Abstract
The concept of exosomes has been progressively changed from the status of cellular trashcans to multitasking organelles involved in many processes, including internalization, transport and transfer of macromolecules such as proteins, lipids and nucleic acids. While underpinning the mechanisms behind neurodegeneration and neuronal loss, exosomes were shown to be involved in carrying pathological misfolded proteins, propagation of β-amyloid protein and hyper-phosphorylated tau proteins across the brain that ultimately leads to the onset of Alzheimer’s disease (AD), the most prevailing multifactorial neurodegenerative disorder. A potential novel therapeutic role of exosomes in AD intervention is suggested by their ability to increase Aβ clearance. This review aims to highlight the important pathological mechanisms as well as therapeutic strategies involving exosomes towards AD prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Alzheimer’s disease (AD) is the common cause of dementia. It is life threatening for elderly population as well as economic and social burden for the families and health-care system [1]. Clinically, AD is a late-onset condition in which subjects, generally above the age of 65 years present themselves with progressive deterioration in cognition including impairment in memory [2, 3]. Besides the two key pathological features, i.e., senile plaques of amyloid- β (Aβ) peptide and neurofibrillary tangles triggered by hyper-phosphorylated tau proteins, cholinergic dysfunctions, vascular, inflammatory and degenerative pathways have been shown to play roles in the etiology of AD [2, 4, 5].
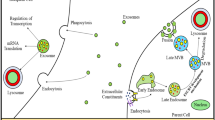
Extracellular vesicles (EVs) represent a heterogeneous group of membrane-bound vesicles containing proteins, nucleic acids, and lipids. Studies suggest that EVs are an essential part of the intercellular communication system and possess critical roles in both physiological and pathological mechanisms [6,7,8]. EVs are categorized into 3 main groups- exosomes, micro-vesicles and apoptotic bodies. Three decades ago, exosomes were first reported in reticulocytes functioning in the disposal of cellular waste components [9]. Exosomes are small lipid bilayer nano-vesicles of 30-100 nm diameters, secreted by almost all cell types, including neurons [10, 11]. The inward budding of endosomes forms multi-vesicular bodies (MVBs) containing intraluminal vesicles (ILVs) [12]. MVBs can either fuse with lysosomes for cargo degradation or with plasma membrane releasing ILVs to the extracellular space as exosomes [13]. Thus, exosomes, depending on their contents, can exert positive or negative effects on a cell upon binding to its cell membrane [14, 15]. This supports the role of exosomes as extracellular transporters- once released they fuse with the membrane of another cell and expel their contents [16]. Exosomes could represent, a good vehicle for drug delivery since they are non-toxic and stable in the circulatory system [17], and may even be able to deliver drugs across the blood brain barrier (BBB) and subsequently into the brain. For example, exosomal siRNA, when intravenously administered to mice, crossed the BBB and entered brain tissue, suggesting a highly potent drug delivery system [18, 19].
Exosomes and Neurodegeneration
Neurodegeneration is a complex biological mechanism often initiated by both hereditary and sporadic factors that trigger dysfunctions of the nervous system. Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD) and Amyotrophic lateral sclerosis (ALS) are the most common neurodegenerative conditions [20]. Their pathogenesis involves the aggregation and propagation of respective misfolded proteins, which are subjected to either refolding or degradation followed by proteolytic clearance [21, 22]. Defects or mutations in the proteolytic clearance system lead to the aggregation of misfolded proteins, whose crosstalk with normal neuronal or glial cells causes them to undergo pathogenesis [23, 24]. The spread of EVs and communication between glial cells and nerves mediated by EVs stay as the main cause of neurodegenerative diseases [25]. Major causative proteins for neurodegenerative diseases are found to be enclosed in CSF-derived EVs [26]. Exosomes derived from neurons, microglia, astrocytes, or oligodendrocytes are known to facilitate synaptic plasticity and tissue regeneration and thereby believed to confer neuroprotection [27]. However, in pathological conditions, exosomes can become active mediators in the progression of neurological diseases, by promoting pathological aggregations and spreading of characteristic misfolded proteins such as β-amyloid and tau proteins in AD, α-synuclein in PD, mutant Huntingtin (mtHtt) in HD and superoxide dismutase (SOD 1) in ALS [28,29,30,31,32] (Fig. 1).
Role of Exosomes in Alzheimer’s Disease
AD is the main cause of dementia and is mounting as a socio-economic hurdle to the health care system around the world, owing to two main facts: the difficulty in early intervention, and lack of therapeutic strategies that reverse or prevent the disease [33, 34]. Pertaining to the group of proteinopathies, AD is characterized by accumulation of Aβ peptide and hyper-phosphorylated tau proteins resulting in neuritic plaques and neurofibrillary tangles respectively, in the memory- associated regions of the brains in AD patients [35]. These proteins aggregate as the disease progresses and evolve as key pathological hallmarks by the later stages of AD [33]. The presence of Aβ peptide and Tau in cerebrospinal fluid and blood of living AD patients expend wide applications in number of clinical studies. However, lack of significant change in both the proteins among healthy and AD patients make them simple but no specific biomarker towards an early asymptomatic stage of AD [34]. This resulted in extended research to identify novel biomarkers. Besides intercellular transmission, cellular communication as well as clearance mediated by exosomes is pivotal in AD [36]. Exosomes represent the leading and the most interesting candidate biomarkers currently under investigation.
Amyloid Pathology and Role of Exosomes
Alzheimer’s disease is the most prominent neurological disorder chiefly characterized by progressive deprivation in cognition including memory [33, 37]. An in vitro study suggested a role of exosomes in propagation of Aβ proteins to extracellular space via exosomes, after the cleavage from amyloid precursor protein (APP) in early endosomes. Excessive accumulation of Alix, a specific marker for exosomes, was found within amyloid plaques of AD brain sections suggesting a role of exosomes in the pathogenesis of AD. The brain of a healthy control was observed negative for plaques as well as Alix [38]. APP undergoes enzymatic cleavage by β and γ secretases to generate Aβ peptide, which is released into the extracellular space. Because its clearance from the brain slows with aging, Aβ peptide gradualy accumulates and ultimately results in the characteristic amyloid plaque formation observed in AD patients [39]. The Aβ1-42 peptide is found predominantly in these plaques. Aβ protein generation and degradation can be controlled by endosome/lysosome activity, but dysfunction of this system elicits early symptoms associated with AD-related neuropathology [40]. Studies have also shown the presence of APP C-terminal fragment in exosomes in cell lines that overexpress APP [41, 42]. Exosomes are involved in the transport of full-length amyloid precursor protein (flAPP), APP metabolites and enzymes cleaving flAPP and C-terminal fragment of APP (APP-CTF) to extracellular space in neuronal cell cultures [41]. More specifically Gonzales et al. (2012) confirmed that amount of flAPP and APP-CTFs secreted out of neuronal cells by exosomes are directly proportional to their amount in the brain. Also brain exosomes have high levels of APP CTFs when compared to flAPP [43]. Moreover, exosomes lacking the tetraspanin protein CD63 with APP and its C-terminal fragments indicate that exosomes secreted from the neuroblastoma cells differ in their cargoes as well as target cell types [44].
Exosomes in the brains of patients with AD possess high quantity of Aβ-oligomers, which act as neuron-neuron transfer vectors. This reinforces the correlation between exosomal neuron-to-neuron transfer of intercellular Aβ peptides and progressive extracellular accumulation of Aβ peptides (Fig. 2). Conversely, it was observed that down regulation of TSG101 (Tumor susceptibility gene 101) and VPS4A (Vacuolar protein sorting –associated protein 4A), proteins responsible for the formation and secretion of exosomes respectively, resulted in inhibition of formation of exosomes. As a result, transfer of Aβ-oligomer gets completely blocked among neurons and the associated toxicity was reduced. These findings strongly demonstrate a central role for exosomes in the etiology of AD [45].
Biogenesis (a) and role of exosomes in AD (b). Early endosomes enclosing cytoplasmic cargo convert into multivesicular bodies (MVBs) filled with many intraluminal vesicles (ILVs). MVBs mature into late MVBs which either undergo lysosomal degradation or fuse with plasma membrane and ILVs as exosomes. MVB associated Aβ peptide formed from amyloid precursor protein (APP) in a neuron enter the adjacent neuron via exosomes and starts propagating there. On the other hand, exosomes involved in transfer of hyper-phosphorylated Tau (p-Tau) from microglia to neurons. Conjointly, role of exosomes is highlighted in dissemination of key pathological protein aggregates across the neurons. (References:- Lee JY and Kim HS, 2017 [27]; Sarko DK and McKinney CE, 2017 [65]
Exosomes on Tau Pathology
Tau is a microtubule associated protein encoded by MAPT (Microtubule-associated protein Tau) gene and is strongly involved in regulation of cytoskeleton. Neurofibrillary tangles (NFTs) elicited from the aggregates of excessively phosphorylated tau considered to be one of the major neuropathological lesions in AD and other tauopathies [2]. According to previous studies blood and CSF samples of AD patients revealed the presence of exosomes associated with phosphorylated tau [46]. Exosome-mediated trans-synaptic transmission of tau through extracellular space suggests a progressive spreading of tauopathy in AD patients’ brain [47]. A recent study indicated that microglia spread mutant tau in brain via their exosomes and inhibition of exosomal secretory pathways and/or depletion of microglia reduce the tau propagation under in vitro and in vivo conditions. Human CSF was also identified for exosome associated tau phosphorylation at Thr-181 [48]. Winston et al. (2016) reported that plasma neuronal derived exosomes (NDEs) with p-tau leads to the generation of highly pathogenic tau aggregation. Brains of normal mice were marked with induced tau pathology when injected with plasma NDEs from patients with Mild Cognitive Impairment (MCI) and MCI converting to AD (ADC). Mice injected with plasma NDEs from ADC patients displayed high concentrations of p-tau suggest the role of tau itself as the spreading agents. The study also points that high expression of p-tau and Aβ1-42 can be useful for predicting the conversion of MCI to AD [49]. Crotti et al. (2019) showed that Bridging Integrator 1 (BiN1) contribute Tau pathology in AD by altering Tau clearance and promote the release of Tau- enriched extracellular vesicles by microglia [50].
Apart from associating with the signatures of AD- Aβ-protein and tau- exosomes could be one of the mediators of neuroinflammation and oxidative stress accompanied with AD. Exosomes being the cargo of inflammatory molecules, they serve as key players in exchanging inflammatory agents between glia and neurons and thereby enhance the neuroinflammation [51]. At the same time, it was reported that MVB release of exosome could be accelerated by oxidative stress, supported by the fact that exosomes released by MVBs are believed to be associated with diseases like, cancer, cerebral ischemia, cardiovascular diseases, multiple sclerosis etc. [52].
Exosomes as Diagnostic and Therapeutic Tools for Alzheimer’s Disease
Brain diseases are generally strenuous to diagnose as well as to treat. Until now, none of the treatment practices have been reported to either prevent or reverse the progression of AD. The global dissemination of AD has created an even larger need for detection, prevention, and/or curative strategies. Bioactive herbal and marine compounds may provide useful in therapeutic interventions via a variety of cellular mechanisms. Since exosomes have been identified as a part of spreading or transporting neurodegenerative disease-related proteins, they may furnish appropriate biomarkers for detecting and diagnosing neurological diseases [53].
AD generally progresses through 3 stages- (1) a pre-symptomatic stage- generally an asymptomatic interval between the onset of neuronal dysfunction and appearance of impaired cognition; (2) a prodromal stage with mild cognitive aberrations and finally (3) a symptomatic stage where patients suffer from dementia [54]. Finding new biomarkers may allow the diagnosis of AD at an earlier stage, which is highly demanding for preventing or delaying progression of the disease. Several appreciable approaches in unveiling the possibilities to identify blood-based biomarkers were on board to open up prospective studies. Fiandaca et al. (2015) reported that P-S396-tau, P-T181-tau and Aβ1-42 in extracts of neural-derived blood exosomes predict development of AD up to 10 years before clinical onset [55].
According to Goetzl et al. (2015) early appearance of neuronal lysosomal dysfunctions in living patients with AD resulted from elevated levels of autolysosomal proteins like cathepsin D, lysosome-associated membrane protein 1 (LAMP-1), and ubiquitinylated proteins, in exosomes than controls, suggested that these proteins can be useful biomarkers to detect AD at an early stage [56]. Decreased levels of transcription factors in plasma neural-derived exosomes of AD patients 2–10 years before the clinical diagnosis of AD was also observed [57]. Immunochemical analysis of neurally derived plasma exosomes also showed altered levels of phosphorylated form of insulin receptor proximal signaling protein, insulin receptor substrate (IRS) in AD in contrast to controls [58]. The cargo components in neurally derived exosomes versus other exosomes put an impact on neuropathogenesis and disease progression.
The results of recent studies suggest that presence of Aβ1-42 or tau in the CSF may act as a diagnostic biomarker for the early diagnosis of AD. CSF and plasma exosomes from AD patients have full-length tau, which is absent in healthy people. Therefore, the presence of exosomes present in the CSF may provide a useful biomarker for the diagnosis of AD [59]. Likewise, detection of Aβ1-42 in CSF exosomes could be a sensitive measure for diagnosing AD. The combined detection of tau and Aβ 1–42 in CSF may help in the diagnosis of AD 10 years prior to clinical onset [60]. Researchers refer to exosomes as “brain fluid biopsy” since they can be easily isolated from CSF [61]. In a recent study by Jia et al. (2019) found highest levels of Aβ42, total tau, and pT181-tau in neuronal exosomes of AD patients when compared to patients with amnestic MCI (aMCI) and healthy controls. Interstingly a strong correlation was observed among the level of each exosomal biomarker with the respective CSF biomarker [62].
High levels of neprilysin, an endopeptidase related to degradation of Aβ, were detected in human adipose tissue derived mesenchymal stem cells (ADSCS) when compared to neuronal cells. Exosomal delivery of neprilysin from mesenchymal stem cell derived from adipose tissues provided a therapeutic tool to reduce Aβ accumulation [63]. Other studies also confirm that exosomes may be able to deliver drugs and nucleic acid fragments (siRNA and miRNA) across the BBB [19, 64]. Erivity AL et al. (2011) demonstrated that siRNA delivered by exosomes relieved some symptoms related to AD pathogenesis [19]. Dendritic cell derived exosomes with siRNA were injected into transgenic AD mouse brains through electroporation. The exosomes were able to cross the BBB and siRNA, which reduced the Aβ expression and deposition. The siRNA caused dose-dependent knockdown of mRNA and protein levels for BACE-1 (Beta-secretase 1), a protease leading to the N-cleavage of APP to release Aβ [19]. Delivery of exosomal miR-124a enhanced GLT-1 (Glutamate transporter 1) on astrocytes resulting in improved synaptic activity, which may alleviate neuronal apoptosis in AD [64] (Fig. 3).
Constructive and destructive roles of exosomes in Alzheimer’s disease (Adapted and modified from Alvarez-Erviti L et al., 2011 [19])
Conclusion
Scientific research over the last decade has progressively changed the view of exosomes from cellular trashcans to potential therapeutic delivery vehicles for broad spectrum of diseases since they are known to carry molecular markers of many diseases. Currently exosomes perform a part of the intercellular communication chain that can carry, transport and transfer biomolecules. Exosomes are considered to be the secure and selective vehicles for delivering therapeutic molecules to the brain and stay as an integral part of drug delivery system. Exosomes in neurodegenerative conditions like AD could participate in aggregation, transmission and effective clearance of pathogenic proteins. Exosomes are evolved as attractive candidates for therapeutic drug delivery in neurodegenerative diseases owing to their natural, non-synthetic origin, stability in circulation and low immunogenicity in the host. Yet, further studies are essential to make long-term safe and persistent application of exosomes in target-specific treatment practises to address the broad range of neurodegenerative conditions such as Alzheimer’s disease.
References
Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M (2016) World Alzheimer report 2016- improving healthcare for people living with Dementia. Coverage, quality and costs now and in the future.
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344
Hampel H, Prvulovic D, Teipel S, Jessen F, Luckhaus C et al (2011) The future of Alzheimer’s disease: the next 10 years. Prog Neurobiol 95:718–728
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Jiang L, Dong H, Cao H, Ji X, Luan S, Liu J (2019) Exosomes in pathogenesis, diagnosis, and treatment of Alzheimer’s Disease. Med Sci Monit 25:3329–3335
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles and friends. J Cell Biol 200:373–383
Yamamoto S, Azuma E, Muramatsu M, Hamashima T, Ishii Y, Sasahara M (2016) Significance of extracellular vesicles: pathobiological roles in disease. Cell Struct Funct 41:137–143
Surgucheva I, Shestopalov VI, Surguchov A (2008) Effect of gamma-synuclein silencing on apoptotic pathways in retinal ganglion cells. J Biol Chem 284:36377–36385
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97:329–339
Simpson RJ, Jensen SS, Lim JW (2008) Proteomic profiling of exosomes: current perspectives. Proteomics 8:4083–4099
Kastelowitz N, Yin H (2014) Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. Chem Bio Chem 15:923–928
Vlassov AV, Magdaleno S, Setterquist R, Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820:940–948
Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73:1907–1920
Pegtel DM, Peferoen L, Amor S (2014) Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos Trans R Soc Lond B Biol Sci 369:20130516
Li X, Tsibouklis J, Weng T, Zhang B, Yin G, Feng G et al (2017) Nano carriers for drug transport across the blood-brain barrier. J Drug Target 25:17–28
Samir EL, Andaloussi SL, Imre M, Wood MJA (2013) Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev 65:391–397
Samira L, Wood MJA (2011) Exosome nanotechnology: an emerging paradigm shift in drug delivery. Bioassays 33:737–741
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Ba S (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharm Res 32:2003–2014
Alvarez-Erviti L, Seow Y, Yin H et al (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
Lakshmi S, Prakash P, Essa MM, Qoronfleh WM, Akbar M, Song BJ, Kumar S, Elumalai P (2018) Marine derived bioactive compounds for treatment of Alzheimer’s disease. Front Biosci 10:537–548
Surgucheva I, Newell KL, Burns J, Surguchov A (2014) New alpha- and gamma-synuclein immunopathological lesions in human brain. Acta Neuropathologica Com 2:132
Surgucheva I, He S, Rich MC, Sharma R, Ninkina NN, Stahel PF, Surguchov A (2014) Role of synucleins in traumatic brain injury—an experimental in vitro and in vivo study in mice. Mol Cell Neurosci 63:114–123
Hipp MS, Park SH, Hartl FU (2014) Proteostasis impairment in protein-misfolding and aggregation diseases. Trends Cell Biol 24:506–514
Lopez-Leal R, Court FA (2016) Schwann cell exosomes mediate neuron- glia communication and enhance axonal regeneration. Cell Mol Neurobiol 36:429–436
Liu W, Bai X, Zhang A, Huang J, Xu S, Zhang J (2019) Role of exosomes in central nervous system diseases. Front Mol Neurosci 12:240
Kawahara H, Hanayama R (2018) The role of exosomes/extracellular vesicles in neural signal transduction. Biol Pharm Bull 41:1119–1125
Lee JY, Kim HS (2017) Extracellular vesicles in neurodegenerative diseases: a double-edged sword. Tissue Eng Regen Med 14:667–678
Howitt J, Hill AF (2016) Exosomes in the pathology of neurodegenerative diseases. J Biol Chem 291:26589–26597
Quek C, Hill AF (2017) The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun 483:1178–1186
Wu X, Zheng T, Zhang B (2017) Exosomes in Parkinson’s disease. Neurosci Bull 33:331–338
Grad LI, Yerbury JJ, Turner BJ, Guest WC, Pokrishevsky E, O’neill MA et al (2014) Intercellular propagated misfolding of wild- type Cu/Zn superoxide dismutase occurs via exosome-dependent and independent mechanisms. Proc Natl Acad Sci USA 111:3620–3625
Jeon I, Cicchetti F, Cisbani G, Lee S, Li E, Bae J et al (2016) Human to mouse prion-like propagation of mutant huntingtin protein. Acta Neuropathol 132:577–592
Reitz C, Mayeux R (2014) Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88:640–651
Pluta R, Utamek-Koziot M, Januszewski S, Stanistaw J (2018) Exosomes as possible spread factor and potential biomarkers in Alzheimer’s disease: current concepts. Biomark Med 12:1025–1033
Frozza RL, Lourenco MV, De Felice FG (2018) Challenges for Alzheimer’s disease therapy: insights from novel mechanisms beyond memory defects. Front Neurosci 12:37
Soria FN, Pampliega O, Bourdenx M, Meissner WG, Bezard E, Dehay B (2017) Exosomes, an unmasked culprit in neurodegenerative diseases. Front Neurosci 11:26
Gupta J, Kulshreshtha M (2017) Memory impairment with reference to Alzheimer’s disease: An Update. Int J Nutr Pharmacol Neurol Dis 7:45–53
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P et al (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA 103:11172–11177
Pluta R, Furmaga-Jablonska W, Maciejewski R, Ulamek-Koziol M, Jablonski M (2013) Brain ischemia activates β-and γ-secretase cleavage of amyloid precursor protein: significance in sporadic Alzheimer’s disease. Mol Neurobiol 47:425–434
Lord A, Kalimo H, Eckman C et al (2006) The Arctic Alzheimer mutation facilitates early intraneuronal Abeta aggregation and senile plaque formation in trans- genic mice. Neurobiol Aging 27:67–77
Vingtdeux V, Hamdane M, Loyens A et al (2007) Alkalizing drugs induce accumulation of amyloid precursor protein byproducts in luminal vesicles of multivesicular bodies. J Biol Chem 282:18197–18205
Sharples RA, Vella LJ, Nisbet RM et al (2008) Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J 22:1469–1478
Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E (2012) The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 287:43108–43115
Laulagnier K, Javalet C, Hemming FJ, Chivet M, Lachenal G, Blot B et al (2018) Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci 75:757–773
Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M (2018) Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 136:41–56
Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287:3842–3849
Lee S, Kim W, Li Z, Hall GF (2012) Accumulation of vesicle- associated human tau in distal dendrites drives degeneration and tau secretion in an in situ cellular tauopathy model. Int J Alzheimers Dis 2012:172837
Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T et al (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18:1584–1593
Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D et al (2016) Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement 3:63–72
Crotti A, Sait HR, McAvoy KM, Estrada K, Ergun A, Szak S et al (2019) BIN1 favors the spreading of Tau via extracellular vesicles. Sci Rep 9:9477
Rajendran L, Bali J, Barr MM, Court FA, Kramer-Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breakefield XO (2014) Emerging roles of extracellular vesicles in the nervous system. J Neurosci 34:15482–15489
Cai ZY, Xiao M, Quazi SH, Ke ZY (2018) Exosomes: a novel therapeutic target for Alzheimer's disease? Neural Regen Res 13:930–935
Kim D, Kim YS, Shin DW, Park CS, Kang JH (2016) Harnessing cerebrospinal fluid biomarkers in clinical trials for treating Alzheimer’s and Parkinson’s diseases: potential and challenges. J Clin Neurol 12:381–392
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau CPJ, Delacourte A et al (2007) Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746
Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB et al (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement 11:600–6071
Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Carlson OD, Mustapic M, Kapogiannis D (2015) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85:40–47
Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Carlson OD, Mustapic M, Kapogiannis D (2015) Low neural exosomal levels of cellular survival factors in Alzheimer's disease. Ann Clin Transl Neurol 2:769–773
Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 29:589–596
lsson B, Lautner R, Andreasson U, et al (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol 15:673–684
Clark CM, Xie S, Chittams J et al (2003) Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol 60:1696–1702
Chiasserini D, van Weering JR, Piersma SR, Pham TV, Malekzadeh A, Teunissen CE, de Wit H, Jiménez CR (2014) Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J proteomics 106:191–204
Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J et al (2019) Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement 15:1071–1080
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K et al (2013) Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3:1197
Morel L, Regan M, Higashimori H et al (2013) Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem 288:7105–7116
Sarko DK, McKinny CE (2017) Exosomes: Origin and therapeutic potential of neurodegenerative disease. Front Neurosci 11:82
Acknowledgements
The authors deeply acknowledge Department of Health Research, New Delhi, for the encouragement and financial assistance (No. R.12013/04/2017-HR) towards HRD fellowship- Women Scientist with break in career to SL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakshmi, S., Essa, M.M., Hartman, R.E. et al. Exosomes in Alzheimer’s Disease: Potential Role as Pathological Mediators, Biomarkers and Therapeutic Targets. Neurochem Res 45, 2553–2559 (2020). https://doi.org/10.1007/s11064-020-03111-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03111-1