarachnoid hemorrhage. In, Li YV and Zhang JH (Eds): Metal Ion in Stroke. Springer New York 2012.
Macdonald RL, Weir BKA. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22:971–82.
Turner CP, Bergeron M, Matz P, et al. Heme-oxygenase-1 (HO-1) is induced in glia throughout the brain by subarachnoid hemoglobin. J Cereb Blood Flow Metab. 1998;18:257–73.
Carbonell T, Rama R. Iron, oxidative stress and early neurological deterioration in ischemic stroke. Curr Med Chem. 2007;14:857–74.
Asano T, Tanishima T, Sasaki T, et al. Possible participation of free radical reactions initiated by clot lysis in the pathogenesis of vasospasm after subarachnoid hemorrhage. In: Wilkins RH, editor. Cerebral arterial spasm. Baltimore, MD: Williams & Wilkins; 1980.
Pyne-Geithman GJ, Nair S, Caudell Stamper DN, et al. Role of bilirubin oxidation products in the pathophysiology of DIND following SAH. In: Zuccarello et al (Eds): Cerebral vasospasm: Neurovascular events after subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:267–73.
Lochhead JL, McCaffrey G, Quigley CE, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occluding during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–36.
Lee JY, Keep RF, He Y, et al. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow and Metab. 2010;30:1793–803.
Kaur D, Rajagopalan S, Chinat S, et al. Chronic ferritin expression within murine dopaminergic midbrain neurons result in a progressive age-related neurodegeneration. Brain Res. 2007;1140:188–94.
Vollmer DG, Hongo K, Ogawa H, et al. A study of the effectiveness of the iron-chelating agent deferoxamine as vasospasm prophylaxis in a rabbit model of subarachnoid hemorrhage. Neurosurgery. 1991;28:27–32.
Horky LL, Pluta RM, Boock RJ, Oldfield EH. Role of ferrous iron chelator 2,2’-dipyridyl in preventing delayed vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg. 1998;88:298–303.
Suzuki H, Muramatsu M, Kojima T, Taki W. Intracranial heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2003;34:2796–800.
Nakashima T, Takenaka K, Fukazawa S, et al. Purification of a factor from CSF in patient after SAH which induces the cytosolic free calcium elevation in vascular smooth muscle cells. Neurol Res. 1997;19:51–6.
Takenaka KV, Sakai N, Murase S, et al. Elevated transferrin concentration in cerebral spinal fluid after subarachnoid hemorrhage. Neurol Res. 2000;22:797–801.
Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–58.
Osaki S, Johnson D, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–57.
Gutteridge JM. Antioxidant properties of ceruloplasmin towards iron- and copper-dependent oxygen radical formation. FEBS Lett. 1983;157:37–40.
Chapman ALP, Mocatta TJ, Shiva S, et al. Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J Biol Chem. 2013;288:6465–77.
Bakhautdin B, Febbraio M, Goksoy E, et al. Protective role of macrophage-derived ceruloplasmin in inflammatory bowel disease. Gut. 2013;62:209–19.
Harris ZL, Klomp LWJ, Gitlin JD. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Am J Nutr. 1998;67(suppl):972S–7S.
Patel BN, Dunn RJ, Jeong SY, et al. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578–86.
Naidech AM, Bendock BR, Bassin SL, et al. Classification of cerebral infarction after subarachnoid hemorrhage impacts outcome. Neurosurgery. 2009;64:1052–8.
Benarroch EE. Brain iron homeostasis and neurodegenerative disease. Neurology. 2009;72:1436–40.
Marrif HI, Alwabel NA, Mousa HM. Brain lactoferrin: an endogenous peptide or merely an intruder. Am J Sci. Res. 2009;6:79–85.
Terent A, Hällgren R, Venge P, Bergström K. Lactoferrin, lysozyme, and beta 2- microglobulin in cerebrospinal fluid. Elevated levels in patients with acute cerebrovascular lesions as indices of inflammation. Stroke. 1981;12:40–6.
Hirsch EC, Faucheux BA. Iron metabolism and Parkinson’s disease. Mov Disord. 1998;13(Suppl 1):39–45.
Frazer DM, Anderson GJ. Hepcidin compared to prohepcidin: an absorbing story. Am J Clin Nutr. 2009;89:475–6.
Wang SM, Fu LJ, Duan XL, et al. Role of hepcidin in murine brain iron metabolism. Cell Mol Life Sci. 2010;67:123–33.
Lavados M, Guillon M, Mujica MC, et al. Mild cognitive impairment and Alzheimer patients display different levels of redox-active CSF iron. J Alzheimer Dis. 2008;13:225–32.
Acknowledgments
The authors wish to thank Prof. Loav Cabantchik (Hebrew University of Jerusalem, Israel) for his guidance with the NTBI and REDOX-Fe tests. The authors wish to acknowledge the assistance provided with statistical analysis by Esteban Walker, PhD (Department of quantitative health sciences, Cleveland Clinic. Cleveland, OH), and Dr. Jennifer Frontera for her help with selection of statistical software. We also wish to thank Valerie Swank for her assistance with sample processing. This study was supported by a grant from the Cerebrovascular Center, Cleveland Clinic. Cleveland, OH, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomes, J.A., Selim, M., Cotleur, A. et al. Brain Iron Metabolism and Brain Injury Following Subarachnoid Hemorrhage: iCeFISH-Pilot (CSF Iron in SAH). Neurocrit Care 21, 285–293 (2014). https://doi.org/10.1007/s12028-014-9977-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-9977-8