Abstract
Subarachnoid hemorrhage (SAH) is the deadliest of all strokes. It mostly affects younger adults. The classic manifestation of SAH is thunderclap headache which is also accounted by patients as “the worst headache of their life”. SAH is associated with neurological impairment, cognitive dysfunction and worse prognosis. There are two most important pathological mechanisms involved; early cerebral injury and cerebral vasospasm. Several pro inflammatory factors and cell death pathways contribute towards the pathogenesis of SAH. Iron deposition has been found to play a prominent role in the acute phase of this disease. Iron related SAH biomarkers for example haptoglobin, hepcidin and total iron binding capacity etc. have been found. These biomarkers can be used to detect early injury and distinguish ruptured aneurysm from an unruptured aneurysm. Iron is also linked to other complications of SAH i.e., acute seizures and hydrocephalus. DFX an iron chelator has shown promising results in the long term for the treatment of SAH. It has improved neurological function outcome in the patients. Several other therapeutic agents have also emerged in the experimental studies. Unfortunately, no drug and therapy has conveyed validated clinical value. More research and larger studies are required in this area.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction to Subarachnoid Hemorrhage (SAH)
SAH has been recognized as the most lethal form of stroke. SAH might not be the prevalent of all strokes but it is significantly responsible for all-stroke associated mortality and disability. High mortality and morbidity rates are frequently associated with the incidence of SAH. These devastating consequences result in prolonged or permanent disability. The patient leads a poor quality of life and it affects the socioeconomic structure of the society (Yu et al., 2021; Heinsberg et al., 2020a).
2 Epidemiology
Previous studies exhibit the prevalence of SAH to be 5% among all types of strokes. SAH relatively affects men in young ages while after 55 years of age, it seems to affect women (de Rooij et al., 2007).
The reported incidences of SAH are found to be 9.1 per 100,000 person years in number (Nieuwkamp et al., 2009). The recorded incidence of subarachnoid hemorrhage (SAH) is comparatively less than that of acute ischemic stroke. In fact, SAH is the rare type of all stroke and accounts for only 1–6% of all incidences of stroke. It is linked to high mortality and morbidity rate. It has poor prognosis and the patient leads a poor quality of life (Veremakis, 1991). In 80% of the cases reported, SAH occurs due to rupture of aneurysm. The other reasons behind this occurrence are; vascular irregularities and vasculitis (Lawton & Vates, 2017).
3 Signs and Symptoms
Patients with SAH frequently report “the worst headache of their life”. This is the classic manifestation of subarachnoid hemorrhage (Connolly et al., 2012).
The presentation of patients after the onset of SAH is highly dependent upon the magnitude and the great speed at which the hemorrhage diffuses in various regions of the brain.
The early manifestations are Jacksonian epilepsy and after sometime, hemiparesis takes place due to mechanical pressure exerted on motor cortex. These findings are not present when the pressure is localized in the base of brain. The manifestations are intermittent because the blood travels from one region to another rapidly. The late manifestations are nausea, vomiting, headache, dizziness and disorientation and there is chance of falling into coma. These late presentations occur due to raised intracranial pressure. A rupture of a small cerebral vessel leads to unconsciousness (Campbell, 1932).
The classic manifestations observed due to extravasated blood are neck rigidity, headache in the occipital region, pain that originates from the back and travels towards limbs, moderate fever with chills and Kernig’s sign (the patient is unable to extend his knee or there is pain on extension of the knee (Alyssa et al., 2022). This presentation is typically seen in basal meningitis (Campbell, 1932).
4 Introduction to Iron
Iron holds immense importance as a nutrient in the body as it engages in various biological processes that includes redox reactions in the cells, formation of myelin, cell multiplication and DNA synthesis. It remains vital to maintain the levels of iron in equilibrium in the brain. The balance is maintained by the delicate process of homeostasis (Kühn, 2015). Although Iron has essential physiological function i.e., formation of myelin and neurotransmitter, electron transport chain and nervous tissue development but free iron in the body leads to disastrous consequences. Free iron causes oxidative stress, production of free radicals and neuronal injury (Helbok et al., 2021).
5 Overview of the Anatomy of Brain
The brain is protected by three coverings namely dura matter, arachnoid matter and pia matter. Dura matter is the outer fibrous tough covering whereas pia matter is the inner most thin fragile covering of the brain. Arachnoid matter is the middle covering which comprises of filaments of connective tissue. These filaments of connective tissue are known as arachnoid trabeculae and they build a connection between pia matter and arachnoid matter. They also form subarachnoid space which is filled with cerebrospinal fluid. Cerebral vessels travel through this space.
6 Homeostasis of Iron in the Brain
Iron can be largely found in the brain in its non-heme form. This indicates increased level of metabolic activity in the brain linked to iron. Substantia nigra pars compacta (SN) and basal ganglia contain high concentration of iron. The Blood Brain Barrier is an intricate one cell thick endothelial cell layer which is polarized and it is held by tight junctions. This molecular structure tightly regulates the transport of iron into the brain from rest of the system.
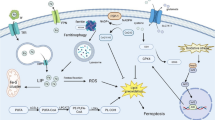
The transport of iron into the brain is controlled by two pathways namely Tf-TfR and DMT1-Fpn pathways. Fpn carries Fe2+ and it is released at the basolateral side of the endothelial cells. Cp expressed on astrocytes foot processes which lines the endothelial layer. Cp oxidizes Fe+2 to Fe+3. The Fe+3 is arrested by Tf which is found in CSF and interstitial fluid of the brain. Citrate, ascorbate and ATP also transport considerable quantity of iron in the brain. An unknown receptor on the apical side of the endothelial cells is also responsible for the transport of iron into the brain as ferritin (Bradbury, 1997; Guo et al., 2016; Moos et al., 2007; Rouault & Cooperman, 2006).
7 Early Brain Injury Post-SAH
Early brain injury (EBI) and cerebrovascular spasm are the fatal consequences of SAH (Liu et al., 2019). Several mechanisms and pathways have been identified that are responsible for these appalling consequences. EBI results in nervous tissue damage, neuronal injury, BBB disruption (blood brain barrier), cerebral edema, cerebral ischemia and hypoxia and significant exposure to oxidative stress. There is complex pathophysiology involved in the development of EBI. There is a dire need to determine accurate pathways associated with EBI so that therapeutic targets can be recognized. As a result, significant detrimental morbidity and mortality linked to SAH can be prevented (Cahill & Zhang, 2009).
8 Cerebral Vasospasm Post-SAH
The most commonly occurring complication of SAH is cerebral vasospasm. This leads to devastating consequences i.e., cerebral hypoxia and ischemia (Bederson et al., 1995). Endothelin(s) are known as the active vasoconstrictors and play a primary role in causing cerebral vasospasm post-SAH (Bickford et al., 2014). Iron bound heme is responsible for causing vasospasm (Joerk et al., 2014). Nitric oxide a vasodilator opposes the vasoconstrictive effects of endothelin. Iron from the heme attaches to NO, the inhibitory effect NO on endothelin is lost and as a result, vasospasm occurs (Pluta & Oldfield, 2008; Stow et al., 2011) (Garton et al., 2016).
9 Role of Iron after SAH
Scientists believe that the initial brain damage occurs within 72 hours of incidence of SAH. Blood builds up in the subarachnoid space which means Hb levels increase in the brain. The bleeding activates heme–hemopexin scavenging system that removes all the extra Hb accumulated in the brain. The activation of this system is significantly involved in the buildup of intracellular iron and subsequently cause damage to the brain after the event of SAH. The expression of CD91; the receptor of hemopexin increases when iron deposits in the brain tissue (Garton et al., 2016).
Three studies prove relationship between iron toxicity and brain damage after SAH. SAH created in a lab setting in the monkeys showed prominent increase in the levels of heme oxygenase-1 in the microglia and iron induced oxidation in the neuron leads to permanent DNA damage. This results in neuronal death and ultimate brain damage (Ono et al., 2000).
The preliminary data obtained from cerebrospinal fluid samples of 12 patients diagnosed with aneurysmal subarachnoid hemorrhage revealed the presence of free iron and iron mediating proteins (Gomes et al., 2014).
SAH induced rats were studied, significant increase in the expression of heme oxygenase 1 was seen in the acute phase after SAH. The expression of iron regulating proteins such as Transferrin factor and Transferrin factor receptor also increase. These proteins are responsible for transportation and storage of iron within the basal region of the brain. Thus, iron toxicity takes place which means generation of oxidative radicals and permanent cell damage (Lee et al., 2010).
Once inside the cell, the oxidative radicals enter the mitochondria. The integrity of the cell is compromised and cell death pathway gets activated. Caspases, a protease primarily involved in programmed cell death, is released from the mitochondria. The process of programmed cell death is called apoptosis. Consequently, programmed neuronal death takes place. Therefore, it can be said that buildup of iron after the SAH event induces apoptosis and ultimately leads to severe brain damage (Garton et al., 2016).
10 Body’s Defense against Unbound Heme
Blood brain barrier provides protection to the brain against various harmful substances. The structure of BBB consists of endothelial cells, pericytes, basement membrane and astrocytes end-foot It plays a prominent role in the stabilization of the internal environment of the brain tissue by maintaining the hemostasis. It usually does that by controlling the transport of various substances inside and outside the brain. It defends the brain tissue from neurotoxic substance i.e., unbound heme, iron etc. (Chen et al., 2020).
A traumatic event like SAH disrupts this barrier and it results in accumulation of heme in the subarachnoid space. Human body has two protective scavenging systems against extracellular heme and iron toxicity namely; Heme-Hpx complex and haptoglobin-CD163-heme oxygenase-1 pathway. Haptoglobin-CD163-heme oxygenase-1 pathway is the major scavenger system and it is known as the first line of defense mechanism against unbound iron toxicity. Haptoglobin has high affinity for Hb dimers. The expression of CD163 (a phagocytic receptor) increases at cellular level and binds avidly with the hemoglobin-haptoglobin complex. CD163 mediates Haptoglobin-heme complex transport into the macrophage and endosomal degeneration occurs. Haptoglobin is recycled. Heme-hpx pathway is the alternative iron scavenging pathway. When haptoglobin becomes concentrated with hemoglobin after SAH, the rate of recycling does not match with the rate of consumption of haptoglobin. As a result, exhaustion of haptoglobin reserves occur and there is no haptoglobin left to clear the extravascular hemoglobin. During this time, the backup hemoglobin scavenging system gets activated. The hemopexin-hemoglobin pathway is known as the second line of defense against iron toxicity. Hemopexin-hemoglobin complex formation neutralizes the redox toxicity produced by unbound heme. The uptake of this complex is facilitated by plasma member receptor called CD91 via endocytosis. (Chen-Roetling et al., 2018a, b; Garland et al., 2016; Kinner-Bibeau et al., 2018).
11 SAH Markers
11.1 Hepcidin
Patient outcomes after SAH events are poor and variable. There is a need to explore and investigate authentic and reliable biomarkers in patients who show poor response. As a result, supportive treatment for these patients can be improved. Two studies have been done studying relationship between iron related genotypes and poor outcome of the patients (Heinsberg et al., 2020a, b).
Hepcidin is a major player in controlling iron hemostasis in the brain tissue. It maintains optimum levels of iron in the brain tissue. A study investigating the pathophysiology of early brain injury in SAH induced rats revealed that iron accumulation in the subarachnoid space cause increased expression of hepcidin. The increased expression of hepcidin intracellularly gives rise to apoptosis and iron dependent oxidative injury. All of these catastrophic events occur via downregulation of iron handling proteins i.e., ceruloplasmin and ferroportin-1. These proteins are involved in the removal of iron from the brain tissue (Tan et al., 2016).
Previous literature has established that increased expression hepcidin in the brain is responsible for the poor prognosis of patients with aSAH (aneurysmal subarachnoid hemorrhage). Hepcidin antimicrobial peptide gene (HAMP) holds instruction for the production of hepcidin. One study presents a hypothesis that the ability to handle iron buildup depends on genetic variability in HAMP. The genetic variability in HAMP is established by DNA methylation. Therefore, genetic variability of HAMP DNA methylation can determine outcome of patients with aSAH. HAMP DNA methylation can be detected in the CSF. Additionally, HAMP can be used as therapeutic target to improve the prognosis of these patients after aSAH. This indicates that hepcidin can be used as a reliable and stable biomarker to predict the poor outcome of patients following SAH event (Heinsberg et al., 2020b).
11.2 Haptoglobin
Haptoglobin is an iron handling protein that binds to the free iron. This way it prevents the damaging oxidative effects of iron. Increased levels of haptoglobin have been observed in patients who suffered from vasospasm after SAH event. Haptoglobin is responsible for the uptake and clearance of hemoglobin. It is also known as acute phase protein. Acute phase proteins produced by the hepatocytes as a body’s response mechanism to systemic disturbance. Therefore, it can be said that haptoglobin modulates the process of inflammation in the body.
11.3 Haptoglobin Genotype
A meta-analysis of six studies reveals that HP1 and HP2; genotypes of haptoglobin have opposite effect on the outcome of SAH. Short-term outcomes and long-term outcomes were investigated. Short-term outcomes include cerebrovascular spasm and delayed cerebral ischemia. Long-term outcomes include functional status assessment through the modified Rankin Scale (mRS) or the Glasgow Outcome Scale. Short-term outcomes are the reason for prolonged hospital stays and prominent disability after SAH. The association between HP2 allele and the high risk of cerebrovascular spasm have been observed. This allele also has associations with cerebral salt wasting and poor functional outcomes. On the other hand, HP1 allele has shown protective effect against early cerebral injury after SAH event. Therefore, the goal of scientists must be to produce therapeutic interventions directed at HP1 allele. To prove the reliability and validity of this hypothesis larger prospective studies (Gaastra et al., 2018; Bulters et al., 2018).
11.4 Total Iron Binding Capacity
The analysis of 366 patients who undertook iron studies has shown that low total iron binding capacity is linked to aSAH. It was also found that serum ferritin levels were high in the short-term post-SAH. One possible explanation for these higher levels is that ferritin is released in the acute phase of inflammatory process. It is necessary to identify biomarkers for ruptured aSAH so that mortality and neurological deficits associated with aSAH can be reduced (Can et al., 2019; Northrop-Clewes, 2008).
12 Other Markers
After SAH, hemoglobin, heme, ferritin and iron levels in the CSF (cerebrovascular fluid) increases. Elevated levels of hemopexin in the CSF have also been observed after SAH. Hemopexin is a glycoprotein and it plays a role in the removal of free heme intracellularly and extracellularly. It transports free heme to the liver where it is broken down into stable products. This indicates that hemopexin provides protection against oxidative and damaging effects of iron (Latunde-Dada, 2016). In a study with 30 patients with SAH and 20 control individuals at tertiary care hospital raised levels of hemopexin have shown worst outcome (Garland et al., 2016).
13 Heme-Oxygenase 1
A link between higher levels of HO-1 (heme oxygenase 1) and higher chance of cerebrovascular spasm was found (Pyne-Geithman et al., 2005). These elevated levels were also associated with reduced functionality in the patient and the study concluded that at Day 7 after SAH event, heme oxygenase-1 levels (HO-1) in the CSF can be used as a biomarker for the indication of poor outcome in the patients with Fischer Grade III aSAH (K. C. Wang et al., 2014). A prospective study conducted on 39 patients with SAH revealed that metabolites of HO-1, ferritin and bilirubin levels were not related to cerebral vasospasm in the hospital setting (Suzuki et al., 2003). These conflicting results warrant further research with larger cohorts in a clinical setting to determine both favorable and unfavorable effects of HO-1 after SAH.
In a study conducted in SAH-induced rats, aerobic capacities and their response to EBI were investigated through MRI, behavioral testing and brain examination. Greater BBB disruption, ventricular damage and brain edema was observed in rats with low aerobic capacities. Upregulation and increased expression of CD163 and HO-1 was seen in the brain tissue of low aerobic capacity rats. This also indicates high neuronal stress in the brain after SAH. Identifying risk factors i.e., aerobic capacity can help to determine therapeutic targets. These risk factors are significantly associated with increased incidence of EBI and consequently, lead to worse neurological and functional outcome in the patient with SAH (Toyota et al., 2021). This study is relevant to human subjects as in a retrospective cohort of more than 60,000 revealed that cardiorespiratory fitness is linked to reduced occurrences of all forms of stroke (al Rifai et al., 2020). In a prospective population-based study with long-term follow up observed that physical activity reduces the incidence of aSAH in both genders and exercise is particularly beneficial in smokers (Lindbohm et al., 2019). Another prospective general population-based study conducted in Norway failed to show any correlation between physical activity and SAH (Lindbohm et al., 2019).
14 Ferroptosis
Several other cellular death pathways have been identified that may be involved in the EBI. The known cell death processes identified after a hemorrhagic stroke are; autophagy, apoptosis, pyroptosis, necrosis, necroptosis and ferroptosis etc. (Fricker et al., 2018). The most accepted cell death pathway via mitochondrial caspases has been associated with neuronal demise in EBI post SAH. Previously, apoptosis, necrosis and autophagy were detected neuronal death pathways in EBI. In animal study, melatonin was proposed as therapeutic agent that can be used to attenuate autophagy and apoptosis in EBI. Therefore, melatonin can prevent neuronal death during EBI post SAH (Edebali et al., 2014; Shi et al., 2018).
A newly emerging cell death pathway has come into spotlight namely ferroptosis. Ferroptosis is a cellular death pathway which is nonapoptotic in nature. The mechanism is iron and lipid oxidative species dependent (Y. Sun et al., 2020).
Ferroptosis is linked to deposits of iron in the brain tissue and it is also triggered by lipid peroxides. The intriguing results found in the experimental SAH study exhibit that ferroptosis is involved in EBI post SAH. This detrimental process initiates due to several reasons namely; accumulation of iron in the tissues, reduction and deactivation of glutathione and glutathione peroxidase respectively, and lipid oxidative species buildup. Fer-1 is a compound that penetrates into the cell and it minimizes the buildup of lipid peroxides. As a result, it blocks the process of ferroptosis and brain tissue demise. It has been determined that ferroptosis participates in the EBI. Subsequently, avoiding EBI in patients with SAH can improve the prognosis and functional outcome (Li et al., 2021).
Another has also suggested ferroptosis as therapeutic target post-SAH. Fer-1 increases the expression of cysteine-glutamate antiporter SLC7A11 and GPX4 which prevents neuronal damages. Combined data of this study revealed that activation of p53 is also involved in the process of ferroptosis. Deactivation of p53 can inhibit ferroptosis (Kuang et al., 2021).
15 Biomarker for Ferroptosis
ACSL4 has been suggested to be the biomarker of ferroptosis. This marker can be used to detect and investigate this pathway for research and therapeutic purposes in future (Yuan et al., 2016). Another recent study has detected human transferrin receptor 1 protein as ferroptosis biomarker (Feng et al., 2020). No absolute biomarker has been recognized till this date to detect the mechanism of ferroptosis. Transmission electron microscopy is the most accepted method of detection for this pathway (Stockwell et al., 2017).
16 Ferroptosis and GPX4
It has been observed that raised iron level and inhibition of glutathione peroxidase 4 (GPX4) induce and enhance the process of ferroptosis (Dixon et al., 2012). An experimental SAH animal study has demonstrated that decreased expression of GPX4 after 24 hours of SAH leads raised levels of lipid peroxidation and cell injury. Thus, an increased expression of GPX4 can prevent radical formation and subsequently, neuronal injury. GPXP4 has also shown to reduce neurological deficit and brain edema 24 hours post-SAH. GPX4 is an antioxidant enzyme and it blocks lipid peroxidation. Lipid peroxidation occurs in the process of ferroptosis which basically is iron dependent regulated cell death pathway. Ferroptosis has been determined to play a significant role in post-SAH brain cell injury. Prominent GPX4 expression has been identified in the animal brain and neuronal cultures. The expression of GPX undergoes many changes due to activation of several inflammatory factors i.e., tumor necrosis factor-alpha, interleukins-1 beta and radical formation. The clinical use of GPX4 can be proposed to inhibit ferroptosis which can help to avoid initial brain injury post SAH. For this reason, this antioxidant can pave a new way to improve functional outcome and prognosis of SAH (Gao et al., 2020).
17 Ferroptosis and Liproxstatin-1
Liproxastin-1 can also be used for the treatment of SAH. It has the following beneficial mechanisms and effects
-
Terminates the process of lipid peroxidation located in the mitochondria
-
reduces the formation of reactive oxidative species
-
enhances the activity of glutathione
-
re-establish the levels of GPX4 (Fan et al., 2021)
In a recent single centered prospective study with human subjects found that CSF lipocalin-2 released in the inflammatory process can be used as prognostic biomarker in SAH patients (Yu et al., 2021).
18 Intracerebral Iron Accumulation
The volume of blood accumulated in the subarachnoid space and intraventricular space. This amount is associated with reduced functional status, prolonged hospital stays and global cerebral ischemia. Additionally, the number of complications also increase and worse outcome is inevitable (Helbok et al., 2021).
An observational study conducted on 32 patients with poor grade SAH found that iron can be measured quantitatively in the extracellular space, iron levels are raised in the white matter, the concentration of iron is associated with intraventricular blood accumulation and raised levels of iron are correlated with secondary brain injury. There is buildup of blood in the white matter during the acute phase of SAH, the patients develop mitochondrial dysfunction in the neurons. The disruption in the normal functioning of mitochondria is the cause of post-SAH cerebral vascular constriction (Helbok et al., 2021).
19 SAH and Acute Seizures
A prospective observation cohort of 554 patients with SAH concludes that lower levels of hemoglobin were associated with acute seizures. The mechanism behind the incidence of seizures after aSAH is still not determined yet. The hypothesized mechanism reveals that post-SAH inflammation and intracranial bleeding disrupts the levels of iron and induces iron deficiency anemia. There is decreased serum iron levels and intracranial hypertension. This causes ischemia and hypoxia of cerebral tissues and eventually, metabolic disturbance and cell injury occur in the neurons which triggers acute seizures (D.-L. Wang et al., 2019). Previous data shows that in acute phase of aSAH, the volume of subarachnoid hemorrhage and cerebral vasospasm might give rise to acute seizures (Hart et al., 1981; Ibrahim et al., 2013). Post-SAH acute seizures are correlated with worse prognosis and neurological deficits. No specific biomarkers have been identified till date to predict acute seizures after aSAH. A follow up study has revealed that the volume of cerebral bleeding has been associated with long-term epilepsy (Huttunen et al., 2015). Therefore, Wang et al. have proposed the use of prophylactic antiepileptic drugs for anemic patients who have suffered from an aSAH event (D.-L. Wang et al., 2019).
20 SAH and Acute Hydrocephalus
9–67% of the patients with SAH have reported the occurrence of hydrocephalus in the acute phase of aSAH (Graff Radford et al., 1989; Paisan et al., 2018; Shishido et al., 2016; Zaidi et al., 2015). The mechanism behind this complication is also linked to iron overload after SAH. Buildup of iron in the arachnoid granulations and leptomeninges. This leads to development of hydrocephalus which occurs due to hindrance in the CSF flow when BBB injury takes place along with deposition of iron from lysed red blood cells (Chen et al., 2017). Zhang et al. in their prospective observational study concluded that low serum iron levels are associated with hydrocephalus after SAH. Low serum iron levels can act as biomarkers to predict the onset of hydrocephalus. Hence, these low iron levels can predict worse functional outcomes in patients with SAH (Zhang et al., 2019).
21 MRI-QSM (Magnetic Resonance Imaging—Quantitative Susceptibility Mapping)
MRI-QSM technology can be used to detect iron deposition located inside the aneurysmal wall. The iron deposition is basically a micro-hemorrhage. The deposition of iron causes the aneurysmal wall to undergo several structural modifications. This gives rise to sentinel headache also called thunderclap headache. The real incidence of sentinel headache varies from 0–40% in SAH patients (Polmear, 2003; Schwedt et al., 2006).
MRI-QSM can be utilized to diagnosis cases with small aneurysms SAH when results of lumber puncture and CT scan are negative. This tool can also be useful to differentiate sentinel headache from all other forms of headache (H. Sun et al., 2018).
22 Nimodipine and Deferoxamine
In an animal study comparing the efficacy of both drugs in the elimination of cerebral vasospasm has found both drugs to be neuroprotective in nature. Nimodipine prevents vasospasm in the acute phase and it fails to improve the prognosis of SAH. On the other hand, deferoxamine (DFX) an iron chelator, penetrates the BBB and decreases iron overload in the brain. Subsequently, it improves cognitive function and prevents neuronal death (Qin et al., 2019).
In another animal study, DFX has shown to target iron deposition and reduced BBB breakdown. In return, this therapeutic effect has ameliorated brain edema, cerebral impairment and cognitive abnormality (Lee et al., 2010; Li et al., 2017).
Fatal lung damage was reported in four patients who were being treated with persistent administration of DFX in a phase 2 trial documented in 1992 (Tenenbein et al., 1992). Another study also suggested increased susceptibility of neuronal tissue to HB with the use of DFX (Peng et al., 2020).
23 Treatment of Neurotoxicity Caused by HB with Vitreous
In an experiment, vitreous consisting of iron deficient transferrin, ferritin, hyaluronan and selenium provided absolute protection to neuronal cell cultures from HB toxicity (Chen-Roetling et al., 2018b).
24 Heat Shock Protein (HSP) and SAH
HSP70, HO-1, HSP20 and HSP27 are responsible for cerebral vasospasm. HSP70, HSP90, HSP20 and HSP27 also play a prominent role in apoptotic cellular death pathway. HSP70 and HO-1 are neuroprotective in nature and save neuronal tissue from HB toxicity. These proteins are also cellular stress markers. HSP90 has shown to cause neurotoxicity and initial EBI (Shao et al., 2019).
25 Conclusion
Keeping in view all the previous and recent findings, it can be said that SAH is a fatal traumatic event. Patients who recover from this event are left with serious disabilities. The therapeutic advances made to treat SAH have been of little clinical value. Therefore, more experiments and larger studies are required to pave innovative ways to treat SAH in its acute phase. In recent times, artificial intelligence has been showing promising revolution in the early detection, investigation and treatment of several cerebrovascular diseases.
References
Alyssa, K., Ali, M. A., Brandis, D. (2022). Kernig sign. StatPearls. https://pubmed.ncbi.nlm.nih.gov/29262005/.
al Rifai, M., Blaha, M. J., Ahmed, A., Almasoudi, F., Johansen, M. C., Qureshi, W., Sakr, S., Virani, S. S., Brawner, C. A., Ehrman, J. K., Keteyian, S. J., & Al-Mallah, M. H. (2020). Cardiorespiratory fitness and incident stroke types: The FIT (Henry ford exercise testing) project. Mayo Clinic Proceedings, 95(7), 1379–1389. https://doi.org/10.1016/J.MAYOCP.2019.11.027
Bederson, J. B., Germano, I. M., & Guarino, L. (1995). Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke, 26(6), 1086–1091. https://doi.org/10.1161/01.STR.26.6.1086
Bickford, J. S., Ali, N. F., Nick, J. A., Al-Yahia, M., Beachy, D. E., Doré, S., Nick, H. S., & Waters, M. F. (2014). Endothelin-1-mediated vasoconstriction alters cerebral gene expression in iron homeostasis and eicosanoid metabolism. Brain Research, 1588, 25–36. https://doi.org/10.1016/J.BRAINRES.2014.09.022
Bradbury, M. W. B. (1997). Transport of iron in the blood-brain-cerebrospinal fluid system. Journal of Neurochemistry, 69(2), 443–454. https://doi.org/10.1046/J.1471-4159.1997.69020443.X
Bulters, D., Gaastra, B., Zolnourian, A., Alexander, S., Ren, D., Blackburn, S. L., Borsody, M., Doré, S., Galea, J., Iihara, K., Nyquist, P., & Galea, I. (2018). Haemoglobin scavenging in intracranial bleeding: Biology and clinical implications. Nature Reviews. Neurology, 14(7), 416–432. https://doi.org/10.1038/s41582-018-0020-0
Cahill, J., & Zhang, J. H. (2009). Subarachnoid hemorrhage: Is it time for a new direction? Stroke, 40(3 Suppl). https://doi.org/10.1161/STROKEAHA.108.533315
Campbell, S. B. B. (1932). Sub-arachnoid hæmorrhage. The Ulster Medical Journal, 1(3), 169. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2478790/
Can, A., Lai, P. M. R., Castro, V. M., Yu, S., Dligach, D., Finan, S., Gainer, V., Shadick, N. A., Savova, G., Murphy, S., Cai, T., Weiss, S. T., & Du, R. (2019). Decreased total iron binding capacity may correlate with ruptured intracranial aneurysms. Scientific Reports, 9(1), 6054. https://doi.org/10.1038/s41598-019-42622-y
Chen, S., Luo, J., Reis, C., Manaenko, A., & Zhang, J. (2017). Hydrocephalus after subarachnoid hemorrhage: Pathophysiology, diagnosis, and treatment. BioMed Research International, 2017. https://doi.org/10.1155/2017/8584753
Chen, S., Xu, P., Fang, Y., & Lenahan, C. (2020). The updated role of the blood brain barrier in subarachnoid Hemorrhage: From basic and clinical studies. Current Neuropharmacology, 18(12), 1266. https://doi.org/10.2174/1570159X18666200914161231
Chen-Roetling, J., Ma, S.-K., Cao, Y., Shah, A., & Regan, R. F. (2018a). Hemopexin increases the neurotoxicity of hemoglobin when haptoglobin is absent. Journal of Neurochemistry, 145(6), 464–473. https://doi.org/10.1111/jnc.14328
Chen-Roetling, J., Regan, K. A., & Regan, R. F. (2018b). Protective effect of vitreous against hemoglobin neurotoxicity. Biochemical and Biophysical Research Communications, 503(1), 152–156. https://doi.org/10.1016/j.bbrc.2018.05.202
Connolly, E. S., Rabinstein, A. A., Carhuapoma, J. R., Derdeyn, C. P., Dion, J., Higashida, R. T., Hoh, B. L., Kirkness, C. J., Naidech, A. M., Ogilvy, C. S., Patel, A. B., Thompson, B. G., & Vespa, P. (2012). Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke, 43(6), 1711–1737. https://doi.org/10.1161/STR.0B013E3182587839
de Rooij, N. K., Linn, F. H. H., van der Plas, J. A., Algra, A., & Rinkel, G. J. E. (2007). Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. Journal of Neurology, Neurosurgery, and Psychiatry, 78(12), 1365. https://doi.org/10.1136/JNNP.2007.117655
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., Patel, D. N., Bauer, A. J., Cantley, A. M., Yang, W. S., Morrison, B., & Stockwell, B. R. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell, 149(5), 1060–1072. https://doi.org/10.1016/J.CELL.2012.03.042
Edebali, N., Tekin, İ. Ö., AçıkgÖz, B., AçıkgÖz, Ş., Barut, F., Sevinç, N., & SümbüloĞlu, V. (2014). Apoptosis and necrosis in the circumventricular organs after experimental subarachnoid hemorrhage as detected with annexin V and caspase 3 immunostaining. Neurological Research, 36(12), 1114–1120. https://doi.org/10.1179/1743132814Y.0000000437
Fan, B. Y., Pang, Y. L., Li, W. X., Zhao, C. X., Zhang, Y., Wang, X., Ning, G. Z., Kong, X. H., Liu, C., Yao, X., & Feng, S. Q. (2021). Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regeneration Research, 16(3), 561. https://doi.org/10.4103/1673-5374.293157
Feng, H., Schorpp, K., Jin, J., Yozwiak, C. E., Hoffstrom, B. G., Decker, A. M., Rajbhandari, P., Stokes, M. E., Bender, H. G., Csuka, J. M., Upadhyayula, P. S., Canoll, P., Uchida, K., Soni, R. K., Hadian, K., & Stockwell, B. R. (2020). Transferrin receptor is a specific ferroptosis marker. Cell Reports, 30(10), 3411. https://doi.org/10.1016/J.CELREP.2020.02.049
Fricker, M., Tolkovsky, A. M., Borutaite, V., Coleman, M., & Brown, G. C. (2018). Neuronal cell death. Physiological Reviews, 98(2), 813–880. https://doi.org/10.1152/PHYSREV.00011.2017
Gaastra, B., Glazier, J., Bulters, D., & Galea, I. (2018). Corrigendum to “haptoglobin genotype and outcome after subarachnoid haemorrhage: New insights from a meta-analysis.”. Oxidative Medicine and Cellular Longevity, 2018. https://doi.org/10.1155/2018/9105120
Gao, S.-Q., Liu, J.-Q., Han, Y.-L., Deji, Q.-Z., Zhaba, W.-D., Deng, H.-J., Liu, X.-L., & Zhou, M.-L. (2020). Neuroprotective role of glutathione peroxidase 4 in experimental subarachnoid hemorrhage models. Life Sciences, 257, 118050. https://doi.org/10.1016/j.lfs.2020.118050
Garland, P., Durnford, A. J., Okemefuna, A. I., Dunbar, J., Nicoll, J. A. R., Galea, J., Boche, D., Bulters, D. O., & Galea, I. (2016). Heme-hemopexin scavenging is active in the brain and associates with outcome after subarachnoid Hemorrhage. Stroke, 47(3), 872–876. https://doi.org/10.1161/STROKEAHA.115.011956
Garton, T., Keep, R. F., Hua, Y., & Xi, G. (2016). Brain iron overload following intracranial haemorrhage. Stroke and Vascular Neurology, 1(4), 172–184. https://doi.org/10.1136/svn-2016-000042
Gomes, J. A., Selim, M., Cotleur, A., Hussain, M. S., Toth, G., Koffman, L., Asi, K., & Provencio, J. J. (2014). Brain iron metabolism and brain injury following subarachnoid hemorrhage: iCeFISH-pilot (CSF iron in SAH). Neurocritical Care, 21(2), 285–293. https://doi.org/10.1007/S12028-014-9977-8
Graff Radford, N. R., Torner, J., Adams, H. P., & Kassell, N. F. (1989). Factors associated with hydrocephalus after subarachnoid hemorrhage: A report of the cooperative aneurysm study. Archives of Neurology, 46(7), 744–752. https://doi.org/10.1001/ARCHNEUR.1989.00520430038014
Guo, S., Frazer, D. M., & Anderson, G. J. (2016). Iron homeostasis: Transport, metabolism, and regulation. Current Opinion in Clinical Nutrition and Metabolic Care, 19(4), 276–281. https://doi.org/10.1097/MCO.0000000000000285
Hart, R. G., Byer, J. A., Slaughter, J. R., Hewett, J. E., & Easton, J. D. (1981). Occurrence and implications of seizures in subarachnoid hemorrhage due to ruptured intracranial aneurysms. Neurosurgery, 8(4), 417–421. https://doi.org/10.1227/00006123-198104000-00002
Heinsberg, L. W., Alexander, S. A., Crago, E. A., Minster, R. L., Poloyac, S. M., Weeks, D. E., & Conley, Y. P. (2020a). Genetic variability in the iron homeostasis pathway and patient outcomes after aneurysmal subarachnoid Hemorrhage. Neurocritical Care, 33(3), 749–758. https://doi.org/10.1007/s12028-020-00961-z
Heinsberg, L. W., Arockiaraj, A. I., Crago, E. A., Ren, D., Shaffer, J. R., Sherwood, P. R., Sereika, S. M., Weeks, D. E., & Conley, Y. P. (2020b). Genetic variability and trajectories of DNA methylation may support a role for HAMP in patient outcomes after aneurysmal subarachnoid Hemorrhage. Neurocritical Care, 32(2), 550–563. https://doi.org/10.1007/s12028-019-00787-4
Helbok, R., Rass, V., Kofler, M., Talasz, H., Schiefecker, A., Gaasch, M., Scherfler, C., Pfausler, B., Thomé, C., Beer, R., Lindner, H. H., & Schmutzhard, E. (2021). Intracerebral iron accumulation may be associated with secondary brain injury in patients with poor grade subarachnoid Hemorrhage. Neurocritical Care. https://doi.org/10.1007/s12028-021-01278-1
Huttunen, J., Kurki, M. I., von Und, Z., Fraunberg, M., Koivisto, T., Ronkainen, A., Rinne, J., Jääskeläinen, J. E., Kälviäinen, R., & Immonen, A. (2015). Epilepsy after aneurysmal subarachnoid hemorrhage: A population-based, long-term follow-up study. Neurology, 84(22), 2229–2237. https://doi.org/10.1212/WNL.0000000000001643
Ibrahim, G. M., Fallah, A., & Macdonald, R. L. (2013). Clinical, laboratory, and radiographic predictors of the occurrence of seizures following aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery, 119(2), 347–352. https://doi.org/10.3171/2013.3.JNS122097
Joerk, A., Seidel, R. A., Walter, S. G., Wiegand, A., Kahnes, M., Klopfleisch, M., Kirmse, K., Pohnert, G., Westerhausen, M., Witte, O. W., & Holthoff, K. (2014). Impact of heme and heme degradation products on vascular diameter in mouse visual cortex. Journal of the American Heart Association, 3, 4. https://doi.org/10.1161/JAHA.114.001220
Kinner-Bibeau, L. B., Pawaria, S., & Binder, R. J. (2018). CD91. Encyclopedia of Signaling. Molecules, 968–974. https://doi.org/10.1007/978-3-319-67199-4_413
Kuang, H., Wang, T., Liu, L., Tang, C., Li, T., Liu, M., Wang, T., Zhong, W., & Wang, Y. (2021). Treatment of early brain injury after subarachnoid hemorrhage in the rat model by inhibiting p53-induced ferroptosis. Neuroscience Letters, 762, 136134. https://doi.org/10.1016/j.neulet.2021.136134
Kühn, L. C. (2015). Iron regulatory proteins and their role in controlling iron metabolism. Metallomics, 7(2), 232–243. https://doi.org/10.1039/C4MT00164H
Latunde-Dada, G. O. (2016). Iron: Biosynthesis and significance of heme. Encyclopedia of Food and Health, 452–460. https://doi.org/10.1016/B978-0-12-384947-2.00402-5
Lawton, M. T., & Vates, G. E. (2017). Subarachnoid hemorrhage. 377(3), 257–266. https://doi.org/10.1056/NEJMCP1605827.
Lee, J. Y., Keep, R. F., He, Y., Sagher, O., Hua, Y., & Xi, G. (2010). Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. Journal of Cerebral Blood Flow & Metabolism, 30(11), 1793. https://doi.org/10.1038/JCBFM.2010.137
Li, Y., Liu, Y., Wu, P., Tian, Y., Liu, B., Wang, J., Bihl, J., & Shi, H. (2021). Inhibition of ferroptosis alleviates early brain injury after subarachnoid hemorrhage in vitro and in vivo via reduction of lipid peroxidation. Cellular and Molecular Neurobiology, 41(2), 263–278. https://doi.org/10.1007/s10571-020-00850-1
Li, Y., Yang, H., Ni, W., & Gu, Y. (2017). Effects of deferoxamine on blood-brain barrier disruption after subarachnoid hemorrhage. PLoS One, 12(3), e0172784. https://doi.org/10.1371/journal.pone.0172784
Lindbohm, J. V., Rautalin, I., Jousilahti, P., Salomaa, V., Kaprio, J., & Korja, M. (2019). Physical activity associates with subarachnoid hemorrhage risk– A population-based long-term cohort study. Scientific Reports, 9, 1. https://doi.org/10.1038/S41598-019-45614-0
Liu, W., Li, R., Yin, J., Guo, S., Chen, Y., Fan, H., Li, G., Li, Z., Li, X., Zhang, X., He, X., & Duan, C. (2019). Mesenchymal stem cells alleviate the early brain injury of subarachnoid hemorrhage partly by suppression of Notch1-dependent neuroinflammation: Involvement of Botch. Journal of Neuroinflammation, 16(1), 1–20. https://doi.org/10.1186/S12974-019-1396-5/FIGURES/11
Moos, T., Nielsen, T. R., Skjørringe, T., & Morgan, E. H. (2007). Iron trafficking inside the brain. Journal of Neurochemistry, 103(5), 1730–1740. https://doi.org/10.1111/J.1471-4159.2007.04976.X
Nieuwkamp, D. J., Setz, L. E., Algra, A., Linn, F. H., de Rooij, N. K., & Rinkel, G. J. (2009). Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. The Lancet Neurology, 8(7), 635–642. https://doi.org/10.1016/S1474-4422(09)70126-7
Northrop-Clewes, C. A. (2008). Interpreting indicators of iron status during an acute phase response--lessons from malaria and human immunodeficiency virus. Annals of Clinical Biochemistry, 45(Pt 1), 18–32. https://doi.org/10.1258/ACB.2007.007167
Ono, S., du Zhang, Z., Marton, L. S., Yamini, B., Windmeyer, E., Johns, L., Kowalczuk, A., Lin, G., & Loch Macdonald, R. (2000). Heme oxygenase-1 and ferritin are increased in cerebral arteries after subarachnoid hemorrhage in monkeys. Journal of Cerebral Blood Flow and Metabolism, 20(7), 1066–1076. https://doi.org/10.1097/00004647-200007000-00006
Paisan, G. M., Ding, D., Starke, R. M., Crowley, R. W., & Liu, K. C. (2018). Shunt-dependent hydrocephalus after aneurysmal subarachnoid Hemorrhage: Predictors and long-term functional outcomes. Neurosurgery, 83(3), 393–402. https://doi.org/10.1093/NEUROS/NYX393
Peng, D., Chen, C. A., Ruhela, D., Li, Y., & Regan, R. F. (2020). Deferoxamine deconditioning increases neuronal vulnerability to hemoglobin. Experimental Cell Research, 390(1), 111926. https://doi.org/10.1016/j.yexcr.2020.111926
Pluta, R., & Oldfield, E. (2008). Analysis of nitric oxide (NO) in cerebral vasospasm after aneursymal bleeding. Reviews on Recent Clinical Trials, 2(1), 59–67. https://doi.org/10.2174/157488707779318062
Polmear, A. (2003). Sentinel headaches in aneurysmal subarachnoid haemorrhage: What is the true incidence? A systematic review. Cephalalgia : An International Journal of Headache, 23(10), 935–941. https://doi.org/10.1046/J.1468-2982.2003.00596.X
Pyne-Geithman, G. J., Morgan, C. J., Wagner, K., Dulaney, E. M., Carrozzella, J., Kanter, D. S., Zuccarello, M., & Clark, J. F. (2005). Bilirubin production and oxidation in CSF of patients with cerebral vasospasm after subarachnoid hemorrhage. Journal of Cerebral Blood Flow and Metabolism, 25(8), 1070–1077. https://doi.org/10.1038/SJ.JCBFM.9600101
Qin, Y., Li, G., Sun, Z., Xu, X., Gu, J., & Gao, F. (2019). Comparison of the effects of nimodipine and deferoxamine on brain injury in rat with subarachnoid hemorrhage. Behavioural Brain Research, 367, 194–200. https://doi.org/10.1016/j.bbr.2019.04.004
Rouault, T. A., & Cooperman, S. (2006). Brain iron metabolism. Seminars in Pediatric Neurology, 13(3), 142–148. https://doi.org/10.1016/j.spen.2006.08.002
Schwedt, T. J., Matharu, M. S., & Dodick, D. W. (2006). Thunderclap headache. The Lancet Neurology, 5(7), 621–631. https://doi.org/10.1016/S1474-4422(06)70497-5
Shao, A., Zhou, Y., Yao, Y., Zhang, W., Zhang, J., & Deng, Y. (2019). The role and therapeutic potential of heat shock proteins in haemorrhagic stroke. Journal of Cellular and Molecular Medicine, 23(9), 5846–5858. https://doi.org/10.1111/jcmm.14479
Shi, L., Liang, F., Zheng, J., Zhou, K., Chen, S., Yu, J., & Zhang, J. (2018). Melatonin regulates apoptosis and autophagy via ROS-MST1 pathway in subarachnoid hemorrhage. Frontiers in Molecular Neuroscience, 11, 93. https://doi.org/10.3389/FNMOL.2018.00093/BIBTEX
Shishido, H., Zhang, H., Okubo, S., Hua, Y., Keep, R. F., & Xi, G. (2016). The effect of gender on acute hydrocephalus after experimental subarachnoid hemorrhage. Acta Neurochirurgica. Supplement, 121, 335–339. https://doi.org/10.1007/978-3-319-18497-5_58
Stockwell, B. R., Friedmann Angeli, J. P., Bayir, H., Bush, A. I., Conrad, M., Dixon, S. J., Fulda, S., Gascón, S., Hatzios, S. K., Kagan, V. E., Noel, K., Jiang, X., Linkermann, A., Murphy, M. E., Overholtzer, M., Oyagi, A., Pagnussat, G. C., Park, J., Ran, Q., et al. (2017). Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell, 171(2), 273–285. https://doi.org/10.1016/J.CELL.2017.09.021
Stow, L. R., Jacobs, M. E., Wingo, C. S., Cain, B. D., Florida, N., & Georgia, S. (2011). Endothelin-1 gene regulation. The FASEB Journal, 25(1), 16–28. https://doi.org/10.1096/FJ.10-161612
Sun, H., Klahr, A. C., Kate, M., Gioia, L. C., Emery, D. J., Butcher, K. S., & Wilman, A. H. (2018). Quantitative susceptibility mapping for following intracranial hemorrhage. Radiology, 288(3), 830–839. https://doi.org/10.1148/radiol.2018171918
Sun, Y., Chen, P., Zhai, B., Zhang, M., Xiang, Y., Fang, J., Xu, S., Gao, Y., Chen, X., Sui, X., & Li, G. (2020). The emerging role of ferroptosis in inflammation. Biomedicine & Pharmacotherapy, 127, 110108. https://doi.org/10.1016/J.BIOPHA.2020.110108
Suzuki, H., Muramatsu, M., Kojima, T., & Taki, W. (2003). Intracranial Heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke, 34(12), 2796–2800. https://doi.org/10.1161/01.STR.0000103743.62248.12
Tan, G., Liu, L., He, Z., Sun, J., Xing, W., & Sun, X. (2016). Role of hepcidin and its downstream proteins in early brain injury after experimental subarachnoid hemorrhage in rats. Molecular and Cellular Biochemistry, 418(1–2), 31–38. https://doi.org/10.1007/S11010-016-2730-1
Tenenbein, M., Kowalski, S., Sienko, A., Bowden, D. H., Adamson, I. Y. R., & Tenenbein, M. (1992). Pulmonary toxic effects of continuous desferrioxamine administration in acute iron poisoning. The Lancet, 339(8795), 699–701. https://doi.org/10.1016/0140-6736(92)90598-W
Toyota, Y., Shishido, H., Ye, F., Koch, L. G., Britton, S. L., Garton, H. J. L., Keep, R. F., Xi, G., & Hua, Y. (2021). Hydrocephalus following experimental subarachnoid Hemorrhage in rats with different aerobic capacity. International Journal of Molecular Sciences, 22, 9. https://doi.org/10.3390/ijms22094489
Veremakis, C. (1991). Subarachnoid hemorrhage. Problems in Critical Care, 5(2), 251–268. https://doi.org/10.1161/01.str.0000014773.57733.3e
Wang, D.-L., Lin, P., Lin, Z.-Y., Zheng, S.-F., Shang-Guan, H.-C., Kang, D.-Z., Chen, G.-R., Zhang, Y.-B., Wen, C.-S., Lin, Y.-X., & Yao, P.-S. (2019). Lower hemoglobin levels are associated with acute seizures in patients with ruptured cerebral aneurysms. World Neurosurgery, 127, e1237–e1241. https://doi.org/10.1016/j.wneu.2019.04.115
Wang, K. C., Tang, S. C., Lee, J. E., Lai, D. M., Huang, S. J., Hsieh, S. T., Jeng, J. S., & Tu, Y. K. (2014). Prognostic value of intrathecal heme oxygenase-1 concentration in patients with fisher grade III aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery, 121(6), 1388–1393. https://doi.org/10.3171/2014.7.JNS131704
Yu, F., Saand, A., Xing, C., Lee, J. W., Hsu, L., Palmer, O. P., Jackson, V., Tang, L., Ning, M., Du, R., Kochanek, P. M., Lo, E. H., & Chou, S. H.-Y. (2021). CSF lipocalin-2 increases early in subarachnoid hemorrhage are associated with neuroinflammation and unfavorable outcome. Journal of Cerebral Blood Flow and Metabolism, 41(10), 2524–2533. https://doi.org/10.1177/0271678X211012110
Yuan, H., Li, X., Zhang, X., Kang, R., & Tang, D. (2016). Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochemical and Biophysical Research Communications, 478(3), 1338–1343. https://doi.org/10.1016/J.BBRC.2016.08.124
Zaidi, H. A., Montoure, A., Elhadi, A., Nakaji, P., McDougall, C. G., Albuquerque, F. C., Spetzler, R. F., & Zabramski, J. M. (2015). Long-term functional outcomes and predictors of shunt-dependent hydrocephalus after treatment of ruptured intracranial aneurysms in the BRAT trial: Revisiting the clip vs coil debate. Neurosurgery, 76(5), 608–615. https://doi.org/10.1227/NEU.0000000000000677
Zhang, Y.-B., Zheng, S.-F., Shang-Guan, H.-C., Kang, D.-Z., Chen, G.-R., & Yao, P.-S. (2019). Lower iron levels predict acute hydrocephalus following aneurysmal subarachnoid hemorrhage. World Neurosurgery, 126, e907–e913. https://doi.org/10.1016/j.wneu.2019.03.009
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zainab, A., Hamid, A. (2023). Iron and Subarachnoid Hemorrhage. In: Mohamed, W., Brogazzi, N.L., Kostrzewa, R.M. (eds) Brain-Iron Cross Talk. Nutritional Neurosciences. Springer, Singapore. https://doi.org/10.1007/978-981-19-7327-7_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-7327-7_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7326-0
Online ISBN: 978-981-19-7327-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)