Abstract
Mango (Mangifera indica L.) is a tropical fruit which is climacteric and highly perishable. Consequently, it is indispensable to address postharvest management techniques by applying eco-friendly technologies to reduce crop losses. Thus, the current study was conducted to evaluate Aloe vera gel’s influence alone and chitosan and calcium chloride (CaCl2) on mango shelf life during the storage time at the ambient temperature (25 ± 2 °C) for 21 days. The results exhibited that A. vera-chitosan coatings were able to remarkably decrease weight loss and ascorbic acid reduction throughout the storage period. Total phenol and antioxidant activity progressively diminished during the storage, and control fruits exhibited the lowest content of the phenol content and antioxidant activity during the storage. The highest correlation (R = 0.95) between antioxidant and ascorbic acid was observed in the A. vera-chitosan treated fruits. Control fruits showed the lowest catalase (CAT) and peroxidase (POD) enzyme activity during the storage time. A. vera-chitosan coating significantly inhibited the activity of polyphenol oxidase (PPO) during the storage period. Oppositely, the coating had no significant effect on total soluble solids (TSS) and titratable acidity (TA) at the end of the experiment. The discoloration trend of the fruits coated with A. vera enriched with chitosan was significantly delayed compared to the control fruits. Finally, A. vera-chitosan coating could be suggested as a suitable coating to preserve the quality of mango fruit throughout storage at the ambient temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mango is one of the most important tropical fruits that has a unique status among other fruits in terms of appearance, taste, favorable aroma, excellent beneficial nutritional values and general eating features [1]. In general, due to the highly perishable nature and sensitivity of mango, a good postharvest practice is required to extend Mango’s shelf life [2].
Nowadays, given the negative consequences of using chemical compounds on human health and the environment, new approaches have been used for the utilization of natural and plant-base compounds in crops’ production and storage. For instance, edible coatings are promising technologies applied in quality improvement and shelf-life extension of fruits. Edible films and coatings have many advantages over other postharvest treatments. They can add commercial value to fruits by enhancing their appearance, safety, quality and act as carriers of functional ingredients, such as antioxidants, antimicrobial agents and nutraceuticals [3]. Edible coatings prevent the exchange of gases and water vapor by forming a modified atmosphere around the crop, thereby reducing the respiration rate of the fruit and preserving the quality of crops [4].
Aloe vera gel is one of the novel edible coatings which has recently attracted many researchers. A. vera gel is clear, odorless, non-sticky having high absorbency. This gel is completely healthy and environmentally friendly, with a pH of about 4.5 which can replace various fruit coverings at the postharvest stage [5]. Approximately 96% of A. vera leaf gel is water and the remaining 4% consist of other compounds such as essential oils, amino acids, vitamins, minerals, enzymes [6]. This polysaccharide gel act as a barrier to moisture and O2, reducing water loss and gas exchange through lenticel coating, resulting in a slow respiration rate and conserving fruit quality [7]. The combination of A. vera with salicylic acid was more effective in maintaining quality and decreasing microbial load compared to the individual substances in orange fruit [8]. In more detail, A. vera gel coatings could significantly delay the firmness, discoloration and weight loss in fruits [9, 10].
Chitosan is considered as an edible coating which has high antimicrobial activity and compatibility with substances such as minerals, vitamins and antimicrobial agents used for products’ shelf life [11]. The ability of chitosan coating in reducing disease incidence of mango has been well acknowledged in many studies [12]. Several studies reported that chitosan, either alone or in combination with other materials, was able to decrease respiration, loss of firmness, weight loss, and disease incidence [13, 14].
It is well known that calcium chloride (CaCl2) could maintain the fruit quality during storage by reducing the metabolism activities and the respiration rate [15]. In an earlier study, calcium chloride combined with gum Arabic coating effectively inhibited the loss of ascorbic acid and phenolic compounds and improved the postharvest quality of fruit during cold storage [16]. Calcium chloride (3%) combined with nano-chitosan preserved ascorbic acid, antioxidant activity and total anthocyanin contents of strawberries stored 15 days at 4 °C [17].
Adding edible materials to coatings improves the functional, nutritional, organoleptic and mechanical properties of coatings and can be considered as a new method to maintain products’ quality. Studies focused on the effect of A. vera and its compounds with calcium and chitosan on mango quality are limited and fruits’ antioxidant system have not been evaluated. Therefore, the purpose of the current study was to investigate the postharvest quality and changes in the antioxidant system of mango fruit stored at room temperature under the influence of A. vera coating enriched with chitosan and calcium chloride.
Materials and methods
Plant materials and treatments
Mango fruits were harvested at the mature-green stage from a commercial orchard located at Minab (Long. 57°05′13″E and Lat. 27°07′51″N) in Hormozgan province, Iran. Fruits immediately transferred to the laboratory, and intact and uniform fruits (in terms of size and shape) were selected and then washed using distilled water. Sodium hypochlorite (0.05%) was also used to sterilize the fruits before coating. A. vera gel was extracted from mature leaves of A. vera plants that were obtained from the field of Hormozgan university and the internal gel was homogenized using an electric blender. Then four concentrations including 5, 10, 20 and 30% were prepared with distilled water. Based on our previous experiment, the best concentration of chitosan and calcium was added to A. vera gel. Chitosan (1.5%) and calcium chloride (1.5%) were incorporated into A. vera gel as reported in Table 1. Fruits were immersed in different treatment liquids for 5 min and then were dried for 2 h at room temperature. Some of the fruits were placed in distilled water as a control treatment. Fruits were subsequently placed in boxes and stored at ambient conditions (25 ± 2 °C temperature and 80 ± 5% relative humidity). The fruits were assessed every 7 days for 21 days of storage.
Weight loss
To measure the percentage of weight loss, the fruits were weighed by a digital balance at the beginning of storage and weighed again in each period. The difference between the initial weight and the final weight was calculated as weight loss and expressed in a percentage.
Ascorbic acid
The amount of ascorbic acid was evaluated using the titration method and with 2,6-dichlorophenolindophenol solution and was expressed as mg of ascorbic acid per 100 g of fruits [18].
Total phenolics content (TPC)
Extraction was performed by homogenizing one gram of mango fruit and five ml of methanol (80%), which was centrifuged for 20 min at 10,000×g. The supernatant was used for TPC and antioxidant activity determination.
Phenolic content was determined with 0.5 ml of the metabolic extract and 2.5 ml of diluted Folin-Ciocalteu (1:10) and 2 ml of 75% sodium bicarbonate. After the incubation at room temperature for two h, phenolic content was read at 750 nm by UV–visible spectrophotometer according to mg of Gallic acid/g Fresh Weight [19].
Antioxidant activity
A volume of 75 μl of supernatant was added to 2925 μl DPPH (2,2-diphenyl-1-picrylhydrazyl) and incubated 30 min in dark condition and then the absorbance was read at 517 nm using UV–visible spectrophotometer (Cecil 2501, England). Antioxidant activity was obtained from the following formula: Antioxidant activity (%) = (At0 − At30) × 100/At0; whereas At0 and At30 are related to the absorption at zero and 30 min, respectively [20].
Enzymatic extraction and assay
The amount of 0.5 g of fruit pulp was homogenized in 5 ml of buffer (50 mM, pH 7.5) comprising 1% PVP (polyvinyl propylene) and 1 ml EDTA (ethylenediaminetetraacetic acid), and then was centrifuged for 20 min at 14,000 rpm and 4 °C. The clear supernatant was taken to measure the enzymes’ activity.
Catalase (CAT)
The catalase (CAT) activity was evaluated based on Chance and Maehly [21] method. The reaction solution contained enzyme extract, H2O2 (15 mM) and phosphate buffer (pH 7.0). Enzyme activity was expressed as a decrease in 240 nm as U/mg FW.
Peroxidase (POD)
The activity of peroxidase was performed according to Chance and Maehl [21]; while, the reaction mixture consisted of guaiacol, phosphate buffer (pH 7.5), H2O2 and enzyme extract. The changes in guaiacol oxidation during two minutes were recorded at 470 nm and the results were expressed as U/mg FW.
Polyphenol oxidase (PPO)
The activity of polyphenol oxidase was measured according to Kar and Mishra [22]; whereas the reaction mixture contained phosphate buffer (50 mM, pH = 7), pyrogallol (20 mM) as a precursor and enzymatic extract. The absorption was read using a UV–visible spectrophotometer at 420 nm after three min and the results were expressed as U/mg FW.
Total soluble solids (TSS) and titratable acidity (TA)
The TSS of the fruit juice extract was determined by a digital refractometer (DBR95, Italy) according to the Brix index [18]. To measure titrated acidity of the juice fruit, five ml of fruit extract was added with 45 ml of distilled water and then titrated with 0.1 N NaOH to reach pH of 8.1 and was expressed as citric acid percentage.
Color parameters
The fruit color was assessed at different points on the surface of the fruit using a colorimeter (Minolta CR-400, Tokyo, Japan) and color characteristics b* (yellow/blue), a* (red/green) and L* (white/black), values were obtained at 400–700 nm range [23]
Statistical analysis
To analyze the data, variance analysis (ANOVA) was applied. The storage times (days) and different coatings were the sources of variation. The mean comparisons were carried out by Duncan’s multiple range test (DMRT) at P ≤ 0.05. Statistical analyses were done using SPSS version 16.0 and the graphs were drawn using SigmaPlot version14.0.
Results
Weight loss
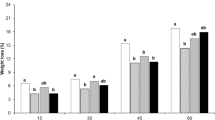
According to Fig. 1, the samples’ weight loss was increased throughout the storage, regardless of the treatment. All coatings had the effective role in weight loss’ prevention compared to the control. After 14 and 21 days of the storage period, A. vera gel treatment combined with chitosan (10% + 1.5%) resulted in the lowest fruit’s weight loss (30.4%) and oppositely the maximum weight loss (48.6%) was found in the control treatment.
The effects of various preservative coatings on weight loss of mango fruit during storage at 25 ± 2 °C and 85 ± 5% RH. Details of the various preservation coatings are given in Table 1. Values are means ± SE from three replicates (n = 3). Statistical analysis was performed using Duncan test. Control = Distilled water, T1 = Aloe vera 5%, T2 = Aloe vera 10%, T3 = Aloe vera 20%, T4 = Aloe vera 30%, T5 = Aloe vera 10% + Calcium chloride 1.5%, T6 = Aloe vera 5% + Calcium chloride 1.5%, T7 = Aloe vera 10% + Chitosan 1.5%
Ascorbic acid
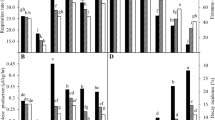
According to Fig. 2a ascorbic acid gradually decreased over time. However, the severity of reduction in A. vera (10%)-calcium (1.5%) and A. vera (10%)-chitosan treatment was significantly (P < 0.05) lower than other treatments. Therefore, at the end of the experimentation, the maximum ascorbic content (40 mg/100 g) was detected in the treated fruits of A. vera-chitosan coating and the lowest ascorbic content (25 mg/100 g) was observed in control fruits.
The effects of various preservative coatings on ascorbic acid (a), total phenol (b) and antioxidant capacity (c) of mango fruit during storage at 25 ± 2 °C and 85 ± 5% RH. Details of the various preservation coatings are given in Table 1. Values are means ± SE from three replicates (n = 3). Statistical analysis was performed using Duncan test. Control = Distilled water, T1 = Aloe vera 5%, T2 = Aloe vera 10%, T3 = Aloe vera 20%, T4 = Aloe vera 30%, T5 = Aloe vera 10% + Calcium chloride 1.5%, T6 = Aloe vera 5% + Calcium chloride 1.5%, T7 = Aloe vera 10% + Chitosan 1.5%
Total phenol and antioxidant activity
In the first 14 days of storage time, the fruit phenol content showed a gradual slight decreasing rate. Thereafter, it suddenly reduced and reached the minimum at the end of the storage duration. The lowest and highest phenol levels were found in the control and fruits treated with A. vera (20%), respectively during the storage time (Fig. 2b).
As shown in Fig. 2c, all treatments demonstrated a significant (P < 0.05) decrease in fruits’ antioxidants compared to the first day of storage. But this decrease was more remarkable in the control fruits. At the end of the storage, the maximum (50.4%) and the minimum (37.4%) antioxidant activity were detected in the fruits treated with A. vera-chitosan and the control fruits, respectively.
Figure 3a, b shows the correlation between ascorbic acid and total phenol with the antioxidant activity of treated and control mango fruits. The highest correlation between ascorbic acid and antioxidant activity was observed in A. vera (10%)-calcium (1.5%) treatment and the lowest was observed in the control. The highest correlation between total phenol and antioxidant activity was observed in A. vera (10%) treatment and the lowest correlation was found in A. vera (10%)-calcium (1.5%) treated mango.
Antioxidant enzymes
Catalase activity showed a similar trend in all samples, with a higher change in control fruit during the same period. The enzyme activity slightly decreased in the first week and then significantly (P < 0.05) increased during the last day of storage periods. At the end of the experiment, control samples significantly (P < 0.05) displayed lower activity rather than other treatments (Fig. 4a).
The effects of various preservative coatings on catalase (CAT) (a), peroxidase (POD) (b) and poly phenol oxidase (PPO) (c) of mango fruit during storage at 25 ± 2 °C and 85 ± 5% RH. Details of the various preservation coatings are given in Table 1. Values are means ± SE from three replicates (n = 3). Statistical analysis was performed using Duncan test. Control = Distilled water, T1 = Aloe vera 5%, T2 = Aloe vera 10%, T3 = Aloe vera 20%, T4 = Aloe vera 30%, T5 = Aloe vera 10% + Calcium chloride 1.5%, T6 = Aloe vera 5% + Calcium chloride 1.5%, T7 = Aloe vera 10% + Chitosan 1.5%
Peroxidase demonstrated a diminishing pattern along with storage period progress irrespective of the treatment. However, the treated fruits showed higher levels of the enzyme. After 21 days of storage, the control fruits showed meaningful differences with other treatments except with the fruits treated with A. vera (20%) as it is presented in Fig. 4b.
In general, polyphenol oxidase activity significantly (P < 0.05) increased during the storage time, nevertheless, the treated fruits with A. vera-chitosan went through lower changes than other treatments. At the end of the experiment, all samples exhibited lower enzyme activity than the control; consequently, the lowest and highest enzyme activities were observed in A. vera-chitosan treatment and the control fruit, respectively (Fig. 4c).
Tithable acidity (TA), TSS
As it is shown in Fig. 5a, the TSS of samples demonstrated an increasing pattern along with storage period progression irrespective of the treatments. After 21 days of storage, the lowest (11.8%) fruit’s TSS was observed in A. vera (5%) treated fruit. However, no significant (P > 0.05) differences were revealed between other treatments and the control group.
The effects of various preservative coatings on total soluble solid (TSS) (a) and titratable acidity (TA) (b) of mango fruit during storage at 25 ± 2 °C and 85 ± 5% RH. Details of the various preservation coatings are given in Table 1. Values are means ± SE from three replicates (n = 3). Statistical analysis was performed using Duncan test. Control = Distilled water, T1 = Aloe vera 5%, T2 = Aloe vera 10%, T3 = Aloe vera 20%, T4 = Aloe vera 30%, T5 = Aloe vera 10% + Calcium chloride 1.5%, T6 = Aloe vera 5% + Calcium chloride 1.5%, T7 = Aloe vera 10% + Chitosan 1.5%
As it is seen in Fig. 5b, the TA gradually reduced throughout the storage; while, the minimum TA was detected at the end of the storage. The A. vera gel combined with chitosan (10% + 1.995%) treatment enjoyed the highest TA rate, equal with 0.93, 0.52 and 0.61 at the 7th, 14th and 21th of the storage period, respectively.
Color parameters
The results revealed a regular reduction in L* value in all samples over time. However, the control fruits showed a significantly (P < 0.05) lower L* value than the coated fruits through the storage. After 21 days of storage, the fruits coated with A. vera (10%) and A. vera (30%) represented higher L* rather than the control and other treatments (Fig. 6a).
The effects of various preservative coatings on color parameters (L*, a* and b*) of mango fruit during storage at 25 ± 2 °C and 85 ± 5% RH. Details of the various preservation coatings are given in Table 1. Values are means ± SE from three replicates (n = 3). Statistical analysis was performed using Duncan test. Control = Distilled water, T1 = Aloe vera 5%, T2 = Aloe vera 10%, T3 = Aloe vera 20%, T4 = Aloe vera 30%, T5 = Aloe vera 10% + Calcium chloride 1.5%, T6 = Aloe vera 5% + Calcium chloride 1.5%, T7 = Aloe vera 10% + Chitosan 1.5%
The a* value of all samples significantly (P < 0.05) increased through the storage time. At the days of 14th and 21st of the storage period, the fruits coated with A. vera gel in combination with chitosan (10% + 1.5%) indicated the lowest a* value (− 15.05 and − 5.86); while, the control fruits enjoyed the highest a* value (− 8.2 and − 1.99) (Fig. 6b).
In general, the b* value had a reducing rate; whereas, all coated fruits showed a higher b* value during the storage duration in comparison with the control. At the end of the storage time, the advanced b* value was detected in the fruits coated with A. vera (5%) and also A. vera (10%) (Fig. 6c).
Principal component analysis (PCA)
The data were evaluated by a PCA to determine the effectiveness of edible coatings after evaluating some biochemical traits and antioxidant enzymes. The data with PCA presented that two principal components (PCs) explained 82.3% of the total variance. PC1 accounted for 61.3% of the variance in the dataset, while PC2 described 21.0% of the variance. The b* value, antioxidant activity, POD enzyme, total phenol and ascorbic acid contents were positively correlated with PC1. However, the a* value, CAT enzyme, PPO enzyme, weight loss, were negatively correlated. PC2 was only positively correlated with L* value, while TSS was negatively correlated (Fig. 7).
Discussion
Weight loss
Fresh products continue to lose their moisture and carbon after the harvest because of metabolic processes such as respiration and transpiration; while, they can be no longer replaced. In fact, fruits and vegetables’ water loss after the harvest is a major problem, which results in shriveling and shrinkage and weight loss [24]. To improve postharvest quality and extend shelf-life of perishable products, the rate of water loss has to be managed. The results of this study showed that coated mango fruit had lower weight loss than the control during the storage. Edible coatings form a semi-compromised and smooth layer on the fruit’s surface, which covers the stomata and guard cells, and thereby reduces water transfer and evaporation as well as gas exchange through the fruit levels, and ultimately decreases the respiration and transpiration [25]. It was confirmed that adding the chitosan and calcium chloride improves A. vera gel coatings’ efficacy and significantly reduces the weight loss of mango fruit. Edible chitosan coating acts as a protective barrier and restricts water transfer and gas exchange from the fruit surface and consequently prevents fruit’s postharvest weight loss [11].
Calcium binding to phospholipids stabilizes the membrane’s lipid bilayers and controls the permeability and integrity of the membrane which eventually leads to the fruit’s weight reduction. Calcium also strengthens the cell wall and particularly the middle lamella by creating new crosslinks among anionic homogalacturonans [26].
In many reports, chitosan has introduced as an effective material on respiration rate, water and weight loss of the crops without any negative impact on the odor, taste and palatability of the fruits and vegetables [27]. Our study is in line with Pinzon et al. [28] who reported that the weight loss of strawberry treated with starch-chitosan A. vera gel was slower than untreated fruit during the postharvest storage time. Similar to our results, Kou et al. [29] discovered that CaCl2 and composite coating can remarkability reduce the weight loss of jujube fruit during the storage period (105 days) at the temperature of 0 °C.
Ascorbic acid
Ascorbic acid which is a nutritional factor of the fruits and vegetables is also considered as one of the most important antioxidants that scavenge reactive oxygen species (ROS) and free radicals during the crops’ ripening. However, due to the oxidation process, the ascorbic acid content of vegetables and fruits decreases throughout the storage [30]. Typically, the oxidation process intensity and ascorbic acid content’ reduction of the products depends very much on their access to oxygen during the storage [31]. In our observations, adding the chitosan significantly prevented mango’s ascorbic reduction during the storage time. In this study, the chitosan effectiveness in controlling the fruit ascorbic acid losses can be attributed to its role in limiting surface permeability of oxygen and carbon dioxide.
The application of A. vera gel and chitosan together can modulate the internal gas atmosphere, in a way that leads to carbon dioxide level increase and oxygen concentration decrease; so that the oxidation process is delayed. Reducing ascorbic acid level in coated fruits can be also because of diminishing ethylene production and respiratory rate as well [25]. Khatri et al. [32] also uncovered that the combination A. vera gel and chitosan preserved the ascorbic acid of tomato fruit during the storage. Agree with the results obtained, Nourozi and Sayyari [33], declared that the combination of basil seed mucilage (BSM) with A. vera edible coatings efficiently preserves ascorbic acid of apricot fruits through cold storage.
Phenol content
Phenolic compounds play a vital role in the nutritional quality (e.g. color, acidity, astringency, bitterness and flavor) of the product. Furthermore, because of their central role in capturing reactive oxygen species that are produced during the fruit ripening, it has a significant effect on fruits and vegetables’ antioxidant potential [34]. Edible coatings have a suppression impact on phenolic compounds’ oxidation through decreasing the gas exchange and respiration [35]. The accumulation of phenolic compounds may be due to increased PAL enzyme activity. Earlier, Romanazzi et al. [36] presented that the activity of the PAL enzyme, which is responsible for the synthesis of phenolic compounds, increased in chitosan-treated grape fruit. The results of the current experiment were consistent with the results of Tzortzakis et al. [37] who acknowledged that the use of A. vera-Sage essential oil coating positively affected preserving phenolic compounds of tomato fruit during the storage. Lo’ay and Taher [38] notified that reducing phenol content consumption of coated fruits was due to declining of polyphenol enzyme activity.
Antioxidant activity
Naturally, the production of reactive oxygen species (ROS), which leads to tissue destruction and decay, would increase in fruits and vegetables during the ripening and storage process. The fruits are able to scavenge ROS through enzymatic [as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX)] and non-enzymatic (phenolic compounds, glutathione and ascorbic acid) antioxidant systems [39]. Therefore, preserving the antioxidants of the products is important in order to maintain the postharvest quality of the products. Previous studies have exhibited that coatings prevent oxidation and reduction of the fruit antioxidant system through gas permeability limitation [40]. It was observed that the A. vera-Sage essential oil used as tomato fruits coating preserved their antioxidants capacity during the storage, in contrast with the control fruits [37]. Khaliq et al. [37] also achieved the same results accentuating the positive effects of A. vera gel on preserving antioxidant activities in banana fruit. Similar to our findings, numerous studies have displayed that there is a positive correlation between fruit’s antioxidant activity and ascorbic acid and its phenolic compounds. Therefore, the greater antioxidant activity of coated fruits can be related to higher levels of ascorbic and total phenol content. Similar to our results, [41] exhibited that A. vera coated guava fruits, which had more antioxidants, also showed higher contents of ascorbic acid, flavonoids (quercetin and rutin), and total phenolics in comparison to control fruits.
Enzyme activities
The catalase (CAT) catalyzes the breakdown of H2O2 accumulated in the cell into the water and molecular oxygen, and thereby reduces the toxic H2O2 that can cause the oxidation of organic substances of the cells of fruits and vegetables [42]. It has been reported that chitosan coating improves the CAT activity [42]. Recently studies have been shown that the use of 80 and 60% Aloe vera coatings in avocado fruit increase the SOD, CAT and APX enzymes activates than control fruits [41]. Ali et al. [43] also showed that the application of A. vera coating increases the catalase, ascorbate peroxidase and superoxide dismutase activities in litchi fruit during the storage period. The POD is a significant oxidoreductase that is normally found in the products and is firmly identified with their physiological and biochemical metabolic processes. Studies have shown that the use of chitosan coatings in orange fruit increases the POD enzyme activity [44]. Previous results also indicated that chitosan treatment decreases the activity of PPO and improves the quality of tomato fruit during the storage time [44]. Similarly, Molamohammadi et al. [45] demonstrated that chitosan-salicylic acid coatings significantly inhibits the PPO activity in fresh pistachio fruit, through maintaining its postharvest quality and extending its shelf life. The inhibitory effect of the chitosan on the PPO activity of the fruit can be attributed to low gas availability especially against O2 [46]. However, no detailed research has been done on the effect of coatings on enzyme s’ activity in treated fruits.
TSS and titratable acidity TA
Generally, some biochemical changes can happen in mango fruit through ripening, including the TA reduction and the TSS increase [2]. The increase in the TSS of the fruit during storage could be attributed to the loss of water or the decomposition of starch to simple sugars and the destruction of cell wall polysaccharides [47]. Acidity reduction could be due to organic acids’ consumption in respiration or its conversion to sugar. The delay in TSS and TA in coated fruits could be associated with the gases (O2 and CO2) barrier property of edible coating and respiration reduction as a result [48]. These effects may be due to the fact that chitosan and A. vera coatings create a semi-impermeable layer on the surface of the mango, which reduces water evaporation and gas exchange and thereby postponing fruit’s ripening evolution process [42]. A similar observation was reported by [32] who showed that there are lower trends of total soluble sugar (TSS) and TA of coated tomato fruit during the cold storage, in comparison with the control fruits. The results are generally consistent with the previous findings that reported a higher value of TSS (14.6%) and TA (49%) in coated apples comparing the control fruits [49]
Color parameters
One of the important appearance features that have a significant impact on customer choice is the peel color of the fruit. The change of the color is basically related to the conversion of chlorophyll into other pigments such as carotenoids and anthocyanins [50]. In our study, the findings revealed that a gradual decrease was occurred in L* value in all samples; whereas, declining in L* value was lower in treated fruits than the control fruits. All coatings considered in this study were able to prevent the greenness (a*) reduction in comparison with the control. According to the above results, A. vera-chitosan treatment could postpone chlorophyll degradation of the fruit peel. This can be attributed to the treatments which played a role as the barrier for gas exchange. As a result of using A. vera coating, the modified atmosphere can lower respiration rate and ethylene production; therefore, it can cause a delay in ripening, chlorophyll degradation, anthocyanin accumulation and carotenoids’ synthesis and eventually in fruits’ discoloration [48]. It was reported that guava fruits coated with A. vera gel 40% and A. vera gel 60% exhibited reduced chlorophyll loss during storage than the controls [41]. Similar to our results, Pinzon et al. (2020) underlined in their research that the least color change was observed in strawberry coated with starch-chitosan- A. vera gel. In addition, the results of Parven et al. [51] represented that A. vera gel coating retards the color change of papaya fruits during storage compared to the control fruits.
Conclusion
The results of this research discovered that incorporating chitosan and calcium could improve the efficacy of A. vera coating and appropriately maintain the postharvest quality of mango and as a result extend its shelf life through controlling the weight loss. The higher ascorbic acid and antioxidant activity were found in A. vera-chitosan coated fruit. These coatings can increase antioxidant enzyme (CAT and POD) activity and inhibit PPO enzyme activity during the storage. Fruits coated with A. vera-chitosan presented minimal discoloration. Thus, considering the positive effects of chitosan in improving the properties of A. vera coating, and also the growing interest for using healthy natural compounds, applying chitosan in combination with A. vera is strongly recommended to preserve the quality of mango fruit during storage at room temperature. However, further studies are necessary to investigate the possible mechanism of A. vera, chitosan and calcium action in activity of antioxidant enzymes.
References
E.B. Esguerra, R. Rolle, Guidance for Horticultural Supply Chain Stakeholders (Food and Agriculture Organization of the United Nations, Rome, 2018).
R.N. Tharanathan, H.M. Yashoda, T.N. Prabha, Food Rev. Int. 22, 95 (2006)
E. Tavassoli-Kafrani, M.V. Gamage, L.F. Dumée, L. Kong, S. Zhao, Crit. Rev. Food Sci. Nutr. 61, 1–28 (2020)
P.K. Raghav, N. Agarwal, M. Saini, Int. J. Sci. Res. Modern Educ. 1, 188 (2016)
J. Misir, F.H. Brishti, M.M. Hoque, Am. J. Food Sci. Technol. 2, 93 (2014)
P. Sharma, A.C. Kharkwal, H. Kharkwal, M.Z. Abdin, A. Varma, Int. J. Pharm. Sci. Rev. Res. 29, 31 (2014)
A.A. Maan, A. Nazir, M.K.I. Khan, T. Ahmad, R. Zia, M. Murid, M. Abrar, J. Herb. Med. 12, 1 (2018)
M. Rasouli, M.K. Saba, A. Ramezanian, Sci. Hortic. 247, 27 (2019)
S. Ali, A.S. Khan, A. Nawaz, M.A. Anjum, S. Naz, S. Ejaz, S. Hussain, Postharvest Biol. Technol. 157, 110960 (2019)
M. Hosseinifarahi, E. Jamshidi, S. Amiri, F. Kamyab, M. Radi, J. Food Process. Preserv. 4, 1–14 (2020)
R.A. Shiekh, M.A. Malik, S.A. Al-Thabaiti, M.A. Shiekh, Food Sci. Technol. Res. 19, 139 (2013)
S. Shah, M.S. Hashmi, Hortic. Environ. Biotechnol. 61, 279 (2020)
S.M. Zahedi, M.S. Hosseini, M. Karimi, A. Ebrahimzadeh, Food Sci. Nutr. 7, 433 (2019)
A. Eshetu, A.M. Ibrahim, S.F. Forsido, C.G. Kuyu, Heliyon 5, e01116 (2019)
Q. Gao, T. Xiong, X. Li, W. Chen, X. Zhu, Sci. Hortic. 253, 412 (2019)
G. Khaliq, M.T.M. Mohamed, H.M. Ghazali, P. Ding, A. Ali, Postharvest Biol. Technol. 111, 362 (2016)
V.T.B. Nguyen, D.H.H. Nguyen, H.V.H. Nguyen, Postharvest Biol. Technol. 162, 111103 (2020)
A. In, W. Horwitz, AOAC International, Arlington VA (1995)
V.L. Singleton, R. Orthofer, R.M. Lamuela-Raventós, Methods Enzymol. 299, 152–178 (1999)
W. Brand-Williams, M.-E. Cuvelier, C. Berset, LWT-Food Sci. Technol. 28, 25 (1995)
B. Chance, A.C. Maehly, Methods Enzymol. 2, 764–775 (1955)
M. Kar, D. Mishra, Plant Physiol. 57, 315 (1976)
R.G. McGuire, HortScience 27, 1254 (1992)
E.M. Yahia, A. Carrillo-Lopez, Postharvest Physiology and Biochemistry of Fruits and Vegetables (Woodhead Publishing, Cambridge, 2018).
V. Falguera, J.P. Quintero, A. Jiménez, J.A. Muñoz, A. Ibarz, Trends Food Sci. Technol. 22, 292 (2011)
B. Hocking, S.D. Tyerman, R.A. Burton, M. Gilliham, Front. Plant Sci. 7, 569 (2016)
D. Chao, M. Xin, M. Jingru, M.I.H. Khan, D. Lei, K. Avik, A. Xingye, Z. Junhua, T. Huq, N. Yonghao, J. Bioresour. Bioprod. 4,11–21 (2019)
M.I. Pinzon, L.T. Sanchez, O.R. Garcia, R. Gutierrez, J.C. Luna, C.C. Villa, Int. J. Food Sci. Technol. 55, 92 (2020)
X. Kou, L. Chai, S. Yang, Y. He, C.E. Wu, Y. Liu, J. Zhou, Z. Xue, Z. Wang, J. Sci. Food Agric. 101 703–717 (2021)
N.A. Akram, F. Shafiq, M. Ashraf, Front. Plant Sci. 8, 613 (2017)
I.T. Agar, J. Streif, F. Bangerth, Postharvest Biol. Technol. 11, 47 (1997)
D. Khatri, J. Panigrahi, A. Prajapati, H. Bariya, Sci. Hortic. 259, 108837 (2020)
F. Nourozi, M. Sayyari, Sci. Hortic. 262, 109041 (2019)
M.S. Swallah, H. Sun, R. Affoh, H. Fu, H. Yu, Int. J. Food Sci. 2020, 1–8 (2020)
B. Hassan, S.A.S. Chatha, A.I. Hussain, K.M. Zia, N. Akhtar, Int. J. Biol. Macromol. 109, 1095 (2018)
G. Romanazzi, F. Nigro, A. Ippolito, D. Divenere, M. Salerno, J. Food Sci. 67, 1862 (2002)
N. Tzortzakis, P. Xylia, A. Chrysargyris, Agronomy 9, 635 (2019)
A.A. Lo’ay, M.A. Taher, Sci. Hortic. 235, 424 (2018)
Z. Chunhua, L. Hongxia, W. Jun, J. Shanghai Agric. Coll. 20, 77 (2002)
S.C. Riva, U.O. Opara, O.A. Fawole, Sci. Hortic. 262, 109074 (2020)
M.A. Rehman, M.R. Asi, A. Hameed, L.D. Bourquin, Foods 9, 1361 (2020)
S. Shah, M.S. Hashmi, Hortic. Environ. Biotechnol. 61, 279–789 (2020)
G. Khaliq, H.T. Abbas, I. Ali, M. Waseem, Hortic. Environ. Biotechnol. 60, 659 (2019)
L. Deng, Y. Zhou, K. Zeng, Crop Prot. 70, 70 (2015)
H. Molamohammadi, Z. Pakkish, H.-R. Akhavan, V.R. Saffari, Food Bioprocess Technol. 13, 121 (2020)
M.S. Pasquariello, D. Di Patre, F. Mastrobuoni, L. Zampella, M. Scortichini, M. Petriccione, Postharvest Biol. Technol. 109, 45 (2015)
X. Sun, Q. Yang, W. Guo, L. Dai, W. Chen, Sci. Hortic. 160, 155 (2013)
G.I. Olivas, J.E. Dávila-Aviña, N.A. Salas-Salazar, F.J. Molina, Stewart Postharvest Rev. 3, 1 (2008)
V. Farina, R. Passafiume, I. Tinebra, E. Palazzolo, G. Sortino, Agronomy 10, 515–533 (2020)
Y. Tadmor, J. Burger, I. Yaakov, A. Feder, S.E. Libhaber, V. Portnoy, A. Meir, G. Tzuri, U. Saar, I. Rogachev, J. Agric. Food Chem. 58, 10722 (2010)
A. Parven, M.R. Sarker, M. Megharaj, IMd. Meftaul, Sci. Hortic. 265, 109228 (2020)
Acknowledgments
This study was supported by the head of research and technology center, University of Hormozgan, Bandar Abbas, Iran.
Author information
Authors and Affiliations
Contributions
RHS: investigation, software, statistical analysis. SR: conceptualization, methodology, resources, project administration, writing—original draft. SF: methodology and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hajebi Seyed, R., Rastegar, S. & Faramarzi, S. Impact of edible coating derived from a combination of Aloe vera gel, chitosan and calcium chloride on maintain the quality of mango fruit at ambient temperature. Food Measure 15, 2932–2942 (2021). https://doi.org/10.1007/s11694-021-00861-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00861-6