Abstract
Parkinson’s disease (PD) is characterized by the presence of insoluble protein clusters containing α-synuclein. Impairment of mitochondria, endoplasmic reticulum, autophagy and intracellular trafficking proper function has been suggested to be caused by α-synuclein toxicity, which is also associated with the higher levels of ROS found in the aged brain and in PD. Oxidative stress leads to protein oligomerization and aggregation that impair autophagy and mitochondrial dynamics leading to a vicious cycle of organelles damage and neurodegeneration. In this review we focused on the role of α-synuclein dysfunction as a cellular stressor that impairs mitochondria, endoplasmic reticulum, autophagy and cellular dynamics culminating with dopaminergic depletion and the pathogenesis of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) was initially described by James Parkinson in 1817 [1], it is the second most common age-related neurodegenerative disease, after Alzheimer’s disease, and the most common motor disease [2]. There are, in 2018, approximately 10 million diagnoses of PD worldwide affecting 2% of the population over 60 years of age, with men being 1.5 times more prone to PD than women do [3].
Clinical symptoms of PD include motor dysfunctions such as muscle rigidity, bradykinesia, balance disturbances, and resting tremor, besides non-motor symptoms such as cognitive decline, depression, gastrointestinal symptoms, and olfactory and gustatory deficits in the early stages of the disease, while mood alteration, sleep disturbances, and dementia typically occur in advanced stages of PD [4].
Studies of post-mortem brains from PD patients revealed the presence of cellular inclusions called Lewy bodies, which contain α-synuclein among other proteins. Indeed, since the identification of α-synuclein as the main protein associated with Parkinson’s disease, in late nineties, it has been the major therapeutic target of synucleinopathies [5], although no major conclusive advances were reached. Quantification of α-synuclein levels is also important for PD diagnosis, however precise clinical diagnosis and evaluation progression of PD is still challenging, since the access to α-synuclein levels in cerebrospinal fluid (CSF) and biopsies are not trivial, due to the invasiveness, which stimulated the investigation of blood and saliva, as alternative source of α-synuclein as a biomarker of PD [6].
Accumulation of α-synuclein in both neurons and glia precedes degeneration of dopaminergic (DA) neurons located in the substantia nigra (SN). Therefore, α-synuclein aggregation is considered a hallmark of both sporadic and familial PD being extensively investigated. Alpha-synuclein evokes a global toxicity to cells that is time-dependent and impairs proper function of mitochondria, lysosome and other organelles and compartments. However, molecular mechanisms involved in α-synuclein toxicity upon cells are still a matter of investigation.
The current review presents an overview of the mechanisms of PD pathogenesis related to α-synuclein toxicity, focusing on intracellular impairment and oxidative stress.
Characteristics of α-Synuclein
Alpha-synuclein is a small acidic protein composed of 140 amino acids divided in three domains: the N-terminus, the non-amyloid component (NAC) and the C-terminus. The lipid-binding α-helixes are formed by seven repeats of 11 amino acids containing the conserved KTKEGV hexameric motif. The NAC is the core of α-synuclein and is reported to form fibrils and aggregation. The C-terminus is an acidic unstructured tail that is important to prevent α-synuclein aggregation. Neutralization of the acidic residues or phosphorylation of serine 129 leads to the annulation of the inhibitory effect of C-terminus over NAC that ending up in α-synuclein aggregation [7, 8] (Fig. 1). A recent study reported that the C-terminus also interacts with the membrane of synaptic vesicles depending on calcium concentrations favouring neurotransmitter release [9].
Amino acid sequence of human α-synuclein evidencing the location of point mutations associated with early-onset PD (A30P, E46K, H50Q, G51D and A53T). The α-synuclein sequence is divided in N-terminus that adopts an α-helix secondary structure; non-amyloid beta component (NAC), that is prone to forming β-sheet aggregates; and the C-terminus that is unstructured, negatively charged and inhibits α-synuclein aggregation. Seven imperfect repeats of 11aa (shaded) are predicted to interact with lipid membranes
Alpha-synuclein is a neuronal presynaptic protein critically involved in recycling vesicles at synapses, including their trafficking, docking, and endocytosis at the presynaptic membrane. It is also found in the soma, nucleus, dendrites and axons. In DA neurons specifically, α-synuclein is important for the synthesis, regulation, storage, and release of dopamine; it is a crucial protein for synaptic plasticity [10]. Moreover, α-synuclein interacts with membrane lipids, such as those from mitochondria and endoplasmic reticulum (ER), impacting on dopamine release and calcium homeostasis. It interacts also with structural proteins, like tubulin, playing a role on microtubule dynamics [11, 12].
Excess of α-synuclein interacts with tyrosine hydroxylase (TH) to inhibit dopamine biosynthesis, and with the dopamine transporter (DAT) impairing dopamine uptake and storage [13]. Overexpression of wild type (WT) α-synuclein or expression of the mutant A53T form reduces dopamine release via covalent binding to dopamine and/or modulation of TH; this disrupted dopamine metabolism leads to cell toxicity in part because the increase dopamine accumulation generates intracellular oxidative stress [14]. The A30P mutation of α-synuclein disrupts the N-terminal α-helix and reduces the ability of α-synuclein to interact with lipid membranes. Its toxicity may be related to the increased mobility from cytoplasm to nucleus and its inhibitory effect on chromatin acetylation. On the other hand, the mutation A53T does not affect the affinity of α-synuclein to membranes, but is associated with an increased propensity to aggregation, which may be due to the increased spreading, leading to early-onset PD [7] (Fig. 1).
Spreading of α-Synuclein
Experiments with both A53T, A30P or other mutant forms of α-synuclein reveal that the oligomeric β-sheet-rich secondary structure of α-synuclein is highly toxic and is also crucial to PD pathology [15]. During PD pathogenesis, soluble oligomers with high membrane-binding affinities may spread among neurons and glia [16] through pores formed in the involved cells. Studies on post-mortem brains of patients with PD who received fetal tissue grafts 10–15 years earlier, have found Lewy body-like structures in the graft tissue, suggesting that α-synuclein entered the graft cells from the surrounding host cells, similarly to the mechanisms of prion spreading [17].
Experiments using animal models to study the prion-like properties of α-synuclein indicate that exposure to α-synuclein fibrils stimulated α-synuclein aggregates and synucleinopathy, including concomitant impairment in synaptic function. Holmqvist and collaborators [18] revealed that monomeric, oligomeric, or fibrillar α-synuclein may be transported from the enteric nervous system to the brain via the vagus nerve, via the slow and fast intracellular microtubule transport systems. These findings suggest an intimate relationship between α-synuclein translocation and aggregate formation.
Oligomerization, Aggregation and Degradation of α-Synuclein
Proteins normally fold to form three-dimensional structures in cells. However, incorrect folding may occur, causing misfolding or incomplete folding. These aberrant proteins may be prone to aggregate and spread [19].
Overexpression or anomalous conformation due to point mutations lead to oligomerization of α-synuclein and formation of amyloidogenic filaments [20]. These events subsequently generate aggregates and Lewy bodies. Furthermore, α-synuclein may form multimers by self-assemblage, which irreversibly produces insoluble aggregates. Mutant A53T α-synuclein appears to be more prone to aggregation (including in dopaminergic neurons) than A30P or WT α-synuclein [21, 22]. The role of protein aggregation is unclear; however, neurotoxic effects of α-synuclein have been demonstrated to result from its propensity to form multiple oligomers and spread since α-synuclein aggregates extracted from patients with synucleinopathy showed high seeding activity when inoculated in mice brains [23]. In contrast, other studies indicate that oligomers (pre-aggregates) of α-synuclein or other proteins involved with neurodegeneration are more toxic than aggregates [24, 25], and aggregate formation has been suggested as a neuroprotective mechanism [26].
The real conformation of functional α-synuclein present inside the cells is a matter of controversy. Dettmer et al. [27] demonstrated the presence of α-synuclein monomers of 14 kDa, tetramers (60 kDa) and other multimers (80 and 100 kDa) in normal human brain, suggesting a physiological role of these forms. It is speculated that the lack of balance between native tetramers and monomers causes the initiation of neurodegeneration [28] due to the accumulation of insoluble α-synuclein forms.
The presence of insoluble α-synuclein in the cells may result from genetic mutations, which would disrupt the balance between α-synuclein forms, deficits in degradation, and exposure to oxidative conditions [29]. Stimulation of degradation pathways prevents the formation of insoluble α-synuclein inclusions [30, 31].
The monomeric form of the protein is predominantly degraded by chaperone-mediated autophagy (CMA); nevertheless, when ser-129 is phosphorylated α-synuclein can be degraded by proteasome [32]. However, mechanisms that favor oligomeric and insoluble α-synuclein formation as a component of PD-associated neurodegeneration are not well understood. The general mechanisms of α-synuclein degradation have been studied and the findings indicate that small inclusions of the protein and puncta aggregates are ubiquitylated and driven to the ubiquitin–proteasome system. As proteasomes do not degrade large cargos, such as aggregates, degradation of aggregated α-synuclein occurs through autophagy [33, 34].
Both mutant and overexpressed wild-type α-synuclein disrupts the ubiquitin–proteasome system and autophagy; interestingly, dysfunction of degradation pathways also leads to accumulation and aggregation of α-synuclein. During aging and in the presence of large amounts of α-synuclein or mutant A53T α-synuclein, CMA and macroautophagy are inhibited [35].
Mitochondrial dysfunction may result from lysosome dysfunction combined with the presence of mutant A53T α-synuclein. Especially in DA neurons, impairment in non-functional mitochondria degradation causes a rapid increase reactive oxygen species (ROS) levels and induces neurodegeneration [36]. Figure 2 summarizes the effects of α-synuclein accumulation on cell degradation pathways, mitochondria function, DA release and ROS levels.
Effects of soluble monomeric, oligomeric and aggregates of α-synuclein in normal aged neuron (a) and PD neurons (b). During the normal aging (a) α-synuclein monomers are found in the cytosol and associated with membranes, especially of synaptic vesicles, it is degraded by the proteasome (1) via autophagy (2) such as organelles (3). During PD (b), the presence of α-synuclein oligomers causes cellular toxicity involving the impairment of degradation pathways via proteasome (1) or lysosome (2), the presence of α-synuclein oligomers is associated with accumulation of synaptic vesicles and decrease of dopamine release (3), and increased free radicals levels worsening the consequences of protein accumulation and aggregation. Aggregation of α-synuclein alleviates the cellular stress caused by oligomers, which partially recovers proteostasis and neurotransmission. However, many PD neurons do not recover from the initial impairment caused by the presence of α-synuclein oligomers and end up dying. Factors such as aging, genetic susceptibility and environment influence neuronal fate
Mitochondria as a Source and Target of Reactive Oxygen Species
During PD, the activities of mitochondrial complexes I and IV are preferentially affected in DA neurons of the SN, thereby increasing ROS production. ROS can be generated by α-synuclein accumulation or the presence of mutant proteins in cellular compartments such as the mitochondria and ER during cellular stress. Notwithstanding, ROS are produced primarily via the electron transport chain, at the inner mitochondrial membrane [37].
Mitochondria are crucial for the maintenance of healthy neurons, since these organelles are the source of ATP. Its DNA encodes 13 essential proteins involved in the respiratory chain and three short peptides (humanin, gau and MOTS-c) [38]. Interestingly, two independent studies using aged post-mortem brain showed that the mitochondrial DNA of DA neurons present more mutations than other neurons [39, 40].
Mitochondria are also involved in regulating apoptosis, calcium and ROS homeostasis. In healthy mitochondria, the electron transport chain induces a gradient of protons over the mitochondrial inner membrane, which is used for ATP synthesis. Electrons are extracted from reduced substrates and are transferred to molecular oxygen (O2), through a chain of enzymatic complexes (I to IV). In the last step of the electron transport chain, cytochrome c oxidase (complex IV) completely reduces O2 in water, with minimum formation of oxygen radicals. However, during mitochondrial dysfunction, partial reduction of O2 occurs more frequently than normal reduction, thereby generating increased radical superoxide anions; approximately 0.1–2% of O2 are partially reduced by mitochondria, although the actual rates in vivo may be less. The radical superoxide anion can be dismutated in H2O2 and O2 by the Cu/Zn-Superoxide dismutase (SOD) 1 enzyme in the intermembrane space or in the mitochondrial matrix by MnSOD2 [41].
Mitochondrial dysfunction occurs during normal aging, leading to higher ROS production that accelerates α-synuclein aggregation and dopamine depletion, thereby initiating neurodegeneration.
Mitochondrial Dysfunction in Parkinson’s Disease
Human DA neurons are considered the greatest consumer of ATP since they form 2.4 million synapses and they are thought to be the neurons with more synapses connections [42]. Thus, mitochondria dysfunction inevitably contributes to neuronal death. Moreover, as stated earlier, given that a percentage of total oxygen consumed in normal conditions is converted to free radicals in the mitochondria, this organelle is a major source of ROS [43].
The presence of unpaired electrons during ATP production facilitates ROS elevation, which can accelerate aging and activate antioxidant enzymes, including SOD and GPx, and antioxidant components such as DAT and vesicular monoamine transporter 2 (VMAT2), which drives dopamine precursor relocation from the intracellular medium to synaptic vesicles. Several studies propose that complex I disruption increases ROS levels in the mitochondrial matrix and the cytosol, leading to glutathione (GSH) depletion and cell death. In addition, ROS levels rapidly increase throughout tissues, including the PD brain and platelets of PD patients in cybrid models [44].
The mitochondrial electron transport chain produces 90% of ROS in cells and is localized close to mtDNA, thereby facilitating mtDNA mutations. Furthermore, mtDNA is not protected by histones and its replication is independent of cell cycle. Recently, mitochondrial DNA damage was linked to complex I deficiency and increased ROS in PD [45].
Compared to other tissues, the brain is more susceptible to mtDNA mutations, especially the SN. A study of healthy 60-year-old participants found that > 40% of all mtDNA deletions occur in the SN [40]. Mutations in mtDNA and/or electron transport chain impairments lead to mitochondrial dysfunction and energy depletion. Damaged or depolarized mitochondria cause electron leakage, generating excessive ROS, and releasing pro-apoptotic factors such as cytochrome C, which initiate cell death [46]. Moreover, overexpression of α-synuclein, or the presence of its mutant forms inhibit mitochondrial complex I activity, increasing ROS production. Curiously, basal expression of α-synuclein has been shown to protect against excessive ROS generation [47].
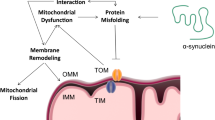
Mitochondria form a highly interconnected network throughout the neuron and its dynamics involves continuous autophagic destruction via mitophagy. Maintenance of healthy mitochondrial functioning involves fusion and fission processes that alter mitochondrial morphology. Deficits in the fusion or fission machinery cause aggregation and loss of directed movement, thereby impairing correct mitochondrial migration to neurites. Furthermore, investigations of impaired fusion and fission have demonstrated spontaneous generation of mtDNA mutations in neurodegenerative disorders such as PD [48].
Mitochondrial membrane damage and changes in mitochondria fission and morphology can result from interactions with α-synuclein. In order to understand α-synuclein toxicity and analyze the consequences for mitochondrial dynamics, several models have been created to overexpress α-synuclein or transfect mutant forms of the protein. It was revealed that overexpression of α-synuclein inhibits mitochondrial membrane fusion and disturbs the mitochondria cycle, which leads to fragmented or swollen mitochondria that contain laminated bodies. Moreover, α-synuclein overexpression increases colocalization of autophagosomes and mitochondria, whereas α-synuclein knockdown results in elongated mitochondria [49].
Mutant α-synuclein may also amplify mitochondrial dysfunction. A53T α-synuclein colocalizes to the mitochondrial membrane disrupting complex I and interfering with the fission process and autophagy machinery [50]. However, mitophagy is blocked, leading to the appearance of fragmented mitochondria. In addition, damaged mtDNA and dysmorphic mitochondria occur in A53T α-synuclein transgenic mice, and may have resulted from altered affinity of mutant α-synuclein to the mitochondrial membrane, given that α-synuclein primarily interacts with the outer mitochondrial membrane. However, during adequate ATP supply and pH changes, α-synuclein can migrate to the inner mitochondrial membrane quickly changing mitochondrial membrane potential, inhibiting complex I, and leading to aggregation of α-synuclein and fragmentation of mitochondria [51, 52].
Interaction Between Mitochondria and ER During α-Synuclein Toxicity
A proper mitochondrial membrane potential is important for maintaining a normal ER morphology. Altered mitochondrial membrane potential is associated with ER fragmentation that allows calcium leakage and produces high intracellular levels of free calcium driving increased ROS and apoptosis [53]. These findings suggest that both ER stress and mitochondrial dysfunction contribute to DA neurons degeneration. Interestingly, mitochondria morphologic changes, caused by interaction of α-synuclein with the mitochondrial membrane, are exacerbated by A53T α-synuclein. In contrast, A30P α-synuclein does not interferes directly with mitochondria morphology, since this mutation makes α-synuclein less interactive with monolayer lipid membranes, as discussed earlier in this review [54].
It is suggested that mitochondria and ER are affected by α-synuclein toxicity; however, further research is needed to understand the details of crosstalk between these organelles in order to elucidate their dysfunction in PD.
Mitochondria and ER possess mutual membrane contact sites, allowing direct contact for metabolite exchange, signaling involved in organelle dynamics, ATP metabolism, protein folding, and autophagy. Moreover, both organelles form contacts at synapses, where they promote calcium flow and synaptic activity [55, 56]. Together, these findings strongly suggest that contacts between mitochondria and ER are essential to neuron survival. Furthermore, mitochondrial fission processes occur near the ER contact sites even in the absence of mitochondrial fission factors. Besides that, the mitofusins, MFN1 and MFN2, which are proteins involved in mitochondrial fusion, depend on Miro1, a crucial protein associated with mitochondrial trafficking and dynamics. Miro1 is localized at contact sites between ER and mitochondria, and participates in mitochondrial and ER fission through interactions with the membrane contact sites between organelles [57].
Mitophagy also appears to be dependent on mitochondrial and ER membrane contact sites. Several ATG proteins, including the Atg8, are found at mitochondrial and ER contact sites. Thus, mitochondrial and ER contact sites form a platform for mitochondrial biogenesis and degradation [58].
Investigations of mitochondria and ER contacts in PD indicate that α-synuclein co-localize to mitochondria and ER contact sites. Furthermore, α-synuclein overexpression decreases mitochondria and ER contacts (Fig. 3) and affects calcium transfer between these organelles. However, in the presence of the A30P or A53T α-synuclein oligomers contacts between both organelles are further inhibited [59, 60]. This diminished organelle contact induces defective mitochondrial fission, blockage of autophagy and accumulation of impaired mitochondria [61].
Effects of prolonged UPR activation by α-synuclein. Illustration of how α-synuclein affects the interaction, mediated by Miro, between the endoplasmic reticulum (ER) and mitochondria in normal healthy or Parkinson’s disease (PD) neurons. During PD, there is accumulation of oligomers and aggregates of α-synuclein, making the levels of chaperone PDI increase while, the levels of BiP decreased, both changes contributes to increase the consumption of reduced glutathione (GSH), leading to increased levels of oxidized glutathione (GSSG), free radicals and release of calcium to cytoplasm, which collaborates to de decrease of mitochondrial membrane potential, culminating with death of DA neurons
There is a reciprocal relationship between mitochondrial dysfunction and ER stress. Post-mortem brains from patients with PD and animal models of PD both showed indications of ER stress. Overexpressed or mutant α-synuclein accumulates in the ER impairing protein folding and evoking ER stress. Furthermore, A53T α-synuclein increases ROS levels by impairing mitochondria and ER function, thereby causing neuron death [62, 63]. Stressed ER also generates ROS by decreasing GSH levels and transferring excessive calcium to mitochondria, which then also generate more ROS. GSH is the main molecule responsible for maintaining redox states in ER and mitochondria. Moreover, GSH oxidizes and activates the unfold protein response (UPR). In order to restore ER homeostasis, inositol-requiring enzyme 1 alpha protein (Ire1α) activates the UPR, which subsequently requires chaperones such as protein disulfide isomerase (PDI); this increases the folding and secretion of proteins to be degraded hence reestablishing the ER redox state. Secretion of α-synuclein also promotes its own accumulation and contributes to Lewy body formation. Furthermore, α-synuclein, disrupt both the ubiquitin–proteasome system and autophagy, leading to ER stress and UPR activation [64] (Fig. 3).
In addition, accumulated protein in the ER favors calcium leakage to cytosol. Aggregation of α-synuclein is accelerated by increased levels of cytosolic calcium, which occur, probably by destabilization of c-terminus region of α-synuclein, after calcium binding, and dislocation of the protein from lipid membranes [9, 65].
Mitochondria also play a role in calcium buffering. As the uptake of cytosolic calcium by mitochondria become excessive after the calcium release from the ER, mitochondrial metabolism is increased and elevates ROS production.
Furthermore, protein folding, which occurs in the ER, requires high levels of ATP. Therefore, prolonged UPR activation promotes high levels of ROS as illustrated in Fig. 3.
In DA neurons, UPR activates XBP1, which plays a role in activating gene expression to promote neuron survival. However, in the long-term presence of excessive or misfolded proteins, Hac1 mRNA activates apoptotic genes such as transcriptional factor CHOP that drives neurons to death. Therefore, UPR plays a paradoxical role in neurons; it initially activates mechanisms to ameliorate ER stress, however, long-term UPR activation induces cell death.
Recent studies of the first steps in neurodegeneration indicate that alterations in intracellular trafficking are essential to neuron survival. Experiments in cells using different concentrations of α-synuclein expression, in the absence of aggregates, revealed that high levels of this protein are associated with hyperphosphorylation of tau protein, which results in destabilization of microtubules and impaired intracellular trafficking of vesicles and organelles [66,67,68]. These findings suggest that alterations in intracellular trafficking are in fact initial steps in neurodegeneration and may promote aggregate formation.
Alpha-Synuclein Impairs Mitochondria Trafficking in PD
Mitochondrial quality control is essential to neuronal survival and involves trafficking mitochondria to neuronal regions that require more energy and returning mitochondria to the soma for recycling and repair, since there is where fusion and fission preferentially occur. The axons of DA neurons in the SN account for 95% of the cellular volume and recruit a significant portion of its energy. Disrupted mitochondrial trafficking impairs the ATP-supply at specific sites, such as synaptic terminals, and impairs new healthy mitochondria generation by fusion and fission processes in the soma [69]. Anterograde mitochondrial trafficking is the axonal transportation of mitochondria from the soma to the synaptic terminals. Retrograde transport to the soma is required during recycling, or in cases of mitochondrial damage and dysfunction.
At the soma, mitophagy involves lysosomes and ubiquitin–proteasome processes to degrade damaged mitochondria [70, 71]. Motor proteins from the kinesin family (KIFs), and other proteins such as dynein and dynactin, are responsible for maintenance of intracellular trafficking along the microtubules [72].
Injuries to the cytoskeleton are responsible for rearrangement and movement of organelles in neurodegenerative diseases, including PD [73]. Microtubules participate in diverse cellular functions including motility, cell division, and transportation of organelles, vesicles, and proteins, and maintenance of cellular morphology and general organization of the cytoplasm. Microtubule dynamics is regulated by the concentration of free tubulin. Intriguingly, in PD, α-synuclein and Lewy bodies are colocalized with free tubulin and with the tubulin polymerization promoting protein (TPPP), suggesting that α-synuclein may disrupt intracellular trafficking by impairing microtubule stabilization [74]. Whilst α-synuclein, in physiological environment, is hypothesized as a microtubule associates protein, as stated earlier in this review.
Accumulating evidence suggests that disrupted axonal transport is critical to PD development. It has been suggested that α-synuclein may impair mitochondrial axonal transport by disturbing motor protein expression such as for dynein. In addition, α-synuclein appears to disrupt interactions of these proteins with microtubules [12, 75]. During neurodegeneration, alterations in motor proteins may have consequences for mitochondrial trafficking. Expression of the anterograde motor proteins KIF1Bα and KIF5 and the retrograde motor proteins dynein, dynactin, and syntaphilin, is altered prior to protein aggregation in cell cultures and in animal models of PD [67, 68]. Together, these studies strongly suggest that altered intracellular trafficking is modulated by α-synuclein being an important component of PD pathogenesis.
To transport cargos such as mitochondria, motor proteins associate with the adaptor proteins TRAK (drosophila ortholog Milton) and Miro (also called RHOT), which are attached to the outer mitochondrial membrane [76]. Increased Miro expression leads to upregulation of mitochondrial trafficking. In addition, loss of Miro results in defective trafficking in both directions, suggesting that Miro is an adaptor for both anterograde transport (via interaction with KIF5) and retrograde transport (via interaction with dynein). Furthermore, studies on mitochondrial fragmentation and interconnectivity showed that disruption in mitochondrial trafficking leads to organelle fragmentation, whereas overexpression of Miro increased mitochondrial trafficking and interconnectivity, thereby increasing mitochondria length in neurons [77].
Miro is a Ca2+ sensor containing 2 calcium-binding EF-hands. Increased calcium dissociates motor proteins from Miro and TRAK, blocking mitochondria trafficking. This process is crucial for the anchoring mitochondria at specific sites where abundant ATP is required, such as at synapses. When ADP decreases, stationary mitochondria move to another site with low ATP levels. However, impaired mitochondrial trafficking can lead to mitochondrial dysfunction at the current anchor site and result in increased ROS generation (Fig. 4) [78,79,80].
Illustration of mitochondria trafficking and anchoring in healthy neurons (normal) and during Parkinson’s disease (PD). In the presence of α-synuclein the mitochondrial anterograde and retrograde trafficking are impaired, mitochondrial membrane potential decreases and the levels of free radicals and calcium increased, culminating with decreased ATP production and impaired axonal trafficking
Miro also interacts with mitofusin (MFN) proteins, which participate in mitochondrial fusion. Intriguingly, mitochondrial trafficking is decreased in neurons in which MFN2 was knocked-out, suggesting that Miro and MFN work together in the regulation of mitochondria trafficking [79]. Miro is associated with the mitochondrial outer membrane; it coordinates the transport of mitochondria moving together with ER and it takes care that mitochondria stay close enough for the initiation of fusion or fission processes [81]. Once in contact, MFN1 and MFN2 interact with Miro and both are required for proper axonal transport, suggesting that association of these proteins and balanced trafficking are essential for the fusion process [82]. Absence of Miro exacerbates mitophagy, and transgenic MFN2 knockdown mice demonstrated blocked mitophagy.
The ER provides lipids to form membrane vesicles during autophagy [83] these lipids are transferred and accumulate at the outer mitochondrial membrane before they are transported to the sites of vesicle formation and mitophagy initiation [84]. However, alterations in Miro or MFN proteins can disturb these processes, revealing that both proteins are required for normal fusion and mitophagy processes.
Damaged mitochondria are targeted for mitophagy via PINK1 signaling. Parkin forms a complex with PINK1 during mitophagy and ubiquitylates substrates at the outer mitochondrial membrane, including Miro, which triggers mitophagy. Miro may function as a receptor for both proteins, since Miro interacts with PINK1 and parkin, thereby allowing their association with the outer mitochondrial membrane. Moreover, damaged mitochondria require rapid Miro ubiquitylation, which is mediated by parkin. In addition, the fibroblasts of patients carrying parkin mutations showed altered Miro turnover, suggesting that Miro is critically involved in regulating fusion, fission, and mitophagy events [85, 86].
Rab-mediated Trafficking and α-Synuclein Toxicity
The specificity of intracellular organelle trafficking among cellular compartments is strictly regulated by small GTPases (Rabs) from the Ras super family of proteins. Furthermore, Rabs are responsible for the correct attachment of motor proteins and cargos and for cargo motility and their delivery to the correct destination. Furthermore, Rabs may be involved in α-synuclein toxicity, such as through formation of Lewy bodies, as discussed above. Cultured cells internalize α-synuclein added to the culture media, which is associated with aggregate formation. Alpha-synuclein may be secreted from cells via exocytosis, and subsequently internalized by other neurons in culture via Rab5-dependent endocytosis, thus initiating a spreading cycle of seeding α-synuclein and aggregate formation [87]. The mechanisms of α-synuclein propagation are unclear; however, α-synuclein seeding and propagation is considered a crucial process in PD development [88]. Interestingly, a recent study show that α-synuclein can be propagated by the traveling of lysosome vesicles along tunneling inter-cellular nanotubules from cell to another cell [89].
Rab5 is a multifunctional protein that regulates the first steps in endocytic pathways and contributing to anchoring, trafficking, fusion of endosomal membranes, and autophagy-mediated recycling [90]. In addition, mutant Rab5 leads to an accumulation of enlarged early and late endosomes/phagosomes and defects in the regulation of endosome/phagosome trafficking to lysosomes, which involves Rab7.
After vesicle maturation, Rab7 coordinates the fusion of late endosomes with autophagosomes and LC3 requirement. Together, these findings indicate that Rab5 is involved in the formation and transportation of immature endosomes/phagosomes and LC3 signaling, thereby contributing to the first steps of autophagy. Rab5, LC3, and Miro have unique roles in endocytosis and trafficking of early endosomes and in autophagy and in the dynamics of mitochondria and ER [91]. However, α-synuclein toxicity related to trafficking and Rabs, Miro, or LC3 have not been elucidated.
Investigations of intracellular trafficking and autophagy dysfunction in neurological disorders have revealed that degradation via lysosomes is crucial to balanced axonal vesicles and lysosome trafficking. Furthermore, intracellular trafficking impairments lead to the accumulation of lysosome vesicles causing axonal swelling and neurite dystrophy. Other studies have shown that endocytic pathway alterations result in accumulation of endolysosomes (endosomes fused to lysosomes), thereby impeding autophagy and resulting in α-synuclein accumulation.
A recent review has highlighted the link between Rabs and PD, including the role of α-synuclein toxicity upon intracellular trafficking during PD and the recently identified PD-associated mutations on Rabs [92], providing significant insight about PD etiology and therapy.
Future Directions
Death of DA neurons during PD is a complex and multifactorial process. Aggregates and oligomers are associated with cell death, but the mechanisms of α-synuclein toxicity remain unclear. Investigations concerning the toxicity of α-synuclein indicate that disturbances of the ubiquitin–proteasome system and the function of lysosomes also are involved. Moreover, concomitant impairment of mitochondrial function generates oxidative stress, which produces excessive ROS and subsequent neurotoxic effects. Interactions between mitochondria and the ER are important for maintaining homeostasis in these organelles and, impaired interactions can also trigger cell death. Moreover, α-synuclein accumulation and mitochondrial dysfunctions are major contributors to trafficking impairments, which further contribute to DA cell death. Overall, the current literature describes several contributors to the pathology of PD, but a comprehensive model of pathogenesis and order of effects has not been established yet.
References
Parkinson J (2002) An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 14:223–236; (discussion 222)
Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA (2012) Parkinson’s disease. Subcell Biochem 65:389–455
Tysnes OB, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 124:901–905
Weintraub D, Comella CL, Horn S (2008) Parkinson’s disease—part 1: pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care 14:S40–S48
Brundin P, Dave KD, Kordower JH (2017) Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol 298:225–235
Atik A, Stewart T, Zhang J (2016) Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol 26:410–418
Vamvaca K, Volles MJ, Lansbury PT Jr (2009) The first N-terminal amino acids of alpha-synuclein are essential for alpha-helical structure formation in vitro and membrane binding in yeast. J Mol Biol 389:413–424
Emamzadeh FN (2016) Alpha-synuclein structure, functions, and interactions. J Res Med Sci 21:29
Lautenschlager J, Stephens AD, Fusco G, Strohl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, George-Hyslop St, Rees P, Phillips E, De Simone JJ, Kaminski A, C. F. & Schierle GSK (2018) C-terminal calcium binding of alpha-synuclein modulates synaptic vesicle interaction. Nat Commun 9:712
Burre J (2015) The synaptic function of alpha-Synuclein. J Parkinsons Dis 5:699–713
Alim MA, Ma QL, Takeda K, Aizawa T, Matsubara M, Nakamura M, Asada A, Saito T, Kaji H, Yoshii M, Hisanaga S, Ueda K (2004) Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein, J Alzheimers Dis. 6, 435–42; (discussion 443-9)
Cartelli D, Aliverti A, Barbiroli A, Santambrogio C, Ragg EM, Casagrande FV, Cantele F, Beltramone S, Marangon J, De Gregorio C, Pandini V, Emanuele M, Chieregatti E, Pieraccini S, Holmqvist S, Bubacco L, Roybon L, Pezzoli G, Grandori R, Arnal I, Cappelletti G (2016) Alpha-synuclein is a novel microtubule dynamase. Sci Rep 6:33289
Khan W, Priyadarshini M, Zakai HA, Kamal MA, Alam Q (2012) A brief overview of tyrosine hydroxylase and alpha-synuclein in the Parkinsonian brain. CNS Neurol Disord Drug Targets 11:456–462
Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA (2002) Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med 8:600–606
Ranjan P, Kumar A (2017) Perturbation in long-range contacts modulates the kinetics of amyloid formation in alpha-synuclein familial mutants, ACS Chem Neurosci 8:2235–2246
Walsh DM, Selkoe DJ (2016) A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat Rev Neurosci 17:251–260
Rey NL, George S, Brundin P (2016) Review: Spreading the word: precise animal models and validated methods are vital when evaluating prion-like behaviour of alpha-synuclein. Neuropathol Appl Neurobiol 42:51–76
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, Wang ZY, Roybon L, Melki R, Li JY (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128:805–820
Mahul-Mellier AL, Vercruysse F, Maco B, Ait-Bouziad N, De Roo M, Muller D, Lashuel HA (2015) Fibril growth and seeding capacity play key roles in alpha-synuclein-mediated apoptotic cell death. Cell Death Differ 22:2107–2122
Lazaro DF, Rodrigues EF, Langohr R, Shahpasandzadeh H, Ribeiro T, Guerreiro P, Gerhardt E, Krohnert K, Klucken J, Pereira MD, Popova B, Kruse N, Mollenhauer B, Rizzoli SO, Braus GH, Danzer KM, Outeiro TF (2014) Systematic comparison of the effects of alpha-synuclein mutations on its oligomerization and aggregation. PLoS Genet 10:e1004741
Moussa CE, Mahmoodian F, Tomita Y, Sidhu A (2008) Dopamine differentially induces aggregation of A53T mutant and wild type alpha-synuclein: insights into the protein chemistry of Parkinson’s disease. Biochem Biophys Res Commun 365:833–839
Marmolino D, Foerch P, Atienzar FA, Staelens L, Michel A, Scheller D (2016) Alpha synuclein dimers and oligomers are increased in overexpressing conditions in vitro and in vivo. Mol Cell Neurosci 71:92–101
Tarutani A, Arai T, Murayama S, Hisanaga SI, Hasegawa M (2018) Potent prion-like behaviors of pathogenic alpha-synuclein and evaluation of inactivation methods. Acta Neuropathol Commun 6:29
Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R (2011) In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA 108:4194–4199
Deas E, Cremades N, Angelova PR, Ludtmann MH, Yao Z, Chen S, Horrocks MH, Banushi B, Little D, Devine MJ, Gissen P, Klenerman D, Dobson CM, Wood NW, Gandhi S, Abramov AY (2016) Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in Parkinson’s disease. Antioxid Redox Signal 24:376–391
Chaves RS, Kazi AI, Silva CM, Almeida MF, Lima RS, Carrettiero DC, Demasi M, Ferrari MFR (2016) Presence of insoluble Tau following rotenone exposure ameliorates basic pathways associated with neurodegeneration. IBRO Rep 1:32–45
Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, Sanderson JB, Jaenisch R, Bartels T, Selkoe D (2015) Parkinson-causing alpha-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun 6:7314
Dettmer U, Selkoe D, Bartels T (2016) New insights into cellular alpha-synuclein homeostasis in health and disease. Curr Opin Neurobiol 36:15–22
Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M (1999) Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem 274:9843–9846
Myohanen TT, Norrbacka S, Savolainen MH (2017) Prolyl oligopeptidase inhibition attenuates the toxicity of a proteasomal inhibitor, lactacystin, in the alpha-synuclein overexpressing cell culture. Neurosci Lett 636:83–89
Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A (2013) TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci USA 110:E1817–E1826
Machiya Y, Hara S, Arawaka S, Fukushima S, Sato H, Sakamoto M, Koyama S, Kato T (2010) Phosphorylated alpha-synuclein at Ser-129 is targeted to the proteasome pathway in a ubiquitin-independent manner. J Biol Chem 285:40732–40744
Ciechanover A, Orian A, Schwartz AL (2000) Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22:442–451
Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ (2012) The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med 2:a009357
Ottolini D, Cali T, Szabo I, Brini M (2017) Alpha-synuclein at the intracellular and the extracellular side: functional and dysfunctional implications. Biol Chem 398:77–100
Redmann M, Darley-Usmar V, Zhang J (2016) The role of autophagy, mitophagy and lysosomal functions in modulating bioenergetics and survival in the context of redox and proteotoxic damage: implications for neurodegenerative diseases. Aging Dis 7:150–162
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson’s disease. Front Neuroanat 9:91
Capt C, Passamonti M, Breton S (2016) The human mitochondrial genome may code for more than 13 proteins. Mitochondrial DNA A DNA Mapp Seq Anal 27:3098–3101
Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38:518–520
Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38:515–517
Arun S, Liu L, Donmez G (2016) Mitochondrial biology and neurological diseases. Curr Neuropharmacol 14:143–154
Haddad D, Nakamura K (2015) Understanding the susceptibility of dopamine neurons to mitochondrial stressors in Parkinson’s disease. FEBS Lett 589:3702–3713
Maharjan S, Sakai Y, Hoseki J (2016) Screening of dietary antioxidants against mitochondria-mediated oxidative stress by visualization of intracellular redox state. Biosci Biotechnol Biochem 80:726–734
Arduino DM, Esteves AR, Swerdlow RH, Cardoso SM (2015) A cybrid cell model for the assessment of the link between mitochondrial deficits and sporadic Parkinson’s disease. Methods Mol Biol 1265:415–424
Giannoccaro MP, La Morgia C, Rizzo G, Carelli V (2017) Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson’s disease. Mov Disord 32:346–363
Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM (2002) Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem 80:207–218
Choong CJ, Say YH (2011) Neuroprotection of alpha-synuclein under acute and chronic rotenone and maneb treatment is abolished by its familial Parkinson’s disease mutations A30P, A53T and E46K. Neurotoxicology 32:857–863
Chen H, Chan DC (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18:R169–R176
Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK (2006) Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci 26:41–50
Pozo Devoto VM, Dimopoulos N, Alloatti M, Pardi MB, Saez TM, Otero MG, Cromberg LE, Marin-Burgin A, Scassa ME, Stokin GB, Schinder AF, Sevlever G, Falzone TL (2017) alphaSynuclein control of mitochondrial homeostasis in human-derived neurons is disrupted by mutations associated with Parkinson’s disease. Sci Rep 7:5042
Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL (2008) Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res 314:2076–2089
Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283:9089–9100
Bao FX, Shi HY, Long Q, Yang L, Wu Y, Ying ZF, Qin DJ, Zhang J, Guo YP, Li HM, Liu XG (2016) Mitochondrial membrane potential-dependent endoplasmic reticulum fragmentation is an important step in neuritic degeneration. CNS Neurosci Ther 22:648–660
Ghio S, Kamp F, Cauchi R, Giese A, Vassallo N (2016) Interaction of alpha-synuclein with biomembranes in Parkinson’s disease—role of cardiolipin. Prog Lipid Res 61:73–82
Mironov SL, Symonchuk N (2006) ER vesicles and mitochondria move and communicate at synapses. J Cell Sci 119:4926–4934
Krols M, van Isterdael G, Asselbergh B, Kremer A, Lippens S, Timmerman V, Janssens S (2016) Mitochondria-associated membranes as hubs for neurodegeneration. Acta Neuropathol 131:505–523
Rowland AA, Voeltz GK (2012) Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13:607–625
Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, Amano A, Yoshimori T (2013) Autophagosomes form at ER-mitochondria contact sites. Nature 495:389–393
Cali T, Ottolini D, Brini M (2011) Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors 37:228–240
Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S (2014) Alpha-synuclein is localized to mitochondria-associated ER membranes. J Neurosci 34:249–259
Manor U, Bartholomew S, Golani G, Christenson E, Kozlov M, Higgs H, Spudich J, Lippincott-Schwartz J (2015) A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division, Elife. 4: e08828
Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, Dawson VL, Dawson TM, Ross CA (2005) Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet 14:3801–3811
Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, Schneider BL, Lee MK (2012) Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J Neurosci 32:3306–3320
Schroder M (2008) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65:862–894
Nath S, Goodwin J, Engelborghs Y, Pountney DL (2011) Raised calcium promotes alpha-synuclein aggregate formation. Mol Cell Neurosci 46:516–526
Oikawa T, Nonaka T, Terada M, Tamaoka A, Hisanaga S, Hasegawa M (2016) Alpha-synuclein fibrils exhibit gain of toxic function, promoting tau aggregation and inhibiting microtubule assembly. J Biol Chem 291:15046–15056
Chaves RS, Melo TQ, D’Unhao AM, Farizatto KL, Ferrari MF (2013) Dynein c1h1, dynactin and syntaphilin expression in brain areas related to neurodegenerative diseases following exposure to rotenone. Acta Neurobiol Exp (Wars) 73:541–556
Melo TQ, D’Unhao A, Martins M, Farizatto SA, Chaves KL, R. S. & Ferrari MF (2013) Rotenone-dependent changes of anterograde motor protein expression and mitochondrial mobility in brain areas related to neurodegenerative diseases. Cell Mol Neurobiol 33:327–335
Phillipson OT (2017) Alpha-synuclein, epigenetics, mitochondria, metabolism, calcium traffic, & circadian dysfunction in Parkinson’s disease. An integrated strategy for management. Ageing Res Rev 40:149–167
Florenzano F (2012) Localization of axonal motor molecules machinery in neurodegenerative disorders. Int J Mol Sci 13:5195–5206
Lehmann G, Udasin RG, Ciechanover A (2016) On the linkage between the ubiquitin–proteasome system and the mitochondria. Biochem Biophys Res Commun 473:80–86
Hirokawa N, Niwa S, Tanaka Y (2010) Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68:610–638
Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH (2012) Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain 135:2058–2073
Szunyogh S, Olah J, Szenasi T, Szabo A, Ovadi J (2015) Targeting the interface of the pathological complex of alpha-synuclein and TPPP/p25. Biochim Biophys Acta 1852:2653–2661
Fang F, Yang W, Florio JB, Rockenstein E, Spencer B, Orain XM, Dong SX, Li H, Chen X, Sung K, Rissman RA, Masliah E, Ding J, Wu C (2017) Synuclein impairs trafficking and signaling of BDNF in a mouse model of Parkinson’s disease. Sci Rep 7:3868
Devine MJ, Birsa N, Kittler JT (2016) Miro sculpts mitochondrial dynamics in neuronal health and disease. Neurobiol Dis 90:27–34
Fransson S, Ruusala A, Aspenstrom P (2006) The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun 344:500–510
Mironov SL (2007) ADP regulates movements of mitochondria in neurons. Biophys J 92:2944–2952
Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G (2008) Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci USA 105:20728–20733
Klosowiak JL, Focia PJ, Chakravarthy S, Landahl EC, Freymann DM, Rice SE (2013) Structural coupling of the EF hand and C-terminal GTPase domains in the mitochondrial protein Miro. EMBO Rep 14:968–974
Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK (2010) ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol 190:363–375
Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30:4232–4240
Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182:685–701
Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J (2010) Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141:656–667
Birsa N, Norkett R, Wauer T, Mevissen TE, Wu HC, Foltynie T, Bhatia K, Hirst WD, Komander D, Plun-Favreau H, Kittler JT (2014) Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase. J Biol Chem 289:14569–14582
Kazlauskaite A, Kelly V, Johnson C, Baillie C, Hastie CJ, Peggie M, Macartney T, Woodroof HI, Alessi DR, Pedrioli PG, Muqit MM (2014) Phosphorylation of Parkin at Serine65 is essential for activation: elaboration of a Miro1 substrate-based assay of Parkin E3 ligase activity. Open Biol 4:130213
Sung JY, Kim J, Paik SR, Park JH, Ahn YS, Chung KC (2001) Induction of neuronal cell death by Rab5A-dependent endocytosis of alpha-synuclein. J Biol Chem 276:27441–27448
Borghammer P (2018) How does parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord 33:48–57
Abounit S, Bousset L, Loria F, Zhu S, de Chaumont F, Pieri L, Olivo-Marin JC, Melki R, Zurzolo C (2016) Tunneling nanotubes spread fibrillar alpha-synuclein by intercellular trafficking of lysosomes. EMBO J 35:2120–2138
Olchowik M, Miaczynska M (2009) Effectors of GTPase Rab5 in endocytosis and signal transduction. Postepy Biochem 55:171–180
Wegner CS, Malerod L, Pedersen NM, Progida C, Bakke O, Stenmark H, Brech A (2010) Ultrastructural characterization of giant endosomes induced by GTPase-deficient Rab5. Histochem Cell Biol 133:41–55
Gao Y, Wilson GR, Stephenson SEM, Bozaoglu K, Farrer MJ, Lockhart PJ (2018) The emerging role of Rab GTPases in the pathogenesis of Parkinson’s disease. Mov Disord 33:196–207
Acknowledgements
MFRF is supported by research grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) (2011/06434-7; 2013/08028-1; 2015/18961-2), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melo, T.Q., Copray, S.J.C.V.M. & Ferrari, M.F.R. Alpha-Synuclein Toxicity on Protein Quality Control, Mitochondria and Endoplasmic Reticulum. Neurochem Res 43, 2212–2223 (2018). https://doi.org/10.1007/s11064-018-2673-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2673-x