Abstract
In order to increase the low-temperature resistance of Nile tilapia, the purpose of this study was to determine the potential effect of ω3 fatty acid incorporation in Oreochromis niloticus diet. To perform this, two experimental diets containing soybean oil (D1) and cod liver oil (D2) have been supplied to juvenile tilapia for 30 days. According to our results, similar improvements in the two diets have been recorded for growth performance of O. niloticus including the final body mass, specific growth rate, and feed conversion ratio. Our results showed that fish fed with diet D2 promoted high polyunsaturated fatty acids mainly n-3 series (PUFA (n-3)) percent, highlighting the increased levels of docosahexaenoic (DHA) and eicosapentaenoic (EPA) as well as the activation of their conversion enzyme ratios D5D and D6D desaturases. The second objective was to assess the effect of the two experimental diets on low water temperature tolerance. This was done by exposing juvenile fish at the end of the first experiment to 16, 14, 12, 10, and 8 °C for 12 h, 24 h, and 48 h. The sub-lethal LT50 of O. niloticus fed with diet D1 was 10.6, 11.4, and 13 °C respectively, after 12 h, 24 h, and 48 h. This pattern was commonly observed for O. niloticus fed with D2, showing that the subLT50 were 10.3, 11.1, and 12 °C during the same period. These results demonstrate that O. niloticus juveniles fed with diet D2 are more tolerant to low temperatures than those fed with diet D1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tilapia is Africa’s aquaculture resource that was introduced in several countries based on several characteristics such as rusticity of breeding, broad ecological valence, and the flexibility of adaptation to extremely wide environmental variations. For that reason, Oreochromis niloticus is considered a basis of freshwater fish farming in many inter-tropical regions of the world (FAO 2014). Indeed, according to the latest statistics on freshwater fish production (FAO, 2020), tilapia production, especially O. niloticus, increased from 3.31 million tons in 2010 to 4.25 million tons in 2018. Temperature is considered one of the most important environmental factors that affect the growth, physiological state, reproduction, and metabolism of fish especially tilapia (Pandit and Nakamura 2010). These effects were more highlighted in temperate and subtropical regions defined by seasonal fluctuations in water temperature. The optimum temperature range depends principally on many biological parameters such as the fish species, size (Hofer and Watts, 2002; Sigurd and Sigurd 2008), and genetic variations (Cnaani et al. 2003). Some optimum temperatures comprised between 25 and 32 °C seem to be necessary for the normal development, reproduction, and growth of tilapia. Known, that for the majority of aquaculture systems, they take place in waters that are characterized by no thermoregulation and are influenced by more or less pronounced daily fluctuations. It should be noted that in intensive breeding in geothermal water, the breeding temperature is stable and optimal for the species. However, in the dam reservoir, it is variable and exhibits seasonal or even daily fluctuations. According to different studies, some authors demonstrated that the thermal condition fluctuations induced slower or lower growth efficiencies than the constant temperatures (Flodmark et al. 2004). However, other authors suggested no significant effect (Dhillon and Fox 2007).
The ability to tolerate low temperatures depends on several ecological (environment and geographic distribution), genetic (strain effect), and nutritional factors (Li et al. 2002). In addition, farming conditions can have a significant impact on species’ tolerance to low temperatures (Cnaani et al. 2003; Charo-Karisa et al. 2006). Charo-Karisa et al. (2006) demonstrated that the acclimatization of O. niloticus at autumnal temperatures increases their thermal tolerance to low temperatures compared to those acclimated at summer temperatures. Thus, fish size, which is considered another parameter of the breeding condition, has no relationship with the tolerance to low temperatures in Nile tilapia.
The essential fatty acids (EFAs) ensure many roles including normal growth, development, and reproduction of fish (Tocher 2010). In freshwater fish, two main polyunsaturated fatty acid families constituting these EFAs such as the n-6 and n-3 series are influenced essentially by the feed diets. Linoleic acid (18: 2n-6, LA) and α-linolenic acid (18: 3n-3, ALA) are the principal substrates produced by the feed diets which are then converted through the desaturase activities (D5D and D6D) to arachidonic acid (20: 4n-6, ARA), eicosapentaenoic acid (20: 5n-3, EPA), and docosahexaenoic acid (22: 6n-3, DHA) (Da Costa et al. 2015). Concerning tilapia, their growth and reproduction depend essentially on LA or ARA levels (Lim et al. 2011). Several authors determined the optimum requirement of dietary n-6 PUFA (LA levels) for many species of tilapia. It was estimated to be about 1.0% for red belly tilapia (Kanazawa et al. 1980), 0.5% for Nile tilapia (Takeuchi et al. 2010), and 1.14% for hybrid tilapia, Oreochromis niloticus × Oreochromis aureus (Li et al. 2013). Moreover, Li et al. (2013) suggested that this requirement could be reduced when ALA was present simultaneously. Similar to other warm water fish, tilapia is further to apt the requirement of the high level of n-6 PUFA compared to n-3 PUFA for maximal growth (NRC 2011), Yet, the growth has been depressed when tilapia fed diets with above 1% of LNA (Stickney and McGeachin 1983) or oils (i.e., 5% Pollock liver oil, 10% or 12% cod liver oil) with high levels of n-3 PUFA (Al-Souti et al. 2012; Kanazawa et al. 1980; Ng et al. 2001). However, some other studies support the necessity of n-3 and n-6 PUFA for growth performance. So, Cou and shiau (2001) have demonstrated that tilapia diets without any cold liver oil had significantly lower growth performance and showed a typical n-3 deficiency. Thus, Ng et al. (2011) have shown that tilapia diets based on fish oil and vegetable oil resulted in the highest EPA, DHA, and n-3/n-6 ratios as well greatest growth.
On other hand, several authors suggested that the lipid composition of fish fillet plays a key role in the ability of fish to adapt the changes in water temperature (Lu et al. 2019). The essential fatty acids mainly ALA and LA provided from diets induce an alteration of the lipid composition of fish when they are placed in colder water and therefore determine their survival (Corrêa et al. 2018). It is known that the PUFA (n-3) interacts with protein membrane in order to maintain the modulation of the fluidity and integrity of the cell membrane (Nemova et al., 2013). In this line, according to Lu et al. (2019), the high level of PUFA (n-3) present in the diet allows an increase in the ability of fish to tolerate the low temperature.
Additionally, fishmeal is generally the major component of feed in aquaculture. Indeed, it is rich in essential amino acids (EAA). Furthermore, some authors confirmed that the complete substitution of vegetable oil by fish oil in tilapia diets improves its growth (Teoh et al. 2011). However, others suggested there are no significant effects between diets (Al-Souti et al. 2012).
As well, in order to improve and ensure the success of breeding operations, the study of the effects of diets on thermal tolerance in tilapia is of capital importance to avoid the risk of mortality during temperature fluctuations in rearing systems where the water temperature is not thermoregulated (Azaza et al. 2010). For that reason, the goal of the present study was to investigate (i) the effects of two experimental diets based on soybean oil and cod liver oil on the growth performance of tilapia after 1 month and (ii) to determine their tolerance capacity to low water temperatures (16, 14, 12, 10, and 8 °C) for 12 h, 24 h, and 48 h.
Materials and methods
Feed formulation and pellet preparation
Two experimental diets D1 with soybean oil and D2 with cod liver oil were tested in our study. For each experimental diet, the food ingredients were weighed and mixed with 10% of vegetable oil (soybean oil, D1) and with 10% of animal oil (cod liver oil, D2). The mineral and vitamin premix was combined with the two mixed. Water was then added to 60% dry matter content in order to obtain a malleable paste which was pelleted through a 3 mm hole in the kitchen meat grinder (TC 22SL) and which was dried in the sun. The dried pellets were fragmented to the desired and suitable size, bagged in polythene bags, until required, and stored at a temperature of − 20 °C until distribution.
Fish and experimental conditions
Acclimation
The juvenile Nile tilapia (Oreochromis niloticus) was obtained from an experimental research station of the Tunisian National Institute of Marine Sciences and Technology (INSTM). Fish were acclimated in experimental aquariums (80 L) for 15 days (Fig. 1). The photoperiod was fixed at a constant (12 h/12 h) light–dark cycle. As previously studied by Azaza et al. (2008), the select temperature (28 ± 2 °C) corresponds to the optimum temperature for tilapia growth.
In each reservoir tank, an immersion thermostatic heater (1 kW) was installed to maintain the preselected water temperature. Using submerged filtration in each aquarium (Rena, Filstar), the fecal matter was removed. In order to maintain dissolved oxygen levels near saturation, a supplemental aeration was conditioned. For all aquaria, water was constantly renewed by continuous flow at the rate of 1L/min to provide oxygen and remove the excess nitrogenous waste.
Experimental diets
After acclimation, 12 fish with an initial body weight of 3.44 ± 0.9 g were taken randomly at the start of the experiment and considered as initial samples. The 120 fish with similar initial body were distributed randomly into six aquaria (80 L for each), and each aquarium was stocked with 20 fish for 30 days. Each experimental diet was tested for triplicates of tanks. The average weight variation between different aquariums was reduced as necessary by the sorting and redistribution of fish with similar mass in order to ensure a uniform starting mass. All fish in each aquarium were anesthetized (MS-222, 50 ppm), to reduce the stress and to ensure reliability of the weighing, and then weighed and measured to the nearest 0.001 g and 0.1 cm, respectively, at the beginning and at the end of the trial. Fish were fed two times daily at 09:00 a.m. and 17:00 p.m. Mortality was controlled daily.
At the end of the experimental period, all fish in each tank were weighted to assess growth and feed utilization efficiency. The fish (n = 12) of each treatment were sacrificed on ice and the muscle fragments which came from the left side of the animal corresponding to the latero-dorsal part were quickly removed for the lipid and fatty acid composition analyses (6 replicates in each experimental diet (n = 2 fish for each replicate)). The other 40 fish from each experimental diet are intended for the temperature experiment.
Temperature experiment
At the end of the first experiment trial, groups of 8 fish from each diet were subjected to temperature tests of 16, 14, 12, 10, and 8 °C. At each test temperature, 2 series of experiments (n = 4) are executed in a plastic tank (100 L) and which are separated by a perforated plastic plate. The test temperatures were reached progressively at a rate of 1 °C every 5 min. The temperature was lowered using a cry-plunger (Huber TC 50 E). During the experiment periods (i.e., 12 h, 24 h, and 48 h), the fish are kept fasted and a constant aeration is controlled in order to maintain the saturated oxygen level. For each temperature treatment, we note the behavior of the fish and the individual survival times for which the fish lose their ability to swim for at least 10 s (Kraïem and Duvernay 1981). A survival number and mortality rate (%) were calculated.
Growth indices
Growth performance and nutrient utilization of fish used in the experiment were measured in terms of final body weight (g), survival rate (%), specific growth rate (SGR, % day−1), and feed conversion ratio (FCR).
Growth performance parameters were calculated as follows:
-
\(\mathrm{Survival rate }(\mathrm{\%}) = 100 \times (\mathrm{final number fish}/\mathrm{initial number fish})\)
-
\(\mathrm{Weight gain }(\mathrm{\%}) = 100 \times (\mathrm{final body weight }-\mathrm{ initial body weight})/\mathrm{final body weight}.\)
-
\(\mathrm{Daily weight gain }(\mathrm{g day}-1) = (\mathrm{final body weight }-\mathrm{ initial body weight})/(\mathrm{duration of the experiment }(\mathrm{day})).\)
-
\(\mathrm{Specific growth rate }(\mathrm{SGR},\mathrm{ \% day}-1) = 100\times ((\mathrm{log final body weight }-\mathrm{ log initial body weight})/\mathrm{duration of the experiment }(\mathrm{day}))\)
-
\(\mathrm{Feed conversion ratio }(\mathrm{FCR}) = (\mathrm{dry feed intake})/(\mathrm{biomass produced }(\mathrm{Bp}))\)
-
\(\mathrm{Biomass produced }(\mathrm{Bp}) =\mathrm{ final biomass }-\mathrm{ initial biomass }+\mathrm{ dead biomass}\)
-
\(\mathrm{Protein efficiency ratio }= (\mathrm{biomass produced})/(\mathrm{proteins ingested}).\)
Biochemical analysis
The total lipids (TL) were extracted using chloroform: methanol (2:1, v/v) solution containing 0.01% butylated hydroxyl toluene (BHT) as an antioxidant following the method described by Folch et al. (1957).
Fatty acid analysis
The total lipid was trans-esterified to methyl esters according to the method of Cecchi et al. (1985). The methyl nonadecanoic acid C19:0 (Sigma) which was absent in our samples was added as an internal standard. Methyl esters were analyzed by gas chromatography using a chromatogram “Agilent Technologies” HP 6890 model equipped with a capillary column INNO-WAX (30 m × 0.25 μm) and supplied by a carrier gas: nitrogen. The temperature program during the injection has been started at a temperature of 50 °C. Thereafter, the T°C was raised to 180 °C (40 °C/min), then to 220 °C (33 °C/min). Finally, it was remained at 220 °C for 5 min. Identification of FAMEs was based on the comparison of their retention times with those of a mixture of methyl esters PUFA-3 (by SUPELCO) and Marine oil (Menhaden oil by SUPELCO). Fatty acid peaks were integrated and analyzed using HP Chemstation software. Fatty acids were expressed in percentages (%).
Desaturase and elongase activities and lipid quality index
Desaturase activities were estimated as the product/precursor ratios of individual fatty acids according to the following formulas: D9D: Δ9-desaturase = stearoyl-CoA-desaturase = C18:1 n-9/18:0; D5D: Δ5-desaturase = C20:5n-3/C20:4n-3. D6D: Δ6-desaturase = C20: 4n-3/C18:3n-3 (Da costa et al. 2015; Rabei et al. 2018). The elongase activity index (ELOVL6) was calculated using (C18:0/C16:0) ratio (Kotronen et al. 2010).
The lipid quality index such as n-3/ n-6, ΣPUFA/ΣSFA, and DHA/ EPA ratios and EPA + DHA were determined according to Marques et al. (2010).
Statistical analysis
Data analysis was performed using the software Statistica version 5.0. The fatty acid composition, lipid quality indices, and desaturase and elongase activities of tilapia fillet were compared between the two experimental diets. Normality was assessed for all datasets using the Shapiro-Wilcoxon test. The significant differences between variables were analyzed using a one-way analysis of variance (ANOVA) followed by a post hoc Tukey’s test (p < 0.05). When the conditions for ANOVA were not satisfied, nonparametric Kruskal–Wallis’s test was used (p < 0.05). The differences between samples were deemed to be significant at p < 0.05. Principal component analysis (PCA) was used to display the effects of two diets based on soybean oil and cod liver oil on the fatty acid composition of tilapia.
Results
Growth performances
Regarding the general physiological state of the fish during the experiment, no pathological signs and no mortality were recorded.
After feeding the fish for 1 month, the fish fed by the soybean oil (D1) and cod liver oil (D2) diets were found to have a similar final weight. The results of all growth performance indices showed similar changes between the two experimental diets with no significant difference (Table 1). The survival rate is estimated at 100% for animals fed on D1 and D2 diets.
Fatty acid composition
In this study, twenty-two fatty acids (FA) were identified and three families were determined.
Experimental diet’s fatty acid profile
The fatty acid compositions of two experimental diets (soybean oil (D1) and cod liver oil (D2)) are summarized in Table 2.
A highly significant difference was recorded for all fatty acids between the two experimental diets. A great level of linoleic acid (C18:2n-6, 37%), polyunsaturated fatty acids (PUFA’s, 43.6%), and n-6 fatty acids (PUFA (n-6), 37%) characterized the soybean oil. However, the cod liver oil has an important level of arachidonic (C20:4n-6, 0.23%), docosahexaenoic (C22:6n-3, 2.73%), EPA: eicosapentaenoic (C20:5n-3, 2.55%) acids; saturated (SFA, 27%), and monounsaturated (MUFA, 20%) fatty acids (Table 1).
The tilapia fillet fatty acid profile after experimental diets
The fatty acid composition of tilapia fillet from initial samples and from samples fed by two experimental diets (soybean oil (D1) and cod liver oil (D2)) is summarized in Table 3.
Remarkable and significant changes in the fatty acid composition of tilapia fillet were recorded in our experiment. These changes were noted in the initial samples and between the experimental diets.
Total SFA in tilapia fillet decreased significantly after the two dietary treatments (D1 and D2) for 1 month when compared to the initial samples (− 11% and − 15%, respectively). However, no significant difference was recorded between the two dietary treatments. A significant decline in the total MUFA level was observed in the tilapia fillet fed with diets (D1 and D2) as compared to the initial fish (− 53% and − 30%, respectively). The total MUFA level in the fillet of tilapia fed with D2 was significantly higher than those fed with D1. These significant elevations (P < 0.001) are observed especially for 16:1n-7, 18:1n-9, and 18:1n-7.
A significant increase in total PUFA and PUFA (n-3) levels was observed in the tilapia fillet fed by D2 (+ 27%, + 111%, respectively) as compared to the initial fish. However, an elevation of PUFA (n-6) level was noted in the tilapia fillet fed by D1 (+ 23%) as compared to the initial animals.
By comparing the two experimental diets, PUFA and PUFA (n-3) levels in the fillet fed with D2 were significantly higher than those fed with D1. Generally, low ALA and high levels of EPA and DHA were recorded in tilapia fillets fed with D2 compared to the initial samples and to those fed by D1. However, the tilapia fed by D1 has demonstrated great levels of LA and ARA.
Lipid quality indices are summarized in Table 4. The DHA/EPA, n-3/n-6 and ΣPUFA/ΣSFA ratios, and EPA + DHA calculated for the fillet of tilapia fed by D1 were significantly lower than those estimated for the fillet from tilapia fed by D2 and the initial fish.
A significant increase (P < 0.001) of D5D and D6D was observed for the fish fed by D2 compared to those fed by D1. However, the fish fed by D1 showed a significant increase in ELOVL6 and a decrease in D9D activities (Table 3).
The results of PCA (Fig. 2) allowed us to retain the first two factorial axes that explained 73.56% of the total variance. Factor 1 displayed 43.54% of the total variance, defined by the PUFA, PUFA (n-3), DHA, and different ratios such as EPA + DHA, (n-3)/(n-6), and D6D activity. The ARA, SFA, MUFA, and ELOV6 and D9D activities characterized factor 2 (30.03%). The ratios of PUFA/SFA, DHA/EPA, and LA and ALA values and D5D activity are the intermediate variables between the two axes. An analysis of fatty acid composition showed significant variations between the two diets. The tilapias are regrouped into three separate groups. The initial sample (IS) group which is represented in the negative parts of two factors of PCA is characterized by high levels of MUFA, SFA, and EPA and low activities of desaturases (D5D and D6D). After 30 days of feeding with two experimental diets, the tilapia fed by diet D2 accumulate high levels of PUFA, PUFA (n-3), and DHA and have great nutritional quality ratios more than the tilapia fed by D1. The low levels of ETA (R = − 0.95), ALA (R = 0.92), and LA (R = 0.87) estimated in the fish fed with D2 are associated with the activations of desaturase enzymes (D5D and D6D), which are responsible for converting these last fatty acids in their substrates such as EPA, DHA, and ARA respectively.
Principal component analysis (PCA) represented by two factors F1 and F2 and produced by the fatty acid composition; estimated desaturase and elongase activities and nutritional indices of Nile tilapia fillets (Oreochromis niloticus) and experimental diets. Projection of the variables and the cases on the factor plane (1–2); D1, soybean oil; D2, cod liver oil; IS, initial samples
Experimental and tolerance temperature
The results relating to the temperature tolerance of O. niloticus are expressed in two different ways: (1) the average survival time in terms of temperature for the two experimental diets (D1 and D2) and (2) sub-lethal temperatures 50% (subLT50) after 12 h, 24 h, and 48 h for the two experimental diets (D1 and D2).
Average survival time
The influence of experimental diets on sub-lethal temperature for 24 h, 48 h, and 72 h expressed by survival time variation is represented in Fig. 3. According to our results, the tilapia fed by D1 has a sub-lethal temperature (subLT) in the order of 9.6, 11.2, and 15 °C after 12 h, 24 h, and 48 h respectively. Those fed on diet D2 have subLT of 8.8, 10.9, and 14.8 °C after 12 h, 24 h, and 48 h respectively. Using to test ANOVA, significant effects of experimental diets were recorded between the subLT50 for 12 h (P = 0.0006) and 24 h (P = 0.021).
Survival time variation of Nile tilapia (Oreochromis niloticus) from experimental diets during 12 h, 24 h, and 48 h at different experimental temperatures. Experimental diet D1: subLT12h = 9.6 °C; subLT24h = 11.20 °C; subLT48h = 15 °C. (Measurements made at 8, 10, 12, 14, and 16 °C on 40 individuals in total.) Experimental diet D2: subLT12h = 8.8 °C; subLT24h = 10.9 °C; subLT48h = 14.8 °C. (Measurements made at 8, 10, 12, 14, and 16 °C on 40 individuals in total.) Each point represents the average of 8 measurements. The regression lines are calculated using the least squares method. D1, soybean oil; D2, cod liver oil
The statistical comparison of these results (Table 5) revealed that the two calculated regression lines corresponding to the two experimental diets are secant (slope test = 5.016 > P = 5% (2365)); showing a significant difference in tolerance of O. niloticus to the lower temperature after the two experimental diets (Fig. 3).
Sub-lethal temperature 50% (subLT50)
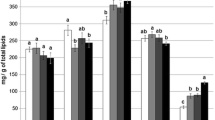
The analysis of the survivor’s fish number from the two experimental diets calculated after 12, 24, and 48 h at the different test temperatures is represented in Fig. 4 and allows us to determine the 50% sub-lethal temperatures. Thus, the fish fed with diet D1 have a subLT50 comprised between 10 and 12 °C at 12 h and 24 h respectively, and more than < 12 °C after 48 h. The animals fed with diet D2 appear more resistant to lowering temperature and the respective subLT50 thresholds are between 10 and 12 °C after 12 h and 24 h and between 12 and 14 °C at 48 h. It should be noted that 100% of these fish survive at 12 °C after 12 h (Fig. 5b).
The analysis of the average mortality in terms of the test temperatures after 12 h, 24 h, and 48 h (Fig. 5) demonstrated that O. niloticus fed with diet D1 has a subLT50 (12 h) at 10.6 °C, subLT50 (24 h) at 11.4 °C, and subLT50 (48 h) at 13 °C (Fig. 5a). In contrast, the values of subLT50 of the batch fed with diet D2 were 10.3 °C, 11.1 °C, and 12 °C after 12 h, 24 h, and 48 h respectively (Fig. 5b).
Discussion
Demand for tilapia has regularly increased in recent decades; accordingly, an important effort to improve the production and the meat quality of these products is necessary. Consequently, several investigations based on the feasibility of partial/complete replacements of dietary fish oil have been carried out to determine the optimal dietary lipid sources for farmed tilapia (Bahurmiz and Ng 2007; Teoh et al. 2011; Ng et al. 2011) (Tables 5 and 6).
The survival rate is estimated at 100% for the soybean oil (D1) and for the cod liver oil (D2). Some observations have been supported by Cou and Shiau (2001) and Al-Souti et al. (2012).
Our results of the growth performance of the experimental diets showed that there was no significant difference between tilapia (Oreochromis niloticus) fed with soybean oil and those fed with cod liver oil for 1 month. It is necessary to indicate that the incorporation of cod liver oil does not lead to an improvement in growth and feed conversion performance. Moreover, the total substitution of vegetable oils by cod liver oil has shown similar results for red tilapia (O. mossambicus x O. aureus) (Bahurmiz and Ng, 2007; Al-Souti et al. 2012) mostly for the replacement (up to 100%) sustains a feed conversion rate (FCR), the protein efficiency coefficient (PCE), and the specific growth rate (SGR). However, a better growth performance of O. niloticus and red tilapia has been mentioned with food containing oil of marine origin (fish) compared to food based on a mixture of vegetable oils (palm oil, linseed oil, sunflower, and olive) (Teoh et al. 2011; Ng et al., 2011). According to Cou and Shiau (2001), hybrid tilapia (Nile tilapia × blue tilapia) fed with a diet composed of 5% of cod liver oil demonstrated a better growth rate (SGR = 2.32%). In this line, our experiment based on the incorporation of 10% of cod liver oil seems to be sufficient for improved growth (SGR = 4.27%).
Tilapia uses essential fatty acids, such as EPA, ARA, and DHA for many biological functions. These fatty acids come respectively from the metabolic conversion of ALA and LA leaders of the two main families of unsaturated fatty acids (n-6 and n-3 series). These last fatty acids cannot be synthesized by tilapia and they must therefore be provided by food (Lim et al. 2011). In our study, the fatty acid composition of the experimental diet (D1) demonstrated high levels of LA and ALA. While the ETA, EPA, and DHA were more abundant in diet D2. Consequently, a remarkable and significant change in tilapia fillet fatty acid composition was recorded after 1 month of feeding. In this line, after the cod liver oil diet, an increase in DHA and EPA levels was coupled with a decrease in their respective precursors, such as ALA and ETA as well as an increase in the activities of the estimated D6 (D6D) and D5 (D5D) desaturase ratios responsible for their conversion (P < 0.001). Our findings confirmed that tilapia has the capacity to bio-convert ETA to EPA and ALA to DHA (Teoh et al. 2011). However, the low level of DHA (P < 0.001) of the fillet from D1 diet in the current study can be explained by its reliance on diet quality and diet duration. Ng et al. (2011) obtained comparable results. Moreover, in our study, the EPA content in diet D2 seems to be higher than that in the tilapia fillet. It can be explained by the advantageous use of EPA as an energy source and/or synthesized into DHA (Montero et al. 2005; Senadheera et al. 2011). Analogous results were reported for other fish species (Ng et al. 2011).
Arachidonic acid is recognized as the major precursor of eicosanoids and is an essential fatty acid for normal growth and juvenile’s development (Bell and Sargen 2003). In the present study, the ARA contents in the tilapia fillet are higher than the contents in diets. Ng et al. (2011) demonstrated similar results after different diets based on fish, soybean, linseed, and crude palm oil. While and Al-Souti et al. (2012) showed that fillets of tilapia feed with experimental diets based on low levels of fish oil contained traces of ARA.
Besides, the tilapias fed D1diet which contained more LA had significantly higher ARA content than D2 dietary treatment. The desaturase activity responsible for converting the LA into ARA might explain these increases in ARA bioavailability in the current study. Similar results are observed by Ng et al. (2011). According to Barthet (2008), C18:1n-9 can be synthesized through two steps: (1) producing C18:0 from C16:0 using elongase (ELVOL6) and subsequently (2) producing C181n-9 from C18:0 using desaturase D9 (D9D). Our results have shown for fillet from two experimental diets a significant decrease in estimated D9D and a significant increase in estimated ELVOL6 explaining the decline of MUFA levels, especially in C18:1n-9 which is associated with an increase in C18:0.
On other hand, myristic acid (C14:0) levels decreased significantly in fillet tilapia from two experimental diets. These declines can be explained by the use of C14:0 as an energy source without deposition in the body (Rioux et al. 2005).
The n-3/n-6 ratio is an important nutritional index. The n-3/n-6 ratio was estimated at 0.48 for fish fed on diet D1 and at 1.44 for fish fed on diet D2. According to Atwood et al. (2003) and Al-Souti et al. (2012), fish fed with diets based on fish oil (Menhaden, cod liver) generally have a higher n-3/n-6 ratio than fish fed with diets based on vegetable oils (corn and/or soy oils). Similar observations have been reported in red hybrid Tilapia fed on vegetable and cod liver oils for 3 months (Ng et al. (2011). Regarding the (n-6) family, the levels are higher in fish fed with a diet containing vegetable oils, and, consequently, they affect the fillet fatty acid composition. Our investigation is in concordance with previous works (Atwood et al. 2003; Bahurmiz and Ng 2007; Ng et al. 2011).
In our study, PUFA/SFA ratio values varied from 0.65 to 0.85 for animals fed on diets D1 and D2, respectively. The low ratio value recommended is 0.45 (HMSO 1994). Another lipid quality index calculated in our experiment is EPA + DHA which is commonly used to assess the nutritional quality of marine animal products. Our results demonstrated that the EPA + DHA index calculated from tilapia fillets fed on diet D2 diet is 4 folds higher than those from fish fed on D1 diet.
According to the literature, diet is known to play an important role in the ability of fish to tolerate low temperatures (Lu et al. 2019). However, for Nile tilapia (O. niloticus), very few studies have addressed this aspect (Atwood et al. 2003; Charo-Karisa et al. 2006). In our investigation, the mortality rate was determined in terms of the test temperatures (8, 10, 12, 14, and 16 °C) after 12 h, 24 h, and 48 h. It has been demonstrated that in fish fed on diet D1, the subLT50 is 10.6, 11.4, and 13 °C after 12 h, 24 h, and 48 h respectively. However, in D2 diet, the values of subLT50 are 10.3 °C, 11.1 °C, and 12 °C after 12 h, 24 h, and 48 h respectively. These results suggest that tilapias fed by D2 diet seem to be more tolerant to low temperatures than those fed by diet D1. Atwood et al. (2003) have demonstrated a little effect of diets (fish oil (Menhaden) and vegetable oil (coconut) for 14 days) on the tolerance of tilapia to low temperatures.
Moreover, some studies have shown that the tolerance of fish to low temperatures is related to the lipid composition of diets and of fish species (Lu et al. 2019). Thus, fish fed on diets with a high level of PUFA (n-3) have shown an increase in their tolerance to low temperatures (Lu et al. 2019). In this context, the high percent of PUFA (n-3) in cod liver oil (D2) sustains the best tolerance and adaptation of animals to the low temperature in our study. (Table 6). Our results are in concordance with those of Corrêa et al. (2018), which suggested the implication of PUFA in the improvement of tilapia tolerance to low temperatures (22 °C) when compared to those maintained at optimal temperatures (28 °C).
There is a direct relationship between the physical properties of cell membranes and their fatty acid composition. The membrane fluidity and integrity depend on MUFA and PUFA levels (Nemova et al. 2013). In our finding, the low levels of MUFA and PUFA in the fish fillet from the D1 diet can reduce the maintenance of membrane functionality during temperature fluctuations, and, therefore, they seem to be less resistant to low temperatures. However, the tilapias fed with D2 rich in PUFA (n-3) have shown a great reserve of these fatty acids, and, accordingly they are able to modify membrane fatty acid composition in order to cope with the lowest temperatures. A similar investigation has been conducted by Corrêa et al. (2018).
Conclusions
Tilapia has crucial economic value and can be produced easily in several environments, including geothermal waters and dam reservoirs. Our work is the first report to assess the improvement of tilapia (Oreochromis niloticus) tolerance to low temperatures following PUFA (n-3) incorporation in the experimental diets. Two experimental diets based on cod liver oil (D1) and on soybean oil (D2) were tested for 1 month (1). For the first time, our results demonstrated that the incorporation of cod liver oil does not lead to an improvement in growth and feed conversion performance compared to soybean oil. However, the fillets of tilapia fed by diet based on cod liver oil accumulate more PUFA levels. For the second time, the mortality rate and subLT50 were significantly different between the two experimental diets suggesting that the tilapias fed by the cod liver oil diet seem to be more tolerant to low temperatures. We can conclude that the improvement of diets based on PUFA (n-3) has a remarkable effect on the tolerance of tilapia to low temperatures. Our finding may provide a new strategy for tilapia production by developing a new economical protocol based on PUFA (n-3) incorporation in tilapia diets. The discovery of these alternative lipid sources will provide for the aquaculture industry a greater array of enhanced PUFA (n-3) incorporation in the human diet by producing PUFA (n-3)-enriched tilapia (1). Finally, when tilapias are produced in environments with high-temperature fluctuations, they reduce their mortality (2). Yet, the use of food additives (e.g., vitamin E) would be reliable to ensure the better conservation of omega 3 against oxidation, and, consequently, to enhance the resistance capacity of fish to low temperatures.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- ARA:
-
Arachidonic acid
- D5D:
-
Δ5-Desaturase
- D6D:
-
Δ6-Desaturase
- D9D:
-
Δ9-Desaturase = stearoyl-CoA-desaturase
- DPA:
-
Docosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- ELOVL6:
-
Elongase
- LA:
-
Linoleic acid
- ND:
-
None determined
- MUFA:
-
Monounsaturated fatty acids
- PUFA:
-
Polyunsaturated fatty acids
- SFA:
-
Saturated fatty acids
- subLT:
-
Sub-lethal temperatures
- subLT50 :
-
Sub-lethal temperatures 50
References
Al-Souti, A., Al-Sabahi, J., Soussi. B and Goddard, S., 2012. The effects of fish oil-enriched diets on growth, feed conversion and fatty acid content of red hybrid tilapia, Oreochromis sp, Food Chemistry,133,723-727.https://doi.org/10.1016/j.foodchem.2012.01.080.
Atwood, H. L., Tomasso, R., Webb, K. and Gatlin, D.M., 2003. Low temperature tolerance of Nile tilapia, 0reochromis niloticus: effects of environmental and dietary factors, Aquaatic Research , 34 (3), 241-251.https://doi.org/10.1046/j.1365-2109.2003.00811.x
Azaza, M.S., Dhraief, M.N. and Kraiem, M.M., 2008. Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. Journal of Thermal Biology, 33, 98–105. https://doi.org/10.1016/j.jtherbio.2007.05.007
Azaza, M.S., Mensi F., Wassim K., Abdelmouleh A., Brini B. and, Kraim M.M., 2009. Nutritional evaluation of waste date fruit as partial substitute for soybean meal in practical diets of juvenile Nile tilapia, Oreochromis niloticus L, Aquaculture Nutrition, 15, 262-272.
Azaza M.S., Legendre M., Kraim M.M. and Baras E., 2010. Size-dependent effects of fluctuating thermal regimes on the growth and size heterogeneity of Nile tilapia, Journal of Fish Biology, 76 (3), 669-683.
Bahurmiz, O.M. and Ng, W.K., 2007. Effects of dietary palm oil source on growth, tissue fatty acid composition and nutrient digestibility of red hybrid tilapia, Oreochromis sp., raised from stocking to marketable size, Aquaculture, 262, 382–392. https://doi.org/10.1016/j.aquaculture.2006.11.023
Barthet, V.J., 2008. (n-7) and (n-9) cis-monounsaturated fatty acid contents of 12 brassica species , Phytochemistry, 69(2), 411-411. https://doi.org/10.1016/j.phytochem.2007.08.016
Bell, J.G. and Sargen, J.R., 2003.Arachidonic acid in aquaculture feeds: current status and future opportunities, Aquaculture, 218, 491-499. doi: https://doi.org/10.1016/S0044-8486(02)00370-8.
Cecchi, G, Basini, S., andCastano, C., 1985. Methanolyse rapide des huiles en solvant, Revue française des corps gras, 4 , 163–164.
Charo-Karisa, H., Komen, H., Rezk, M. A., Ponzoni, R.W., Van Arendonk, J. and Bovenhuis, H., 2006 Heritability estimates and response to selection for growth of Nile tilapia (Oreochromis niloticus) in low-input earthen ponds, Aquaculture, 261(2), 479-486.
Cnaani, A., Hallerman, E.M., Ron, M., Weller, J. I., Indelman, M., Kashi, Y., Gall, G.A.E. and Hulata, G., 2003. Detection of a chromosomal region with two quantitative trait loci, affecting cold tolerance and fish size, in an F2 tilapia hybrid, Aquaculture, 223,117- 128. https://doi.org/10.1016/S0044-8486(03)00163-7
Cou, B.S.and Shiau, S.Y., 2001. Effect of Dietary Cod Liver Oil on Growth and Fatty Acids of Juvenile Hybrid tilapia, North American Journal of Aquaculture, 63, 277-284. https://doi.org/10.1577/15488454(2001)063<0277:EODCLO>2.0.CO;2
Corrêa, C.F., Nobrega, O.R., Block, J.M. and Fracalossi, D.M., 2018. Mixes of plant oils as fish oil substitutes for Nile tilapia at optimal and cold suboptimal temperature.Aquaculture, 497,82-90. https://doi.org/10.1016/j.aquaculture.2018.07.034
Da Costa, F., Robert, R., Quéré, C. and Wikfors, G., Soudant, P., 2015. Essential Fatty acid assimilation and synthesis in larvae of the bivalve Crassostrea gigas. Lipids, 50(5), 503-511. https://doi.org/10.1007/BF00004290.
Dhillon, R. S. and Fox, M. G., 2007. Growth-independent effects of temperature on age and size at maturity in Japanese Medaka (Oryzias latipes). Copeia, http://doi.org/10.1643/CI-02-098R1
FAO., 2014. The state of world fisheries and aquaculture p. 223.
Folch, J., Lees, M. and Stanley, G., 1957. A simple method of the isolation and purification of total lipids from animal tissues, Journal of Biological Chemistry, 226, 497- 509. https://doi.org/10.1016/S0021-9258(18)64849-5
FAO’s Fisheries and Aquaculture Department., 2020 Statistical Collections Capture Production and Aquaculture Production Datasets 1950–2019 (Release Date: March 2020). Available online: https://www.fao.org/shery/topic/16073. Accessed 25 March 2020
Flodmark , L. E. W., Vollestad, L. A. and Foresth, T.,2004. Performance of juvenile brown trout exposed to fluctuating water level and temperature. Journal of Fish Biology, 65, 460–470. https://doi.org/10.1111/j.1095-8649.2004.00463.x.
HMSO, U.K., 1994. Nutritional aspects of cardiovascular disease (report on health and social subject’s No. 46), London: HMSO.
Hofer SC, and Watts SA, 2002. Cold tolerance in genetically male tilapia (GMT registered), Oreochromis niloticus.World Aquaculture, 33,19–21
Kanazawa, A., Teshima, S., Sakamoto, M. and Awal, M.A., 1980. Requirement of Tilapia zilii for essential fatty acids. Bulletin of the Japanese Society for the Science of Fish , 46, 1353-1356. https://doi.org/10.2331/suisan.49.1127
Kotronen, A., Laakso, T.S., Westerbacka, J., Kiviluoto, T., Arola, J., Ruskeepää, A.L., Järvinen, H.Y. and Oresic; M., 2010. Comparison of lipid and fatty acid composition of the liver, subcutaneous and intra-abdominal adipose tissue, and serum, Obesity, 18(5), 937–944. https://doi.org/10.1038/oby.2009.326
Kraїem, M.M., Duvernay, J., 1981. Comparaison des températures limites de nage chez deux populations d’Ombres Communs Thymalus thymalus (L.), d’origine différente (Bavière et Scandinavie), Cybium, 5 (3), 45–49.
Li, S., Li, C., Madan,D., Florabelle,G. and Rex. D., 2002. Cold tolerance of three strains of Nile tilapia, Oreochromis niloticus, in China. Aquaculture, 213(1–4),123-129.
Li, E., Lim, C., Klesius, P.H. and Welker, T.L., 2013. Growth, body fatty acid composition, immune response, and resistance to Streptococcus iniae of hybrid tilapia, Oreochromis niloticus × Oreochromis Aureus, fed diets containing various levels of linoleic and linolenic acids. Journal of World Aquaculture Society, 44, 42–55. https://doi.org/10.1111/jwas.12014.
Lim, C., Yildirim-Aksoy, M. and Klesius, P. 2011. Lipid and fatty acid requirements of tilapias, North American Journal of aquaculture, 73, 188–193. https://doi.org/10.1080/15222055.2011.579032
Lu, D.L., Ma, Q., Wang, J., Li, L.Y., Han, S. L., Limbu, S.M., Li, D.L., Chen, L.Q., Zhang, M.L. and Du, Z.Y., 2019. Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy. Journal of Physiology, 597(6), 1585–1603. https://doi.org/10.1113/JP277091
Marques, A., Teixeira, B., Barrento, S., Anacleto, P., Carvalho, M.L .and Nunes, M.L., 2010. Chemical composition of Atlantic spider crab Maja brachydactyla: human health implication. Journal of Food Composition and Analysis, 23, 230-237.
Montero, D., Robaina, L., Caballero, M.J., Ginés, R. and Izquierdo, M.S., 2005. Growth, feed utilization and flesh quality of European sea bass (Dicentrarchus labrax) fed diets containing vegetable oils: a time-course study on the effect of a re-feeding period with a 100% fish oil diet, Aquaculture, 248, 121-134.
National Research Council (NRC)., 2011. Nutrient Requirement of Fish. Committee on Animal Nutrition, Board on Agriculture,( National Academic Press. Washington, DC), pp, 102–134.
Nemova, N.N., Fokina, N.N., Nefedova, Z.A., Ruokolainen, T.R. and Bakhmet, I.N., 2013. Modifications of gill lipid composition in littoral and cultured blue mussels Mytilus edulis L. under the influence of ambient salinity, Polar Record, 49 (250), 272–277. https://doi.org/10.1017/S0032247412000629
Ng, W.K., Lim, P.K. and Sidek, H., 2001. The influence of a dietary lipid source on growth, muscle fatty acid composition and erythrocyte osmotic fragility of hybrid tilapia, Fish Physiology Biochemistry, 25, 301-310. https://doi.org/10.1023/A:1023271901111
Pandit, N. P. and Nakamura, M., 2010. Effect of High Temperature on Survival, Growth and Feed Conversion Ratio of Nile Tilapia, Oreochromis Niloticus, Our Nature, 8, 219-224. https://doi.org/10.3126/on.v8i1.4331
Rioux, V., Catheline, D., Bouriel, M., Legrand, P., 2005. Dietary myristic acid at physiologically relevant levels increases the tissue content of C20:5 n-3 and C20:3 n-6 in the rat. Reproduction Nutrition and Developpement, 45, 599-612. https://doi.org/10.1051/rnd:2005048
Rabei, A., Hichami, A., Beldi, H., Bellenger, S., Khan, N.A. and. Soltani,.N., 2018. Fatty acid composition, enzyme activities and metallothioneins in Donax trunculus (Mollusca, Bivalvia) from polluted and reference sites in the Gulf of Annaba (Algeria): pattern of recovery during transplantation. Environmental Pollution, 237, 900–907. https://doi.org/10.1016/j.envpol.2018.01.041
Senadheera, S.D., Turchini, G.M., Thanuthong. and T., Francis, D.S., 2011. Effects of dietary α-linolenic acid (18:3n-3)/linoleic acid (18:2n-6) ratio on fatty acid metabolism in Murray cod (Maccullochella peelii peelii). Journal of Agriculture Food Chemistry, 59, 1020-1030. https://doi.org/10.1021/jf104242y
Sigurd, O.H. and Sigurd O.S., 2008.The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture, 283 (1-4), 36-42. https://doi.org/10.1016/j.aquaculture.2008.06.042
Stickney, R.R. and McGeachin, R.B. 1983. Responses of Tilapia aurea to Semipurified Diets of Differing Fatty Acid Composition. In: L. Fishelson and Z. Yaron (Eds.), Proceedings of the International Symposium on Tilapia in Aquaculture, (Tel Aviv University Press,Tel Aviv, Israel), 346–355.
Takeuchi, Y., Yahag, N., Izumida, Y., Nishi, M., Kubota, M., Teraoka, Y., Yamamoto,T., Matsuzaka, T., Nakagawa, Y., Sekiya,M., Iizuka, Y., Ohashi, K., Yamada N., Kadowaki, T. and Shimano, H., 2010. Polyunsaturated fatty acids selectively suppress sterol regulatory element-binding protein-1 through proteolytic processing and autoloop regulatory circuit, Journal of Biology Chemistry, 285, 11681-11691
Teoh, C.Y., Turchini, G. M. and Ng, W.K., 2011. Genetically improved farmed Nile tilapia and red hybrid tilapia showed differences in fatty acid metabolism when fed diets with added fish oil or a vegetable oil blend, Aquaculture, 312, 126-136. https://doi.org/10.1016/j.aquaculture.2010.12.018
Tocher, D.R., 2010. Fatty acid requirements in ontogeny of marine and freshwater fish, Aquaculture Research, 41, 717-732. https://doi.org/10.1111/j.1365-2109.2008.02150.x.
Author information
Authors and Affiliations
Contributions
IC (investigation; visualization; writing—original draft; writing—review and editing); FG (investigation; visualization; writing—original draft; writing—review and editing); SB (investigation); SH (formal analysis); MEC (resources, funding acquisition); MSA (conceptualization, methodology, supervision).
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chetoui, I., Ghribi, F., Bejaoui, S. et al. Incorporation of ω3 fatty acids in the diets of Nile tilapia juvenile (Oreochromis niloticus L.): effects on growth performance, fatty acid composition, and tolerance to low temperature. Trop Anim Health Prod 54, 401 (2022). https://doi.org/10.1007/s11250-022-03394-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03394-2