Abstract
Layered double hydroxide (LDH) nanomaterials have recently become immense research area as it is used widely in industries. So, it’s chance of their release into natural environment and risk assessment to nontarget aquatic invertebrate increasing. So, the present study aimed to synthesize and confirm the crystalline formation of Co-Cd-Fe LDHs and Co-Cd-Fe/PbI2 (LDH) and then to investigate the toxic impact of the two LDH on the adult freshwater snails (Biomphalaia alexandrina). Results showed that Co-Cd-Fe/PbI2 LDH has more toxic effect to adult Biomphalaria than Co-Cd-Fe LDHs (LC50 was 56.4 and 147.7 mg/L, 72 h of exposure, respectively). The effect of LC25 (117.1 mg/L) of Co-Cd-Fe LDHs exposure on the embryo showed suppression of embryonic development and induced embryo malformation. Also, it showed alterations in the tegmental architectures of the mantle-foot region of B. alexandrina snails as declared in scanning electron micrograph. Also, exposure to this sublethal concentration caused abnormalities in hemocyte shapes and upregulated IL-2 level in soft tissue. In addition, it decreased levels of nonenzymatic reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), caspase-3 activity, and total protein content in significant manner. Glutathione S-transferase (GST) activity was not affected by LDH exposure. It caused histopathological damages in both glands of snails and also caused a genotoxic effect in their cells. The results from the present study indicated that LDH has risk assessment on aquatic B. alexandrina snails and that it can be used as a biological indicator of water pollution with LDH.
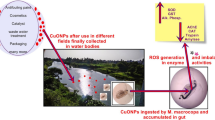
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have been applied in many biomedical researches due to their unique optical, electronic, and magnetic characteristics (Tarafdar et al. 2013; Bazrafshan et al. 2017; Corsi et al. 2018). Layered double hydroxide (LDH) is one of two-dimensional layered inorganic nanomaterials. It is one of various cheap nanoparticles bearing positive charge (Malakar et al. 2021). Layered materials have been extensively used in the application of catalysis, polymer nanocomposites, and sensors (Zhao et al. 2007; Manzi-Nshuti et al. 2009; Han et al. 2011), in medicine and pharmacy (Ladewig et al. 2009; Choi and Choy 2011). Additionally, it is used as fertilizers, herbicides, growth regulators, and in removing environmental chemical pollution (Li et al. 2016; Peligro et al. 2016; Benício et al. 2020; Daniel and Thomas 2020). Their unique uses in many applications depend on the host molecule. These have been attributed to their exchange capacity of anionic and capability to accommodate in the interlayer region different types of functional anions/molecules (metals, halocomplexes, polymers, proteins, drugs, etc.). The wide spread utilization of LDHs may lead to increase the chance of their release into the aquatic ecosystem, which has not been investigated. Toxicological evaluation of LDH has been gained environmental and human health care, since it may cause a negative impact to nontarget aquatic fauna.
Many articles elucidate the toxicological impact of nanomaterials to aquatic organism such as zooplankton, fish, algae, freshwater rotifers, and snails (Zhu et al. 2009; Kim et al. 2012; Long et al. 2012; Myer et al. 2017; Amorim et al. 2019; Martins et al. 2020). However, toxicological impact of inorganic nanomaterial (LDH) to snails does not study until now.
B. alexandrina snails are widely accepted invertebrate models to study the toxicity and toxico-kinetic of inorganic nanomaterial for aquatic ecosystem (Oliveira-Filho et al. 2017; Kaloyianni et al. 2020). It is the intermediate host of Schistosoma mansoni that is wildly disseminated throughout tropical and subtropical highly polluted canals and in the Nile River (DeJong et al. 2001). Biomphalaria characterized by their availability, easy way for collection, acclimate to laboratory conditions, sensitivity to water, and chemical pollutant. All the previous characters nominate it to use as laboratory monitoring in ecotoxicological studies and for analyzing multiples biomarkers. (Duft et al. 2007; OECD 2016; Oliveira-Filho et al. 2017; Ruppert et al. 2017). Many studies in immunology, reproductive, and developmental biology used Biomphalaria as paradigm ( Khangarot and Das 2010; Boisseaux et al. 2017; Pirger et al. 2018). Nanomaterials (NMs) such as carbon nanotubes, silver nanoparticles, have potential effects to B. alexandrina as conducted in many studies (Moustafa et al. 2018). The toxicities of LDH-NPs depend on their chemical compositions and concentrations used. These LDH-NPs might generate reactive oxygen species (ROS) and so inducing oxidative stress, the expression of antioxidant enzymes (like catalase, glutathione reduced, and super oxide dismutase) and inflammation (Choi et al. 2015). Caixeta et al. (2020) stated that the toxicity of NMs has been attributed to reactive oxygen species (ROS) generated that subsequently by lipid peroxidation, DNA, and protein damage. Also, Choi et al. (2009) stated that nanoparticle induced oxidative stress and might negatively alter the physiological responses, such as carcinogenesis, inflammation, fibrosis, and genotoxicity. These nanomaterials could find their ways to the natural environment of snails by water runoff and drainage canals and caused negative effects on the organisms lived in these environments, and so, the present study aimed to evaluate the toxicity of LDHs NMs on B. alexandrina and how it affected their biological processes, and to donate well knowledge about biological behavior and risk assessment of Co-Cd-Fe LDHs in aquatic environments.
Material and method
Preparation of two types of LDHs
Co-Cd-Fe LDH
NaOH (5M) was dissolved in 200 mL of distilled water. Another 200 mL aqueous solution of 1Fe (NO3)3.19H2O (0.1M), Co (NO3)2.16H2O (0.1M), and Cd(NO3)2.4H2O (0.1M) was prepared. This later solution was stirred for 24h. A pH 10 of the reaction is adjusted by using sodium hydroxide solution. At pH 10, the solution was divided into two solutions; one of them is stirred for 24h and the second one is put in the autoclave for 3h. A washing process using DI water is carried out for the resulting precipitate to reduce the pH to 7. Finally, the product is dried at 80°C for 1 day.
T- LDHs/PbI2 NC
In a general synthesis technique, in situ growth of the metal cations, typically, NaOH (5M) in 200mL of distilled H2O is prepared. Another solution of Fe(NO3)3.9H2O (0.1M), Co(NO3)21.6H2O (0.1M), Cd(NO3)2.4H2O (0.1M), and 2.5g PbI2 was prepared. This later solution was stirred for 24h. A pH 10 of the reaction is adjusted by using the sodium hydroxide solution. After reaching pH 10, the solution was remained under continuous stirring for 24h. A washing process using DI water is carried out for the resulting precipitate to reduce the pH to 7. After washing, a drying process is carried out at 80°C for 1 day.
Characterization of LDH
The XRD patterns of Co-Cd-Fe LDH and Co-Cd-Fe LDH/PbI2 NC were obtained by Philips1 XˋPert1-MRD1 X-ray diffraction (λCuKa = 0.15418 nm). Sample morphology is investigated using a 1field-emission1 1scanning electron11 microscope (FESEM, TEM, Zeiss SUPRA/55VP with GEMINI/ column). Fourier transform infrared spectroscopy (FTIR) was performed by A Shimadzu1-FTIR-3401-Jasco1 spectrometer to obtain the important functional groups of the samples. Finally, the optical absorbance behaviors of the products are investigated by Lambda 900-UV/Vis/IR Perkin Elmer spectrophotometer up to 1200 nm.
Snail source and maintenance
Adult B. alexandrina, snails (810 mm in diameter; 0.26g weight) have been obtained from Theodor Bilharz Research Institute (TBRI), (Giza, Egypt). Snails were transferred to Medical Malacology Lab and kept in plastic tank with dechlorinated aerated tap water (10 snails/L) with a photoperiodicity of 12h light/12 h dark cycle, a temperature of 25 ±3 °C, pH: 7± 0.2 and fed on ovendry lettuce leaves (1gm/10 snails) and Tetramin. The tank water was changed every 3 days. For collecting egg masses, pieces of polyethylene sheets (5 × 10 cm) were used (OECD 2016).
Toxicity study
Acute toxicity test in adult B. alexandrina
The toxicity of the two layered materials Co-Fe-Cd and Co-Fe-Cd/PbI2 LDH against adult mature snails (10–12 mm; 150 snails) was determined. Stock solution of two layered materials was prepared using dechlorinated tap water (1000 mg/L). A series of concentrations of Co-Fe-Cd LDH (100, 75, 50, 25, and 20) and of Co-Fe-Cd/PbI2 LDH (200, 150, 100, 75, and 50) were prepared to calculate LC50 and LC90 at laboratory temperature (22–25°C). Three replicates were conducted for each concentration and the control group (30 snails per experimental group): 72 h after, the snails were transferred from the exposure concentrations and maintained in dechlorinated tap water for another 24 h of recovery. Mortality percent of snails was recorded and lethal concentration and slope values were analyzed by Probit analysis (WHO 1965).
Bioassay
After calculation of the sublethal concentration, snails were exposed to LC25 of LDH for 24h followed by a recovery period of another 24 h in dechlorinated water. Then, the following experiments were done:
Embryotoxicity test
According to Rapado et al. (2011), pieces of polyethylene sheets (5 × 10 cm) containing egg clutches (100 eggs) were collected for the embryotoxicity assay. The egg masses were transferred to Petri dishes contains LC25 of LDH for 24h, subsequently washed with filtered and dechlorinated water (pH 7.0). Seven days after exposure, the embryos were examined for unviability (malformed embryos or dead) by stereomicroscope. Another egg clutches was transferred to dechlorinated aerated tap water as a control. Assays were performed in triplicate.
Scanning electron microscope of the mantle-foot region
The mantle-foot regions of snails were separated under a stereomicroscope. Then, the specimens were fixed, dehydrated, critically dried, and coated as recommended by Ibrahim and Abdel-Tawab (2020). Finally, they were analyzed by JSM-6510 LA.
Immunocytotoxicity
Cytotoxicity assay in hemocytes of B. alexandrina
According to Nduku and Harrison (1980), hemolymph of ten snails was collected from the snail heart by insertion a capillary tube into the snail shell that is directly over the heart: 10 μl of hemolymph was spared on a glass slide to prepare hemocytes monolayers and leave to air-dry for 15 min at laboratory temperature. Hemocytes were fixed with 100% methanol for 5 min and then stained with 10% Giemsa stain (Aldrich) for 20 min, then examined under the light microscope. This assay was done in triplicate for each group to study the outer morphological changes in the hemocytes.
Measurement of IL-2 level and Caspase-3 activity
IL-2 in tissue homogenate (1gm/10 mL phosphate buffer) of five snails was measured by enzyme-linked immunosorbant assay (ELISA). Cytokine levels were determined by commercially available ELISA kits for IL-2 (OptEIA™ Kits; BD Biosciences). The depth of the color can then be measured spectrophotometrically at appropriate wave length. The intensity of colored end product provided a measure of the cytokine concentration (Hemdan et al. 2007). Caspase-3 activity was determined according to Bonomini et al. (2004). The released p-nitroaniline (pNA) moiety concentration was measured colorimetrically at 405 nm.
Tissue preparation for oxidant/antioxidant biomarker and biochemical studies
The soft tissues of five snails were removed from the exposure group and the control one, weighted (1gm/10 mL phosphate buffer), and then homogenized in ice cold, twice-distilled water using a glass Dounce homogenizer. The supernatants were separated using high speed centrifuged (3000 rpm for 10 min) and stored at − 80 °C until used; then experiments were done according to the pamphlet of each kit.
Oxidant/antioxidant defense biomarker
These biomarkers have been measured in the supernatant of the tissue homogenate of five snails for LDH exposure group and control one. The enzymatic responses SOD, CAT, and GST were measured according to Aebi 1984 (Mannervik and Guthenberg 1981), and nonenzymatic responses GSH was determined according to the method of Ellman (1959). For biochemical studies, the snails’ total protein was done according to the method of Gornall et al. (1949). All parameters determined using biodiagnostic kits (Biodiagnostic Dokki, Giza, Egypt).
Genotoxicity evaluation was done by detecting of DNA single-strand breaks (comet assay)
DNA single-strand damage of snails exposed to LC25 of LDH for 48 h was detected by single-cell gel assay as previously described by Singh et al. (1988) and Grazeffe et al. (2008). The hemolymphs of twenty snails were collected by inserting a capillary tube into the heart of each snail. The DNA fragment migration patterns of 100 cells were evaluated with a fluorescence microscope at 510 nm. The comet tail lengths were measured from the middle of the nucleus to the end of the tail with 40× increase for the count and measure the size of the comet. Visualization of DNA damage was observed by Ethidium Bromide Staining using a 40× objective. Slides were scored blindly.
Histological evolution of the digestive and hermaphrodite glands
Ten adult B. alexandrina snails (8–10 mm) were exposed to LC25 of LDH for 24h followed by another 24 h of recovery for 2 weeks. The digestive and hermaphrodite glands of the surviving B. alexandrina snails were removed and fixed in Bouin’s solution. The glands dehydrated, embedded in paraffin wax. Then, they both sectioned and stained with hematoxylin and eosin (Mohamed and Saad 1990). Five slides/each gland/snail were examined by light microscopy for any alterations in the histological architecture compared to the control snails and photographed by using a microscopic camera.
Statistical analysis
Data analysis were performed by t-test to determine the significant difference between exposure and control group and expressed as mean ± SME of mean (Graph Pad Prism 6.04 software). The lethal concentration (LC10, LC25, LC50, and LC90) values, slope, and respective 95% confidence limit (CL) of LC50 was calculated by Probit analysis (Finney 1971).
Result
Characterization of Co-Cd-Fe LDH, and T-LDH/PbI2 NC
Function groups identification
The FTIR charts of (Co-Cd-Fe) LDH and its composite are displayed in Fig. 1A (A, B) and Table 1. After combination of PbI2, there are red shifts in absorption bands and some peaks changed in intensity and other broads Fig. 1A (B).
Structural properties
The structure and crystalinity of (Co-Cd-Fe) LDH was confirmed by XRD diffract gram. Its chart displays highly matching of hydrotalcite LDH with hexagonal phase (Fig. 1B). XRD peaks referred to diffractions (003), (006), (101), (009), (107), (018), (110), and (113). It is noticed that these peaks have high intensity which was reflected the high crystallinity of the studied LDH.
Their crystal sizes were calculated using Scherrer’s relation (R). The mean size was ~23.5 nm. In addition to their average microstrain value was ~ 0.7% and its dislocations density was 0.0018 that evaluates the density of defects and the quality of the crystal. This result gives a reflection to high quality of the synthesized LDH crystal.
Morphological properties
The morphological properties were examined through FESEM and TEM, at Fig. 1C (A, B). The morphology of Co-Cd-Fe LDH was characterized with the agglomeration of the particles which have plate-like morphology. This behavior was similar for all hydrotalcites prepared by coprecipitation method. TEM clarified the plate like of LDH layers and proved the morphology of the LDH.
Zeta potential and particle size distribution
The value of zeta potential of the fabricated LDH after dilution is depicted from Fig. 1D to be −8.46 mV. Also, the value the conductivity was 2.47ms/cm. In addition, LDH had a large particle size distribution (1911 nm), as calculated by DLS measurements. This value is larger than that reported using SEM or TEM images and this could be attributed to the aggregate LDH in aqueous solution through DLS technique in contrast to SEM or XRD techniques which do not allow for aggregation.
Toxic impact of LDH on adult B. alexandrina
In the present study, Co-Cd-Fe LDHs and Co-Cd-Fe LDHs/PbI2(LDH) were tested for its toxic effect against B. alexandrina. Snails were exposed to different concentrations of Co-Cd-Fe LDHs and Co-Cd-Fe LDHs/PbI2 (LDH) for 72 h of exposure followed by another 24 h for recovery. Probit analysis showed that the LC50 of Co-Cd-Fe LDHs was 147.7 with confidence limits 110.99–188 mg/L. While Co-Cd-Fe /PbI2 LDH showed more toxic effect, LC50 was 56.4 with confidence limits 21.52–85.07 mg/L (Table 2).
Embryotoxicity
Exposure of embryos of Biomphalaria alexandrina snails to Co-Cd-Fe LDHs caused delay of embryonic development (30%), embryo malformation (20%), and accumulation of LDH NPs in the egg clutches leading to death of some embryos (50%) (Fig. 2B).
Morphological abnormalities in Biomphalaria embryos after exposure to Co-Fe-Cd LDH. (2A) Normal control embryos of 7-days aged where the snails completely formed (E eye; HF head foot; S shell). (2B) After exposure of the egg mass to LC25 Co-Fe-Cd LDH (DE dead embryo, 50%; MF malformed embryo, 20%; DD development delay, 30%)
Effect of LDH on B. alexandrina ultrasturacture
The scanning electron micrographs of the soft part of B. alexandrina snails showing the normal foot plantaris with notable surface fold and covered with fine and smooth cilia (Fig. 3A), and tegmental surface of mantle with microvilli and fine spines (Fig. 3D). Following the exposure to LC25, the foot cilia became tangled, adherent, and ultimately peeled off (Fig. 3A and 3B). Also, the tegmental surface of mantle became rough, most microvilli completely destroyed, nipples and erosion (Fig. 3E and 3F).
Scanning electron micrographs (SEM) of B. alexandrina snails (soft part), (3A) Normal ultrastructure of foot with smooth and regular cilia, (3B) foot plantaris after exposure to Co-Fe-Cd LDH, the cilia became tangled and adherent, (3C) higher magnification of 3B, (3D) normal mantle showing smooth tegmental surface and microvilli, (3E) mantle after exposed to Co-Fe-Cd LDH showing tortuosity, nipples, erosion, and accumulation of LDH NMs in tegmental surface, (3F) higher magnification of 3E
Impact of LDH on hemocytes of B. alexandrina
In control group, microscopical examinations of B. alexandrina hemocytes showed three types of cell that differentiated morphologically. The first type is hyalinocytes; the second is granulocytes (spreading hemocytes), and the third is round small (undifferentiated) (Fig. 4A, 4B, 4C). After exposure to the LDH at sublethal concentration (LC25), hyalinocytes nucleus showed shrinkage and others had two separate nuclei; also, aggregations of hyalinocytes were more evident after exposure to LC25. While granulocytes having irregular cell membrane aggregate and form either pseudopodia or filopodia (Fig. 4D, 4E).
Light micrographs show hemocytes of adult Biomphalaria alexandrina snails. 4A Hyalinocyte (22.32%); 4B granulocyte (37.5%); 4C small (40.17%) (×40); 4D, 4E, and 4F show the abnormalities following exposure to LC25 of Co-Fe-Cd LDH for 48h, 4D hyalinocytes increased in number (43.58%), some forming aggregations, some have two separate nuclei (2N) and vacuoles (V). 4E some granulocytes (35.9%) forming either pseudopodia (PP) or filopodia (FP) and aggregation (AG), 4F some hyalinocytes forming pseudopodia (PP), while small cells formed 20.51%. C cytoplasm, PS pseudopodia, G granulocyte, GR granules, H hyalinocyte, N nucleus, S round small
Influence of LDH on IL-2 level and caspase-3 activity
In the present study, there are a marked increase in expression of IL-2 in LDH exposure group (p< 0.001) in compared to non-exposure one (152.14±0.1 and 72.04±0.21 Pg/mL, respectively) (Fig. 5B). Also, caspase-3 activity was slightly increased (p < 0.05) in exposed snails compared to control one (60.95±0.11 and 54.8±1.8 nmol pNa min−1 mg−1 protein, respectively) (Fig. 5A).
Impact of LDH on oxidant/antioxidant defense biomarker and biochemical studies
In the present study, exposing of snails to LC25 of LDH induced significant decreased (p<0.001) in SOD and CAT (p<0.01) activity compared to the non-exposer group (control), (Fig. 6A and 6B) with no change in GST activity (p˃0.05), (Fig. 6C). Concomitantly, a significant decrease of GSH levels and total protein content (p<0.001) in tissue homogenate was observed in LDH exposure group compared with their time-matched controls (Fig. 6D and 6E).
Influence of LDH on DNA
The present results showed that the olive tail moment (OTM) of snails subjected to sublethal concentrations of LDH was highly increased (p < 0.01) than control snails (5.63±0.1 and 3.25±0.12 μm, respectively) (Fig. 7A, 7B, and 7C).
Light micrograph shows the extent of DNA migration by comet assay. (7A) Control B. alexandrina; (7B) snails exposed to sublethal concentration of Co-Fe-Cd LDH for 48 h with high DNA migration (p < 0.01) than control snails; (7C) showed the increase in OTM in exposed snails than control one (5.63±0.1 and 3.25±0.12 μm, respectively).
Impact of LDH on digestive and hermaphrodite gland of B. alexandrina
Examination of the histological sections through digestive gland showed many tubular glands with single layer of secretory cells (SC) and digestive cells (DC) (Fig. 8A). Treatment these snails, with LC25 of LDH, showed rupturing, vacuolation, and a significant increase in the number of SC. Also, the lumen (L) increased; most of the DC and SC were degenerated and ruptured while the tubular glands lose their confirmed shape (Fig. 8B). Meanwhile, the histological sections of B. alexandrina snails of the control group through the hermaphrodite gland revealed female oogenic cells with normal oocytes and mature ova and male reproductive cells with normal spermatocytes, and sperms (Fig. 8C). The treatment of snails with a dose of LC25 caused degenerations and destruction of some oocytes, mature ova, spermatocytes, and sperms (Fig. 8D).
Light micrographs of the hermaphrodite and the digestive glands of B. alexandrina snails (H&E) (×40): (8A) normal digestive gland of B. alexandrina snails (8B) snails exposed to LC25 of Co-Fe-Cd LDH (8C) normal hermaphrodite gland of B. alexandrina snails (8D) snails exposed to LC25 of LDH. MO mature ovum, OC oocytes, SP sperms, SPR spermatocytes, OC oocyte, DOC degenerated oocytes, DSPR degenerated spermatocytes, DC digestive cells, SC secretory cells, L lumen, TG tubular gland, CT connective tissue, RDC ruptured digestive cells, DDC degenerated digestive cells, RTG ruptured tubular gland, RSC ruptured secretory cells
Discussion
Layered double hydroxide (LDH) gains significant attention in life science applications due to their extremely governable synthesis and high biocompatibility. But few studies highlight toxicity and toxico-kinetic of LDH. In the present study, we engineered Co-Cd-Fe LDH and T-LDH/PbI2 NC, and its toxicological impact was evaluated. The results of Co-Cd-Fe LDH characterization by XRD were matched with a usual LDH with crystallinity mean size ~23.5 nm (Lu et al. 2015; Mohamed et al. 2018). Also, their FTIR spectra were similar to that previously recorded (Shaban et al. 2018; Mohamed et al. 2018; Parida and Mohapatra 2012), while FSEM and TEM clarified the plate like of LDH layers (Tedim et al. 2011; Chen et al. 2017). The zeta potential reflected the stability of nanoparticles in suspension and is also the major factor in the initial adsorption of nanoparticles onto the cell membrane or the uptake inside these cells. The zeta potential and size could affect nanoparticle toxicity (Mohamed et al. 2021).
The present study confirmed that Co-Cd-Fe LDH and T-LDH/PbI2 NMs showed toxic effect against B. alexandrina and that T-LDH/PbI2 NM more toxic to adult Biomphalaria than Co-Cd-Fe LDHs. This result is in good accordance with Cardinale et al. (2012) who stated that TiO2 nanoparticles showed more inhibition in the growth rate in Scenedesmus quadricauda than Al2O3nano-powder and concluded that the toxicity of LDH on algae was time- and concentration-dependent. Also, it was proven that LDH has toxic impact against human cell line (Choi et al. 2007) and green algae Scenedesmus quadricauda(Ding et al. 2018).
Enzymatic (GST, SOD, and CAT) and nonenzymatic (GSH) antioxidant markers play a vital role in protection of the organisms from oxidative stress and suppression of its cellular damage as it reduce and converted H2O2 and superoxide anion radical. While GSH act as a reducing agent in conjugation with xenobiotics (Pena-Llopis et al. 2001). Disturbance of oxidant/antioxidant system has been the main toxic impact induced by NMs in snails. LDH increased the ROS production and subsequently altered the enzymatic and nonenzymatic antioxidant enzyme, such as SOD, CAT, GSH (Ali 2014; Ali et al. 2012; Bao et al. 2018). In addition, this reduction can be elucidated to the direct combination of metal with active site of enzyme and its biotransformation. The present data showed significant decrease in SOD, CAT, GSH and this in agreement with Gnatyshyna et al. (2020) who reported that the nonenzymatic marker activity decreased in Lymnaea stagnalis after exposure to Cu (10 μg L−1), Zn (130 μg L−1), Cd (15 μg L−1), and thiocarbamate fungicide (91 μg L−1) for 14 days.
Also, a decrease of catalase activity was seen previously in snail exposed to herbicides (Bhagat et al. 2016). In addition, exposure of B. alexanderina snails to ZnONPs showed significant inhibition of GSH and CAT ((Fahmy et al. 2014). In contrast with the present result, Atli and Grosell (2016) reported that exposing L. stagnalis to only the highest concentrations of Cu caused an increase in antioxidant enzyme.
Exposed snails showed no significant variation in GST activity compared to control. This finding agreed with those obtained by Sánchez-Marín et al. (2020) as they indicated that this enzyme is not activated in response to organophosphate flame retardants, tris (1,3-dichloro-2-propyl) phosphate in mussels.
Also, the present study declared a marked decrease in the total protein content (p< 0.001) compared with controls. Fahmy et al. (2014) recorded a significant decrease in B. alexanderina snail protein content after exposure to ZnONPs and related the differences in the activity of the antioxidant markers with the tissue type and the metal concentrations. SOD and CAT activities in Daphnia magna exposed to Cd and Cu varied according to metal concentration. Also, L. natalensis snails collected from polluted dams in Zimbabwe showed variation in SOD and CAT activities (Siwela et al. 2010). In addition, Achatina fulica showed reduction in CAT and SOD activities after exposure to Cd and Zn. This variation could be attributed to the excess production of ROS (Chandran et al. 2005). Similarly, such enzyme reductions were also observed in the present study in response to LDH exposure.
A slightly increase of caspase-3 activity was detected as unspecific response to LDH stress. Its elevation was observed previously in apoptotic cells (Elmore 2007; Florentin and Arama 2012), and this elevation may be due to cytological changes in the digestive gland of LDH-stressed snails (Zaldibar et al. 2007a, 2007b; Hödl et al. 2010; Benito et al. 2017). Previously, increasing in caspase-3 activity has been detected in L. stagnalis in response to pollutant stress (Gnatyshyna et al. 2020). Also, caspases-3 levels increased in Helix aspersa snails after exposure to iron oxides nanoparticles (Sidiropoulou et al. 2018).
In the declared data, LDH at sublethal concentration (LC25), caused abnormalities in hyalinocytes and granulocytes shapes as nucleus shrinkage, divided to two separate nuclei, aggregate or formed pseudopodia. The immuno-cell responses and molecular aspects in B. alexandrina snails considered as important biomarkers of exposure to environmental pollutants (Mohamed 2011). Biomphalaria snails immunology can be attributed to hemocyte which are the critical line of cellular defense (Larson et al. 2014), where they contributed in many defense mechanisms against several pathogens as it is responsible for the phagocytosis, cytotoxic reactions (Fried 2016), and release soluble compounds including agglutinins and antimicrobial peptides (Ottaviani 2006; Mitta et al. 2000).
Chronic exposure of the T. pisana to Ag NPs caused alterations in hemocytes, such as micronuclei, binucleated cell, and kidney-like nuclei (Radwan et al. 2019). Also, B. glabrata exposed to CdTe quantum dot showed altered hemocytes binucleates, micronuclei, and apoptosis (de Vasconcelos et al. 2019). Cell–cell aggregation was considered as an immunological response for host defense. Cellular aggregation of the invertebrates’ hemocytes prevented the accidental blood loss by the formation of a biological plug at the site of the wound and resisted the entry of pathogenic microorganism (Guria et al. 2016).
Hughes et al. (1990) and Ottaviani et al. (1993) were detected cytokine-like molecules in marine and freshwater mollusks. IL-2 was one of the cytokines assayed. It is responsible for phagocytosis and provokes the strongest response in the synthesis of biogenic amines, nitric oxide (NO), or oxygen radicals (Ottaviani et al. 1995a, 1995b). In the present study, there are marked increase in expression of IL-2 in LDH exposure group (p< 0.001) in compared to non-exposure one. (IL)-2–like peptide was also detected in sea mussel which may be involved in the regulation of responses to different types of stress (Cao 1998; Barcia et al. 1999).
On the level of DNA damage, as an important biomarker of NM toxicity in snails, comet assay is a sensitive tool to detect DNA damages like DNA single-strand breaks (SSBs)(Ibrahim et al. 2018). The present results showed that the olive tail moment (OTM) of snails exposed to sublethal concentrations was increased than control snails. This in agreement with Ibrahim and Ghoname (2018) who demonstrated that the OTM of snails exposed to LC10 (27.5 mg L−1) or LC25 (32.4 mg L−1) of the aqueous leaves extract of Anagalis arvensis was significantly higher than the control group. Such genotoxic effects might be due to either oxidation of DNA bases or covalent binding to DNA resulting in strand breaks. Some studies link DNA SSBs in aquatic animals to effects on the immune system, reproduction, growth, and population dynamics (Lee and Steinert 2003). Exposure to inorganic nanomaterial as Ag NPs, CuO NPs, IONPs, MgO NPs, TiO2 NPs, and ZnO NPs induced genotoxic effects in snails (Caixeta et al. 2020). Ye et al. (2012) stated that the amount of DNA strand breaks were higher after exposure to DNA damaging chemicals compared with controls.
The embryotoxicity observed after exposure has been attributed to ROS production, oxidative stress, and damage. Also, penetration of LDH NPs to gelatinous capsule and cross the egg membrane reduces essential growth metabolism aspects, changes in its permeability, consuming energy for the development, and finally interrupting the mechanics of hatching (de Chavez and de Lara 2003; de Vasconcelos et al. 2019).
Our result is in agreement with Besnaci et al. (2016), who state morphological changes and precipitation of Fe2O3 NPs in the egg mass. Also, morphological abnormality and hatchability hinder was seen in B. pfeifferi embryos following exposure to curcumin-nisin polylactic acid NPs for 96 h. In addition, hydrophilic nanosilica induced embryotoxic effects in B. alexandrina snail at concentration 590 ppm for 6 h and 980 ppm for 48 h (Attia et al. 2017). Similarly, the growth and hatching rate reduction was seen in B. glabrata embryos exposed to CdTe NPs for 24 h (de Vasconcelos et al. 2019). In contrast, dimer captosuccinic acid (DMSA)-functionalized Fe2O3 NPs did not induce embryo mortality, morphological alterations, and hatching inhibition due to their physical properties and limited internalization in the egg clutches (Oliveira-Filho et al. 2017). LDH posed a significant suppression in the growth of S. quadricauda algae after 72 h of incubation and a complete growth inhibition (100%) at higher LDH concentration. LDH had a higher inhibitory effect to growth than the other NPs (Ding et al. 2018).
In the present study, the foot and mantle of B. alexandrina snails showed bioaccumulation of LDH in its surface and some morphological disturbances after the exposure to LDH for 24 h followed by 24-h recovery as was detected by scanning electron microscope. LDH can interact, accumulated in foot and digestive gland of snails, and distributed to the mantle. Both Ag NPs and CuO NPs accumulation in mantle, foot and digestive gland of B. aeruginosa(Bao et al. 2018; Oliver et al. 2014; Croteau et al. 2014; Ma et al. 2017). Also, NMs possessed a highly adhesive property to a cell membrane; therefore, it could affect the membrane structures and its macromolecules (Rasel et al. 2019). In addition this damage in ultrastructure could lead to snail death (Ibrahim and Abdel-Tawab2020).
The deformation declared in the hermaphrodite gland of B. alexandrina histological sections after exposure to LC25 of LDH was accompanied with a great damage in the gonadal cells where degenerations of some mature ova, spermatocytes, oocytes, and sperms. Also, the connective tissue was dissolved and replaced by vacuoles. Saad et al. (2019) reported similar histological alterations in the hermaphrodite glands of B. alexandrina snails treated with copper oxide nanocomposite (CuO NC), where the ova and sperms degenerated and there were loss in the connective tissues between acini (Saad et al. 2019). The exposure of the snail to LDH may be lead to metabolic changes, destruction of gametogenic cells and damage of hermaphrodite glands which possibly resulting from a decrease in tissue proteins, apoptosis, or degeneration of cells of these vital organs (Omobhude et al. 2017).
The digestive gland was the main organ analyzed in studies concerning oxidative stress induced by NM due to its higher accumulation capacity and role in the metal detoxification. Exposing of the digestive gland of B. alexandrina snails to LC25 of the LDH showed significant increase in the number and degeneration of the SC. The DC ruptured and vacuolated in addition, the tubular glands lose their confirmed shape. In like manner, Saad et al. reported histological alterations in the digestive gland of Coelatura aegyptiaca following treatment with ZnONPs for 6 consecutive days, where there were gradual hypertrophy and hyperplasia in the glandular cells (Fahmy and Sayed 2017).
Conclusion
The data of the current study consider the first toxicological evaluation of LDH nanomaterial on freshwater snail B. alexandrina. In light of the above, LDH induces disturbance in both enzymatic and nonenzymatic antioxidant marker in the tissues of Biomphalaria following exposure to sublethal concentration, suppression the embryonic development. It caused alteration in mantle foot ultrastructure, immune response, histopathology of gland, and finally genotoxic effect. This result reflects the possible ecological implications of LDH release in aquatic ecosystems and its risk assessment to aquatic invertebrate.
Data availability
The data that supports the findings of this study are available in the material of this article.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ali D (2014) Oxidative stress-mediated apoptosis and genotoxicity induced by silver nanoparticles in freshwater snail Lymnea luteola L. Biol Trace Elem Res 162:333–341
Ali D, Alarifi S, Kumar S, Ahamed M, Siddiqui MA (2012) Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquat Toxicol 124:83–90
Amorim J, Abreu I, Rodrigues P, Peixoto D, Pinheiro C, Saraiva A, Carvalho AP, Guimarães L, Oliva-Teles L (2019) Lymnaea stagnalis as a freshwater model invertebrate for ecotoxicological studies. Sci Total Environ 669:11–28
Atli G, Grosell M (2016) Characterization and response of antioxidant systems in the tissues of the freshwater pond snail (Lymnaea stagnalis) during acute copper exposure. Aquat Toxicol 176:38–44. https://doi.org/10.1016/j.aquatox.2016.04.007
Attia MM, Soliman SM, Khalf MA (2017) Hydrophilic nanosilica as a new larvicidal and molluscicidal agent for controlling of major infectious diseases in Egypt. Vet World 10:1046–1051. https://doi.org/10.14202/vetworld.2017.1046-1051
Bao S, Huang J, Liu X, Tang W, Fang T (2018) Tissue distribution of Ag and oxidative stress responses in the freshwater snail Bellamya aeruginosa exposed to sediment-associated Ag nanoparticles. Sci Total Environ 644:736–746
Barcia R, Cao A, Arbeteta J, Ramos-Martinez JI (1999) The IL-2 receptor in hemocytes of the sea mussel Mytilus galloprovincialis Lmk. IUBMB Life 48:419–423
Bazrafshan AA, Ghaedi M, Hajati S, Naghiha R, Asfaram A (2017) Synthesis of ZnO-nanorod-based materials for antibacterial, antifungal activities, DNA cleavage and efficient ultrasound-assisted dyes adsorption. Ecotoxicol Environ Saf 142:330–337
Benício LPF, Pinto FG, Tronto J (2020) Layered double hydroxide nanocomposites for agricultural applications. In: Layered Double Hydroxide Polymer Nanocomposites. Elsevier, pp 715–741. https://doi.org/10.1016/B978-0-08-101903-0.00017-9
Benito D, Niederwanger M, Izagirre U, Dallinger R, Soto M (2017) Successive onset of molecular, cellular and tissue-specific responses in midgut gland of Littorina littorea exposed to sub-lethal cadmium concentrations. Int J Mol Sci 18:1815
Besnaci S, Bensoltane S, Braia FMH et al (2016) Embryotoxicity evaluation of iron oxide Fe2O3 on land snails: Helix aspersa. J Entomol Zool Stud 4:317–323
Bhagat J, Ingole BS, Singh N (2016) Glutathione S-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as biomarkers of oxidative stress in snails: a review. Invertebr Surviv J 13:336–349
Bonomini M, Dottori S, Amoroso L, Arduini A, Sirolli V (2004) Increased platelet phosphatidylserine exposure and caspase activation in chronic uremia. J Thromb Haemost 2:1275–1281
Caixeta MB, Araújo PS, Gonçalves BB, et al (2020) Toxicity of engineered nanomaterials to aquatic and land snails: A scientometric and systematic review. Chemosphere 260:127654. https://doi.org/10.1016/j.chemosphere.2020.127654
Cao A (1998) El receptor de IL-2 en hemocitos deMytilus galloprovinciali s Lmk. International Union of Biochemistry and Molecular Biology Life 48(4):419–423. https://doi.org/10.1080/713803540
Cardinale BJ, Bier R, Kwan C (2012) Effects of TiO2 nanoparticles on the growth and metabolism of three species of freshwater algae. J Nanopart Res 14. https://doi.org/10.1007/s11051-012-0913-6
Chandran R, Sivakumar AA, Mohandass S, Aruchami M (2005) Effect of cadmium and zinc on antioxidant enzyme activity in the gastropod, Achatina fulica. Comp Biochem Physiol C Toxicol Pharmacol 140:422–426
Chen C, Yu W, Liu T, Cao S, Tsang Y (2017) Graphene oxide/WS2/Mg-doped ZnO nanocomposites for solar-light catalytic and anti-bacterial applications. Sol Energy Mater Sol Cells 160:43–53
Choi S-J, Choy J-H(2011) Layered double hydroxide nanoparticles as target-specific delivery carriers: uptake mechanism and toxicity. Nanomedicine 6:803–814
Choi SJ, Oh JM, Choy JH (2009) Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J Inorg Biochem 103:463–471. https://doi.org/10.1016/j.jinorgbio.2008.12.017
Choi SJ, Paek HJ, Yu J (2015) Oxidative stress by layered double hydroxide nanoparticles via an SFK-JNK and p38-NF-κB signaling pathway mediates induction of interleukin-6 and interleukin-8 in human lung epithelial cells. Int J Nanomedicine 10:3217–3229. https://doi.org/10.2147/IJN.S82061
Corsi I, Winther-Nielsen M, Sethi R, Punta C, Della Torre C, Libralato G, Lofrano G, Sabatini L, Aiello M, Fiordi L, Cinuzzi F, Caneschi A, Pellegrini D, Buttino I (2018) Ecofriendly nanotechnologies and nanomaterials for environmental applications: key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotoxicol Environ Saf 154:237–244
Croteau M-N, Misra SK, Luoma SN, Valsami-Jones E (2014) Bioaccumulation and toxicity of CuO nanoparticles by a freshwater invertebrate after waterborne and dietborne exposures. Environ Sci Technol 48:10929–10937
Daniel S, Thomas S (2020) Layered double hydroxides: fundamentals to applications. In: Layered Double Hydroxide Polymer Nanocomposites. Elsevier, pp 1–76. Woodhead Publishing.
de Chavez ERC, de Lara AV (2003) Effects of zinc (Zn2+) and lead (Pb2+) on the early development of the freshwater snail, Radix quadrasi. J Med Appl Malacol 12:59–68
de Vasconcelos LM, de Andrade Pereira MI, Cabral Filho PE et al (2019) Studies on toxicity of suspensions of CdTe quantum dots to Biomphalaria glabrata mollusks. Environ Toxicol Chem 38:2128–2136
DeJong RJ, Morgan JAT, Paraense WL et al (2001) Evolutionary relationships and biogeography of Biomphalaria (Gastropoda: Planorbidae) with implications regarding its role as host of the human bloodfluke, Schistosoma mansoni. Mol Biol Evol 18:2225–2239
Ding T, Lin K, Chen J, Hu Q, Yang B, Li J, Gan J (2018) Causes and mechanisms on the toxicity of layered double hydroxide (LDH) to green algae Scenedesmus quadricauda. Sci Total Environ 635:1004–1011
Duft M, Schmitt C, Bachmann J, Brandelik C, Schulte-Oehlmann U, Oehlmann J (2007) Prosobranch snails as test organisms for the assessment of endocrine active chemicals––an overview and a guideline proposal for a reproduction test with the freshwater mudsnail Potamopyrgus antipodarum. Ecotoxicology 16:169–182
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Fahmy SR, Sayed DA (2017) Toxicological perturbations of zinc oxide nanoparticles in the Coelatura aegyptiaca mussel. Toxicol Ind Health 33:564–575. https://doi.org/10.1177/0748233716687927
Fahmy SR, Abdel-Ghaffar F, Bakry FA, Sayed DA (2014) Ecotoxicological effect of sublethal exposure to zinc oxide nanoparticles on freshwater snail Biomphalaria alexandrina. Arch Environ Contam Toxicol 67:192–202
Finney DJ (1971) Probit Analysis. Cambridge University Press, London
Florentin A, Arama E (2012) Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J Cell Biol 196:513–527
Fried B (2016) An update on hemocytes in Biomphalaria snails. J Hematol Oncol Res 2, 26(20). https://doi.org/10.14302/issn.2372-6601.jhor-14-401
Gnatyshyna L, Falfushynska H, Stoliar O, Dallinger R (2020) Preliminary study of multiple stress response reactions in the pond snail Lymnaea stagnalis exposed to trace metals and a thiocarbamate fungicide at environmentally relevant concentrations. Arch Environ Contam Toxicol 79:89–100
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Grazeffe VS, de Freitas TL, de Sa PA et al (2008) Establishment of the comet assay in the freshwater snail Biomphalaria glabrata (Say, 1818). Mutat Res Toxicol Environ Mutagen 654:58–63
Guria S, Parveen S, Goswami DS (2016) Alteration of morphology, phagocytic behaviour and aggregation of insect haemocytes exposed to contaminated food with arsenic and lead. Int J PharmTech Res 9:177–186
Han J, Xu X, Rao X, Wei M, Evans DG, Duan X (2011)Layer-by-layer assembly of layered double hydroxide/cobalt phthalocyanine ultrathin film and its application for sensors. J Mater Chem 21:2126–2130
Hemdan NYA, Lehmann I, Wichmann G, Lehmann J, Emmrich F, Sack U (2007) Immunomodulation by mercuric chloride in vitro: application of different cell activation pathways. Clin Exp Immunol 148:325–337
Hughes TK, Smith EM, Chin R, Cadet P, Sinisterra J, Leung MK, Shipp MA, Scharrer B, Stefano GB (1990) Interaction of immunoactive monokines (interleukin 1 and tumor necrosis factor) in the bivalve mollusc Mytilus edulis. Proc Natl Acad Sci 87:4426–4429
Ibrahim AM, Abdel-Tawab H (2020) Cystoseira barbata marine algae have a molluscicidal activity against Biomphalaria alexandrina snails supported by scanning electron microscopy, hematological and histopathological alterations, and larvicidal activity against the infective stages of Schistosoma mansoni. Biologia 75(11):1945–1954
Ibrahim AM, Ghoname SI (2018) Molluscicidal impacts of Anagallis arvensis aqueous extract on biological, hormonal, histological and molecular aspects of Biomphalaria alexandrina snails. Exp Parasitol 192:36–41. https://doi.org/10.1016/j.exppara.2018.07.014
Ibrahim MA, Ahmed et al (2018) Hematological, physiological and genotoxicological effects of Match 5% EC insecticide on Biomphalaria alexandrina snails. Ecotoxicol Environ Saf 147:1017–1022. https://doi.org/10.1016/j.ecoenv.2017.09.059
Kaloyianni M, Dimitriadi A, Ovezik M, Stamkopoulou D, Feidantsis K, Kastrinaki G, Gallios G, Tsiaoussis I, Koumoundouros G, Bobori D (2020) Magnetite nanoparticles effects on adverse responses of aquatic and terrestrial animal models. J Hazard Mater 383:121204
Khangarot BS, Das S (2010) Effects of copper on the egg development and hatching of a freshwater pulmonate snail Lymnaea luteola L. J Hazard Mater 179:665–675
Kim K-T, Jang M-H, Kim J-Y, Xing B, Tanguay RL, Lee BG, Kim SD (2012) Embryonic toxicity changes of organic nanomaterials in the presence of natural organic matter. Sci Total Environ 426:423–429
Ladewig K, Xu ZP, Lu GQ (2009) Layered double hydroxide nanoparticles in gene and drug delivery. Expert Opin Drug Deliv 6:907–922
Larson MK, Bender RC, Bayne CJ (2014) Resistance of Biomphalaria glabrata 13-16-R1 snails to Schistosoma mansoni PR1 is a function of haemocyte abundance and constitutive levels of specific transcripts in haemocytes. Int J Parasitol 44:343–353
Lee RF, Steinert S (2003) Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat Res Mutat Res 544:43–64
Li R, Wang JJ, Zhou B, Awasthi MK, Ali A, Zhang Z, Gaston LA, Lahori AH, Mahar A (2016) Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci Total Environ 559:121–129
Long Z, Ji J, Yang K, Lin D, Wu F (2012) Systematic and quantitative investigation of the mechanism of carbon nanotubes’ toxicity toward algae. Environ Sci Technol 46:8458–8466
Ma T, Gong S, Tian B (2017) Effects of sediment-associated CuO nanoparticles on Cu bioaccumulation and oxidative stress responses in freshwater snail Bellamya aeruginosa. Sci Total Environ 580:797–804
Mannervik B, Guthenberg C (1981) [28] Glutathione transferase (human placenta). Methods Enzymol 77:231–235
Manzi-Nshuti C, Songtipya P, Manias E, Jimenez-Gasco MM, Hossenlopp JM, Wilkie CA (2009) Polymer nanocomposites using zinc aluminum and magnesium aluminum oleate layered double hydroxides: effects of LDH divalent metals on dispersion, thermal, mechanical and fire performance in various polymers. Polymer (Guildf) 50:3564–3574
Martins N, Pradhan A, Pascoal C, Cássio F (2020) Effects of metal nanoparticles on freshwater rotifers may persist across generations. Aquat Toxicol 229:105652
Mitta G, Vandenbulcke F, Noël T et al (2000) Differential distribution and defence involvement of antimicrobial peptides in mussel. J Cell Sci 113:2759–2769
Mohamed AH (2011) Sublethal toxicity of Roundup to immunological and molecular aspects of Biomphalaria alexandrina to Schistosoma mansoni infection. Ecotoxicol Environ Saf 74:754–760
Mohamed SH, Saad AA (1990) Histological studies on the hermaphrodite gland of Lymnaea caillaudi and Biomphalaria alexandrina upon infection with certain larval trematodes. Egypt J Histol 13:47–53
Mohamed F, Abukhadra MR, Shaban M (2018) Removal of safranin dye from water using polypyrrole nanofiber/Zn-Fe layered double hydroxide nanocomposite (Ppy NF/Zn-Fe LDH) of enhanced adsorption and photocatalytic properties. Sci Total Environ 640–641:352–363. https://doi.org/10.1016/j.scitotenv.2018.05.316
Mohamed F, Bhnsawy N, Shaban M (2021) Reusability and stability of a novel ternary (Co–Cd–Fe)-LDH/PbI2, photoelectrocatalytst for solar hydrogen production. Sci Rep 11:5618
Moustafa MA, Mossalem HS, Sarhan RM, Abdel-Rahman AA, Hassan EM (2018) The potential effects of silver and gold nanoparticles as molluscicides and cercaricides on Schistosoma mansoni. Parasitol Res 117:3867–3880. https://doi.org/10.1007/s00436-018-6093-2
Myer MH, Henderson WM, Black MC (2017) Effects of multiwalled carbon nanotubes on the bioavailability and toxicity of diphenhydramine to Pimephales promelas in sediment exposures. Environ Toxicol Chem 36:320–328
Nduku WK, Harrison AD (1980) Cationic responses of organs and haemolymph of Biomphalaria pfeifferi (Krauss), Biomphalaria glabrata (Say) and Helisoma trivolvis (Say)(Gastropoda: Planorbirdae) to cationic alterations of the medium. Hydrobiologia 68:119–138
Oliveira-Filho EC, Nakano E, de Tallarico LF (2017) Bioassays with freshwater snails Biomphalaria sp.: from control of hosts in public health to alternative tools in ecotoxicology. Invertebr Reprod Dev 61:49–57
Oliver AL-S, Croteau M-N, Stoiber TL, Tejamaya M, Römer I, Lead JR, Luoma SN (2014) Does water chemistry affect the dietary uptake and toxicity of silver nanoparticles by the freshwater snail Lymnaea stagnalis? Environ Pollut 189:87–91
Omobhude ME, Morenikeji OA, Oyeyemi OT (2017) Molluscicidal activities of curcumin-nisin polylactic acid nanoparticle on Biomphalaria pfeifferi. PLoS Negl Trop Dis 11:e0005855. https://doi.org/10.1371/journal.pntd.0005855
Ottaviani E (2006) Molluscan immunorecognition. Invertebr Surviv J 3:50–63
Ottaviani E, Franchini A, Franceschi C (1993) Presence of several cytokine-like molecules in molluscan hemocytes. Biochem Biophys Res Commun 195:984–988
Ottaviani E, Caselgrandi E, Franceschi C (1995a) Cytokines and evolution: in vitro effects of IL-1α, IL-1β, TNF-α and TNF-β on an ancestral type of stress response. Biochem Biophys Res Commun 207:288–292
Ottaviani E, Franchini A, Cassanelli S, Genedani S (1995b) Cytokines and invertebrate immune responses. Biol Cell 85:87–91
Parida KM, Mohapatra L (2012) Carbonate intercalated Zn/Fe layered double hydroxide: a novel photocatalyst for the enhanced photo degradation of azo dyes. Chem Eng J 179:131–139
Peligro FR, Pavlovic I, Rojas R, Barriga C (2016) Removal of heavy metals from simulated wastewater by in situ formation of layered double hydroxides. Chem Eng J 306:1035–1040
Pena-Llopis S, Pena JB, Sancho E et al (2001)Glutathione-dependent resistance of the European eel Anguilla anguilla to the herbicide molinate. Chemosphere 45:671–681
Pirger Z, Zrinyi Z, Maász G et al (2018) Pond snail reproduction as model in the environmental risk assesment: reality and doubts. In: Ray S (ed) Biol Resour Water. IntechOpen, London, pp 33–53
Radwan MA, El-Gendy KS, Gad AF et al (2019) Responses of oxidative stress, genotoxicity and immunotoxicity as biomarkers in Theba pisana snails dietary exposed to silver nanoparticles. Chem Ecol 35:613–630
Rapado LN, Nakano E, Ohlweiler FP, Kato MJ, Yamaguchi LF, Pereira CAB, Kawano T (2011) Molluscicidal and ovicidal activities of plant extracts of the Piperaceae on Biomphalaria glabrata (Say, 1818). J Helminthol 85:66–72
Rasel MAI, Singh S, Nguyen TD, Afara IO, Gu Y (2019) Impact of nanoparticle uptake on the biophysical properties of cell for biomedical engineering applications. Sci Rep 9(9):5859. https://doi.org/10.1038/s41598-019-42225-7
Ruppert K, Geiß C, Askem C, Benstead R, Brown R, Coke M, Ducrot V, Egeler P, Holbech H, Hutchinson TH, Kinnberg KL, Lagadic L, le Page G, Macken A, Matthiessen P, Ostermann S, Schimera A, Schmitt C, Seeland-Fremer A et al (2017) Development and validation of an OECD reproductive toxicity test guideline with the mudsnail Potamopyrgus antipodarum (Mollusca, Gastropoda). Chemosphere 181:589–599
Saad AEHA, Ragab FMA, Abdel Fatah HM, Abdel-Wareth MTA, Ibrahim NK (2019) Effect of Cystoseira barbata and Dictyota dichotoma-algae on reproduction and protein pattern of Biomphalaria alexandrina snails. Molluscan Res 39:82–88. https://doi.org/10.1080/13235818.2018.1524740
Sánchez-Marín P, Vidal-Liñán L, Fernández-González LE, Montes R, Rodil R, Quintana JB, Carrera M, Mateos J, Diz AP, Beiras R (2021) Proteomic analysis and biochemical alterations in marine mussel gills after exposure to the organophosphate flame retardant TDCPP. Aqua Toxicol 230:105688
Shaban M, Mohamed F, Abdallah S (2018) Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO nanocatalyst. Sci Rep 8:1–15
Sidiropoulou E, Feidantsis K, Kalogiannis S, Gallios GP, Kastrinaki G, Papaioannou E, Václavíková M, Kaloyianni M (2018) Insights into the toxicity of iron oxides nanoparticles in land snails. Comp Biochem Physiol C Toxicol Pharmacol 206–207:1–10. https://doi.org/10.1016/J.CBPC.2018.02.001
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Siwela AH, Nyathi CB, Naik YS (2010) A comparison of metal levels and antioxidant enzymes in freshwater snails, Lymnaea natalensis, exposed to sediment and water collected from Wright Dam and Lower Mguza Dam, Bulawayo, Zimbabwe. Ecotoxicol Environ Saf 73:1728–1732
Tarafdar JC, Sharma S, Raliya R (2013) Nanotechnology: Interdisciplinary science of applications. Afr J Biotechnol 12
Tedim J, Zheludkevich ML, Salak AN, Lisenkov A, Ferreira MGS (2011) Nanostructured LDH-container layer with active protection functionality. J Mater Chem 21:15464–15470
WHO (1965) Molluscicide screening and evaluation. Bull WHO 33:567–581
Ye J, Wu H, Wu Y, Wang C, Zhang H, Shi X, Yang J (2012) High molecular weight hyaluronan decreases oxidative DNA damage induced by EDTA in human corneal epithelial cells. Eye 26:1012–1020
Zaldibar B, Cancio I, Marigómez I (2007a) Reversible alterations in epithelial cell turnover in digestive gland of winkles (Littorina littorea) exposed to cadmium and their implications for biomarker measurements. Aquat Toxicol 81:183–196. https://doi.org/10.1016/j.aquatox.2006.12.007
Zaldibar B, Cancio I, Soto M, Marigómez I (2007b) Digestive cell turnover in digestive gland epithelium of slugs experimentally exposed to a mixture of cadmium and kerosene. Chemosphere 70:144–154
Zhao Y, Jiao Q, Li C, Liang J (2007) Catalytic synthesis of carbon nanostructures using layered double hydroxides as catalyst precursors. Carbon N Y 45:2159–2163
Zhu X, Zhu L, Chen Y, Tian S (2009) Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. J Nanopart Res 11:67–75
Malakar A, Kanel SR, Ray C, Snow D, Juna M, Nadagouda N (2021) Nanomaterials in the environment, human exposure pathway, and health effects: A review. Sci Total Environ 759:143470
OECD (2016) Test No. 243: Lymnaea stagnalis reproduction test, OECD guidelines for the testing of chemicals, Section 2. OECD Publishing, Paris
Boisseaux P, Noury P, Thomas H, Garric J (2017) Immune responses in the aquatic gastropod Lymnaea stagnalis under short-term exposure to pharmaceuticals of concern for immune systems: Diclofenac, cyclophosphamide and cyclosporine A. Ecotoxicol Environ Saf 139:358–366
Lu H, Zhu Z, Zhang H, Zhu J, Qiu Y (2015) Simultaneous removal of arsenate and antimonate in simulated and practical water samples by adsorption onto Zn/Fe layered double hydroxide. Chem Eng J 276:365–375
Choi SJ, Oh JM, Park T, Choy JH (2007) Cellular toxicity of inorganic hydroxide nanoparticles. J Nanosci Nanotechnol 7(11):4017–4020
Hödl E, Felder E, Chabicovsky M, Dallinger R (2010) Cadmium stress stimulates tissue turnover in Helix pomatia: increasing cell proliferation from metal tolerance to exhaustion in molluscan midgut gland. Cell Tissue Res 341:159–171. https://doi.org/10.1007/s00441-010-0980-x
Code availability
Not applicable
Author information
Authors and Affiliations
Contributions
Conceived and designed experiments; Heba Abdel-Tawab, Amina M. Ibrahim
Data curation; Taghreed Hussein, Fatma Mohamed
Formal analysis; Taghreed Hussein, Fatma Mohamed
Methodology; Heba Abdel-Tawab, Amina M. Ibrahim
Software; Taghreed Hussein, Fatma Mohamed
Supervision; Heba Abdel-Tawab, Amina M. Ibrahim
Validation; Heba Abdel-Tawab, Amina M. Ibrahim
Visualization; Heba Abdel-Tawab, Amina M. Ibrahim
Roles/writing-original draft; Taghreed Hussein, Fatma Mohamed
Writing—review & editing; Heba Abdel-Tawab, Amina M. Ibrahim
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest/competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Tawab, H., Ibrahim, A.M., Hussein, T. et al. Mechanism of action and toxicological evaluation of engineered layered double hydroxide nanomaterials in Biomphalaria alexandrina snails. Environ Sci Pollut Res 29, 11765–11779 (2022). https://doi.org/10.1007/s11356-021-16332-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16332-w