Abstract
Protein aggregation is a hallmark of many neurodegenerative diseases. In Parkinson’s disease protein misfolding of \(\upalpha \)-synuclein involves conformational changes in the protein structure that often results in self-association and aggregation leading to accumulation of \(\upalpha \)-synuclein in neuronal cells. The underlying mechanisms by which aggregations can lead to impaired cellular functions are often not understood. Meanwhile, there is growing evidence that links mitochondrial dysfunction to Parkinson’s disease. As both mitochondria and protein aggregation of \(\upalpha \)-synuclein have been shown to play a major role in Parkinson’s disease, it seems likely that a converging mechanism exists that links the two pathways.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD), a degenerative disorder of the central nervous system is characterized by the loss of dopaminergic neurons, and the presence of large protein inclusion bodies termed Lewy bodies. Although neither dopaminergic neuron loss nor the appearance of Lewy bodies are phenomena that are unique to PD, together they suffice for diagnosis of idiopathic PD (Poewe et al. 2017). The loss of dopaminergic neuronal cells can occur prior to the appearance of Lewy bodies (Dijkstra et al. 2014) and results in dopamine deficiency in the central nervous system. Notable loss in neurons (>50%), even in early stages of the disease is suggested to precede impaired communication between muscles and the brain that leads to manifestations of motor symptoms (Poewe et al. 2017; Cheng et al. 2010; Dauer and Przedborski 2003).
Protein folding is essential to cellular function. The disruption of protein-folding homeostasis leads to misfolding and can result in proteins that oligomerize and accumulate, inducing cytotoxic effects (Kaganovich 2017; Brielle et al. 2015; Kaganovich et al. 2008). Aggregation of proteins is associated with an increasing number of neurodegenerative diseases such as Alzheimer disease (AD), PD, Huntington disease (HD) and Amyotrophic lateral sclerosis (ALS). In PD’s point mutation and multiplications of synuclein alpha (SNCA), the gene that encodes \(\upalpha \)-synuclein results in gain of function and cytosolic accumulation, and aggregation into large deposits called Lewy bodies. It is not entirely clear how \(\upalpha \)-synuclein aggregation is linked to neuronal degeneration and if \(\upalpha \)-synuclein misfolding is a cause or a byproduct of PD pathology, however the emerging paradigm suggests that misfolded monomers and low molecular weight oligomers of \(\upalpha \)-synuclein are cytotoxic (Lashuel et al. 2013), while larger inclusion bodies that appear in late stages of the disease may play a protective role in cellular function (Tanaka et al. 2004). By sequestering reactive, often hydrophobic, misfolded proteins to specialized cellular compartments, cells can restrict their interaction with other proteins, membranes and organelles. Further, in response to accumulation of misfolded proteins, cells relocate quality control machinery, such as molecular chaperones and proteases, to inclusions bodies allowing cells to efficiently process misfolded and aggregated proteins (Dijkstra et al. 2014; Kaganovich et al. 2008).
\(\upalpha \)-Synuclein
SNCA is the first gene to be associated with hereditary PD, although pathogenic mutations in SNCA are responsible for only a small fraction of cases of hereditary PD (Farrer et al. 1998). Yet the discovery of these mutations was a stepping stone in unravelling the molecular pathways associated with PD and establishing \(\upalpha \)-synuclein’s role in both hereditary and sporadic forms of PD. The first evidence linking \(\upalpha \)-synuclein to PD was obtained with the discovery of familial missense mutation A53T, in the gene SNCA that was responsible for the disease onset in families with autosomal dominant inheritance of PD (Polymeropoulos et al. 1996, 1997; Krüger et al. 1998; Nussbaum 2017). This identification pathogenic SNCA mutation has led to the discovery of several other disease-related mutations: A30P, E46K, G51D, and H50Q in SNCA (Krüger et al. 1998; Zarranz et al. 2004; Lesage et al. 2013; Appel-Cresswell et al. 2013).
Shortly after the identification of SNCA mutations in hereditary PD, it was found that \(\upalpha \)-synuclein is one the main components of Lewy bodies in idiopathic PD (Spillantini et al. 1997). Since the detection of \(\upalpha \)-synuclein in Lewy bodies, there is a growing interest in molecular mechanisms that link \(\upalpha \)-synuclein, Parkinson and protein aggregation and numerous works that studied the cytotoxic effects of \(\upalpha \)-synuclein’s aggregation. Early studies in \(\upalpha \)-synuclein’s aggregation properties demonstrated that expression of both wild type and mutant human \(\upalpha \)-synuclein in transgenic mice leads to the progressive accumulation of \(\upalpha \)-synuclein in cytoplasmic inclusions in neuronal cells in the neocortex, hippocampus, and substantia nigra (Masliah et al. 2000; Breydo et al. 2012). The appearance of these inclusions was associated with loss of dopaminergic neurons and loss motor functions. Similar results were also been reported in a Drosophila Parkinson model and in Caenorhabditis elegans expressing mutant \(\upalpha \)-synuclein (Feany and Bender 2000; Kuwahara et al. 2006). In accordance with these results, there have been numerous studies reporting that expression of mutant \(\upalpha \)-synuclein in cell culture models, results in the formation of oligomers and amyloid fibrils including a systemic comparison of the aggregation properties of different \(\upalpha \)-synuclein mutations in cell culture models (Lázaro et al. 2014).
The molecular mechanisms that leads to the formation of oligomers and amyloid fibrils have been studied extensively both in vitro and in vivo. \(\upalpha \)-Synuclein is considered as an intrinsically disordered protein which possesses no ordered structure under physiological conditions and therefore has striking conformational plasticity under various conditions (Uversky 2007). In vitro \(\upalpha \)-synuclein can adopt various conformations including: unfolded native state, \(\upalpha \)-helical and \(\upbeta \)-sheet species that can include both monomeric and oligomeric states as well as amorphous aggregates and amyloid-like fibrils (Breydo et al. 2012). In vitro studies into the aggregation kinetics of \(\upalpha \)-synuclein have demonstrated that the formation of \(\upalpha \)-synuclein fibrils are nucleation dependent. Further, the induction of fibril formation by aggregated \(\upalpha \)-synuclein appears to follow first order kinetics with respect to the concentration of \(\upalpha \)-synuclein. Interestingly, incubation of wild-type \(\upalpha \)-synuclein with mutant \(\upalpha \)-synuclein nuclei results in the induction of wild-type \(\upalpha \)-synuclein aggregation (Wood et al. 1999), suggesting that \(\upalpha \)-synuclein aggregation is promoted by a process of template seeding. Indeed, in a more recent study, it was demonstrated that neuronal cells can form inclusion after being cocultured with neuronal cells overexpressing \(\upalpha \)-synuclein (Desplats et al. 2009).
Although the question of how of \(\upalpha \)-synuclein different aggregates species exert neurotoxicity is still under debate, the current paradigm suggests that oligomeric intermediates of \(\upalpha \)-synuclein are responsible for cytotoxicity rather than large inclusion or amyloid-like fibrils. In vitro studies of the aggregation dynamics of the pathogenic \(\upalpha \)-synuclein A30P have shown that while this mutant form of \(\upalpha \)-synuclein inhibits the formation of the fibrillar \(\upalpha \)-synuclein compared to wild-type \(\upalpha \)-synuclein, the formation of oligomeric species of \(\upalpha \)-synuclein is slightly accelerated. Similarly, the pathogenic \(\upalpha \)-synuclein A53T was also demonstrated to accelerate the formation of oligomeric species, however in this case, it accelerated the formation of fibrillar \(\upalpha \)-synuclein. Together, these results suggest that pathogenic mutants of \(\upalpha \)-synuclein promote the formation of oligomers rather than fibrils (Conway et al. 2000). The toxicity of oligomeric \(\upalpha \)-synuclein has also been demonstrated in several cell culture models. In primary neuronal cells, that were exposed to samples containing different \(\upalpha \)-synuclein species, cells exposed to oligomeric but not monomeric or fibril \(\upalpha \)-synuclein showed increased oxidative stress (Cremades et al. 2012). In addition, a systemic comparison of the effects of \(\upalpha \)-synuclein mutations on oligomerization and aggregation in cell culture showed that familial mutants linked to PD have similar propensities to form oligomers yet differ in their propensities to form aggregates (Lázaro et al. 2014).
Structure and functions of \(\upalpha \)-synuclein
There are currently three known proteins belonging to the synuclein family: \(\upalpha \)-synuclein, \(\upbeta \)-synuclein, and \(\upgamma \)-synuclein. All proteins belonging to this family contain a highly conserved lipid-binding domain that is found only in vertebrates and have some similarity to the binding motif of apolipoprotein \(\hbox {A}_{2}\), a class of protein that can reversibly bind and transport lipids (Eliezer et al. 2001; Jo et al. 2000). \(\upalpha \)-Synuclein which is best known for its association PD is composed of only 140 amino acids: the membrane-binding domain of \(\upalpha \)-synuclein is located in the N-terminus section (1–95), which contains seven segments each 11-resiudes in length. Each of the seven segments in the N-terminus section contains a repeating consensus sequence KTKEGV (Ulmer et al. 2005). In aqueous solution, the N-terminus of \(\upalpha \)-synuclein is considered to be intrinsically disordered but upon association with a membrane surface it can adopt a helical form with an 11/3 periodicity (11 residues over three turns). Located in the central region of \(\upalpha \)-synuclein (61–95), and over lapping with the N-terminus \(\upalpha \)-helix, is a highly hydrophobic segment of 35 residues termed the nonA\(\upbeta \) component (NAC). Interestingly, this hydrophobic section of \(\upalpha \)-synuclein was reported to be abundant in plaques associated with AD. Finally, the C-terminus of \(\upalpha \)-synuclein is considered unstructured and is enriched with acidic residues glutamate and aspartate. Recently, C-terminus truncated forms of \(\upalpha \)-synuclein were reported to be enriched in \(\upalpha \)-synuclein inclusions and may enhance the aggregation of full-length \(\upalpha \)-synuclein (Lashuel et al. 2013).
The function of \(\upalpha \)-synuclein under normal physiological conditions is not well understood yet its abundance in presynaptic terminals of neuronal cells would suggest its involvement in synaptic maintenance. Several works have shown that overexpression of \(\upalpha \)-synuclein in transgenic mice can cause functional defects in the handling the synaptic vesicles such as inhibition of synaptic vesicle exocytosis (Burré 2015; Bendor et al. 2013; Nemani et al. 2010). In addition, some studies that examined neuronal cells of \(\upalpha \)-synuclein knockout mice have suggested that \(\upalpha \)-synuclein may inhibit dopamine synaptic release in neuronal cells (Cabin et al. 2002) and that increase in the rate of dopamine release may decrease the stores of dopamine in the striatum of knockout mice (Abeliovich et al. 2000). In accordance with these studies, it was reported that hippocampal neurons from \(\upalpha \)-synuclein knockout mice have a significant reduction (\(\sim \)50%) in undocked synaptic vesicles but the same number of docked vesicles in the presynaptic terminal of neurons compared to wild-type mice (Murphy et al. 2000). Taken together, these finding suggest that \(\upalpha \)-synuclein has a role in regulating and upkeep of synaptic vesicles. Other studies in support of \(\upalpha \)-synuclein role in the synaptic dynamics have shown that synucleins are required for maintenance of presynaptic SNARE-complex, specifically, \(\upalpha \)-synuclein was reported to bind to the SNARE protein synaptobrevin-2/vesicle-associated membrane protein 2 (VAMP2) and promoted the assembly of the SNARE-complex (Burré et al. 2010). \(\upalpha \)-Synuclein was also implicated in the inhibition of phospholipase D2 (PLD2), an enzyme responsible for catalysing the hydrolysis of phosphatidylcholine (PC) to phosphatidic acid, a precursor for the biosynthesis of many cellular lipids. Phosphatidic acid is also essential for inducing membrane curvature and vesicle budding. It has been proposed that mutation in \(\upalpha \)-synuclein could influence the regulation of PLD2 and interfere with synaptic membrane biogenesis and turnover (Payton et al. 2004).
Membrane interactions of \(\upalpha \)-synuclein
Although \(\upalpha \)-synuclein is a soluble cytosolic protein, it is known to bind a variety of cellular membranes. The N-terminus of \(\upalpha \)-synuclein forms an amphiphilic \(\upalpha \)-helix that can associate with negatively charged membranes (Davidson et al. 1998; Pineda and Burré 2017). Amphiphilic \(\upalpha \)-helices are common in membrane-binding proteins as they energetically favour the hydrophilic–hydrophilic interface of membranes due to the hydrophobic effect (Khandelia et al. 2008). In addition, in case of \(\upalpha \)-synuclein electrostatic, an interactions exist between positively charged lysine residues within the helix structure and negatively charged lipid head groups that can stabilize the association of \(\upalpha \)-synuclein with membrane surfaces. Because of their asymmetrical segregation to hydrophobic and hydrophilic faces, amphiphilic \(\upalpha \)-helices tend to aggregate in aqueous solution. Indeed, deletion of a 12 amino acid section within the amphiphilic \(\upalpha \)-helix in the hydrophobic stretch of the NAC abrogates the ability of \(\upalpha \)-synuclein to aggregate (Giasson et al. 2001). In accordance with these results, all identified familial mutations in \(\upalpha \)-synuclein (A30P, E46K, A53T, G51D and H50Q) reside within the amphiphilic \(\upalpha \)-helix region (Lázaro et al. 2014). Further, as aggregation kinetics is often a function of expression level, it is interesting to note that increased expression of \(\upalpha \)-synuclein in patients with SNCA triplication results in early-onset Parkinsonism (Singleton et al. 2003) whereas patients with locus duplication results in a late onset. Although SNCA duplications are not a common cause of PD (Johnson et al. 2004) the study of SNCA multiplication indicated that \(\upalpha \)-synuclein pathogenesis is dosage dependent and that increased expression levels of \(\upalpha \)-synuclein can accelerate pathogenesis (Farrer et al. 2004).
It is interesting to note that \(\upalpha \)-synuclein preferentially binds to small liposomes (20–25 \(\mu \)m) rather than larger liposomes and also membranes that contain packing defects (Davidson et al. 1998; Drin and Antonny 2010). Due to its ability to bind liposomes based on size, \(\upalpha \)-synuclein is classified as a membrane curvature sensor. The ability to selectively bind vesicles with small curvature might be related to \(\upalpha \)-synuclein localization to synaptic vesicles (Drin and Antonny 2010). It also important to note that while the association of \(\upalpha \)-synuclein with membranes might be related to its endogenic function, it might also drastically affect its tendency to oligomerize by locally increasing the concentration of \(\upalpha \)-synuclein in the vicinity of membranes. Further, due to \(\upalpha \)-synuclein inclination to oligomerize, and because of its favourable association with membranes, it is likely that \(\upalpha \)-synuclein has a role in membrane remodelling.
Mitochondrial dysfunction in PD
By producing energy (ATP) mitochondria plays an essential role in all cell types. Besides energy production, mitochondria also plays a major role in variety of other cellular functions, such as the regulation of the intrinsic pathway of apoptosis, ion buffering, metabolism of lipids and production and regulation of reactive oxygen species (ROS). Mitochondrial impairment is often characterized by a number of damaging effects such as impaired oxidative phosphorylation that leads to decreased levels of ATP, an increase in the production of reactive oxygen species that can lead to a state of oxidative stress and cytochrome c leakage which can lead to apoptosis. All of which can have deleterious effect on cellular functions and even more so in the nervous system where metabolic requirements are more demanding. The constantly changing flux of \(\hbox {Ca}^{+2}\) that is crucial to maintaining neuronal excitability as well as the production and shipment of neurotransmitter vesicles are responsible alone for about 20% of the human body energy consumption (Kann and Kovács 2007). Further, neuronal cells that can span up to 1 m in length require efficient and highly dynamic transport of energy as well as metabolites (Hollenbeck 2005). With an increased rate of energy production postmeiotic neuronal cells must also tightly regulate the production and maintenance of reactive oxygen species which are a byproduct of oxidative phosphorylation and can lead to oxidative stress.
In almost all cell types, mitochondria forms a highly connected and dynamic tubular network that rapidly cycles between mitochondrial fusion and fission (Schrepfer and Scorrano 2016; Westermann 2010). Mitochondrial fusion and fission are both extremely important for mitochondrial fidelity and function: mitochondrial fission enables the proper radial distribution of the mitochondrial network in cells as well as the distribution of mitochondrial DNA in the mitochondrial network. Mitochondrial fission is also critical for the isolation and clearance of damaged mitochondria. Mitochondrial fission is mediated by Drp1, a dynamin related GTPase. Drp1 is a profission protein which is able to form constricting rings on the surface of the outer mitochondrial membrane by GTP hydrolysis. The rings apply mechanical strain on both the outer and inner membranes allowing the tubular mitochondria to separate (Westermann 2010; Smirnova et al. 1998). Mitochondrial fusion, on the other hand, e.g. is used for complementation of mitochondrial proteins. As mitochondrial DNA can become damage over time, mutated mitochondrial genes can lead to respiratory dysfunction; mitochondrial complementation increases the fidelity of aged mitochondria by exchanging proteins products between damaged and healthy mitochondria. The mechanism of mitochondrial fusion requires two homologue GTP-binding proteins to fuse the outer mitochondrial membranes: mitofusin 1 (MFN1) and mitofusin 2 (MFN2) while optic atrophy 1 (OPA1), a Dynamin-like protein, is required for fusion of the inner membrane of the mitochondria (Chen et al. 2003). The regulation of mitochondrial fusion and fission by direct interaction with the mitochondria or with the fusion or fission machinery is tightly linked to many mitochondrial functions and pathways. Consequently, mitochondrial morphology in neuronal cells is central to the pathogenesis of many neurodegenerative disorders (Lin and Beal 2006; Burté et al. 2015).
Toxin-based mitochondrial dysfunction associated with PD
The first evidence linking mitochondrial dysfunction and PD came to focus in the late seventies when accidental exposure to a material called 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a byproduct of an illicit drug 1-methyl-4-phenyl-4-propionoxy-piperidine (MPPP), resulted in four people developing features strikingly similar to Parkinson (Langston et al. 1983). An autopsy later reveled these patients displayed degeneration of dopaminergic neurons but without the presence of Lewy bodies. Subsequently, it was found that the onset of their symptoms was caused by the inhibition of complex I of the mitochondrial respiratory chain in dopaminergic neurons (Nicklas et al. 1987; Dauer and Przedborski 2003). Since then a number of mitochondria inhibitors such as rotenone and 6-OHDA have been reported to induce Parkinson-like symptoms in animal models (Betarbet et al. 2000). Although the death of neuronal cells is not only restricted to dopaminergic neurons, it has been suggested that catecholamine-secreting neurons are more susceptible to mitochondrial dysfunction as the production of dopamine is an additional source for the production of reactive oxygen species.
Mitochondrial functions of genes associated with PD
Tough only 5–10% of Parkinson patients carry a heritable form of PD, the identification of the pathogenic genes associated with PD have provided crucial clues to the pathways involved in idiopathic PD. Specific mutation in more than 10 genes so far has been associated with the familial form of PD including: of interest, mutations in the genes that encode PINK1, Parkin, and DJ-1 that result in autosomal recessive Parkinsonism, and mutations in the genes encoding leucine-rich repeat kinase 2 (LRRK2), \(\upalpha \)-synuclein (SNCA) and the recently identified: vacuolar protein sorting 35 (VPS35) cause the onset of the dominant form of PD (Trinh 2013; Farrer 2006). Of special interest in recent years are the genes: LRRK2, mutations in this gene are present in 13% of familial dominant inheritance PD cases and are responsible for up to 50% of all cases in some population (Farrer 2006), the genes that encode PINK1 and Parkin, two mitochondrial proteins that participate in autophagosomal clearance of dysfunctional mitochondria (Pickrell and Youle 2015), and DJ-1 which has been suggested to have a role as a sensor for oxidative stress and has been shown to act as a chaperone that prevents the aggregation of \(\upalpha \)-synuclein (Zhou et al. 2006).
PARKIN and PINK1
In recent years multiple findings have linked the disruption of mitochondrial dynamics to neurodegenerative disorders. In Parkinson, multiple pathways and proteins (table 1) associated with the disease have been implicated in modulating mitochondria morphology, autophagy, and fission or fusion of mitochondria (Poole et al. 2008; Michel et al. 2016; Bertholet et al. 2016). Both PINK1 and Parkin which are associated with autosomal recessive PD contribute to the mitochondria quality control by regulating the removal of dysfunctional mitochondria by mitophagy (Narendra et al. 2008; Pickrell and Youle 2015). Under normal physiological conditions, PINK1 is imported into the inner mitochondrial membrane by the mitochondrial import complexes TOM and TIM. Following the import of PINK1, a protease protein, presenilin-associated rhomboid-like protein (PARL) attaches to PINK1 and cleaves its N-terminus section. The truncated form of PINK1 is then released into the cytosol where it degraded by the proteasome system. As a consequence of the continuous importation and degradation of PINK1 under normal conditions, PINK1 levels on the outer mitochondrial membrane are maintained relatively low. In response to mitochondrial stress, protein import through the TOM complex is less efficient or blocked and therefore PARL cannot cleave PINK1 which accumulates on the outer mitochondrial membrane. The accumulation of PINK1 signals the cytosolic E3 ubiquitin-protein ligase, Parkin to ubiquitinate a wide range of mitochondrial and cytoplasmic (Yamano and Youle 2013; Jin et al. 2010). More than 36 proteins on the outer mitochondrial membrane are subjected to ubiquitination and are later degraded by the proteasome pathway (Chan et al. 2011). The removal and degradation of mitochondrial proteins appears to be necessary for mitophagy, however the signal for mitophagy might not be substrate specific but instead dependent on the density and the type of ubiquitin chains that Parkin attaches (Sarraf et al. 2013).
DJ-1
Mutations in the gene coding DJ-1 are associated with early onset of autosomal recessive Parkinsonism (Bonifati et al. 2003) and are linked to DJ-1 loss of function. DJ-1 is a 189 amino acids protein which forms a homodimer and that is expressed in all tissues including neuronal tissues (Bonifati et al. 2003). The precise biochemical functions of DJ-1 remain elusive, however DJ-1 has been suggested to participate in multiple pathways including the maintenance of reaction oxygen species homeostasis (Wilson 2011), regulation of gene expression (Biosa et al. 2017), chaperone activity (Shendelman et al. 2004) and the regulation of mitochondrial function (Hao et al. 2010). In cell models, knockdown of DJ-1 has been reported to render cells susceptible to oxidative stress (Taira et al. 2004). The regulation of oxidative stress by DJ-1 have been associated with DJ-1’s multiple cysteine residues (C46, C53 and C106) that enable DJ-1 to sense oxidative stress by undergoing oxidization, resulting in DJ-1 localization to the mitochondria (Canet-Avilés et al. 2004). The substitution of residue DJ-1’s C106 with another amino acid have been reported to abrogate the protective role of DJ-1 against neuronal death in response to oxidative stress (Canet-Avilés et al. 2004). In addition, the structure of DJ-1 was shown to be similar to that of Escherichia coli’s stress response chaperone, HSP31 (Lee et al. 2003). Due to its structural similarity to HSP31, it has been suggested that DJ-1 may act as a chaperone that is activated in response to oxidative stress (Shendelman et al. 2004) and might even be involved in inhibiting \(\upalpha \)-synuclein’s oligomerization (Zhou et al. 2006; Zondler et al. 2014). In addition, DJ-1 has been shown to be involved in regulation of the master transcription factor, Nrf2, which is associated with oxidative stress response (Moscovitz et al. 2015). In response to oxidative stress DJ-1 can bind to the 20S proteasome and inhibit its activity, stabilizing partially unfolded proteins (Moscovitz et al. 2015). In both Drosophila and human dopaminergic cells, deletion of DJ-1 result in mitochondrial swelling and dysfunction, poorly coupled mitochondria and shorted lifespan. Similar defects have been reported in flies with deletion of both Parkin and PINK1, interestingly, upregulation of DJ-1 has been reported to improve the mutant phenotypes in PINK1, but not Parkin mutants, suggesting that DJ-1 is important for mitochondrial quality control and may act parallel to PINK1 (Hao et al. 2010; Thomas et al. 2010).
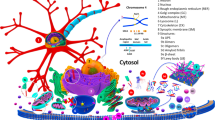
Overview of potential interaction between mitochondria and \(\upalpha \)-synuclein. Mitochondrial dysfunction have been shown to cause accumulation of protein misfolding in the cytosol. \(\upalpha \)-synuclein may interact with the TIM/TOM mitochondrial protein import complex and inhibit its function resulting in mitochondrial dysfunction. The outer mitochondrial membrane (OMM) may interact with misfolded \(\upalpha \)-synuclein resulting in membrane modeling leading to mitochondrial fission. Membrane interaction of \(\upalpha \)-synuclein may also increase misfolding of \(\upalpha \)-synuclein.
LRRK2
LRRK2 is large (286 kDa) cytosolic protein with multiple functional domains which is a member of the leucine-rich repeat kinase family. LRRK2 has been shown to interact and colocalize on the outer mitochondrial membrane with multiple members of the dynamin GTPase family that modulate the mitochondrial fusion/fission mechanism, including Drp1, Mfn1, Mfn2 and OPA (Stafa et al. 2013). Interestingly, overexpression of LRRK2 or PD-associated mutants of LRRK2 (R1441C or G2019S) has been demonstrated to increase mitochondrial fission in primary neuron cells. The mitochondrial fragmentation induced by LRRK2 can be blocked by overexpression of a dominant negative variant of DRP1, suggesting that PD-associated mutants of LRRK2 enhance the profission function of DRP1 (Wang et al. 2012). Neuronal cells model that expressed the most common pathogenic mutant of LRRK2, G2019S, in cells derived from induced pluripotent stem cells (iPSC) have been reported to have increased expression of stress-response genes and \(\upalpha \)-synuclein and high sensitivity to stress agents, such as hydrogen peroxide, MG-132, and 6-hydroxydopamine (Nguyen et al. 2011). Mutated LRRK2-G2019S has also been reported to inhibit chaperone-mediated autophagy and cause the accumulation of \(\upalpha \)-synuclein, which is a known substrate of the chaperone-mediated autophagy pathway (Orenstein et al. 2013).
VPS35
VPS35 is a gene which belongs to a group of vacuolar protein sorting (VPS) genes which encode components of the retromer complex. The retromer complex is involved in recycling of transmembrane proteins from endosomes to the trans-Golgi network and endosomes to the plasma membrane. VPS35 has recently emerged as a gene associated with autosomal-dominant PD. The mechanisms by which mutations in VSP35 lead to pathogenesis are still unclear, however several potential mechanisms have been suggested, including impaired binding of the WASH complex and inhibition of autophagy, disruption of receptor trafficking in dendritic spines that result in altered synaptic transmission, and induction of mitochondrial fragmentation by altering the turnover of the pro-fission, DRP1 protein (Williams et al. 2017). VPS35-deficient mice showed impaired recycling of lysosome-associated membrane glycoprotein 2a (Lamp2a), a receptor protein thought to be involved in chaperone-mediated autophagy of \(\upalpha \)-synuclein (Tang et al. 2015). VSP35 has also been implicated in vesicle transport between the mitochondria and peroxisomes (Braschi et al. 2010). In a recent study, mutation in VSP35 have been shown to increase the interaction of VPS35 with DRP1 resulting in its enhanced turnover leading to mitochondrial fragmentation and neurodegeneration (Wang et al. 2016).
Interaction of \(\upalpha \)-synuclein with the mitochondria
Under normal condition \(\upalpha \)-synuclein is found in a soluble state in the cytosol and only weakly associates with cellular membranes: such as the endoplasmic reticulum, synaptic vesicles and mitochondrial membrane. Nevertheless, there is evidence that suggests that \(\upalpha \)-synuclein (figure 1) is directly involved in mitochondrial dysfunction (Li et al. 2007; Devi et al. 2008; Nakamura et al. 2011). In both cultured cells and animal models overexpression of \(\upalpha \)-synuclein have been shown to cause the fragmentation of the mitochondrial network (Kamp et al. 2010; Nakamura et al. 2011). Although the mechanism leading to this morphology is not yet understood, it is interesting to note that mitochondrial fragmentation appears to occur independently of Drp1, the protein responsible for mitochondrial fission in mammalian cells. Instead, \(\upalpha \)-synuclein was reported to be able to alter the shape of mitochondria by directly associating with the mitochondrial membrane suggesting that \(\upalpha \)-synuclein has the ability to remodel mitochondrial membranes (Nakamura et al. 2011, 2013). Completing these finding are studies that showed that mice expressing mutant \(\upalpha \)-synuclein develop intraneuronal inclusion, mitochondrial DNA damage and fragmented mitochondria (Martin et al. 2006; Xie and Chung 2012). In addition, primary neuronal cells that overexpressed mutant \(\upalpha \)-synuclein, A53T, exhibited mitochondrial loss and increased mitochondrial mitophagy. Interestingly, cells in which mitochondrial fission was inhibited by the expression of a dominant negative form of the profission protein Drp1 or by overexpressing the profusion protein MFN2 mitochondrial loss was not observed. Similar results were reported for cells in which mitophagy pathway was inhibited by silencing Parkin indicating that mitochondrial morphology is linked mitochondrial autophagy (Choubey et al. 2011). Correspondingly, it was reported that knock-down of \(\upalpha \)-synuclein in C. elegans results in elongated mitochondria and that mitochondrial fragmentation induced by overexpression of \(\upalpha \)-synuclein can be rescued by overexpression of PINK1, Parkin or DJ-1 (Kamp et al. 2010).
In a study conducted on a rat model, chronic exposure to rotenone, which is a complex I inhibitor that causes oxidative stress and is toxin-model for PD, caused oxidative modification and redistribution of DJ-1 to the mitochondria and downregulation of proteasome activity that resulted in the aggregation of \(\upalpha \)-synuclein in both in vivo and in vitro models (Betarbet et al. 2006). More recently, it was reported that post-translationally dopamine modified species of \(\upalpha \)-synuclein could bind to the TOM20 complex, a mitochondrial receptor complex which together with TOM22 is responsible for the recognition and import of mitochondrial proteins from the cytosol. It was reported that binding of modified \(\upalpha \)-synuclein to the TOM20 complex resulted in its inability to interact with TOM22 and inhibited the import of mitochondrial protein causing a decrease in mitochondrial fidelity, loss of mitochondrial potential and an increase in reactive oxygen species in cultured cells (Di Maio et al. 2016). As mitochondrial targeting signals are often N-terminus amphiphilic \(\upalpha \)-helices, the authors suggested that under certain conditions, the amphiphilic \(\upalpha \)-helix located in the N-terminus of \(\upalpha \)-synuclein can interact and interfere with the TOM20 complex. Interestingly, it was reported that only small molecular weight oligomeric species but not monomeric or fibrillar forms of dopamine modified \(\upalpha \)-synuclein were cytotoxic. This point is also sustained by previous studies that showed that catecholamines related to dopamine can inhibit synuclein fibrillization therefore suggesting an explanation as to why dopaminergic neurons are more susceptible to damage in PD.
Regarding \(\upalpha \)-synuclein’s involvement in the processing of mitochondrial protein, it is interesting to note that recently other multiple studies have linked proteostatic stress to mitochondrial dysfunction. A study recently published in yeast cells showed that protein aggregates that form in the cytosol under stress interact with the mitochondrial import complex and molecular chaperones such as Hsp104 to enter the mitochondria via import receptors such as Tom70 followed by degradation by mitochondrial proteases. In addition, blocking mitochondrial import appeared to prevent the dissolution of aggregates. These findings suggest a protective role for mitochondria by maintaining cytosolic proteostasis (Conway et al. 2001). Another study done in yeast showed that in response to mitochondrial dysfunction and inefficient mitochondrial protein import can lead to cytotoxic accumulation of mitochondrial proteins that results in protein aggregation, subsequently leading to cellular degeneration (Ruan et al. 2017).
Several other potential mechanisms have been suggested that link \(\upalpha \)-synuclein to mitochondrial dysfunction and should be considered: Peroxisome proliferator-activated receptor \(\upgamma \) coactivator 1\(\upalpha \) (PGC-1\(\upalpha )\), is a master regulator of mitochondrial biogenesis and mitochondrial energy metabolism that has been reported to be downregulated in human PD brain as well in transgenic animal model and cultured cell expressing mutant \(\upalpha \)-synuclein (A30P). Correspondingly, downregulation of PGC-1\(\upalpha \) in cell culture neurons and PGC-1\(\upalpha \) deficient mice resulted in induction of cytotoxic \(\upalpha \)-synuclein oligomerization (Zheng et al. 2010). Other studies have suggested that \(\upalpha \)-synuclein might have a role in disrupting the mitochondria-associated endoplasmic reticulum membrane (MAM) subdomain. The MAM subdomain tethers mitochondria to the surface of the endoplasmic reticulum by a set of proteins that bridge the outer mitochondrial membrane to the endoplasmic reticulum membrane. The MAM domain is associated with regulating several fundamental cellular processes such as mitochondrial biogenesis, mitochondrial fission and fusion, and calcium buffering (Paillusson et al. 2016). Moreover, tau (microtubule-associated protein tau) has been shown to interact with \(\upalpha \)-synuclein and their interaction may lead to the aggregation of both proteins. For this reason, \(\upalpha \)-synuclein oligomers have been suggested to impair axonal transport machinery by decreasing microtubule stability (Oikawa et al. 2016).
Concluding remarks
In addition to providing most of the cellular ATP, mitochondria also play a central role in a wide variety of metabolic pathways and cellular functions. As a result, disruption of mitochondrial homeostasis may lead to cellular dysfunction and cell death. In this review, we have discussed different aspects of two major pathological hallmarks of PD: mitochondrial dysfunction and protein aggregation. These two seemingly unrelated responses to stress have had mounting genetic and biochemical evidence over the last two decades that linked them to PD. However, it is only recently that possible functional links between the aggregation of \(\upalpha \)-synuclein and mitochondrial function have come to light. Future studies will have to explore the molecular mechanisms that involve \(\upalpha \)-synuclein and mitochondria interaction and different pathways that relate the both mitochondrial dysfunction and protein quality control.
References
Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W. H., Castillo P. E. et al. 2000 Mice lacking \(\upalpha \)-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252.
Appel-Cresswell S., Vilarino-Guell C., Encarnacion M., Sherman H., Yu I., Shah B. et al. 2013 Alpha-synuclein p. H50Q a novel pathogenic mutation for Parkinson’s disease. Mov. Disord. 28, 811–813.
Bendor J. T., Logan T. P. and Edwards R. 2013 The function of \(\upalpha \)-synuclein. Neuron 79, 1044–1066.
Bertholet A. M., Delerue T., Millet A. M., Moulis M. F., David C., Daloyau M. et al. 2016 Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol. Dis. 90, 3–19.
Betarbet R., Sherer T. B., MacKenzie G., Garcia-Osuna M., Panov A. V. and Greenamyre J. T. 2000 Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neurosci. 3, 1301.
Betarbet R., Canet-Aviles R. M., Sherer T. B., Mastroberardino P. G., McLendon C., Kim J. H. et al. 2006 Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1 \(\upalpha \)-synuclein and the ubiquitin–proteasome system. Neurobiol. Dis. 22, 404–420.
Biosa A., Sandrelli F., Beltramini M., Greggio E., Bubacco L. and Bisaglia M. 2017 Recent findings on the physiological function of DJ-1: Beyond Parkinson’s disease. Neurobiol. Dis. 108, 65–72.
Bonifati V., Rizzu P., Van Baren M. J., Schaap O., Breedveld G. J. et al. 2003 Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259.
Braschi E., Goyon V., Zunino R., Mohanty A., Xu L. and McBride H. M. 2010 Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr. Biol. 20, 1310–1315.
Breydo L., Wu J. W. and Uversky V. N. 2012 \(\upalpha \)-Synuclein misfolding and Parkinson’s disease. Biochim. Biophys. Acta 1822, 261–285.
Brielle S., Gura R. and Kaganovich D. 2015 Imaging stress. Cell Stress Chaperones 20, 867–874.
Burré J. 2015 The synaptic function of \(\upalpha \)-synuclein. J. Parkinson Dis. 5, 699–713.
Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M. R. and Südhof T. C. 2010 \(\upalpha \)-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667.
Burté F., Carelli V., Chinnery P. F. and Yu-Wai-Man P. 2015 Disturbed mitochondrial dynamics and neurodegenerative disorders. Nature Rev. Neurol. 11, 11.
Cabin D. E., Shimazu K., Murphy D., Cole N. B., Gottschalk W., McIlwain K. L. et al. 2002 Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking \(\upalpha \)-synuclein. J. Neurosci. 22, 8797–8807.
Canet-Avilés R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C. Bandyopadhyay S. et al. 2004 The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 101, 9103–9108.
Chan N. C., Salazar A. M., Pham A. H., Sweredoski M. J., Kolawa N. J., Graham R. L. et al. 2011 Broad activation of the ubiquitin–proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 20, 1726–1737.
Chen H., Detmer S. A., Ewald A. J., Griffin E. E., Fraser S. E. and Chan D. C. 2003 Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200.
Cheng H. C., Ulane C. M. and Burke R. E. 2010 Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 67, 715–725.
Choubey V., Safiulina D., Vaarmann A., Cagalinec M., Wareski P., Kuum M. et al. 2011 Mutant A53T \(\upalpha \)-synuclein induces neuronal death by increasing mitochondrial autophagy. J. Biol. Chem. 286, 10814–10824.
Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Williamson R. E. and Lansbury P. T. 2000 Acceleration of oligomerization not fibrillization is a shared property of both \(\upalpha \)-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA 97, 571–576.
Conway K. A. Rochet J. C. Bieganski R. M. and Lansbury P. T. 2001 Kinetic stabilization of the \(\upalpha \)-synuclein protofibril by a dopamine-\(\upalpha \)-synuclein adduct. Science 294, 1346–1349.
Cremades N., Cohen S. I., Deas E., Abramov A. Y., Chen A. Y., Orte A. et al. 2012 Direct observation of the interconversion of normal and toxic forms of \(\upalpha \)-synuclein. Cell 149, 1048–1059.
Dauer W. and Przedborski S. 2003 Parkinson’s disease: mechanisms and models. Neuron 39, 889–909.
Davidson W. S., Jonas A., Clayton D. F. and George J. M. 1998 Stabilization of \(\upalpha \)-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273, 9443–9449.
Desplats P., Lee H. J., Bae E. J., Patrick C., Rockenstein E., Crews L. et al. 2009 Inclusion formation and neuronal cell death through neuron-to-neuron transmission of \(\upalpha \)-synuclein. Proc. Natl. Acad. Sci. USA 106, 13010–13015.
Devi L., Raghavendran V., Prabhu B. M., Avadhani N. G. and Anandatheerthavarada H. K. 2008 Mitochondrial import and accumulation of \(\upalpha \)-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 283, 9089–9100.
Di Maio R., Barrett P. J., Hoffman E. K., Barrett C. W., Zharikov A. Borah A. et al. 2016 \(\upalpha \)-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 8, 342ra78–342ra78.
Dijkstra A. A., Voorn P., Berendse H. W., Groenewegen H. J., Rozemuller A. J. and Berg W. D. 2014 Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov. Disord. 29, 1244–1251.
Drin G. and Antonny B. 2010 Amphipathic helices and membrane curvature. FEBS Lett. 584, 1840–1847.
Eliezer D., Kutluay E., Bussell Jr R. and Browne G. 2001 Conformational properties of \(\upalpha \)-synuclein in its free and lipid-associated states1. J. Mol. Biol. 307, 1061–1073.
Farrer M., Vrieze W. D., Crook R., Boles L., Perez-Tur J., Hardy J. et al. 1998 Low frequency of \(\upalpha \)-synuclein mutations in familial Parkinson’s disease. Ann. Neurol. 43, 394–397.
Farrer M., Kachergus J., Forno L., Lincoln S., Wang D. S., Hulihan M. et al. 2004 Comparison of kindreds with parkinsonism and \(\upalpha \)-synuclein genomic multiplications. Ann. Neurol. 55, 174–179.
Farrer M. J. 2006 Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet. 7, 306.
Feany M. B. and Bender W. W. 2000 A Drosophila model of Parkinson’s disease. Nature 404, 394.
Giasson B. I., Murray I. V., Trojanowski J. Q. and Lee V. M. Y. 2001 A hydrophobic stretch of 12 amino acid residues in the middle of \(\upalpha \)-synuclein is essential for filament assembly. J. Biol. Chem. 276, 2380–2386.
Hao L. Y., Giasson B. I. and Bonini N. M. 2010 DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc. Natl. Acad. Sci. USA 107, 9747–9752.
Hollenbeck P. J. 2005. Mitochondria and neurotransmission: evacuating the synapse. Neuron 47, 331–333.
Jin S. M., Lazarou M., Wang C., Kane L. A., Narendra D. P. and Youle R. J. 2010 Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942.
Jo E., McLaurin J., Yip C. M., George-Hyslop P. S. and Fraser P. E. 2000 \(\upalpha \)-Synuclein membrane interactions and lipid specificity. J. Biol. Chem. 275, 34328–34334.
Johnson J., Hague S. M., Hanson M., Gibson A., Wilson K. E., Evans E. W. et al. 2004 SNCA multiplication is not a common cause of Parkinson disease or dementia with Lewy bodies. Neurology 63, 554–556.
Kaganovich D. 2017 There is an inclusion for that: material properties of protein granules provide a platform for building diverse cellular functions. Trends Biochem. Sci. 42, 765–776.
Kaganovich D., Kopito R. and Frydman J. 2008 Misfolded proteins partition between two distinct quality control compartments. Nature 454, 1088.
Kamp F., Exner N., Lutz A. K., Wender N., Hegermann J., Brunner B. et al. 2010 Inhibition of mitochondrial fusion by \(\upalpha \)-synuclein is rescued by PINK1 Parkin and DJ-1. EMBO J. 29, 3571–3589.
Kann O. and Kovács R. 2007 Mitochondria and neuronal activity. Am. J. Physiol. Cell Physiol. 292, C641–C657.
Khandelia H., Ipsen J. H. and Mouritsen O. G. 2008 The impact of peptides on lipid membranes. Biochim. Biophys. Acta. 1778, 1528–1536.
Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M. Sigfried K. et al. 1998 AlaSOPro mutation in the gene encoding \(\upalpha \)-synuclein in Parkinson’s disease. Nat. Genet. 18, 101–108.
Kuwahara T., Koyama A., Gengyo-Ando K., Masuda M., Kowa H., Tsunoda M. et al. 2006 Familial Parkinson mutant \(\upalpha \)-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J. Biol. Chem. 281, 334–340.
Langston J. W., Ballard P., Tetrud J. W. and Irwin I. 1983 Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980.
Lashuel H. A., Overk C. R., Oueslati A. and Masliah E. 2013 The many faces of \(\upalpha \)-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 14, 38.
Lázaro D. F., Rodrigues E. F., Langohr R., Shahpasandzadeh H., Ribeiro T., Guerreiro P. et al. 2014 Systematic comparison of the effects of alpha-synuclein mutations on its oligomerization and aggregation. PLoS Genet. 10, e1004741.
Lee S. J., Kim S. J., Kim I. K., Ko J., Jeong C. S., Kim et al. 2003 Crystal structures of human DJ-1 and Escherichia coli Hsp31 which share an evolutionarily conserved domain. J. Biol. Chem. 278, 44552–44559.
Lesage S., Anheim M., Letournel F., Bousset L., Honoré A., Rozas N. et al. 2013 G51D \(\upalpha \)-synuclein mutation causes a novel Parkinsonian–pyramidal syndrome. Ann. Neurol. 73, 459–471.
Li W. W., Yang R., Guo J. C., Ren H. M., Zha X. L., Chenget al. 2007 Localization of \(\upalpha \)-synuclein to mitochondria within midbrain of mice. Neuroreport 18, 1543–1546.
Lin M. T. and Beal M. F. 2006 Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787.
Martin L. J., Pan Y., Price A. C., Sterling W., Copeland N. G., Jenkins et al. 2006 Parkinson’s disease \(\upalpha \)-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 26, 41–50.
Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A. et al. 2000 Dopaminergic loss and inclusion body formation in \(\upalpha \)-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269.
Michel P. P., Hirsch E. C. and Hunot S. 2016 Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 90, 675–691.
Moscovitz O., Ben-Nissan G., Fainer I., Pollack D., Mizrachi L. and Sharon M. 2015 The Parkinson’s-associated protein DJ-1 regulates the 20S proteasome. Nat. Commun. 6, 6609.
Murphy D. D., Rueter S. M., Trojanowski J. Q. and Lee V. M. Y. 2000 Synucleins are developmentally expressed and \(\upalpha \)-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 20, 3214–3220.
Nakamura K. 2013 \(\upalpha \)-Synuclein and mitochondria: partners in crime? Neurotherapeutics 10, 391–399.
Nakamura K., Nemani V. M., Azarbal F., Skibinski G., Levy J. M., Egami K. et al. 2011 Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein \(\upalpha \)-synuclein. J. Biol. Chem. 286, 20710–20726.
Narendra D., Tanaka A., Suen D. F. and Youle R. J. 2008 Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803.
Nemani V. M., Lu W., Berge V., Nakamura K., Onoa B., Lee M. K. et al. 2010 Increased expression of \(\upalpha \)-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65, 66–79.
Nguyen H. N., Byers B., Cord B., Shcheglovitov A., Byrne J., Gujar P. et al. 2011 LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8, 267–280.
Nicklas W. J., Youngster S. K., Kindt M. V. and Heikkila R. E. 1987 IV. MPTP MPP+ and mitochondrial function. Life Sci. 40, 721–729.
Nussbaum R. L. 2017 The identification of alpha-synuclein as the first Parkinson disease gene. J. Parkinsons Dis. 7(s1), S43–S49.
Oikawa T., Nonaka T., Terada M., Tamaoka A., Hisanaga S. I. and Hasegawa M. 2016 \(\upalpha \)-Synuclein fibrils exhibit gain of toxic function promoting tau aggregation and inhibiting microtubule assembly. J. Biol. Chem. 291, 15046–15056.
Orenstein S. J., Kuo S. H., Tasset I., Arias E., Koga H., Fernandez-Carasa I. et al. 2013 Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 16, 394.
Paillusson S., Stoica R., Gomez-Suaga P., Lau D. H., Mueller S., Miller T. et al. 2016 There’s something wrong with my MAM; the ER–mitochondria axis and neurodegenerative diseases. Trends Neurosci. 39, 146–157.
Payton J. E., Perrin R. J., Woods W. S. and George J. M. 2004 Structural determinants of PLD2 inhibition by \(\upalpha \)-synuclein. J. Mol. Biol. 337, 1001–1009.
Pickrell A. M. and Youle R. J. 2015 The roles of PINK1 parkin and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273.
Pineda A. and Burré J. 2017 Modulating membrane binding of \(\upalpha \)-synuclein as a therapeutic strategy. Proc. Nat. Acad. Sci. USA 114, 1223–1225.
Poewe W., Seppi K., Tanner C. M., Halliday G. M., Brundin P. Volkmann J. et al. 2017 Parkinson disease. Nat. Rev. Dis. Primers 3, 17013.
Polymeropoulos M. H., Higgins J. J., Golbe L. I., Johnson W. G., Ide S. E., Di Iorioet al. 1996 Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23. Science 274, 1197–1199.
Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A. et al. 1997 Mutation in the \(\upalpha \)-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047.
Poole A. C., Thomas R. E., Andrews L. A., McBride H. M., Whitworth A. J. and Pallanck L. J. 2008 The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Nat. Acad. Sci. USA 105, 1638–1643.
Ruan L., Zhou C., Jin E., Kucharavy A., Zhang Y., Wen Z. et al. 2017 Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 543, 443.
Sarraf S. A., Raman M., Guarani-Pereira V., Sowa M. E., Huttlin E. L., Gygi S. P. et al. 2013 Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496, 372.
Schrepfer E. and Scorrano L. 2016 Mitofusins from mitochondria to metabolism. Mol. Cell 61, 683–694.
Shendelman S., Jonason A., Martinat C., Leete T. and Abeliovich A. 2004 DJ-1 is a redox-dependent molecular chaperone that inhibits \(\upalpha \)-synuclein aggregate formation. PLoS Biol. 2, p.e362.
Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kacherguset al. 2003 \(\upalpha \)-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841.
Smirnova E., Shurland D. L., Ryazantsev S. N. and van der Bliek A. M. 1998 A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 143, 351–358.
Spillantini M. G., Schmidt M. L., Lee V. M. Y., Trojanowski J. Q., Jakes R. and Goedert M. 1997 \(\upalpha \)-Synuclein in Lewy bodies. Nature 388, 839.
Stafa K., Tsika E., Moser R., Musso A., Glauser L., Jones A. et al. 2013 Functional interaction of Parkinson’s disease-associated LRRK2 with members of the dynamin GTPase superfamily. Hum. Mol. Genet. 23. 2055–2077.
Taira T., Saito Y., Niki T., Iguchi-Ariga S. M., Takahashi K. and Ariga H. 2004 DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 5, 213–218.
Tanaka M., Kim Y. M., Lee G., Junn E., Iwatsubo T. and Mouradian M. M. 2004. Aggresomes formed by \(\upalpha \)-synuclein and synphilin-1 are cytoprotective. J. Biol. Chem. 279, 4625–4631.
Tang F. L., Erion J. R., Tian Y., Liu W., Yin D. M., Ye J. et al. 2015 VPS35 in dopamine neurons is required for endosome-to-Golgi retrieval of Lamp2a a receptor of chaperone-mediated autophagy that is critical for \(\upalpha \)-synuclein degradation and prevention of pathogenesis of Parkinson’s disease. J. Neurosci. 35, 10613–10628.
Thomas K. J., McCoy M. K., Blackinton J., Beilina A., van der Brug M., Sandebring A. et al. 2010 DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum. Mol. Genet. 20, 40–50.
Trinh J. and Farrer M. 2013 Advances in the genetics of Parkinson disease. Nat. Rov. Neurol. 9, 445.
Ulmer T. S., Bax A., Cole N. B. and Nussbaum R. L 2005 Structure and dynamics of micelle-bound human \(\upalpha \)-synuclein. J. Biol. Chem. 280, 9595–9603.
Uversky V. N. 2007 Neuropathology biochemistry and biophysics of \(\upalpha \)-synuclein aggregation. J. Neurochem. 103, 17–37.
Wang W., Wang X., Fujioka H., Hoppel C., Whone A. L., Caldwell M. A. 2016 Parkinson’s disease–associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat. Med. 22, 54.
Wang X., Yan M. H., Fujioka H., Liu J., Wilson-Delfosse A., Chen S. G. et al. 2012 LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 21, 1931–1944.
Westermann B. 2010 Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 11, 872.
Williams E. T., Chen X. and Moore D. J. 2017 VPS35 the retromer complex and Parkinson’s disease. J. Parkinsons Dis. 7, 219–233.
Wilson M. A. 2011 The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid. Redox Signal. 15, 111–122.
Wood S. J., Wypych J., Steavenson S., Louis J. C., Citron M. and Biere A. L. 1999 \(\upalpha \)-Synuclein fibrillogenesis is nucleation-dependent implications for the pathogenesis of Parkinson\(\prime \) s disease. J. Biol. Chem. 274, 19509–19512.
Xie W. and Chung K. K. 2012 Alpha-synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson’s disease. J. Neurochem. 122, 404–414.
Yamano K. and Youle R. J. 2013 PINK1 is degraded through the N-end rule pathway. Autophagy 9, 1758–1769.
Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R. Ampuero I. et al. 2004 The new mutation E46K of \(\upalpha \)-synuclein causes parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173.
Zheng B., Liao Z., Locascio J. J., Lesniak K. A., Roderick S. S., Watt M. L. et al. 2010 PGC-1\(\upalpha \) a potential therapeutic target for early intervention in Parkinson’s disease. Sci. Transl. Med. 2, 52ra73–52ra73.
Zhou W., Zhu M., Wilson M. A., Petsko G. A. and Fink A. L. 2006 The oxidation state of DJ-1 regulates its chaperone activity toward \(\upalpha \)-synuclein. J. Mol. Biol. 356, 1036–1048.
Zondler L., Miller-Fleming L., Repici M., Gonçalves S., Tenreiro S., Rosado-Ramos R. et al. 2014 DJ-1 interactions with \(\upalpha \)-synuclein attenuate aggregation and cellular toxicity in models of Parkinson’s disease. Cell Death Dis. 5, e1350.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Brielle, S., Kaganovich, D. Mitochondrial dysfunction in protein conformational disorders. J Genet 97, 703–713 (2018). https://doi.org/10.1007/s12041-018-0958-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-018-0958-0