Abstract
The aim of this study was to evaluate the effects of increasing dietary arachidonic acid (ARA) levels (from 1 to 6% of total fatty acids) on European sea bass (Dicentrarchus labrax) juveniles’ growth performance, tissue fatty acid profile, liver morphology as well as long-chain polyunsaturated fatty acids (LC-PUFA) biosynthesis, triglyceride and cholesterol synthesis and lipid transport. A diet with total fish oil (FO) replacement and defatted fish meal (FM) containing a 0.1-g ARA g−1 diet was added to the experimental design as a negative control diet. Dietary ARA inclusion levels below 0.2 g ARA g−1 diet significantly worsened growth even only 30 days after the start of the feeding trial, whereas dietary ARA had no effect on fish survival. Liver, muscle and whole body fatty acid profile mainly reflected dietary contents and ARA content increased accordingly with ARA dietary levels. Tissue eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) levels were positively correlated among them. Hepatic lipid vacuolization increased with reduced dietary ARA levels. Expressions of fatty acyl desaturase 2 and 3-hydroxy-3-methylglutaryl-coenzyme genes were upregulated in fish fed the negative control diet compared to the rest of the dietary treatments denoting the influence of ARA on lipid metabolism. Results obtained highlight the need to include adequate n-6 levels and not only n-3 LC-PUFA levels in European sea bass diets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary long-chain polyunsaturated fatty acids (LC-PUFA) are necessary for fish growth and development, as well as for an efficient functioning of fish lipid metabolism and immune system (Higgs and Dong 2000; Kiron et al. 2011; Tian et al. 2014). However, during the last decades, a plethora of studies has focused on the effects of n-3 LC-PUFA deposition in fish (Izquierdo and Koven 2011; Morais et al. 2011; Betancor et al. 2015a, b, 2016a, b) and limited attention has been paid to the effects that low levels of n-6 LC PUFA, in particular arachidonic acid (ARA; 20:4n-6), can have on teleost fish species (Montero and Izquierdo 2010; Izquierdo and Koven 2011). Furthermore, the optimum ARA inclusion levels have not been estimated for juveniles of marine fish as most of the requirement studies have been performed on larvae (Bessonart et al. 1999; Atalah et al. 2011; Yuan et al. 2015).
ARA is an essential fatty acid for fish as appropriate levels of this FA are required to achieve proper growth performance, development and survival (Carrier et al. 2011; Boglino et al. 2014; Lund et al. 2007, 2010; Rombenso et al. 2016), lipid metabolism (Martins et al. 2012; Norambuena et al. 2012), reproduction (Furuita et al. 2003; Norambuena et al. 2012, 2013), stress resistance (Koven et al. 2001, 2003; Rezek et al. 2010), immune system function (for review see Montero and Izquierdo 2010) as well as resistance to disease (Xu et al. 2010). It also is the main precursor for the synthesis of two-series prostaglandins (Smith and Murphy 2002) and is known to affect signalling pathways and membrane-associated enzyme activities by modifying the fatty acid profile of cell membranes (Waagbø 2006; Calder 2008). Furthermore, not only the individual amounts of ARA have an effect on fish, but an interaction also exists between this and the n-3 LC-PUFA eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3). In this sense, a recent study in Atlantic salmon (Salmo salar) showed that combined dietary inclusion of ARA and EPA improved fish performance and changed tissue fatty acid composition (Norambuena et al. 2016). On the other hand, a high EPA/ARA ratio caused reduced growth in gilthead sea bream (Sparus aurata) juveniles fed feeds containing a de novo oil derived from a GM-oilseed crop (Betancor et al. 2016b). This fact is even more obvious in carnivorous fish species given that these have limited availability to biosynthesize n-3 and n-6 LC-PUFA from their precursors in the feeds by the action of fatty acid desaturases and elongases (Mourente et al. 2005). Additionally, the marginal deficiency of essential fatty acids can lead to health-related problems in farmed fish including alterations on stress resistance (Montero et al. 2015), gut integrity (Caballero et al. 2002; Torrecillas et al. 2016) or lipid metabolism (Caballero et al. 2006). Indeed, ARA is known to modify metabolic functions in fish by altering the expression of genes belonging to several pathways (Betancor et al. 2014; Salini et al. 2016) and thus is worthy to evaluate the effect that increasing ARA levels can have on genes involved in LC-PUFA biosynthesis, lipid transport and synthesis as well as cholesterol metabolism.
Thus, the present study aimed to examine the effect of graded levels of dietary supplementation of ARA-rich oil on fish growth, muscle, liver and whole body fatty acid distribution, liver morphology and lipid metabolism in European sea bass (Dicentrarchus labrax) as well as to evaluate the potential interactions with either EPA or DHA.
Materials and methods
Diets
Five isolipidic and isoproteic experimental dry pelleted diets were prepared to contain graded levels of arachidonic acid (g fatty acid g−1 diet (dry weight)) as follows: 0.1 (ARA0.1; considered as negative control; C-), 0.2 (ARA0.2), 0.4 (ARA0.4), 0.8 (ARA0.8) and 1.4 (ARA1.4). The desired ARA content was completed with commercially available ARA oil obtained from Mortierella alpina (Vevodar®, DSM Food Specialties, the Netherlands). Supplementation of DHA and EPA was done using DHA50 and EPA50 (CRODA, East Yorkshire, UK). Diet ingredients, fatty acid profile and proximate composition are detailed in Tables 1 and 2.
Experimental conditions
Eight hundred and forty European sea bass juveniles were maintained in stocking tanks and fed a commercial diet for 4 weeks before starting the feeding trial. Afterwards, with an initial weight and length of 13.4 ± 0.3 g and 9.9 ± 0.1 cm (mean ± SD), animals were randomly allocated in 15 indoor cylindroconical 200 L tanks (56 fish/tank; 3.7 kg m−3 initial stocking density). Tanks were supplied with filtered sea water at a temperature of 22.8–24.9 °C in a flow-through system and natural photoperiod (12L/12D). Water dissolved oxygen ranged between 7.3 and 8.1 ppm. Fish were manually fed until apparent satiation with one of the five experimental diets for 70 days (three times a day, 6 days a week). Each dietary treatment was assayed in triplicate.
Sampling was performed after 30 and 70 days of feeding. The whole fish population was individually sampled for final weight and length after 24 h of starvation. At the end of the feeding trial, eight fish per tank were sampled for somatic indexes calculation and livers and fillets pooled and used for lipid content and fatty acid analyses. Besides, three pools of five fish at the beginning of the experiment and five fish per tank at the end of the feeding trial were sampled for whole body nutrient retention calculations. Five fish per tank were sampled individually for liver morphological analyses. Additionally, livers of five fish per tank were taken and quickly kept in RNA later and frozen at − 80 °C until gene expression analysis. The animal experiments described comply with the guidelines of the European Union Council (86/609/EU) and Spanish legislation (RD 1201/2005) for the use of laboratory animals and have been approved by the Bioethical Committee of the University of Las Palmas de Gran Canaria.
Proximate composition and fatty acid analyses
Feeds, whole body and tissue proximate composition analyses were conducted following standard procedures (AOAC 2000). Crude protein content (N × 6.25) was determined by Kjeldahl method, and crude lipid was analysed as described by Folch et al. (1957). Moisture was determined by thermal dehydration to constant weight at 110 °C. Ash content was determined by combustion at 600 °C for 12 h. Fatty acid methyl ester profiles were prepared by transmethylation (Christie 2003) and separated by gas chromatography (GC-14A, Shimadzu, Japan) following the conditions described by Izquierdo et al. (1992). Fatty acid methyl ester quantification was performed by a flame ionizator detector and identification by comparison with external and well-characterized fish oil standards (EPA 28, Nippai, Ltd. Tokyo, Japan). All analyses were conducted in triplicate.
Liver morphological studies
Individual liver samples were fixed in 4% neutral-buffered formalin, embedded in paraffin and stained with haematoxylin and eosin (H&E) for optical examination (Martoja and Martoja-Pearson 1970). Liver steatosis level was evaluated by two scientists unaware of the dietary treatments based on hepatocyte vacuolization level and nuclei displacement and alignment around sinusoidal spaces grade. Micrographs were taken at a final magnification of × 40 using an Olympus Cx41 microscope and an Olympus XC50 camera.
RNA extraction and real-time PCR analysis
Total RNA of fish liver was extracted using TRI reagent (Sigma-Aldrich, Saint Louis, MO, USA), quantified by measuring absorbance at 260 nm in a spectrophotometer (Nanodrop 1000, Thermo Fisher Scientific Inc., USA). The RNA integrity was verified by Gel Red™ staining (Biotium Inc., Hayward, CA) on a 1% agarose gel. The reverse transcription (RT) reactions were performed using the iScript™ complementary DNA (cDNA) Synthesis Kit (Bio-Rad Hercules, CA) in 20 μl final volume containing 1 μg of total RNA. fads2, fabp7, hmgrc and gck gene expressions were analysed by real-time PCR, using elongation factor-1 alpha (ef-1a) as housekeeping under the conditions described by Geay et al. (2011). Specific primers used and annealing temperatures (°C) are indicated in Table 3. The PCR program consisted of an initial DNA denaturation of 94 °C for 90 s, followed by 45 cycles at 95 °C for 30 s and 60 °C for 60 s. All PCR reactions were carried out in a I-cycler with optical module (Bio-Rad Hercules, CA, USA) in a final volume of 20 μl, containing 10 μl Brilliant SYBR Green QPCR Master Mix (Bio-Rad Hercules, CA, USA), 0.6 μl of each primer (10 mM) and 6.6 μl of cDNA (1:10 dilution).
Each run was ended with a melting curve analysis resulting in a melting peak profile specific for the amplified target DNA. All reactions were performed in duplicate for each template cDNA. Blank control reactions replaced cDNA with water. Relative gene expression was estimated by the Δ-Δ method (Livak and Schimittgen 2001) in relation to fish fed ARA0.2 diet.
Statistical analysis
All data were tested for normality and homogeneity of variance. Statistical analyses followed the methods described by Sokal and Rolf (1995). The significant level for all analyses was set at 5%, and results are presented as means and standard deviations (SD), with exception of gene expression data that is presented as means and standard error (SE). If necessary, transformation of the data was performed. Data was submitted to a one-way analysis of variance (ANOVA). Two different statistical analyses were performed for all the parameters evaluated, including and excluding the negative control diet (ARA0.1). When F values showed significance, individual means were compared using post hoc tests for multiple means comparison. When variances were not homogeneous, non-parametric tests were applied. All analyses were performed using SPSS 21 software package for Windows (IBM, Chicago, IL, USA).
Results
Biological parameters
On one side, feeding European sea bass with ARA0.1 along the trial (30 and 70 days) clearly reduced (P < 0.05) fish total weight and length as well as specific growth rate (SGR) compared to fish fed higher ARA dietary levels (Table 4); however, all fish presented similar condition factor (K) with exception of fish fed ARA0.1 and ARA0.2 after 70 days of feeding (Table 4). Besides, fish fed ARA0.1 presented lower final perivisceral fat index (PFI) and hepatosomatic index (HSI) than fish fed diets ARA0.2 and ARA0.4, although it was similar to that presented by fish fed diets ARA0.8 and ARA1.4.
On the other hand, for fish fed ARA dietary levels from 0.2 to 1.4 (g fatty acid g−1 diet), no significant differences (P > 0.05) were found for final weight, length, K and SGR. For somatic indexes, fish fed ARA0.2 presented higher viscerosomatic index (VSI), HSI and PFI than fish fed higher ARA levels; however, HSI and PFI were not significantly different (P > 0.05) from fish fed ARA0.4 (Fig. 1). Indeed, VSI (R = − 0.803, n = 12, P = 0.002), HSI (R = − 0.727, n = 12, P = 0.024) and PFI (R = − 0.709, n = 12, P = 0.002) were significantly negatively correlated with dietary ARA content. Fish fed ARA0.2 diet presented the highest (P < 0.05) final VSI compared to fish fed the rest of the dietary treatments (Table 4).
Survival was high along the feeding trial for all the dietary treatments ranging from 93.3 ± 4.6 to 97.6 ± 1.0%, with no significant differences observed (Table 4).
Tissue lipid content and fatty acid profiles
Liver fatty acid profiles
Dietary ARA levels did not significantly affect final liver lipid content (P > 0.05), and liver fatty acid profile mostly mirrored that of the diet.
On one hand, when comparing liver fatty acid profile of fish fed the negative control treatment (ARA0.1) with livers of fish the rest of the dietary treatments, fish fed diet ARA0.1 presented the lowest (P < 0.05) saturated and monoenes and the highest (P < 0.05) Ʃn-3 fatty acid levels (Table 5). No differences (P > 0.05) were found for total liver n-3 LC-PUFA or DHA contents among livers of fish fed diets ARA0.1 up to ARA0.8, regardless of the 50–60% lower dietary intake of total n-3 LC-PUFA for fish fed diet ARA0.1 (Tables 2 and 5). Besides, livers of fish fed ARA0.1 showed a relative reduction of a 50–58% EPA in relation to the lower 70–80% EPA dietary percentage when compared with livers of fish fed the rest of the dietary treatments (Table 5). ARA hepatic content in fish fed ARA0.1 was similar to that presented by fish fed diet ARA0.2, despite that dietary content was relatively lower in a 45%. Certainly, 20:3n-6 and 18:4n-3 levels in liver of fish fed ARA0.1 were similar to those presented by fish fed ARA0.2 diet, regardless of the lower dietary content (Tables 2 and 5). Liver n-3/n-6 ratio tends to decrease as ARA liver content increases, presenting fish fed ARA1.4 diet the lowest (P < 0.05).
On the other hand, when comparing among profiles of hepatic fatty acid from fish fed ARA0.2 up to ARA1.4, fish fed ARA0.2 presented higher (P < 0.05) monoenes and lower (P < 0.05) Ʃn-6 fatty acids content than fish fed diet ARA0.8 and ARA1.4 diets, but not significantly different (P > 0.05) from those presented by fish fed ARA0.4 diet (Table 5). No differences in saturated, Ʃn-3, n-3 LC-PUFA, EPA, docosapentaenoic acid (DPA) or DHA fatty acids were detected. However, dietary ARA amount was positively correlated with liver n-3 LC-PUFA (R = 0.72, n = 12, P = 0.009), liver DHA (R = 0.77, n = 12, P = 0.003) and liver DPA (R = 0.58, n = 12, P = 0.047) contents. Moreover, liver EPA content was positively correlated with liver DPA (R = 0.97, n = 12, P = 0.001) and DHA (R = 0.88, n = 12, P = 0.001), as well as liver DPA and DHA amounts found (R = 0.95, n = 12, P = 0.001). Ratio n-3/n-6 tended to decrease as ARA dietary content increased, with fish fed ARA1.4 presenting lower (P < 0.05) values than fish fed ARA0.2 diet.
Muscle fatty acid profiles
Dietary ARA levels did not significantly affect muscle lipid content (P > 0.05) after 70 days of feeding and, in general terms, fatty acid profile mirrored that of the diet.
On one hand, when comparing muscle fatty acid profile of fish fed the negative control treatment (ARA0.1) with muscle of fish the rest of the dietary treatments, fish fed diet ARA0.1 presented the lowest (P < 0.05) saturated, monoenes and Ʃn-3 LC-PUFA and highest (P < 0.05) Ʃn-3 levels. Ʃn-6 fatty acid muscle contents of fish fed negative control diet were similar to that presented by fish fed ARA1.4 diet, but higher (P < 0.05) than fish fed the rest of the dietary treatments (Table 6). In contrast to the liver, the muscle of fish fed ARA0.1 diet presented an approximately 25% less Ʃn-3 LC-PUFA content than the muscle of fish fed higher levels of dietary ARA. Indeed, the muscle of fish fed ARA0.1 presented a relative reduction of a 4–15% in DHA and a 50–55% in EPA content in relation to the lower 25–40% DHA and 72–80% EPA dietary percentage (Tables 2 and 6). Muscle ARA content in fish fed diet ARA0.1 was reduced by a 15, 54, 76, and 84% compared to fish fed diets ARA0.2 up to ARA1.4 respectively, although dietary content was relatively lower in a 45, 71, 85, and 91%, respectively (Tables 2 and 6). Actually, fish fed diet ARA0.1 presented higher (P < 0.05) muscle 20:3n-3 content compared to fish fed other dietary ARA percentages, despite similar dietary levels (Table 6). Muscle n-3/n-6 ratio tends to decrease as ARA dietary content increases, presenting fish fed ARA1.4 diet the lowest (P < 0.05).
On the other hand, by comparing dietary ARA levels from 0.2 to 1.4 (g fatty acid g−1 diet), no differences (P > 0.05) among dietary treatments were found for saturates, Ʃn-3 and Ʃn-3 LC-PUFA levels. Muscle of fish fed diet ARA0.2 presented the highest (P < 0.05) monoenes and lowest (P < 0.05) Ʃn-6 fatty acid contents (Table 6). Similarly to liver, muscle EPA content was positively correlated with muscle DPA (R = 0.99, n = 12, P = 0.001) and DHA (R = 0.95, n = 12, P = 0.001), as well as liver DPA and DHA amounts found (R = 0.97, n = 12, P = 0.001). Muscle n-3/n-6 tends to decrease as ARA liver content increases; however, fish fed ARA0.2 and ARA0.4 diets presented similar values (P > 0.05).
Whole body fatty acid profiles
Dietary ARA levels did not significantly affect whole body lipid content (P > 0.05) and whole body fatty acid profile generally reflected that of the diet.
On one hand, by comparing dietary ARA levels from 0.1 to 1.4 g fatty acid g−1 diet, fish fed diet ARA0.1 presented the lowest (P < 0.05) saturated, monoenes and Ʃn-3LC-PUFA and the highest (P < 0.05) Ʃn-3 and Ʃn-6 fatty acids levels (Table 7). Fish fed ARA0.1 and ARA0.2 diets presented similar whole body ARA and DHA contents, despite of a relative lower 45 and 40% dietary percentage, respectively. Indeed, whole body DHA relative content in fish fed ARA0.1 was only a 5–17% lower than DHA percentages in fish fed higher dietary ARA levels, regardless of the lower 25–40% dietary percentage fed. Similarly, whole body composition of fish fed ARA0.1 diet resulted in an EPA relative reduction of approximately a 50% compared to fish fed the rest of the dietary treatments, despite the lower 70–80% EPA dietary content. Whole body n-3/n-6 ratio tends to decrease as ARA dietary content increases, presenting fish fed ARA1.4 diet the lowest (P < 0.05).
On the other hand, excluding negative control treatment, fish fed diet ARA0.2 presented the highest (P < 0.05) monoenes and the lowest Ʃn-6 levels. Indeed, whole body monoenes and Ʃn-3LC-PUFA levels were positively correlated (R = 0.77, n = 12, P = 0.003) among them. No differences (P > 0.05) among dietary treatments were found for Ʃn-3 levels and fish fed diet ARA0.2 presented lower Ʃn-3LC-PUFA level than fish fed diet ARA0.4, but it was not significantly different (P < 0.05) from that presented by fish fed diets ARA0.8 and ARA1.4 (Table 7). Whole body ARA content increased accordingly with ARA dietary levels and similarly to the muscle and the liver, whole body EPA content was positively correlated with muscle DPA (R = 0.94, n = 12, P = 0.001) and DHA (R = 0.78, n = 12, P = 0.003), as well as liver DPA and DHA amounts found (R = 0.84, n = 12, P = 0.001). Whole body n-3/n-6 tended to decrease as ARA dietary content increased; however, fish fed ARA0.2 and ARA0.4 diets presented similar values (P > 0.05) as detected in muscle.
Liver morphology
Qualitative morphological evaluation revealed an effect of dietary ARA level on the liver morphology of European sea bass fed the different dietary treatments. Fish fed the negative control diet presented the highest steatosis level when compared to fish fed diets containing higher ARA dietary levels, which presented a moderate to low steatosis level (Fig. 2). This pattern was more evident in fish fed diets ARA0.4, ARA0.8 and ARA1.4 which, in general terms, presented a more regular-shaped morphology around sinusoidal spaces and a reduction of intracytoplasmic lipid vacuolization resulting in decreased number of hepatocytes with the nuclei displaced to the cellular periphery (Fig. 2b). Fish fed diet ARA0.2 presented an intermediate morphological pattern.
Liver lipid metabolism related gene expression
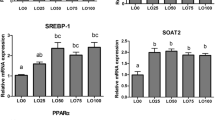
In general terms, an upregulation in the expression of the studied genes could be observed in livers of fish fed ARA0.1 in relation to livers of fish fed the rest of the dietary treatments. Particularly, fish fed diet ARA0.1 presented a significant upregulation (P < 0.05) of hmgcr and fads2 compared to the rest of the dietary treatments (Fig. 3). When comparing fish fed diets ARA0.2 up to ARA1.4, no significant (P > 0.05) differences were observed for the genes studied.
Discussion
Dietary supplementation of ARA from 0.2 up to 1.4 (g fatty acid g−1 diet) did not affect European sea bass growth performance and survival after 70 days of feeding when supplemented in a high FM/FO based diet (n-3 LC-PUFA 4.5–5.7 and n-6 LC-PUFA 0.3–1.6 (g fatty acid g−1 diet). This finding is in agreement with previous studies in juvenile Atlantic salmon fed dietary ARA levels from 0.2 to 0.8 (g fatty acid g−1 diet) for 12 weeks supplemented in low FO diets (Dantagnan et al. 2017), in young gilthead sea bream (S. aurata) fed for 54 days increased levels of dietary ARA (0.03 up to 1.68 (g fatty acid g−1 diet) in 100% VO diet (Fountoulaki et al. 2003) and in gobid (Synechogobius hasta) fed for 8 weeks levels from 0.9 to 6.5 (g fatty acid g−1 diet) in a 48%FM/0%FO based diet (Luo et al. 2012). However, fish fed the negative control diet, which totally replaced FO content and contained defatted FM, presented reduced growth just only 30 days after the start of the feeding trial. Similarly, decreased growth has been observed in European sea bass fed total replacement of dietary FM and FO by plant based ingredients (Geay et al. 2011; Le Boucher et al. 2011, 2013; Torrecillas et al. 2017a, b). Particularly, Torrecillas et al. (2017a) found a clear reduction of weight gain in juvenile European sea bass fed for 90 days a diet without FM and FO (n-3 LC-PUFA 0.14% and n-6 LC-PUFA 0.02 g fatty acid g−1 diet). However, supplementation with LC-PUFA (n-3 LC-PUFA 1.1% and n-6 LC-PUFA 0.16 g fatty acid g−1 diet) led to increased growth (Torrecillas et al. 2017a) denoting the important role of LC-PUFA on fish growth (Izquierdo and Koven 2011). Not only a deficiency but also an excess of dietary LC-PUFA may lead to reduced fish growth performance. For instance, Japanese sea bass (Lateolabrax japonicus) fed ARA dietary supplementation greater than 0.7% g fatty acid g−1 diet in zero FO diets for 12 weeks resulted in reduced growth performance despite containing similar n-3 LC-PUFA percentages (Xu et al. 2010). It is not clear why ARA affects growth in fish, but it has been speculated that ARA can modulate the growth through altering the ratio of prostaglandin E2 (PGE2) and prostaglandin F2a (PGF2a; Palmer 1990) related with alterations on muscle fibre formation and protein degradation (Bell and Sargent 2003). Indeed, it has been suggested that some of the discrepancies reported for dietary ARA effects on fish growth performance may be related to low EPA dietary levels (Norambuena et al. 2016). In this sense, Atlantic salmon fed proportions of ARA/EPA around 1:4.2 significantly increased weight gain when compared to fish fed low or high ratios of ARA and EPA (1:1.5 and 1:9) after 14 weeks of feeding (Norambuena et al. 2016). However, when supplemented for a total of 20 weeks, fish fed the lowest ARA/EPA ratio were similar in weight gain to fish fed 1:4.2 ARA/EPA ratio. In the present trial, no differences were found in growth performance despite the dietary difference in the ARA/EPA ratio among fish fed diet ARA0.2 (1:8.8) and fish fed higher dietary ARA levels; however, the effect of ARA/EPA ratio on European sea bass growth productivity in a long-term feeding study must be evaluated before concluding in relation to this particular subject.
When comparing among ARA-supplemented groups, fish fed ARA0.2 presented higher levels of lipid deposition as denoted by higher PFI, HSI and VSI indexes; meanwhile, there was a reduction in lipid deposition with enhanced levels of dietary ARA. Indeed, there was a strong linear correlation between dietary ARA levels and the indexes (HSI − 0.73, n = 12, P = 0.024; VSI − 0.80, n = 12, P = 0.002; PFI − 0.79, n = 12, P = 0.002) when the feed absent in ARA was exempted from the analysis. Similar results have been observed in other teleost species where increasing levels of dietary ARA levels were negatively correlated with lipid deposition in whole body and individual tissues (Castell et al. 1994; Fountoulaki et al. 2003; Xu et al. 2010; Luo et al. 2012). In fact, it has been suggested that dietary ARA may reduce lipid accumulation and inhibit biosynthesis of fatty acids (Norambuena et al. 2016). In this sense, grass carp (Ctenopharyngodon idellus) fed diets containing intermediate levels of ARA (0.3 g fatty acid g−1 diet) showed higher PFI than fish fed lower or higher ARA levels (0.03 and 0.6 g fatty acid g−1 diet; Tian et al. 2014). Additionally, Japanese sea bass showed a significant decrease in HSI and a trend to reduced VSI with increasing dietary levels of ARA (0.36 up to 2.24 g fatty acid g−1 diet; Xu et al. 2010) in FM/VO supplemented with EFA enriched oil diet, similarly to what was observed in the present trial, with the exemption of fish fed negative control diet, where the reduced HSI could be probably attributed to the nutritional status of the fish. Indeed, the trend to higher accumulation of perivisceral fat and increased VSI and HSI, observed in fish fed ARA0.2, is in concordance with the trend to the greater lipid vacuolization of the cytoplasm observed in relation to fish fed higher dietary ARA levels and lower ARA/EPA ratios.
Generally, the fatty acid profiles of fish tissues reflect those of the diets, being this particularly true in marine fish species with a reduced LC-PUFA biosynthesis activity. In this sense, differences in the fatty acid profile among tissues could indicate physiological relevance of that fatty acid for that particular tissue. In the present study, the incorporation of ARA in tissue total lipids did not vary among the evaluated tissues, ARA increasing when increasing in diet. Previous studies with differing dietary ARA levels showed a selective incorporation into several organs other than liver and muscle, which are of special importance on immune defence, such as the gills, kidney, head kidney leucocytes and plasma (Castell et al. 1994; Betancor et al. 2014: Torrecillas et al. 2017b). The role of ARA on immune system function involves effects on cell membrane fluidity and stability, ion transport, trafficking and vesicular transport, membrane-associated enzymes activities, alterations of gene-encoding proteins involved in lipid metabolism or activation of NADPH oxidase activity (Torrecillas et al. 2017b). Besides, eicosanoids derived from ARA increase vascular permeability and vasodilatation, induce leucocytes chemotaxis and promote generation of reactive oxygen species (ROS) (Calder 2006).
Muscle is a storage tissue and as expected, its fatty acid profile reflected dietary contents in agreement with previous studies (Izquierdo et al. 2003; Montero et al. 2005; Torrecillas et al. 2016; Betancor et al. 2016b), although levels of some fatty acids such as EPA, DHA and ARA were found in higher proportion in fish flesh than in the feeds for fish fed the negative control diet. Indeed, ARA and DHA contents in flesh and whole body for fish fed diets ARA0.1 and ARA0.2 were similar regardless of the different dietary contents, despite that ARA is a minor component of membranes in fish compared to EPA and DHA and is mainly stored in polar lipids (Tocher 2010; Norambuena et al. 2016). Besides, EPA content was positively correlated with muscle and whole body DPA and DHA content when negative control was exempted from the analysis. Altogether, it may be indicating not only a selective accumulation of DHA but also pointing to a functional n-3 LC-PUFA biosynthesis towards DHA.
The liver is considered an important site for LC-PUFA synthesis and lipid metabolism in fish (Monroig et al. 2010) and is expected that differences in feed fatty acid profile may elicit changes in this tissue. For fish fed reduced levels of n-3 LC-PUFA in combination with increased levels of C18 fatty acids (negative control diet), the evident liver morphological pattern alteration observed is in agreement with previous studies in this (Torrecillas et al. 2016, 2017a, b) and other Mediterranean fish species (Caballero et al. 2003). Despite of seeming to indicate that the negative control diet did not fulfil European sea bass nutritional requirements for a proper growth performance with consequences in liver health status, signs of a selective accumulation of DHA and an active n-3 LC-PUFA biosynthesis could be observed in their liver as indicated by the lack of differences in the DHA contents among fish fed diets ARA0.1 up to ARA 0.8 as well as enhanced liver contents of the intermediate product 18:4n-3, which may indicate an certain activity of elongases and desaturases involved in LC-PUFA biosynthesis. Indeed, when negative control diet was exempted from the analysis, n-3LC-PUFA, DPA and DHA liver contents were positively correlated with dietary ARA and liver DPA and liver EPA was positively correlated with liver DPA and DHA. Additionally, increased expression of fads2 was significantly higher in ARA0.1-fed fish particularly when compared to fish fed ARA0.8. In order to achieve isolipidic feeds, high levels of VO were used in the formulation of ARA0.1, which consequently generated high levels of C18 fatty acids in the feeds and reduced levels of not only ARA but also EPA and DHA. This is the reason why it was expectable to find an upregulation in the expression of fads2 in fish fed the lowest LC-PUFA levels as observed in previous studies substituting FO by VO blends in European sea bass (González-Rovira et al. 2009; Geay et al. 2010; Castro et al. 2015). As fads2 also participates in the desaturation of 18:2n-6 to 18:3n-6 (Monroig et al. 2010), enhanced levels of this intermediate product were also found in the liver of fish fed ARA0.1 feeds compared to dietary content, indicating additional biosynthesis towards n-6 LC-PUFA. Indeed, liver ARA content was positively correlated with liver 18:3n-6 (R = 0.98, n = 16; P < 0.001). The reason why levels of ARA differed greatly in the liver among fish fed the different dietary treatments whereas DHA levels were the same could be due to higher preference of fads2 towards 18:3n-3 rather than 18:2n-6 as has been proved in vitro (14.5 versus 5.6%; González-Rovira et al. 2009). It must be noted though that the European sea bass, as a carnivorous marine fish, has a marine fish pattern in the metabolism of LC-PUFA meaning that it has limited capacity to convert C18 fatty acids to ARA, EPA and DHA (González-Rovira et al. 2009). Thus, the small rates of LC-PUFA produced are not enough to enhance whole body or flesh levels of DHA, EPA or ARA. Another lipid metabolism related gene that was upregulated in fish fed negative control diet was hmgcr, the rate-limiting enzyme in cholesterol biosynthesis (Le Martelot et al. 2009). Upregulation in genes belonging to the cholesterol pathway has been previously described in fish fed more sustainable feeds (Geay et al. 2011; Betancor et al. 2015a), particularly those containing high levels of VO as this is naturally low in cholesterol and can contain cholesterol-lowering phytosterols (Gilman et al. 2003). Thus, the enhancement in the expression of hmgcr in fish fed ARA0.1, low in dietary n-3 and n-6 PUFA content and with high percentages 18:2n-6 and 18:3n-3 as consequence of not contain FO may correspond to a compensatory mechanism in order to produce the cholesterol levels necessary to fulfil fish requirements rather than a direct effect of the lack of ARA. These findings are in agreement with previous studies in Atlantic salmon, in which FO replacement by VO elicits not only liver compositional changes but also metabolic alterations in HUFA and cholesterol biosynthetic pathways in order to compensate for deficiencies in the diet, being fads2, srebp-1 and srebp-2 especially upregulated (Leaver et al. 2008; Morais et al. 2011).
The expression of fabp7, a gene related to fatty acid transport and uptake, tended to be upregulated in the liver of sea bass fed ARA0.1, although no differences could be observed among the other dietary treatments. In a similar trial where sea bass were fed diets containing high levels of terrestrial ingredients showed an upregulation in this gene (Geay et al. 2011) and sea bream and cod fed plant-based feed showed upregulation in fabp2 when fed on more sustainable feeds (Lilleeng et al. 2007; Betancor et al. 2016b). Additionally, these fish also showed the lowest performance, what could be suggesting that the upregulation in fabp7 expression may be associated with the lower growth of these fish, which in turn could elicit compensatory mechanisms such as fatty acid mobilization. ARA did not show to regulated the hepatic expression of gck, a phosphotransferase enzyme involved in triglycerides and glycerophospholipids synthesis although there was a trend for an upregulation in the liver if fish were fed with the negative control diet. Previous studies in salmonids have also found an upregulation in the expression of gck when fish were fed terrestrial-ingredient diets (Panserat et al. 2009; Morais et al. 2011), and it was hypothesized that it was related to higher lipid biosynthesis in liver. In the present trial, the higher lipid vacuolization degree in liver was observed in fish fed the lower dietary ARA levels and thus seems the result of activation in triglyceride synthesis although not directly elicited by gck as no differences were found in terms of gene expression.
In summary, dietary ARA levels up to a 0.2-g fatty acid g−1 diet seem to be necessary to fulfil juvenile sea bass requirements for this fatty acid and guarantee proper growth. Increasing ARA levels between a 0.2- and a 1.4-g fatty acid g−1 diet did not enhance fish growth or survival, but levels over a 0.2-g fatty acid g−1 diet seemed to inhibit triglyceride biosynthesis as reduced PFI, HSI and VSI were observed. An upregulation in the hepatic expression of fads2 in fish fed the lowest ARA levels (0.1 g fatty acid g−1 diet) seemed to be related to desaturation not only from 18:2n-6 to 18:3n-6 but also from 18:3n-3 to 18:4n-3, as also levels of n-3 LC-PUFA were low in this feed. Additionally, an upregulation in the expression of hmgcr, the rate-limiting enzyme in cholesterol biosynthesis, was observed in the liver of ARA0.1-fed fish what seems to indicate the response of fish to low levels of dietary cholesterol although a direct effect of ARA on the regulation of hmgcr cannot be excluded as has been found in mammals. Results from the present study point out the necessity to include adequate levels of n-6 LC-PUFA in diets for European sea bass juveniles. Therefore, the new alternatives to FO investigated should aim to contain not only adequate levels of n-3 LC-PUFA but also n-6 LC-PUFA.
Abbreviations
- LC-PUFA:
-
Long-chain polyunsaturated fatty acid
- DHA:
-
Docosahexaenoic acid
- ARA:
-
Arachidonic acid
- EPA:
-
Eicosapentaenoic acid
- DPA:
-
Docosapentaenoic acid
- HSI:
-
Hepatosomatic index
- PFI:
-
Perivisceral fat index
- VSI:
-
Viscerosomatic index
- K:
-
Condition factor
- SGR:
-
Specific growth rate
- FCR:
-
Feed conversion ratio
- fads2 :
-
Fatty acid desaturase gene
- gck :
-
Glycerol kinase gene
- hmgrc :
-
3-Hydroxy-3-methylglutaryl-coenzyme A reductase gene
- fabp7 :
-
Fatty acid binding protein gene
References
AOAC (2000) Official methods of analysis. Association of Official Analytical Chemists, Washington, DC
Atalah E, Hernández-Cruz CM, Benítez-Santana T, Ganga R, Roo J, Izquierdo M (2011) Importance of the relative levels of dietary arachidonic acid and eicosapentaenoic acid for culture performance of gilthead seabream (Sparus aurata) larvae. Aquac Res 42:1279–1288. https://doi.org/10.1111/j.1365-2109.2010.02716.x
Bell JG, Sargent JR (2003) Arachidonic acid in aquaculture feeds: current status and future opportunities. Aquaculture 218:491–499. https://doi.org/10.1016/S0044-8486(02)00370-8
Bessonart M, Izquierdo MS, Salhi M, Hernández-Cruz CM, González MM, Fernández-Palacios H (1999) Effect of dietary arachidonic acid levels on growth and survival of gilthead sea bream (Sparus aurata L.) larvae. Aquaculture 179:265–275. https://doi.org/10.1016/S0044-8486(99)00164-7
Betancor MB, Howarth FJE, Glencross BD, Tocher DR (2014) Influence of dietary docosahexaenoic acid in combination with other long-chain polyunsaturated fatty acids on expression of biosynthesis genes and phospholipid fatty acid compositions in tissues of post-smolt Atlantic salmon (Salmo salar). Comp Biochem Physiol 172-173B:74–89. https://doi.org/10.1016/j.cbpb.2014.04.007
Betancor MB, Sprague M, Usher S, Sayanova O, Campbell PJ, Napier JA, Tocher DR (2015a) A nutritionally-enhanced oil from transgenic Camelina sativa effectively replaces fish oil as a source of eicosapentaenoic acid for fish. Sci Rep 5:8104. https://doi.org/10.1038/srep08104
Betancor MB, Sprague M, Sayanova O, Usher S, Campbell PJ, Napier JA, Caballero MJ, Tocher DR (2015b) Evaluation of a high-EPA oil from transgenic Camelina sativa in feeds for Atlantic salmon (Salmo salar L.): effects on tissue fatty acid composition, histology and gene expression. Aquaculture 444:1–12. https://doi.org/10.1016/j.aquaculture.2015.03.020
Betancor MB, Sprague M, Sayanova O, Usher S, Metochis C, Campbell PJ, Napier JA, Tocher DR (2016a) Nutritional evaluation of an EPA-DHA oil from transgenic Camelina sativa in feeds for post-smolt Atlantic salmon (Salmo salar L.) PLoS One 11:e0159934. https://doi.org/10.1371/journal.pone.0159934
Betancor MB, Sprague M, Montero D, Usher S, Sayanova O, Campbell PJ, Napier JA, Caballero MJ, Izquierdo M, Tocher DR (2016b) Replacement of marine fish oil with de novo omega-3 oils from transgenic Camelina sativa in feeds for gilthead sea bream (Sparus aurata L.) Lipids 51:1171–1191. https://doi.org/10.1007/s11745-016-4191-4
Boglino A, Wishkerman A, Darias MJ, de la Iglesia P, Andree KP, Gisbert E, Estévez A (2014) Senegalese sole (Solea senegalensis) metamorphic larvae are more sensitive to pseudo-albinism induced by high dietary arachidonic acid levels than post-metamorphic larvae. Aquaculture 433:276–287. https://doi.org/10.1016/j.aquaculture.2014.06.012
Caballero MJ, Obach A, Rosenlund G, Montero E, Gisvold M, Izquierdo MS (2002) Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 214:253–271. https://doi.org/10.1016/S0044-8486(01)00852-3
Caballero MJ, Gallardo G, Robaina L, Montero D, Fernández A, Izquierdo M (2006) Vegetable lipid sources affect in vitro biosynthesis of triacylglycerols and phospholipids in the intestine of sea bream (Sparus aurata). Br J Nutr 95:448–454. https://doi.org/10.1079/BJN20051529
Caballero MJ, Izquierdo MS, Kjorsvik E, Montero D, Socorro J, Fernández A, Rosenlund G (2003) Morphological aspects of the intestinal cells from gilthead seabream (Sparus aurata) fed diets containing different lipid sources. Aquaculture 225:325-340. https://doi.org/10.1016/S0044-8486(03)00299-0
Calder PC (2006) N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83(6):S1505–S119S
Calder PC (2008) The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fat Acids 79:101–108. https://doi.org/10.1016/j.plefa.2008.09.016
Carrier JK, Watanabe WO, Harel M, Rezek TC, Seaton PJ, Shafer TH (2011) Effects of dietary arachidonic acid on larval performance, fatty acid profiles, stress resistance, and expression of Na+/K+ ATPase mRNA in black sea bass Centropristis striata. Aquaculture 319:111–121. https://doi.org/10.1016/j.aquaculture.2011.06.027
Castell JD, Bell JG, Tocher DR, Sargent JR (1994) Effects of purified diets containing different combinations of arachidonic and docosahexaenoic acid on survival, growth and fatty acid composition of juvenile turbot (Scophthalmus maximus). Aquaculture 128:315–333. https://doi.org/10.1016/0044-8486(94)90320-4
Castro C, Corraze G, Panserat S, Oliva-Teles A (2015) Effects of fish oil replacement by a vegetable oil blend on digestibility, postprandial serum metabolite profile, lipid and glucose metabolism of European sea bass (Dicentrarchus labrax) juveniles. Aquac Nutr 21:592–603. https://doi.org/10.1111/anu.12184
Christie WW (2003) Lipid Analysis, 3rd edn. Oily Press, Bridgwater
Dantagnan P, González K, Hevia M, Betancor MB, Hernández AJ, Borquez A, Montero D (2017) Effect of the arachidonic acid/vitamin E interaction on the immune response of juvenile Atlantic salmon (Salmo salar) challenged against Piscirickettsia salmonis. Aquac Nutr 23:710–720. https://doi.org/10.1111/anu.12438
Folch J, Lees N, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fountoulaki E, Alexis MN, Nengas I, Venou B (2003) Effects of dietary arachidonic acid (20:4n-6) on growth, body composition and tissue fatty acid profile of gilthead sea bream fingerlings (Sparus aurata L.) Aquaculture 225:309–323. https://doi.org/10.1016/S0044-8486(03)00298-9
Furuita H, Yamamoto T, Shima T, Suzuki N, Takeuchi T (2003) Effect of arachidonic acid levels in broodstock diet on larval and egg quality of Japanese flounder Paralichthys olivaceus. Aquaculture 220:725–735. https://doi.org/10.1016/S0044-8486(02)00617-8
Geay F, Santigosa E, Corporeau C, Boudry P, Dreano Y, Corcos L, Bodin N, Vandeputte M, Zambonino-Infante JL, Mazurais D, Cahu CL (2010) Regulation of FADS2 expression and activity in European sea bass (Dicentrarchus labrax) fed a vegetable oil. Comp Biochem Physiol Part B: Biochem Mol Biol 156:237–243. https://doi.org/10.1016/j.cbpb.2010.03.008
Geay F, Ferraresso S, Zambonino-Infante JL, Bargelloni L, Quentel C, Vandeputte M, Kaushik S, Cahu CL, mazurais D (2011) Effects of the total replacement of fish-based diet with plant-based diet on the hepatic transcriptome of two European sea bass (Dicentrarchus labrax) half-sibfamilies showing different growth rates with the plant-based diet. BMC Genomics 12:522. https://doi.org/10.1186/1471-2164-12-522
Gilman CI, Leusch FD, Breckenridge WC, MacLatchy DL (2003) Effects of a phytosterol mixture on male fish plasma lipoprotein fractions and testis P450scc activity. Gen Comp Endocrinol 130:172–184. https://doi.org/10.1016/S0016-6480(02)00590-7
González-Rovira A, Mourent G, Zheng X, Tocher DR, Pendon C (2009) Molecular and functional characterization and expression analysis of a delta-6 fatty acyl desaturase cDNA of European sea bass (Dicentrarchus labrax L.) Aquaculture 298:90–100. https://doi.org/10.1016/j.aquaculture.2009.10.012
Higgs DA, Dong FM (2000) Lipids and fatty acids. The encyclopedia of aquaculture. John Wiley and Sons, New York, pp 476–496
Izquierdo MS, Koven (2011) Lipids. In: Holt J (ed) Larval fish nutrition. Wiley-Blackwell, John Wiley and Sons Publisher, Oxford, pp 47–82
Izquierdo MS, Arakawa T, Takeuchi T, Haroun R, Watanabe T (1992) Effect of n-3 HUFA levels in Artemia on growth of larval Japanese flounder (Paralichthys olivaceus). Aquac Nutr 2:183–191. https://doi.org/10.1016/0044-8486(92)90163-F
Izquierdo MS, Obach A, Arantzamendi L, Montero D, Robaina LE, Rosenlund G (2003) Dietary lipid sources for seabream and sea bass: growth performance, tissue composition and flesh quality. Aquac Nutr 9:397–407. https://doi.org/10.1046/j.1365-2095.2003.00270.x
Kiron V, Thawonsuwan J, Panigrahi A, Scharsack J, Satoh S (2011) Antioxidant and immune defences of rainbow trout (Oncorhynchus mykiss) offered plant oils differing in fatty acid profiles from early stages. Aquac Nutr 17:130–140. https://doi.org/10.1111/j.1365-2095.2009.00715.x
Koven W, Barr Y, Luzky S, Ben-Atia I, Weiss R, Harel M et al (2001) The effect of dietary arachidonic acid (20:4n-6) on growth, survival and resistance to handling stress in gilthead seabream (Sparus aurata) larvae. Aquaculture 193:107–122. https://doi.org/10.1016/S0044-8486(00)00479-8
Koven W, Anholt RV, Lutzsky S, Atia IB, Nixon O, Ron B et al (2003) The effect of dietary arachidonic acid on growth, survival, and cortisol levels in different-age gilthead seabream larvae Sparus auratus exposed to handling or daily salinity change. Aquaculture 228:307–320. https://doi.org/10.1016/S0044-8486(03)00317-X
Le Boucher R, Vandeputte M, Dupont-Nivet M, Quillet E, Mazurais D, Robin J, Vergnet A, Médale F, Kaushik S, Chatain B (2011) A first insight into genotype × diet interactions in European sea bass (Dicentrarchus labrax L. 1756) in the context of plant-based diet use. Aquac Res 42:583–592
Le Boucher R, Vandeputte M, Dupont-Nivet M, Quillet E, Ruelle F, Vergnet A, Kaushik S, Allamellou M, Médale F, Chatain B (2013) Genotype by diet interactions in European sea bass (Dicentrarchus labrax L.): nutritional challenge with totally plant-based diets. J Anim Sci 91:44–56
Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornman, Lo Sasso G, Moschetta A, Schibler U (2009) REV-ERBa participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol 7(9):e1000181. https://doi.org/10.1371/journal.pbio.1000181
Leaver MJ, Villeneuve LAN, Obach A, Jensen L, Bron JE, Tocher DR, Taggart JB (2008) Functional genomics reveals increases in cholesterol biosynthetic genes and highly unsaturated fatty acid biosynthesis after dietary substitution of fish oil with vegetable oils in Atlantic salmon (Salmo salar). BMC Genomics 9:299. https://doi.org/10.1186/1471-2164-9-299
Lilleeng E, Frøystad MK, Vekterud K, Valen EC, Krogdahl Å (2007) Comparison of intestinal gene expression in Atlantic cod (Gadus morhua) fed standard fish meal or soybean meal by means of suppression subtractive hybridization and real-time PCR. Aquaculture 267:269–283. https://doi.org/10.1016/j.aquaculture.2007.01.048
Livak KJ, Schimittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lund I, Steenfeldt SJ, Hansen BW (2007) Effect of dietary ARA, EPA and DHA on the fatty acid composition, survival, growth and pigmentation of larvae of common sole (Solea solea L.) Aquaculture 273:532–544. https://doi.org/10.1016/j.aquaculture.2007.10.047
Lund I, Steenfeldt SJ, Hansen BW (2010) Influence of dietary arachidonic acid combined with light intensity and tank colour on pigmentation of common sole (Solea solea L.) larvae. Aquaculture 308:159–165. https://doi.org/10.1016/j.aquaculture.2010.08.004
Luo Z, Tan XY, Li XD, Yin G-J (2012) Effect of dietary arachidonic acid levels on growth performance, hepatic fatty acid profile, intermediary metabolism and antioxidant responses for juvenile Synechogobius hasta. Aquac Nutr 18:340–348. https://doi.org/10.1111/j.1365-2095.2011.00906.x
Martins DA, Rocha F, Martínez-Rodríguez G, Bell G, Morais S, Castanheira F, Bandarra N, Coutinho J, Yúfera M, Conceição L (2012) Teleost fish larvae adapt to dietary arachidonic acid supply through modulation of the expression of lipid metabolism and stress response genes. Br J Nutr 108:864–874. https://doi.org/10.1017/S0007114511006143
Martoja R, Martoja-Pearson M (1970) Técnicas de Histología Animal. Toray-Masson SA (ed), Barcelona, p350
Monroig O, Zheng X, Morais S, Leaver MJ, Taggart JB, Tocher DR (2010) Multiple genes for functional Δ6 fatty acyl desaturases (Fad) in Atlantic salmon (Salmo salar L.): gene and cDNA characterization, functional expression, tissue distribution and nutritional regulation. Biochim Biophys Acta 1801:1072–1081. https://doi.org/10.1016/j.bbalip.2010.04.007
Montero D, Izquierdo MS (2010) Welfare and health of fish fed vegetable oils as alternative lipid sources to fish oil. In: Turchini G, Ng W, Tocher D (eds) Fish oil replacement and alternative lipid sources in aquaculture feeds. CRC Press, Cambridge, pp 439–485
Montero D, Robaina LE, Caballero MJ, Gin_es R, Izquierdo MS (2005) Growth, feed utilization and flesh quality of European sea bass (Dicentrarchus labrax) fed diets containing vegetable oils. A time-course study on the effects of re-feeding period with a 100% fish oil diet. Aquaculture 248:121–134. https://doi.org/10.1016/j.aquaculture.2005.03.003
Montero D, Terova G, Rimoldi S, Betancor MB, Atalah E, Torrecillas S, Caballero MJ, Zamorano MJ, Izquierdo M (2015) Modulation of the expression of components of the stress response by dietary arachidonic acid in European sea bass (Dicentrarchus labrax) larvae. Lipids 50:1026–1041. https://doi.org/10.1007/s11745-015-4057-1
Morais S, Pratoomyot J, Taggart JB, Bron JE, Guy DR, Bell JG, Tocher DR (2011) Genotype-specific responses in Atlantic salmon (Salmo salar) subject to dietary fish oil replacement by vegetable oil: a liver transcriptomic analysis. BMC Genomics 12:255. https://doi.org/10.1186/1471-2164-12-255
Mourente G, Dick JR, Bell JG, Tocher DR (2005) Effects of partial substitution of dietary fish oil by vegetable oils on desaturation and β-oxidation of [1-14-C] 18:3n-3 (LNA) and [1-14C] 20:5n-3 (EPA) in hepatocytes and enterocytes of European sea bass (Dicentrarchus labrax L.) Aquaculture 248:173–186. https://doi.org/10.1016/j.aquaculture.2005.04.023
Norambuena F, Estévez A, Sánchez-Vázquez FJ, Carazo I, Duncan N (2012) Self-selection of diets with different content of arachidonic acid by Senegalese sole (Solea senegalensis) broodstock. Aquaculture 364:198–205. https://doi.org/10.1016/j.aquaculture.2012.08.016
Norambuena F, Lewis M, Hadid NKA, Hermon K, Donald JA, Turchini GM (2013) Fish oil replacement in current aquaculture feed: is cholesterol a hidden treasure for fish nutrition? PLoS One 8:e81705. https://doi.org/10.1371/journal.pone.0081705
Norambuena F, Rombenso A, Turchini GM (2016) Towards the optimization of Atlantic salmon reared at different water temperatures via the manipulation of dietary ARA/EPA ratio. Aquaculture 450:48–57. https://doi.org/10.1016/j.aquaculture.2015.06.044
Palmer RM (1990) Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fatty Acids 39:95–104. https://doi.org/10.1016/0952-3278(90)90017-F
Panserat S, Hortopan GA, Plagnes-Juan E, Kolditz C, Lansard M, Skiba-Cassy S, Esquerré D, Geurden I, Médale F, Kaushik S, Corraze G (2009) Differential gene expression after total replacement of dietary fish meal and fish oil by plant products in rainbow trout (Oncorhynchus mykiss) liver. Aquaculture 294:123–131. https://doi.org/10.1016/j.aquaculture.2009.05.013
Rezek TC, Watanabe WO, Harel M, Seaton PJ (2010) Effects of dietary docosahexaenoic acid (22:6n-3) and arachidonic acid (20:4n-6) on the growth, survival, stress resistance and fatty acid composition in black sea bass, Centropristis striata (Linnaeus 1758) larvae. Aquac Res 41:1302–1314. https://doi.org/10.1111/j.1365-2109.2009.02418.x
Rombenso AN, Trushenki JT, Jirsa D, Drawbridge M (2016) Docosahexaenoic acid (DHA) and arachidonic acid (ARA) are essential to meet LC-PUFA requirements of juvenile California Yellowtail (Seriola dorsalis). Aquaculture 463:123–134. https://doi.org/10.1016/j.aquaculture.2016.05.004
Salini MJ, Wade NM, Araújo BC, Turchini GM, Glencross BD (2016) Eicosapentaenoic acid, arachidonic acid and eicosanoid metabolism in juvenile Barramundi Lates calcarifer. Lipids 51:973–988. https://doi.org/10.1007/s11745-016-4167-4
Smith WL, Murphy RC (2002) The eicosanoids: cyclooxygenase, lipoxygenase, and epoxygenase pathways. In: Vance DE, Vance JE (Eds) Biochemistry of Lipids, Lipoproetins and Membranes (4th Ed). Elsevier Sicence BV. 36:341–371
Sokal RR, Rolf SJ (1995) Biometry. In: Taylor, Francis (eds) The principles and practice of statistics in biological research, 3rd edn. Freeman, New York, p 419
Tian JJ, Ji H, Oku H, Zhou JS (2014) Effects of dietary arachidonic acid (ARA) on lipid metabolism and health status of juvenile grass carp, Ctenopharyngodon idellus. Aquaculture 430:57–65. https://doi.org/10.1016/j.aquaculture.2014.03.020
Tocher DR (2010) Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac Res 41:717–732. https://doi.org/10.1111/j.1365-2109.2008.02150.x
Torrecillas S, Caballero MJ, Montero D, Sweetman J, Izquierdo MS (2016) Combined effects of dietary mannan oligosaccharides and total fish oil substitution by soybean oil on European sea bass (Dicentrarchus labrax) juvenile diets. Aquac Nutr 22:1079–1090. https://doi.org/10.1111/anu.12322
Torrecillas S, Robaina L, Caballero MJ, Montero D, Calandra G, Mompel D, Karalazos V, Kaushik S, Izquierdo MS (2017a) Combined replacement of fishmeal and fish oil in European sea bass (Dicentrarchus labrax): production performance, tissue composition and liver morphology. Aquaculture 474:101–112. https://doi.org/10.1016/j.aquaculture.2017.03.031.
Torrecillas S, Román L, Rivero-Ramírez F, Caballero MJ, Pascual C, Robaina L, Izquierdo MS, Acosta F, Montero D (2017b) Supplementation of arachidonic acid rich oil in European sea bass juveniles (Dicentrarchus labrax) diets: effects on leucocytes and plasma fatty acid profiles, selected immune parameters and circulating prostaglandins levels. Fish Shellfish Immunol 64:437–445. https://doi.org/10.1016/j.fsi.2017.03.041
Waagbø R (2006) Chapter 13 Feeding and disease resistance in fish. In: Mosenthin R, Zentek J and Żebrowska T (eds) Biology of Growing Animals 4, p 387–415. https://doi.org/10.1016/S1877-1823(09)70100-6
Xu H, Ai Q, Mai K, Xu W, Wang J, Ma H, Zhang W, Wang X, Liufu Z (2010) Effects of dietary arachidonic acid on growth performance, survival, immune response and tissue fatty acid composition of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture 307:75–82. https://doi.org/10.1016/j.aquaculture.2010.07.001
Yuan YH, Li SL, Mai KS, Xu W, Zhang YJ, Ai QH (2015) The effect of dietary arachidonic acid (ARA) on growth performance. Fatty acid composition and expression of ARA metabolism-related genes in larval half-smooth tongue sole (Cynoglossus semilaevis). Br J Nutr 113:1518–1530. https://doi.org/10.1017/S0007114515000781
Funding
This work was funded by the Spanish Ministry of Economy and Competitiveness (MINECO). Project AGL2012-39919 (PROINMUNOIL). Complementarily, it has been financed through a ULPGC postdoctoral fellowship (PICULPGC-2013-CIENCIAS, concept 643.00.06) and a predoctoral fellowship (ULPGC-2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experiments described comply with the guidelines of the European Union Council (86/609/EU) and Spanish legislation (RD 1201/2005) for the use of laboratory animals and have been approved by the Bioethical Committee of the University of Las Palmas de Gran Canaria.
Rights and permissions
About this article
Cite this article
Torrecillas, S., Betancor, M.B., Caballero, M.J. et al. Supplementation of arachidonic acid rich oil in European sea bass juveniles (Dicentrarchus labrax) diets: effects on growth performance, tissue fatty acid profile and lipid metabolism. Fish Physiol Biochem 44, 283–300 (2018). https://doi.org/10.1007/s10695-017-0433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0433-5