Abstract
The paper reviews the problem due to the use and disposal of synthetic polymers to the environment and its solutions; in particular poly (ethylene terphthalate). Wide spread application and non-biodegradability of the PET creates huge amounts of waste and disposal, tend to a serious problem. The most important cause for recycling and reprocessing the waste PET has arisen from the awareness and concern for environmental pollution. To manage this various methods of polymer recycling has been proposed. Among them chemical recycling, i.e. hydrolysis, methanolysis, glycolysis and aminolysis are reviewed in detail. Appropriate technology and waste disposal procedures based on the socio-economic aspect to solve this problem are suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In search of a better material for our advancing technologies the polymer scientist have all these years invested much efforts, energy and money to develop man-made polymers that are strong, stable and durable. Polymers have become the important materials for science and technology development, and high standard of living. The modern society cannot live or progress without polymers. Synthetic polymers have become very versatile and useful materials for modern technology. Because polymers are low cost and can be easily fabricated to consumer products by fast automated machines, they have been widely used in form of packaging materials for farm, forest, dairy products and other consumer items. The usual strength and durability of polymers, however desirable they may be create threat to environmental pollution when these are discarded after use. That the synthetic polymers, unlike natural ones, do not rust or rot or not easily degraded in the outdoor environment, they accumulate in the garbage dump site and cause litter. Thus most of the applications of synthetic polymers are based on their relative resistance to environmental degradation including biodegradation.
Extensive use of polymeric materials, lead to the waste disposal management difficulties. Incineration helps produce energy but has problem of emission of toxic fumes and gases due to the decomposition of polymer chain molecules and particular additives present. Land filling of plastics is not preferred because of space constraints and land pollution. On the other hand recycled polymers are also not a permanent solution either since recycling led to the poor quality product.
Due to this problem, polymer industry, both manufacturing as well as processing, poses a positive threat to the clean environment. If these industrial operations are not carefully carried out and controlled there exists a very serious problem of pollution of the environment. Polymer scientists are conscious about it and have seized the problem face to face. The problem of disposal of waste plastics is complex, and requires active participation of industry, government and the public.
Though the most important cause for recycling and reprocessing the waste plastics has arisen from the awareness and concern for environmental pollution, it is also important due to the urge of conservation of costly and scarce energy and feedstock. Also discarded polymers represent a colossal waste of energy embodied in them. If these are recovered, even partly, a large amount of scarce and costly resources for energy and chemical feed stocks can be conserved. Also polymer recycling is related to the national economy where the hydrocarbons feed stocks is both scarce and costly. The high price of virgin polymers and cheap labor are the driving forces for recycling of waste plastics.
The paper reviews the problem due to the use and disposal of poly (ethylene terphthalate) to the environment. To solve this problem various method of polymer recycling has been presented.
PET
PET is an acronym for polyethylene terephthalate, which is a long-chain polymer belonging to the generic family of polyesters [1]. PET is formed from the intermediates, terephthalic acid (TPA) and ethylene glycol (EG), which are both derived from oil feedstock. There are other polyesters based on different intermediates but all are formed by a polymerisation reaction between an acid and an alcohol. PET, in its purest form, is an amorphous glass-like material. Under the influence of direct modifying additives it develops crystallinity. Also, crystallinity can be developed by heat treatment of the polymer melt. Originally patented and exploited by DuPont during the search for new fibre-forming polymers [2, 3], polyester fibre applications have developed to such an extent that PET represents over 50% of world synthetic fibre manufacture [4]. PET is used alone or blended with cotton or wool to impart better wash/wear and crease resistant properties to textiles. In the late 1950s PET was developed as a film. It was first used for video, photographic and X-ray films in addition to uses in flexible packaging. Later PET was modified for use in injection moulded and extruded articles, primarily reinforced with glass fibre. In the early 1970s PET was stretched by blow moulding techniques which produced the first oriented three dimensional structures initiating the rapid exploitation of PET as lightweight, tough, unbreakable bottles [5]. Many companies produce virgin PET globally giving it different trade name [6, 7]. Some of the common trade names of commercially available PET are summarized in table.
Trade name | Manufacture |
|---|---|
Arnite | DSM Engineering Plastics |
Diolen | ENKA-Glazstoff |
Eastapac | Eastman chemical company |
Hostadur | Farbwerke Hoechst AG |
Mylar | E.I.Du Point de Nemours & Co. Inc |
Melinex | Imperial Chemical Indusatries Ltd |
Rynite | Du Point de Nemours & Co. Inc |
The Manufacture of PET
Polyesters are made by the reaction of bi-functional acids and alcohols, in the presence of a metal catalyst. The key polymerization step is known as a condensation reaction in which molecules react and release a simple by-product. This is followed by a second polymerization reaction, which occurs in the solid phase. For manufacture of PET the intermediates, pure terephthalic acid (TPA) and ethylene glycol (ethanediol), are derived from crude oil. When heated together the first product is a monomer (BHET - bis-hydroxyethyl-terephthalate) mixed with low molecular weight polymers (oligomers). The mixture then reacts further, distils out excess ethylene glycol and forms the PET (Fig. 1). At this stage the PET is a viscous molten liquid. It is extruded, and water quenched to form a glasslike amorphous material. Some PET is also manufactured using technology based on the dimethyl ester of terephthalic acid (DMT). The required high molecular weight PET is manufactured by a second polymerisation stage carried out in the solid state at lower temperatures. This effectively removes all volatile impurities, like acetaldehyde, free glycols and water. The high molecular weight is essential for good mechanical properties providing stiffness, toughness and creep resistance while, at the same time, giving sufficient flexibility to resist bursting and breaking under pressure.
Once the polymer is formed it is very difficult to purify and for this reason the purity of the starting materials is the key factor. Vacuum distillation processes easily purify ethylene glycol whilst terephthalic acid is purified by repeated crystallization. Such high purity and high molecular weight materials are needed for food packaging applications. Catalysts are used at extremely low concentrations to promote the reactions and ensure practical economics. The most common catalyst is antimony trioxide but salts of titanium, germanium, cobalt, manganese, magnesium and zinc are also used and small amounts remain encapsulated into the polymer matrix or in the polymer chain itself.
But in the laboratory, PET is made by other reactions. Terephthalic acid and ethylene glycol can polymerize to make PET when it is heated with an acid catalyst. It’s possible to make PET from terephthoyl chloride and ethylene glycol. This reaction is easier, but terephthoyl chloride is more expensive than terephthalic acid, and it’s a lot more dangerous.

The Physical and Chemical Properties of PET
PET exhibits interesting physical properties (morphology). PET, in its purest form, is an amorphous glass-like material. Under the influence of direct modifying additives it develops crystallinity. Also, crystallinity can be developed by heat treatment of the polymer melt. PET is classed as a semi-crystalline polymer and when heated above 72 °C changes from a rigid glass-like state into a rubbery elastic form where the polymer molecular chains can be stretched and aligned in either one direction to form fibers, or in two directions to form films and bottles. Because of its rather high transition temperature only a limited amount of crystallization can occur during cooling after injection molding poly (ethylene terphthalate). Such moldings are transparent and amorphous and are of little value. If the material melt is cooled quickly, while still held in the stretched state, then the chains are frozen, with their orientation remaining intact. Once set in this stretched state the material is extremely tough and confers the properties we see in a typical PET bottle. If the PET is held in the stretched form at temperatures above 72 °C it slowly crystallizes and the material starts to become opaque, more rigid and less flexible. It is then known as crystalline PET or CPET. In this form it is capable of withstanding higher temperatures and can be used for trays and containers capable of withstanding moderate oven temperatures. It is this ‘heat setting’ technique which also develops the crease and wash resistance properties of polyester textiles. Careful manipulation between each of these forms generates a wide range of different products, which are all variants of the same basic chemical formula of PET.
The PET described so far is the simplest typical product. However, many modifications are introduced to develop specific properties for the various packaging applications and to suit particular manufacturing equipment. Usually the modifications are of a chemically nature to make manipulation of the PET between different crystalline forms easier. For example, small concentrations of an appropriate co-monomer (isophthalic acid—IPA or 1, 4-cyclohexanedimethanol) slow down the rate of crystallization and allow the manufacture of thicker bottle walls, sheets and films. A typical example would be in the heavier, thicker bottles used for refillable container systems. There is also a requirement to extend the rate of crystallization to restrict movement and deformation at elevated temperatures, for example in oven able food trays. In this case a nucleating agent or crystallization promoter is employed and the molecular weight is increased. PET is becoming the package of choice for many food products, particularly beverages and mineral waters. The main reasons for its popularity are the properties of glass-like transparency coupled with adequate gas barrier properties for retention of carbonation. Also it exhibits a high toughness/weight property ratio which allows lightweight, large capacity safe unbreakable containers.
Application of PET
For more than 100 years, plastic products have revolutionized the way we live. Polyethylene terephthalate, or PET, is a particularly notable example. Global consumption of PET for packaging is valued at $17 billion this year, and is forecast to reach $24 billion by 2011. Asia Pacific, central and eastern Europe, and parts of Latin America showed the strongest growth between 2001 and 2006. The United State is the largest user of PET packaging, followed by China and Mexico. Polyethylene terephthalate is a thermoplastic polymer resin of the polyester family that produced by the chemical industry and is used in synthetic fibers; beverage, food and other liquid containers; thermoforming applications; and engineering resins often in combination with glass fiber. It is one of the most important raw materials used in man-made fibers. It also used for microwave food trays and food packaging films. This is due in part to its inherent properties that are well suited for lightweight, large-capacity and shatter-resistant containers. Because it provides an excellent barrier against oxygen and carbon dioxide and due to consumer trend favoring healthier beverage options, the carbonated soft drink sector has been growing more rapidly than other applications in the past five years.
Because of this wide spread application the PET waste disposal poses a serious problem to maintain a clean environment. However, the most important cause for recycle and reprocess the waste PET has arisen from the awareness and concern for environmental pollution. PET recycling represents one of the most successful and widespread examples of polymer recycling. The main driving force responsible for this increased recycling of post-consumer PET is its widespread use, particularly in the beverage industry. A very important feature of PET, decisive in the choice of its wide application in the manufacture of packaging for the food industries is that it does not have any side effects on the human beings. It should be pointed out, that PET does not create a direct hazard to the environment, but due to its substantial fraction by volume in the waste stream and its high resistance to the atmospheric and biological agents, it is seen as a noxious material. Therefore, the recycling of PET does not only serve as a partial solution to the solid waste problem but also contributes to the conservation of raw petrochemical products and energy. Products made from recycled plastics can result in 50–60% energy saving as compared to making the same product from virgin resin.
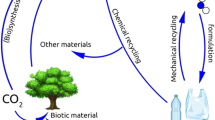
The recycling of waste polymers including PET can be carried out in many ways. Four main classes have been proposed.
Classification of Polymer Recycle
Polymer recycle can be classified into four categories e.g. Primary, secondary, tertiary and quaternary recycling.
-
1.
Primary recycling (pre-consumer industrial scrap)
It is the recycling of clean, uncontaminated single-type waste which remains the most popular, as it ensures simplicity and low cost, especially when done “in-plant” and feeding with scrap of controlled history [8]. The recycled scrap or waste is either mixed with virgin material to assure product quality or used as a second-grade material [9]. Primary recycling of industrial scrap produced during the manufacture of food-contact articles is not expected to pose a hazard to the consumer.
-
2.
Mechanical recycling (secondary recycling)
In this approach, the polymer is separated from its associated contaminants and it can be readily reprocessed into granules by conventional melt extrusion. Mechanical recycling includes the sorting and separation of the wastes, size reduction; melt filtration and reforming of the plastic material. The basic polymer is not altered during the process. The main disadvantage of this type of recycling is the deterioration of product properties in every cycle. This occurs since the molecular weight of the recycled resin is reduced due to chain-scission reactions caused by the presence of water and trace acidic impurities. A secondary recycling process presents some unique problems that may cause it to be inappropriate for the production of food-contact articles, particularly if the recycler had little or no control over the waste stream entering the recycling facility [10–12].
-
3.
Chemical recycling (tertiary recycling)
Unlike physical recycling, chemical recycling involves transformation of polymer chain. The polymer backbone under the recycling process is degraded into monomer units (i.e. depolymerisation) or randomly ruptured into larger chain fragments (i.e. random chain scission) with associated formation of gaseous products. The chemical recycling is carried out either by solvolysis or by pyrolysis; the former through degradation by solvents including water, and the latter through degradation by heat in absence of oxygen or air, or vacuum. Chemical recycling yields monomers, petroleum liquids and gases. Monomers are purified by distillation and drying, and used for manufacture of polymers.
Energy Recovery (Quaternary Recycling)
The energy content of the plastics waste can be recovered by incineration. When the collection, sorting and separation of plastics waste are difficult or economically not viable, or the waste is toxic and hazardous to handle, the best waste management option is incineration to recover the chemical energy stored in plastics waste in the form of thermal energy. This is carried out in special type of reactors called incinerators, to burn wastes in the presence of air in a controlled manner to convert hydrocarbons of the plastic into carbon dioxide and water. The heat produced by burning plastics in the waste in the form of superheated steam can be utilized for generating electricity through turbine generators, and the residual heat from the waste stream for heating residential and industrial buildings. The melt residue from the incinerator is free from toxicity hazards and may be disposed off by landfill.
Although polymers are actually high yielding energy sources, this method has been widely accused of being ecologically unacceptable owing to the health risk from air borne toxic substances such as dioxins (in the case of chlorine containing polymers). It should admit that it is not possible to have zero-emission in the incineration of waste plastic. Apart from the aforementioned methods, direct reuse of a plastic material (i.e., PET) could be considered as a ‘‘zero-order’’ recycling technique. [8] In a lot of countries, it is a common practice for PET bottles to be refilled and reused. However, this should be done with great care since plastic bottles are more likely than glass to absorb contaminants that could be released back into food when the bottle is refilled. Moreover, refilling of a PET bottle with a drink with high alcohol content may lead to degradation of the macromolecular chains with unexpected results. Worldwide, the main end-use of post-consumed PET is for the production of fibers (almost 70%), with only 4% of PET recycled with chemical methods.
Among the above recycling techniques, the only one acceptable according to the principles of sustainable development (development that meets the needs of the present generation without compromising the ability of future generations to meet their needs) is chemical recycling, since it leads to the formation of the raw materials (monomers) from which the polymer is made [9]. In this way the environment is not surcharged and there is no need for extra resources (monomers) for the production of PET.
PET Chemical-Recycling Techniques
The world’s most recyclable polymer is polyester. PET is polyester with functional ester groups that can be cleaved by some reagents, such as water (hydrolysis), alcohols (alcoholysis), acids (acidolysis), glycols (glycolysis), and amines (aminolysis). The recycled PET is mostly used in the form of fibres, films, foams, sheets, bottles etc. Thus, chemical-recycling processes for PET are divided as follows: (i) hydrolysis, (ii) glycolysis, (iii) methanolysis and (iv) other processes.
The chemical recycling of PET is discussed in detail, below.

According to the reagent used, different products are obtained. The main depolymerization processes that have reached commercial maturity up to now are glycolysis and methanolysis.
Hydrolysis
Nowadays there is growing interest in hydrolysis for the chemical recycling of PET, since it is the only method with the reaction products terphthalic acid (TPA) and ethylene glycol (EG), i.e. the monomer from which PET is produced. This is associated with the trend in the new factories for PET synthesis to produce it directly from TPA and EG, thus replacing dimethyl terphthalate (the traditional monomer) from the technological process. The main disadvantage of this method is the use of high temperature (200–250 °C) and pressure (1.4–2 MPa) as well as long time needed for complete depolymerisation. Commercially, hydrolysis is not widely used to produce food-grade recycled PET, because of the cost associated with purification of the recycled TPA.
Hydrolysis of PET can be carried out as (a) alkaline hydrolysis, (b) acid hydrolysis and (c) neutral hydrolysis.
Alkaline Hydrolysis
Alkaline hydrolysis of PET is usually carried out with the use of an aqueous alkaline solution of NaOH or KOH, of a concentration of 4–20 wt.% [13, 14]. The reaction products are EG and the disodium or dipotassium terephthalate salt, according to the chemical reaction shown below.

The mixture is heated up to 340 °C to evaporate and recover the EG by distillation. Pure TPA can be obtained by neutralization of the reaction mixture with a strong mineral acid (e.g., H2SO4), as shown below.

The detailed mechanism of degradation in an alkali environment is shown below.

The process runs for 3–5 h at temperatures of 210–250 °C, under a pressure of 1.4–2 MPa [15]. Very good results of PET alkaline hydrolysis are achieved using an aqueous ammonia solution at 200 °C. In this case a solution of TPA diammonium salt is formed, from which, after filtration and acidification with sulfuric acid, TPA of high purity (99 wt%) is obtained [16].
Furthermore, detailed reaction kinetics of PET depolymerization in a KOH solution were investigated using a pressurized autoclave [17]. Kinetic data at reaction temperatures below the PET melting point were obtained and a possible reaction mechanism was proposed.
Yoshioka investigated the chemical recycling of PET flakes to TPA and oxalic acid by simultaneous hydrolysis and oxygen oxidation in concentrated NaOH [18]. PET flakes were hydrolyzed to sodium terephthalate and ethylene glycol (EG) in NaOH solutions before oxygen introduction. Because sparingly soluble sodium terephthalate in concentrated NaOH solutions was stable to the oxidation, the TPA yield was approximately 100 mol% under all conditions. In contrast, EG was oxidized to oxalate and CO2, and the maximum oxalic acid yields Thus, apart from the monomer (TPA) a valuable byproduct (oxalic acid) was obtained.
Factors such as temperature, time and alkali concentration influencing the kinetics of the alkaline depolymerization of PET in NaOH solution. Also the choice of solvent plays an important role in the alkaline depolymerisation of PET, were also investigated by Ramsden and Phillips [19].
The main advantage of alkaline hydrolysis is that it can tolerate highly contaminated, post-consumer PET such as magnetic recording tape, metallized PET film, or photographic film (X-ray film) [14]. The process is relatively simple and less costly than methanolysis.
PET hydrolysis in aqueous alkaline solute ion was investigated by Karayannidis [20]. PET decomposition was conducted in a 2 L stainless-steel autoclave reactor equipped with a digital temperature-control system, an agitator, and a manometer as a pressure indicator. The reaction took place with a constant NaOH concentration and different reaction-time intervals and temperatures. At the appropriate time the reactor was cooled and the reaction mixture was neutralized to pH 6.5 with H2SO4 and filtered to remove unreacted PET solids. The TPA in the mixture was precipitated by acidification with H2SO4 to pH 2.5 and the mixture was filtered and washed with methanol. The solid TPA produced was dried in an oven at 80 °C and weighed. A great increase in the TPA yield on increasing the reaction temperature was observed. This is expected if the chemical reaction is the rate-determining step. At the highest-studied temperature of 200 °C a TPA yield of 98% was obtained in only 1 h.
PET Hydrolysis in a Non-Aqueous Alkaline Solution
It has been observed that addition of an ether (such as dioxane or THF) in nonaqueous alkali solutions accelerates the rate of chemical degradation of PET [21]. One possible explanation is that ethers accelerate the percolation of hydroxide ions and increase the ionic strength of the hydroxide ion and, therefore, the decomposition of PET is increased. Methyl Cellulose is a chemical compound combining the properties of ether together with those of an alcohol. It is for this reason that this substance was selected for this study. The ether part will lead to swelling of the PET solid and the alcoholic part will support the action of KOH in destroying the chemical structure of PET during depolymerization (PET surface is easily attacked by alcohols). The addition of an ether (such as dioxane, or tetrahydrofuran (THF)) as a mixed solvent with an alcohol (methanol, or ethanol) accelerated the chemical degradation of PET. It is for this reason that the alkaline hydrolysis of PET at 110–120 °C with a non-aqueous solution of KOH in methyl cellulose was selected to be studied by Karayannidis [20]. Pellets of potassium hydroxide were dissolved in methyl cellulose and the alkali hydroxide solution was added to a glass reaction vessel equipped with a reflux condenser, an inert-gas flow, a stirrer and a heating device. The reactor was immersed in a silicon-oil bath in order to obtain high enough temperatures. PET flakes were added into the reactor and the inert-gas flow was started, together with the agitation. After a certain period the inert-gas flow stopped and the temperature was set to the desired point. After reaching the desired temperature, the reaction time started and the PET decomposition was followed for a specified time period. After that time, the reaction mixture was cooled rapidly by immersing the flask in cold water. The mixture was then filtered to remove the un-decomposed PET solids and dried in an oven at 110 °C. In the final product, 500 mL of distilled water were added, in order to dissolve all of the potassium terephthalate. The solution was filtered again and the procedure described previously for the isolation of TPA was followed.
Pitat have patented a method of PET alkaline hydrolysis by an 18 wt% solution of NaOH [22]. The most advantageous results are achieved at a PET NaOH weight ratio of 1:20, at about 100 °C in 2 h. Sodium salt of terephthalic acid formed in the reaction is relatively well-soluble in aqueous solutions of alkaline hydroxides; however, by maintaining the concentration of NaOH at a constant level of 18 wt%, it is possible to achieve its complete precipitation. After separation it is dissolved in a small amount of water so as to obtain a nearly saturated solution. After acidification, TPA is precipitated from the solution, filtered off, rinsed, and dried. Other than TPA, EG formed during the reaction remains in the aqueous phase. This is recycled into the process after its enrichment in NaOH. The EG content in a solution increases, and therefore its recovery by vacuum distillation becomes feasible. An aqueous alcoholic solution instead of an aqueous solution of alkaline hydroxide should be used in order to decrease the solubility of TPA sodium salt in the reaction mixture. The process can be run either in high pressure conditions or in pressure less conditions as well as in conditions using lower hydroxide concentrations [22].
Lazarus described a process allowing the recovery of TPA and other monomeric components from PET/polyamide polymeric mixtures [23]. In the first stage, the mixture is heated in an aqueous solution of sodium or potassium hydroxide; most favorable results are achieved at temperatures of 210–250 °C under autogenic pressure. Technologically the most advantageous is a weight ratio of the polymer mixture to water within a range of 1:2–1:3. If hydroxide solutions of a concentration of 3–10 wt% are used, then the reaction time amounts to 3–5 h. The quantity of alkali used is dependent on the polyester content of the polymer mixture. After the termination of the reaction, the mixture is filtered in order to remove the insoluble residue, and then a strong mineral acid is added so that the dicarboxylic acid being formed is released. The generated caprolactam and EG are separated by distillation or are salted out using NaCl.
A significant reduction of TPA impurities generated in alkaline processes of PET hydrolysis can be achieved by introducing an additional stage consisting of the oxidation of impurities and thereby converting them into insoluble forms [24]. The process can be additionally improved through the application of quaternary ammonium hydroxide or nonionic surface-active agents which accelerate the depolymerization reaction. After the hydrolysis is finished, the post reaction mixture is diluted and the precipitate is separated; the remaining solution is supersaturated with air. The precipitated hardly soluble impurities are filtered off, and the filtrate is subsequently acidified in order to separate TPA.
Very good results of PET alkaline hydrolysis are achieved using an aqueous ammonia solution at 200 °C. In this case a solution of TPA diammonium salt is formed, from which, after filtration and acidification with sulfuric acid, TPA of high purity (99 wt%) is obtained [16].
An interesting solution is the alkaline hydrolysis of a mixture of PET waste and methyl benzoate formed as a byproduct of the oxidation of p-xylene to TPA [25]. In the first stage PET is treated with methyl benzoate at temperature of 190–200 °C. The mixture obtained undergoes hydrolysis by an aqueous solution of alkali-metal hydroxide with a concentration of 2–7 wt% for 30 min at temperatures of 95–100 °C. The process allows the recovery of TPA and benzoic acid with yields of 87–95% and 84–89%, respectively.
Treatment processes based on partial PET alkaline hydrolysis are widely used in the polyester fiber industry. The effect of such processes on the mechanical properties of fibers [26–28], oligomer content and change of the molecular weight distribution [28, 29], or loss of fiber mass [27, 29] has been investigated. From the point of view of research on PET chemical recycling, an interesting relationship between PET mass loss, reaction time, and the concentration of the NaOH aqueous solution used [26], as well as between oligomer contents and the molecular weight distribution of degraded PET [29] has been observed. Collins and Zeronian have demonstrated that NaOH solutions in methanol react with PET significantly faster than analogous aqueous solutions.
Namboori and Haith have compared the reactivity of NaOH aqueous solutions, as well as solutions of sodium tert-butoxide in tert-butanol, sodium isopropoxide in isopropyl alcohol, sodium methoxide in methanol, and sodium ethoxide in ethanol with PET. They have demonstrated that, of the above-mentioned solutions, sodium ethoxide in ethanol is the most reactive and an aqueous solution of sodium hydroxide is the least reactive.
In the recycling of PET to terephthalates of alkali metals or alkaline-earth metals, a process described by Benzaria may be crucial [30]. The depolymerization is carried out in a mixer-extruder with the use of solid NaOH at temperatures of 100–200 °C. After the distillation of EG from the post reaction mixture under reduced pressure, a corresponding salt of terephthalic acid in the form of a powder is obtained. In this method the necessity of separating the glycol and water mixture is eliminated, which is undoubtedly its essential advantage. The degree of polyester saponification achieved a level of about 97%.
Alkaline Hydrolysis in the Presence of a Phase Transfer Catalyst
Phase transfer catalyzed alkali decomposition of PET taken from post-consumer soft-drink bottles was revealed to be an efficient method for the reproduction of pure terephthalic acid. The kinetics of the depolymerization reaction was extensively studied. The effects of temperature, alkali concentration, PET particle size, PET concentration and catalyst to PET ratio on the TPA yield were investigated. This method had been applied in PET fibers as well as Nylon-46 and Nylon-66 fibers [31]. Very good results were obtained for the depolymerization of PET and the yield of TPA was as high as 93%. Kosmidis [32] extended the use of the phase-transfer catalyst in the depolymerization of PET flakes taken from waste soft-drink bottles and the reaction kinetics was extensively studied [32].
Acid Hydrolysis
Acid hydrolysis is performed most frequently using concentrated sulfuric acid, although other mineral acids such as nitric or phosphoric acid have also been employed. In order to avoid high pressures and temperatures in the reaction vessel, a concentrated sulfuric acid (>14.5 M) has been proposed by Pusztaszeri, Brown, O’Brien and Sharma [33–35]. However, the process becomes very costly due to the need to recycle large amounts of concentrated H2SO4 and the purification of EG from the sulfuric acid. TPA recovery from PET scrap material in concentrated sulfuric acid at 60–93 °C has been also described [34] (acid concentration of, at least, 87 wt.%). EG was recovered from the final filtrate through extraction with organic solvents such as trichloroethylene [34]. In another patent, the production of pure TPA was described by acid hydrolysis of PET in a 90 wt.% H2SO4 solution at 85–90 °C [35]. A substantial drawback of PET hydrolysis by concentrated sulfuric acid is the high corrosiveness of the reaction system and the generation of large quantities of inorganic salts and aqueous wastes. Yoshioka [36] proposed an acid hydrolysis of waste PET powder in relatively dilute sulfuric acid (<10 M) and the reuse of the sulfuric acid by recovery methods such as dialysis. However, this requires long reaction times (5 h) and an increase in the reaction temperature (150 °C) [36, 37]. Yoshioka [36] also described a process for the depolymerization of PET powder from waste bottles using nitric acid (7–13 M) at 70–100 °C for 72 h. TPA and EG were produced and the resultant EG was simultaneously oxidized to oxalic acid. The proposed method had the advantage of resulting in value-added products such as oxalic acid, which is more expensive than TPA and EG. Hydrolysis of PET was also carried out in concentrated sulfuric acid (96 wt.%) and at room temperature in order to investigate the structure of the materials obtained [38]. An increase in crystallinity with reaction time was observed. Acidic hydrolysis of PET was also reported by Mehrabzadeh [39]. The effect of different parameters, such as acid concentration, time, temperature and PET particle size, on the decomposition and reaction yield was investigated there.
Acid hydrolysis of PET in sulfuric acid at different temperatures and solution concentrations was reported by Achilias and Karayannidis [20]. The depolymerization reaction was carried out in a 0.5 L reactor equipped with a reflux condenser and a magnetic agitator. The required amount of the sulfuric-acid solution (70–83 wt.%), together with the PET flakes, was added into the reactor and heated to the desired reaction temperature (between 30 and 90 °C). The agitation started in order to keep the mixture homogeneous and the reflux condenser set. The reaction time started and the mixture was allowed to react for 3–5 h. afterwards, the mixture was filtered to separate the TPA produced and the unreacted PET. A solution of KOH was added to the solid product. In this way the TPA reacted to form the dipotassium salt, while PET remained unreacted. Finally, the mixture was filtered again, dried in an oven until it had a constant weight and weighed in order to calculate the percentage of unreacted PET. The depolymerization reaction of PET with water in an acid (H2SO4) environment proceeds according to the reaction in below Scheme 1.
The detailed mechanism of acid depolymerisation is as under.

The effect of the sulfuric acid concentration on the degree of PET degradation tell us that at 90 °C an almost complete decomposition of the PET to its monomers is achieved only with a very concentrated acid solution (greater than 80 wt.%). If the acid concentration is less than 76 wt.-percent the depolymerization reaction is very slow. This means that if one wanted to carry out the reaction at temperatures lower than 100 °C, acid-corrosion resistant equipment is required. Thus, if the appropriate acid concentration for the dissolution at the pre-specified temperature is met, then the reaction occurs much more easily. The effect of depolymerization temperature on the amount of unreacted PET tell us that for an 80 wt.% H2SO4 solution It can be seen that the amount of unreacted PET begins to decrease at a temperature of 60 °C and much more at 70 °C (27 wt.%), while if the reaction is carried out at temperatures lower than 50 °C, PET degradation does not occur to a great extent. Hence, at this acid concentration, a temperature of 70 °C would be adequate for the degradation of PET.
Neutral Hydrolysis
Neutral hydrolysis is carried out with the use of water or steam. In spite of this, the pH of the post reaction mixture amounts to 3.5–4.0, which according to Michalski [40] is caused by the formation of TPA monoglycol ester during the reaction. The process usually runs at a pressure of 1–4 MPa at temperatures of 200–300 °C [41–44]. The ratio by weight of PET to water is from 1:2 to 1:12.
Launay [45] have described the process’ kinetics at temperatures of 100 °C, whereas Campanelli [46] have described the application of the process in PET recycling. It has been confirmed that PET hydrolysis proceeds significantly faster in the molten state than as a solid; therefore, it is advantageous to carry out recycling using this method at temperatures higher than 245 °C. The application of common transesterification catalysts is possible; however, the recommended ones are alkali-metal acetates [23].
Michalski has conducted studies on the influence of polymer synthesis catalysts contained in commercial PET, i.e., the first stage (transesterification), the acetates of calcium, manganese, and zinc; the second stage (polycondensation), antimony trioxide as well as the stabilizers blocking transesterification (added between the first and second stage), i.e., phosphorus compounds [47]. Michalski’s investigations have proved the accelerating action of the transesterification catalysts. No inhibiting effects of stabilizers were observed, and in some cases their action had an accelerating effect.
Campanelli have described the catalytic effect of zinc catalysts at temperatures of 250–265 °C [44]. They have found the rate constant to be about 20% greater than in the uncatalyzed system. The catalytic effect of zinc salt as well as sodium salt is attributed to the electrolytic destabilization of the polymer-water interface in the hydrolysis process. During the PET hydrolysis, monoester of glycol and terephthalic acid is formed as a byproduct. It dissolves well in water at temperatures of 95–100 °C; at these temperatures TPA is practically insoluble. Owing to this, the separation of TPA from the post reaction mixture does not create any problems [48].
Using statistical methods, it has been proved that appropriate control of reaction conditions limits to not more than 2% the quantity of monoester obtained [48, 49]. The neutral hydrolysis method is exempt from the primary drawbacks characteristic for acid or alkaline hydrolysis. The formation of substantial quantities of inorganic salts difficult to dispose of is avoided; also problems connected with the corrosion of apparatus due to the use of concentrated acids and alkalis do not occur. An undoubted advantage of neutral hydrolysis is its high ecological purity, and therefore growing interest in this technology can be expected. Its drawback is that all mechanical impurities present in the polymer are left in the TPA; thus, the product has a considerably worse purity than the product of acid or alkaline hydrolysis. Consequently, a much more sophisticated purification process is necessary. Possible product contaminations are removed by filtration of the solution of TPA dissolved in caprolactam or in an aqueous solution of sodium hydroxide [48]. The crystallization of TPA from caprolactam makes it possible to obtain a product with a purity of at least 99% [50]. During the hydrolysis of PET a substantial volume of diluted EG is generated, which can be recovered through extraction or by distillation. An effective five-stage process of neutral hydrolysis of PET to EG and TPA of a purity required for the synthesis of the new polymer has been patented by Tustin [51]. PET is hydrolyzed at temperatures of 200–280 °C. After cooling the post reaction mixture to 70–100 °C, the solid product of the process is filtered and dried at temperatures of 25–199 °C. EG is recovered from the filtrate as a result of two-stage distillation. The solid product of hydrolysis is heated with water at temperatures of 310–370 °C, and after cooling TPA is obtained. The purity of the recovered TPA and EG allows their application in the production of high-quality homo- and copolymers and does not exclude their use in the manufacture of bottles and fibers.
Kamal have presented an efficient process of continuous hydrolysis in which they have used a twin-screw extruder as a reactor [52]. Using this method, it is possible to obtain in an efficient manner PET oligomers containing 2–3 repeating units. Those products with end carboxyl groups have higher melting temperatures in comparison with virgin PET, while those with one carboxyl group and one or two hydroxyl groups have lower melting points. It has been demonstrated that utilization of cold or even hot water in the process does not give a satisfying degree of depolymerization. There is a difference when high-pressure saturated steam of temperatures close to that of molten PET is injected into the reaction zone of the extruder. The maintenance of adequate high pressures requires the application of suitable throttling systems, allowing the control of backleakage of the postreaction mixture from the extruder.
Aminolysis
Aminolysis processes have seldom been used for PET chemical recycling, at least for deep polymer degradation. The superficial partial aminolytic degradation of PET fibers has been the subject of numerous research studies [27, 53–59], and is currently applied on an industrial scale. Such modification processes improve the quality of fiber coloration and other technical and application parameters of the fibers. From the available literature, it can be seen that in most cases the aminolytic modification processes of PET fiber surfaces are conducted using primary amines in aqueous solutions [27, 53–58] or less commonly in the gas form [58]. The most frequently used amines are methylamine [54], [55–57] ethylamine [54, 57], and butylamine [57, 58]. Other amines used are ethanolamine [57] and triethylenetetramine [59].
Poppola proposed a mechanism of aminolytic degradation of PET based on the example of n-butylamine [56]. Literature references concerning the deep aminolytic degradation of PET (or other polyesters) are very few [60], and applications of the products of such a process on a commercial scale are unknown.
There are some known examples of polymer solvolysis using glycols in conjunction with alkanolamines [61, 62], but these are often used in low amounts as catalytic agents.
Aminolysis is another method of chemical degradation of PET, which has been little explored as compared to other techniques. Depolymerisation of the PET waste using different amines such as allylamine [63], morpholine and hydrazine [64], and polyamines [65] has been investigated at the Polish laboratories. PET waste when treated for 2 h with an excess of allylamine at 170 °C under pressure of 2 MPa gave the product N, N′-bisallyl terephthalamide. This product with high melting temperature 217 to 219 °C may be considered as a high-temperature solid cross linking agent for unsaturated polyester compositions. Deep aminolysis of PET yields corresponding diamides of TPA and EG. There are no known reports concerning the utilization of this process on a commercial scale in PET recycling. However, it is known that partial aminolysis has found its application in the improvement of PET properties [66], in the manufacture of fibers with defined processing properties. In the majority of PET aminolysis processes described, the polymer was in the form of powder or fibers. The reaction was usually carried out using primary amine aqueous solutions, most frequently methylamine [55, 56, 66], ethylamine [27, 66], and ethanolamine [66] in the temperature range of 20–100 °C. Anhydrous n-butylamine was also applied as an aminolytic agent at a temperature of 21 °C [66].
The simple chemicals like glacial acetic acid or simple salts are found to be capable of depolymerising PET fibre waste through aminolysis was studied by S.R. Shukla, Ajay M. Harad [67]. With this process, the yield and purity of the chemically reactive product BHETA are of high order with much less reaction time. The product of depolymerisation has the potential of recycling it into useful products through various chemical reactions. They have used ethanolamine for the aminolysis of PET waste materials in the molar ratio 1:6 (PET:ethanolamine) under reflux in the presence of different catalysts for time periods varying up to 8 h. The catalysts, namely glacial acetic acid, sodium acetate and potassium sulphate, were used in concentrations ranging between 0.3 and 1.5% by weight of polymer. At the end of the reaction, distilled water was added in excess to the reaction mixture with vigorous agitation to precipitate out the product, bis(2-hydroxy ethylene)terephthalamide (BHETA). The filtrate contained mainly unreacted ethanolamine and little quantities of a few water soluble PET degradation products. The precipitate obtained was filtered and dissolved in distilled water by boiling for about 30 min. White crystalline powder of BHETA was obtained by first concentrating the filtrate by boiling and then chilling it. It was further purified by recrystallisation in water. It was then dried in an oven at 80 °C and weighed for estimating the yield. Different techniques of analysis were used for its characterization. In this reaction, ethanolamine has two nucleophilic centers. Nitrogen is more electronegative than oxygen. The amine group of ethanolamine attacks on the ester linkage of PET. The catalysts used are glacial acetic acid, sodium acetate and potassium sulphate, which form complexes with the carbonyl group [68, 69] and increase its polarity.
Zahn and Pfeifer [70] carried out aminolysis of PET with solutions of hydrazine, benzyl amine, ethylene diamine, hexamethylene diamine, piperidine and aniline. They obtained different reaction products as the diamides of terephthalic acid, which do not possess any potential for further chemical reactions. According to Popoola [56], the basicity of an amine relative to water as well as its steric hindrance due to size decides the rate of degradation of PET. During aminolysis of PET with methylamine, the methyl terephthalamide is obtained, which is not enough reactive for its recycling into any useful product through further reactions.
Most of these investigations have been focused on selective PET degradation by an aqueous solution of methylamine so as to determine its morphology. The presumption was that, under the influence of primary amine aqueous solutions, the amorphous region would first undergo rapid degradation, and subsequently a significantly slower attack would take place on the crystalline regions [53].
Ammonolysis
TPA amide is produced by the action of anhydrous ammonia on PET in an ethylene glycol environment. This can be converted into terephthalic acid nitrile and further to p-xylylenediamine or 1,4-bis(aminoethyl)cyclohexane [71]. Very good results were obtained from the ammonolysis of PET waste from postconsumer bottles; the process was carried out under a pressure of about 2 MPa in a temperature range of 120–180 °C for 1–7 h. After the reaction was completed, the amide produced is filtered, rinsed with water, and dried at a temperature of 80 °C. The product has a purity of not less than 99%, and the yield is above 90% [71]. A low-pressure method of PET ammonolysis, in which the degradation agent is ammonia in an ethylene glycol environment, is also known. The process is catalyzed by zinc acetate in a quantity of 0.05 wt%, conducted at a temperature of 70 °C and a ratio of PET-NH3 of 1:6. TPA amide was produced with a yield of about 87%.
Methanolysis
This process consists of the degradation of PET by methanol at high temperatures and under high-pressure conditions. The main products of PET methanolysis are dimethyl terephthalate (DMT) and ethylene glycol (EG) [72], which is raw materials necessary for the production of this polymer. Catalysts such as zinc acetate, magnesium acetate, cobalt acetate, and lead dioxide enhance the reaction; however, the most commonly used catalyst is zinc acetate [73, 74]. There are examples of using arylsulfonic acid salts as catalysts for methanolytic degradation of PET [75].
Methods for the conduction of methanolysis have similar basic parameters, e.g., pressures of 2–4 MPa and temperatures of 180–280 °C [76–78]. The polymer degradation takes place with the release of ethylene glycol. After the termination of the reaction, it is necessary to deactivate the catalyst. Otherwise, in subsequent stages of the process, there could occur is possible DMT losses as a result of transesterification with ethylene glycol. The DMT obtained is precipitated from the post reaction mixture after its previous cooling and then is centrifuged and crystallized.

The Kodak Co. possesses a patent describing a process of PET methanolysis and its optimum properties [79]. Another approach to methanolysis was presented in a patent concerning a continuous, two stage process for terephthalic acid (TPA) reclamation from PETW [78]. There are solutions proposing a combination of high temperature PET methanolysis with esterification of TPA or with products of p-xylene oxidation [80]. It has been observed that the yield of DMT from PET methanolysis processes does not exceed 90% [77]. Researchers [81] have presented a method of methanolysis of polymer blends. This allows recovery of dimethyl esters of the corresponding dicarboxylic acid and glycol. A method was developed in which residue from EG rectification is utilized in PET methanolysis processes in the presence of a catalyst [82–85].
The methanolysis process is used by large PET manufacturers such as Hoechst [86] and Eastman [87] as well as lesser manufacturers [88]. The main advantage of this method is that an installation of methanolysis can be located in the polymer production line, since the DMT produced has a product quality identical to virgin DMT. Also, ethylene glycol and methanol can be easily recovered and recycled. In this way, waste PET arising in the production cycle is used and the monomers recovered can be re-used in the manufacture of a full value polymer. Disadvantages of the method include the high cost associated with the separation and refining of the mixture of the reaction products (glycols, alcohols and phthalate derivatives). If water perturbs the process, it poisons the catalyst and forms various azeotropes. However, the main disadvantage is associated with the trend of all of the new PET production processes to use TPA instead of DMT as the raw material. The conversion of the DMT produced by hydrolysis to TPA adds considerable cost to the methanolysis process.
Supercritical fluids have been focused on for depolymerization of PET because of their reactivity. The supercritical fluid over its critical point has high density, such as in a liquid state, and high kinetic energy as in a gas molecule. Therefore the reaction rate is expected to be higher than the reaction under liquid state conditions. In recent years, the supercritical fluids, supercritical water (Tc = 674.3 K, Pc = 22.0 MPa) [89] and supercritical methanol (Tc = 512.3 K, Pc = 8.09 MPa) [90], have been introduced to the depolymerization of PET. PET is depolymerized in supercritical fluids quickly by solvolysis. PET, having an ether bond between terephthalic acid and ethylene glycol (EG), is easily decomposed to its monomers by solvolysis in supercritical water, supercritical methanol, or supercritical ethanol [90–99]. PET hydrolysis with supercritical water has very high reaction rate, but this process is not easy to operate in practice due to the sever reaction conditions (above 670 K, 30 MPa). In addition, the hydrolysis leads to low yield of ethylene glycol (about 20%). Compared with supercritical hydrolysis, supercritical methanolysis can be operated at relatively mild conditions. Sako et al. [90] proposed the methanolytic depolymerisation of PET to DMT and ethylene glycol under the supercritical state of methanol, and investigate the influence of the reaction pressure and time on the yield of decomposition products at relative higher reaction temperature (573 K). Subsequently, they determined the rate constant of decomposition of PET with methanol from 453 to 623 K and reported that the methanolysis in supercritical methanol produced both monomers, dimethyl terephthalate (DMT) and EG, with almost 100% yield in 30 min without a catalyst [93].
The kinetics of poly (ethylene terephthalate) (PET) depolymerization in supercritical methanol was investigated by to de Motonobu Goto [100], develop a chemical recycling process for postconsumer PET bottles. PET with a high molecular weight was depolymerized in a batch reactor at temperatures between 543 and 573 K under estimated pressures of 0.1–15 MPa. In addition to PET with high molecular weight, PET with low molecular weight, such as its oligomer (trimer), bis-hydroxyethyl terephthalate (BHET), and methyl-(2-hydroxyethyl) terephthalate (MHET), was used as a model reactant to clarify the depolymerization pathway of poly(ethylene terephthalate) in supercritical methanol.
Kumazawa [101] studied the depolymerization of polyethyleneterephthalate (PET) in supercritical methanol was carried out using a batch-type autoclave reactor. The total conversion and the yield of dimethylterephthalate (DMT) increased with rising temperature. The final yield of DMT at 300 °Cand 310 °C reached 97.0% and 97.7%, respectively. The yield of DMT was markedly increased when the methanol density was 0.08 g/cm3, and leveled off at higher densities. A kinetic model to describe the depolymerization of PET in supercritical methanol was proposed, where the scission of one ester linkage in PET by a methanol molecule produces one carboxymethyl different temperatures were determined by comparing the observed time dependence of carboxymethyl group concentration with that calculated by the proposed model.
Hongwei Xiang also studied the depolymerisation of PET polyester rapidly and completely into its monomer DMT under the super critical state of methanol. The degree of depolymerisation and selectivity for dimethyl terphthalate increased with an increase of the ratio of methanol to PET, reaction temperature, and reaction time, while pressure had little influence on either of them when the pressure of the reaction exceeds the critical pressure of methanol. According to results of their experiments, the optimal reaction conditions are reaction time of 40–60 min, temperature of 533–543 K pressure of 9.0–11.0 MPa and weight ratio (methanol to PET) of 6–8. Under the optimal reaction conditions PET wastes can readily depolymerised to monomers [102].
Glycolysis
Another most important method in chemical processing of PET is glycolysis. This process is used widely on a commercial scale. The glycolysis reaction is the molecular degradation of PET polymer by glycols, in the presence of trans-esterification catalysts, mainly metal acetates, where ester linkages are broken and replaced with hydroxyl terminals.

PET degradation is carried out most frequently using ethylene glycol [103–109], diethylene glycol [110–112], propylene glycol [111–113], and dipropylene glycol [108, 113] etc. Research concerning this process has been mainly conducted from the point of view of the utilization of the products obtained; very few works have been devoted to the description of the kinetics of glycolysis reactions [105, 107, 114]. The process is conducted in a wide range of temperatures 180–250 °C [114–118], during a time period of 0.5–8 h. Usually 0.5% by weight of catalyst (most often zinc acetate) in relation to the PET content is added. Results of research on the catalytic influence of NaCl, urea, and BHET on the process are given by Nevrekar and Sheth [106]. The best results were achieved when BHET was used as the catalyst.
Much attention has been devoted to glycolysis by EG. In this system, the effect of the reaction parameters, i.e., temperature (190–240 °C), pressure (0.1–0.6 MPa) and PET to EG ratio on the reaction rate has been investigated [107]. It has been observed that the rate of the reaction is proportional to the square of the EG concentration at constant temperature, pressure, and PET concentration.
PET glycolyzates find application in the manufacture of unsaturated polyester resins [111, 112, 116, 118–123], polyurethane foams [109, 113, 115, 124–127], and polyisocyanurate foams [103, 105–108, 114, 125, 127].
One of the first methods of the synthesis of unsaturated polyester resins (UPR), in which a product of partial PET glycolysis was used, was developed by Ostrysz [128]. The product of partial PET glycolysis was applied together with maleic anhydride and propylene glycol so as to obtain unsaturated polyester.
After dissolving the synthesized polyester alkyd in styrene, UPR resin was obtained. During the following years The Industrial Chemistry Research Institute in Warsaw developed technology for the production of UPR with built-in segments of oligo(ethylene terephthalate) obtained as a result of partial PET waste glycolysis with propylene glycol in ratios 0.25–1.0 mol/mol of PET at a temperature of 200 °C (240–250 °C) and a reaction time of 2 h [110, 118]. Due to difficulties in obtaining a glycolyzate with reproducible properties, a new type of unsaturated polyester has been evolved, containing ethylene-diethylene diester obtained as a result of PET degradation by diethylene glycol as its terephthalic part. This resin was used in the production of polyester molding compounds [129].
Recently there is an increased interest in the manufacture of UPR, utilizing PET waste. In one of the patents [123], PET glycolysis products undergo a reaction with maleic anhydride and subsequently a reaction with di-cyclopentadiene. The polyesters obtained have wide possibilities of application, e.g., for gel coats, casting marble, bath fixtures, car elements, etc.
Interesting research results on the synthesis and viscosity of UPR obtained by polycondensation of PET glycolysis product (with propylene or dipropylene glycol) and maleic anhydride have been published by Kim [116]. It has been observed that the molecular weights of UPR increase with an increase of PET content in the reaction system or an increase of the dicarboxylic acid/glycol ratio as well as in the case of the application of dipropylene glycol instead of propylene glycol in the same conditions of glycolysis.
Methods of synthesis of cross-linked polyesters have also been developed; PET glycolyzate and dimethyl glutarate were used as raw materials [130], and the products are subsequently used in polyurethane production. The syntheses of polyester polyols containing polyterephthalate segments for the production of some types of polyurethanes require, in the case of direct utilization of pure terephthalic acid, the solution of the problem of TPA sublimation. This can be avoided with simultaneous ecological advantages, by using oligomeric products of PET glycolysis, which subsequently react with adipic acid [109, 113, 117]. The kinetics of this reaction has been described by Vaidya and Nadkarni [113]. Such polyester polyols in reaction with di-phenyl methanediisocyanate (MDI) [113, 117] or toluene-2, 4-diisocyanate (TDI) [117] enable the manufacture of polyurethanes with various properties.
Another approach was described by Speranza [126], who presented a method of manufacture of stable clear polyester polyols from the product of PET glycolysis reacted with alkylene oxides, e.g., propylene oxide.
Polyester polyols obtained during the reaction of PET with alkylene oxides, in the presence of a catalyst are used in the production of polyurethane or polycyanurate foams as an admixture to the traditional polyols, thus obtaining materials with enhanced fire resistance [131].
In some works biphenols are used for PET degradation, e.g., Bisphenol-A at temperatures of 190–230 °C, in autoclave conditions [132]. The influence of the process parameters and the reaction mechanism composed of the six elementary equilibration reactions was investigated.
The physicochemical properties of about 100 polyesterdiols obtained by glycolysis of post-consuming poly (ethylene terphthalate) waste were studied by Myriam Billiaue-Loreau to correlate the properties of the polyols and their chemical composition [133].
PET waste can be depolymerized by glycolysis to obtain oligomeric diols and polyols, or glycolyzed into its monomeric units, bis(2-hydroxyethyl) terephthalate (BHET) or dimethyl terephthalate [32, 134–136].
Jean-Jacques Robin studied the synthesis of a new type of glycolysates using an oligoester type of compound instead of a diol as described in numerous papers. The physical and thermal properties of the resulting glycolysates are linked to the nature of the diacid and diol segment involved in the oligoesters [137].
The influence of various parameters on the kinetics of poly (ethylene terephthalate) (PET) glycolysis by diethylene glycol (DEG), namely temperature (from 190 to 220 °C), temperature profile, catalysis and PET morphology has been studied by Francis Pardal [138]. The results showed a strong influence of some experimental conditions (temperature and catalysis) on the mixture evolution during depolymerisation. The temperature study showed a critical temperature between 210 and 220 °C which seems to be the consequence of a better diffusion of DEG in PET, allowing easier reactions in solid phase. The initial morphology of PET scraps does not affect the rates of reactions much, in contrast to the temperature profile which has a great importance: time of PET dissolution at 220 °C is considerably shorter by heating PET and DEG separately at 220 °C before mixing, than by heating a cold mixture of the two reagents to 220 °C.
Poly(ethylene terephthalate) [PET] fibre wastes from an industrial manufacturer was depolymerised using excess ethylene glycol [EG] in the presence of metal acetate as a transesterification catalyst. The glycolysis reactions were carried out at the boiling point of ethylene glycol under nitrogen atmosphere up to 10 h. Influences of the reaction time, volume of EG, catalysts and their concentrations on the yield of the glycolysis products were investigated by M. Ghaemy [139].
The methods of glycolytic depolymerization with catalyst optimization technique described by A.S Goje and S. Mishra reveal that it is possible to obtain almost complete and optimal conversion of PET into value added monomeric products (EG and DMT). Optimal reactant size is recorded as 127.5 mm. Depolymerization of PET was increased with increase in the reaction time and temperature. Yields (%) of value added monomeric products (DMT and EG) are almost equal to PET conversion. Results suggest that EG does not have a significant role as an internal catalyst in glycolysis of PET. Depolymerization of PET was decreased with increase in the particle size of PET. Zinc salt as well as cobalt salt show identical results and the numerical values were greater than that of lead salt and manganese salt during glycolytic depolymerization of PET. Zinc salt and cobalt salt appear to have more catalytic effect on glycolysis seem to influence rates at atmospheric pressure [140].
In another studies postconsumer PET bottles including water and soft-drink bottles were depolymerized by glycolysis in excess glycols, such as ethylene glycol, propylene glycol, and diethylene glycol, in the presence of a zinc acetate catalyst by V. Pimpan. The obtained glycolyzed products were reacted with maleic anhydride and mixed with a styrene monomer to prepare unsaturated polyester (UPE) resins. These resins were cured using methyl ethyl ketone peroxide (MEKPO) as an initiator and cobalt octoate as an accelerator. The physical and mechanical properties of the cured samples were investigated. It was found that the type of glycol used in glycolysis had a significant effect on the characteristics of the uncured and cured UPE resins [141].
Waste PET was successfully depolymerised by glycolysis with starch derived glycol-glycosides and polyols prepared from estrification of depolymerised PET oligomers with SOFA can be used as a base component for formulating polyurethane system [142].
In another experiment we have depolymerised PET by glycol glycoside obtained from glycosylation of starch. Depolymerised oligomers obtained were esterified with dehydrated castor oil and coco fatty acid to give polyester polyols and prepared polyurethane adhesive and polyurethane coating [143, 144].
In another approach we have depolymerised PET using PEG of different molecular weights. The oligoesters obtained were transesterified with castor oil to obtained hydroxylfunctional polyester polyols. Two-pack coating systems were formulated using these resin as base component and melamine formaldehyde resin as hardner component. Cured film were tested for their mechanical and chemical performance which shows an excellent properties due to incorporation of PET into resin [145].
In the past few decades, governments and international agencies have been placing more emphasis on the improvement of production technique, working conditions and reduction of the toxic emission to the atmosphere. In this context we have synthesized aqueous polyurethane dispersion depolymerised polyethylene terphthalate (PET) waste. 1, 4-Butanediol was used in PET depolymerisation. Thus incorporation of PET waste in polyurethane dispersion was an added advantage in waste management and produced better quality polyurethane dispersion [146].
Conclusion
Since PET is light in weight, its feedstock are readily available and cheap, and the energy requirement for PET processing and fabrication for consumer articles is the lowest of those for the other materials, PET products for mass consumptions are affordable even to the poorest of the poor. This has resulted in the single use of PET products for mass consumption, and consequently a large volume of such products are being thrown into the garbage. It is true that disposal of PET waste, if done in the same way as followed for other materials, may create environmental problems. This is essentially because of the non-biodegradability and non-biomassimillibity of polymers. That PET is made durable according to the market demand, is the reason for their persistence in the environment causing litter problem and pollution. Thus PET become a red herring to the environmentalists, and by their pressure, governments are forced to enact laws which are not conducive to the growth and development of polymer industry. Some of the over-enthusiastic groups call for a ban of use of some of the mass consumed PET items. If we agree to their demand, the economically background population will suffer most because plastics have become the poor man’s useful materials.
But PET enjoys the advantage of easy reprocessability and recyclability. Also the waste polymers can be used to recovery energy, at least to a significant extent that was used for their production. So, waste plastics disposal should be handled carefully by adopting appropriate technologies. Waste plastics should be considered as valuable resources, particularly in countries where hydrocarbons feed stocks is scare and costly. Steps taken by industry are economy-driven, that adopted by government are legislative, and that by the public responsive. The integrated approach of waste management with the participation of industry, government and public can solve the waste disposal problem without damage to both environments and industry. Appropriate technology, legislation and waste disposal procedures should be adopted based on the socio-economic aspect of the country to solve this problem.
In addition to the environmental incentive, recycling of PET as an industry is getting its driving force from the increasing value and applications of virgin and modified PET. Publications review as reported above indicates that many scientific findings were made in the field of recycling of PET.
Particular focus in this review was on publications exploring different recycling technologies with emphasis on industrial applications. According to our evaluation, PET waste recycling is economically most viable in the case of degradation in very large quantities of defined industrial polymer waste, specially waste occurring at the place of manufacture, and manufacture of special products of low or medium tonnage, i.e., polyols often used in situ in the synthesis of polyurethanes, unsaturated polyester resins, saturated polyesters, paints, and additives for various applications (usually through glycolysis).
References
The Willey encyclopaedia of packaging technology, 2nd edn. Pubs. John Wiley & Sons, New York, pp 742–745 (1997)
Collected papers of Wallace Hume Carothers. In: Mark H, Whitby GS (eds) Interscience Publishers Inc., New York & London (1940)
DuPont Publications: technical data sheets on Melinar PET resins (1997)
Chemical Fibers International 47(4):248–250 (1997)
Wyeth N, Roseveare RN. US Patents 3,733,309 (1973) & 3,845,576 (1974)
Olabisi O (1997) Handbook of thermoplastics. Marcel Dekker Inc., New York, USA
Carraher CE (2000) Polymer chemistry. Marcel Dekker Inc., New York, USA
Warren LM, Burns R (1988) Plast Technol 6:41
Neale CW, Hilyard NC, Barber P (1983) Conserv Recyc 6:91
Schut JH (1993) Plast Technol, May 80
Snyder J (1994) Mod Plast Int, October 73
Jensen JW, Holman JL, Stephenson JB (1974) Recycling and disposal of waste plastics. Ann Arbor Science, chap 7
Paszun D, Spychaj T (1997) Ind Eng Chem Res 36:1373
Scheirs J (1998) Recycling of PET. In: Polymer recycling: science, technology and applications. Wiley Series in Polymer Science, Wiley, Chichester, UK
Alter H (1986) Disposal and reuse of plastics. In: Mark HF, Bikales N, Overberger CG, Menges G (eds) Encyclopedia of polymer science and engineering, vol 5. Wiley, New York, p 103
Datye KV, Raje HM, Sharma ND (1984) Resour Conserv 11:136
Wan B-Z, Kao C-Y, Cheng W-H (2001) Ind Eng Chem Res 40:509
Yoshioka T, Ota M, Okuwaki A (2003) Ind Eng Chem Res 42:675
Ramsden MJ, Phillips JA (1996) J Chem Technol Biotechnol 67:131
Karayannidis GP, Chatziavgoustis AP, Achilias DS (2002) Adv Polym Technol 21:250
Hu L-C, Oku A, Yamada E, Tomari K (1997) Polymer J 29(9):708
Pitat J, Holcik V, Bacak M (1959) A method of processing waste of polyethylene terephthalate by hydrolysis. GB Patent 822,834
Lazarus SD, Twilley JC, Snider OE (1967) US Patent 3,317,519
Rollick KL (1995) WO Patent 95,10499; CA 123:257937
Kozlov NS, Korotysko GP, Kashinskii AV, Gavrilenko ND (1984) Vesti Akad Nauk BSSR, Ser Khim Nauk 5:91; CA 102:113984s
Namboori CGG, Haith MS (1968) J Appl Polym Sci 12:1999
Ellison MS, Fisher LD, Alger KW, Zeronian SH (1982) J Appl Polym Sci 27:247
Filipowska B, Kubacki Z (1985) Przegl Wlok 39:118 (in Polish)
Collins MJ, Zeronian SH (1992) J Appl Polym Sci 45:797
Benzaria J, Durif-Varambon B, Dawans F, Gaillard JB (1994) Eur Patent 597,751; CA 121:281489v
Polk M, Leboeuf L, Shah M, Won C-Y, Hu X, Ding W (1999) Polym-Plast Technol Eng 38:459
Kosmidis VA, Achilias DS, Karayannidis GP (2001) Macromol Mater Eng 286:640
Pusztaszeri SF (1982) US 4,355,175
Brown GE Jr, O’Brien RC (1976) US Patent 3,952,053
Sharma ND, Vaidya AA, Sharma P (1985) Ind Patent 163,385; CA 112:76613d
Yoshioka T, Motoki T, Okuwaki A (2001) Ind Eng Chem Res 40:75
Yoshioka T, Sato T, Okuwaki A (1994) J Appl Polym Sci 52:1353
Glatzer H, Doraiswamy LK (2000) Chem Eng Sci 55:5149
Mehrabzadeh M, Shodjaei ST, Khosravi M (2000) Iranian Polym J 9:37
Michalski A (1993) Ekoplast 2:52 (in Polish)
Mandoki JW (1986) US Patent 4,605,762
Rosen BJ (1991) US Patent 5,095,145; CA 116:195064t
Royall DJ, Harvie JL (1993) Eur Patent 550,979; CA 120:9127a
Campanelli JR, Kamal MR, Cooper DG (1994) J Appl Polym Sci 54:1731
Launay A, Thominette F, Verdu J (1994) Polym Degrad Stab 46:319
Campanelli JR, Cooper DG, Kamal MR (1992) ANTEC 50:270
Michalski A (1990) Wl Chem 63:387 (in Polish)
Michalski A (1987) Wl Chem 49:144 (in Polish)
Doerr ML (1986) US Patent 4,578,510
Michalski A (1987) Polish Patent 140015
Tustin GC, Pell TM Jr, Jenkins DA, Jernigan MT (1995) US Patent 5,413,681; CA 123:170507w
Kamal MR, Lai-Fook RA, Yalcinyuva T (1997) Ind Eng Chem Res 36(4):1381
Farrow G, Ravens DAS, Ward IM (1962) Polymer 3:17–25
Overton JR, Haynes SK (1973) J Polym Sci Polym Symp 43:9
Awodi Y, Johnson A, Peters RH, Poppola AV (1987) J Appl Polym Sci 33:2503
Poppola AV (1988) J Appl Polym Sci 36:1677
Collins MJ, Zheronian SG, Marshall ML (1991) J Macromol Sci A28:775
Holmes SA (1996) J Appl Polym Sci 61:255
Mangovska B, Bogeva-Gaceva G, Pohlers A (1996) J Appl Polym Sci 62:605
Kastierina TN, Kalinina LS (1965) Chemical analysis of plastics (in Polish). WNT, Warsaw, p 163
Ulrich H, Odinak A, Tucker B, Sayigh A (1978) Polym Eng Sci 18:844
Van der Wal HR (1993) Advances in recovery and recycling. In: Henstock ME, Skov HR (eds) Collected papers at RCC’93, vol II. International Recycling Congress, Geneva, Hexaton, pp 290–294
Spychaj T, Paszun D (1975) Pol Pat 179018
Sulkowski W, Ossowski J, Makarucha B (2000) Ser conferences, Plastic recycling. Wroclaw, p 117
Fabrycy E, Leistner A, Spychaj T (2000) Adhesion 44(4):35
Collins MJ, Zeronian SH, Marshall ML (1991) J Macromol Sci Chem A28:775
Shukla SR, Ajay Harad M (2006) Polym Degrad Stab 91:1850
Tomita K (1976) Polymer 17:221
Shukla SR, Harad AM (2005) J Appl Polym Sci 97(2):513
Zahn H, Pfeifer H (1963) Polymer 4:429
Blackmon KP, Fox DW, Shafer SJ (1988) Eur Patent 365,842
Chandra R, Adab A (1994) Rubber and plastic waste: recycling, reuse and future demand, 2nd edn. CBS Publishers & Distributors, New Delhi
GB Patent 748,248 (1957)
Dimov K, Terlemezyan E (1972) J Polym Sci Part I 10:3133
GB Patent 806,269 (1958)
Marathe MN, Dabholkar DA, Jain MK (1980) GB Patent 2,041,916
Michel RE (1992) Eur Patent 484,963
Socrate C, Vosa R (1995) Eur Patent 662,466; Chem Abstr 123:257959u
Debruin BR, Naujokas AA, Gamble WJ (1995) US Patent 5,432,203; Chem Abstr 123:229404r
Pitat J, Holcik V, Bacak MA (1959) GB Patent 822,834
Sato K, Sumitani K (1995) Japan Patent 07,196,578; Chem Abstr 123:257777h
Mikolajczyk B, Lubawy A, Deerskin M, Smoczynski P, Pozniak A, Boebel H (1985) Polish Patent 126,009
Mishra S, Goje AS, Zope VS (2001) Proc Int Conf Plastic Waste Manage Environ, Mar 15–16, New Delhi, p 163
Kint D, Munoz-Guerra S (1999) Polym Int 48:346
Ostrysz R, Kicko-Walczak E, Milunski P, Jakubas T, Mental Z, Okorowski J, Koscielski K (1990) Polish Patent 150,126
Brandrup J (1975) Kunststoffe 65:881
Muhs P, Plage A, Schumann HD (1992) Kunststoffe 82:289
Kapelanski A, Kurek P, Rozanski A (1995) Production and Recycling of PET. In: Elana SA (ed) Proceedings of the National Conference on polymers Environment Recycling, Szczecin-Miedzyzdroje (in Polish)
Kishimoto Y, Kajihara T, Kato S (1994) Polym Bull 42(3):295
Sako T, Sugeta T, Otake K, Nakazawa N, Sato M, Namiki K, Tsugumi M (1997) J Chem Eng Jpn 30:342
Genta M, Yano F, Kondo Y, Matsubara W, Oomoto S (2003) Development of chemical recycling process for post-consumer PET bottle by methanolysis in supercritical methanol; Technical review, 40, Extra No. 1, Mitsubishi Heavy Industries, Ltd., Tokyo, Japan, pp 1–4
Genta M, Uehara R, Yano F, Kondo Y, Matsubara W (2003) Development of chemical recycling process for post-consumer pet bottle by methanolysis in supercritical methanol. 6th ISSF Proc, p 1381
Sako T, Sugeta T, Otake K, Takebayashi Y, Kamizawa C, Tsugumi M, Hongo M (1998) Kobunshi Ronbunshu 55:685
Yamamoto S, Aoki M, Yamagata M (1996) R-D Kobe Steel Eng Rep 46(1):60
Adschiri T, Sato O, Machida K, Saito N, Arai K (1997) Kagakukogaku Ronbunshu 23(4):505
Sako T, Okajima I, Sugeta T, Otake K, Yoda S, Takebayashi Y, Kamizawa C (2000) Polym J 32:178
Goto M, Koyamoto H, Kodama A, Hirose T, Nagaoka S (1999) MWD Analysis in decomposition of PET in supercritical methanol. Proc 1st Int Symp Feedstock Recycl Plast, pp 255–258
Goto M, Koyamoto H, Kodama A, Hirose T, Nagaoka S, McCoy B (2002) AIChE J 48(2):136
Goto M, Genta M (2003) Supercritical methanol for chemical recycling of PET bottle. Proc 2nd Int Symp Supercrit Fluid Technol Energy Environ Appl, pp 64–73
Genta M, Iwaya T, Sasaki M, Goto M, Hirose T (2005) Ind Eng Chem Res 44:3894
Kim B-K, Hwang G-C, Bae S-Y, Yi S-C, Kumazawa H (2001) J Appl Polym Sci 81:2102
Yang Y, Lu Y, Xiang H, Xu Y, Li Y (2002) Polym Degrad Stab 75:185
Fujita A, Sato M, Murakami M (1985) Japan Patent 06,248,646; CA 105:7063t
Fujita A, Sato M, Murakami M (1986) US Patent 4,609,680
Baliga S, Wong WT (1989) J Polym Sci Part A: Polym Chem 27:2071
Nevrekar NA, Sheth NS (1990) Man-Made Text India 33:7; CA 114:104132h
Chen JY, Ou YC, Lin CC (1991) J Appl Polym Sci 42:1501
Johnson PL, Teeters DA (1991) Polym Prepr (Am Chem Soc Div Polym Chem) 32:144
Tersac G, Laurencon G, Roussel H (1991) Caoutch Plast 68:81
Ostrysz R (1969) Polimery 14:203 (in Polish)
Vaidya UR, Nadkarni VM (1987) Ind Eng Chem Res 26:194
Vaidya UR, Nadkarni VM (1987) J Appl Polym Sci 34:235
Vaidya UR, Nadkarni VM (1988) J Appl Polym Sci 35:775
Vaidya UR, Nadkarni VM (1989) J Appl Polym Sci 38:1179
Vaidya UR, Nadkarni VM (1990) Polym Mater Sci Eng 63:1029
Kim JH, Lee DS, Park TS, Kim J, Kim KU (1995) Pollimo 19:353; CA 123:84712c
Lee SC, Sze YW, Lin CC (1995) J Chin Inst Chem Eng 26:289; CA 123:201608 m
Ostrysz R (1970) Polimery 15:406 (in Polish)
Rebeiz KS (1993) Diss Abstr Int B 53:4269; CA 120:246841m
Rebeiz KS (1995) Cem Concr Compos 17:119; CA 123:11142g
Rebeiz KS, Flower DW, Paul DR (1992) J Appl Polym Sci 44:1649
Rebeiz KS, Flower DW, Paul DR (1993) Trends Polym Sci 1:315; CA 120:324729x
Pepper TP (1995) US Patent 5,380,793; CA 123:34414m
Grigsby RA Jr, Speranza GP, Brennan ME, Yeakey EL (1985) US Patent 4,536,522; CA 104:6585g
Hallmark RK, Skowronski MJ, Stephens WD (1985) Eur Patent 152,915; CA 104:35078z
Speranza GP, Grigsby RA Jr, Brennan ME (1984) US Patent 4,485,196
Carlstrom WL, Stoehr RT, Svoboda GR (1985) Mod Plast 62:100
Ostrysz R, Klosowska Z, Jankowska F (1964) Polish Patent 76,005
Nowaczek W, Krolikowski W, Pawlak M, Klosowska-Wolkowich Z, Mieczkowski W (1992) Polish Patent 153,520
Penczek P (1996) Private information
Brennan ME (1984) US Patent 4,439,549
Wang DC, Chen LW, Chiu WY (1995) Angew Makromol Chem 230:47
Billiau-Loreau M, Durand G, Tersac G (2002) Polymer 43:21
Malik AI, Most EE (1978) US Patent 4,078,148
Lagorati F, Anglietti, G, Nova VE (1972) Ger Offen 2,158, 560
Hemmi H, Nagashima H, Kimura Y, Teresaki I, Satani M (1975) Japan Kokai 7, 562, 732
Colomines G, Robin J-J, Tersac G (2005) Polymer 46:3230
Pardal F, Tersac G (2007) Polym Degrad Stab 92:611
Ghaemy M, Mossaddegh K (2005) Polym Degrad Stab 90:570
Goje AS, Mishra S (2003) Macromol Mater Eng 288(4):326
Pimpan V, Sirisook R, Chuayjuljit S (2003) J Appl Polym Sci 88:788
Patel JV, Soni PK, Sinha V (2001) Int J Polym Mater 49:204
Desai SD, Patel JV, Patel MR, Sinha VK (2005) Indian J Chem Technol 12:82
Patel MR, Patel JV, Sinha VK (2005) Polym Degrad Stab 90:111
Patel MR, Patel JV, Sinha VK (2006) Polym Int 55:1315
Patel MR, Patel JV, Sinha VK (2007) J Polym Environ 15:97
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinha, V., Patel, M.R. & Patel, J.V. Pet Waste Management by Chemical Recycling: A Review. J Polym Environ 18, 8–25 (2010). https://doi.org/10.1007/s10924-008-0106-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-008-0106-7