Abstract
Six pharmaceuticals of different categories, such as nonsteroidal anti-inflammatory drugs (ibuprofen, ketoprofen, naproxen, diclofenac), anti-epileptic (carbamazepine), and anti-microbial (trimethoprim), were investigated in wastewater of the urban areas of Ghaziabad and Lucknow, India. Samples were concentrated by solid phase extraction (SPE) and determined by high-performance liquid chromatography (HPLC) methods. The SPE-HPLC method was validated according to the International Conference on Harmonization guidelines. All the six drugs were detected in wastewater of Ghaziabad, whereas naproxen was not detected in Lucknow wastewater. Results suggest that levels of these detected drugs were relatively higher in Ghaziabad as compared to those in Lucknow, and diclofenac was the most frequently detected drug in both the study areas. Detection of these drugs in wastewater reflects the importance of wastewater inputs as a source of pharmaceuticals. In terms of the regional distribution of compounds in wastewater of two cities, higher spatial variations (coefficient of variation 112.90–459.44 %) were found in the Lucknow wastewater due to poor water exchange ability. In contrast, lower spatial variation (162.38–303.77 %) was observed in Ghaziabad. Statistical analysis results suggest that both data were highly skewed, and populations in two study areas were significantly different (p < 0.05). A risk assessment based on the calculated risk quotient (RQ) in six different bioassays (bacteria, duckweed, algae, daphnia, rotifers, and fish) showed that the nonsteroidal anti-inflammatory drugs (NSAIDs) posed high (RQ >1) risk to all the test species. The present study would contribute to the formulation of guidelines for regulation of such emerging pharmaceutical contaminants in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
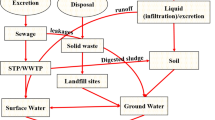
In recent years, increasing attention has been directed toward the discharge, presence, and potential effects of pharmaceuticals in the environment. Pharmaceuticals are a large and diverse group of compounds designed to prevent, cure, and treat diseases and improve health. Furthermore, they often have low biodegradability. Thousands of tons of pharmacologically active substances are used yearly to prevent illness (Kosjek et al. 2005). The pharmaceutical contamination is due to the consumption and the excretion via urine and feces in wastewater. Indeed, many of ingested pharmaceuticals are excreted in the same form or in a conjugated or slightly transformed form. The unused part of medicine could be also a source of sewage contaminations (Tauxe-Wuersch et al. 2005). The type and concentrations of pharmaceuticals in the environment vary among areas and countries, depending on consumption and use patterns (Kolpin et al. 2002). In some industrialized countries, wastewater treatment plant effluents containing pharmaceuticals used in human medicine are major source of these compounds in the aquatic environment (Wiegel et al. 2004). Although substances of pharmaceutical origin have been in the environment for a long time, they have been classified in the group of emerging contaminants because large amount enters the environment as a result of increasing consumption both for human use and in veterinary practice (Ascar et al. 2013). Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the pharmaceutical drugs most widely used by the humans all over the world. Among them, the acidic NSAIDs, ibuprofen (IBF), diclofenac (DCF), ketoprofen (KTF), and naproxen (NPX) received great research interest due to their worldwide high consumption and their reported physicochemical and toxicological properties. The anti-microbials are also one of the most important groups of pharmaceuticals, employed in both human and veterinary medicines. Anti-microbials constitute more than 6 % of all prescriptions in human medicine and more than 70 % of all consumed pharmaceuticals in veterinary medicines (Thiele-Bruhn 2003). Occurrence of NSAIDs and anti-microbials has been documented through several studies around the world with concentration levels ranging from few nanograms per liter to micrograms per liter in wastewater (Samaras et al. 2011), surface water (Stasinakis et al. 2008; Iglesias et al. 2013), groundwater (Latorre et al. 2003), and drinking water (Kleywegt et al. 2011). Out of the diverse pool of pharmaceuticals, over the counter or nonprescription drugs are extensively consumed (Ziylan and Ince 2011). Approximately 90 % of them belong to the commonly administered therapeutic group of NSAIDs (Crouse et al. 2012). NSAIDs are mostly carboxylic acid derivatives which act by inhibiting the prostaglandin synthesis by cyclooxygenase (COX) enzymes responsible for cell to cell signaling in body tissues (Derle et al. 2006). Carbamazepine (CARB) is an anti-epileptic drug used to control seizures. It has complex pharmokinetic properties leading to wide fluctuation in their plasma concentration interaction potential with other drugs and narrow therapeutic index which can lead to toxic effect or loss of therapeutic efficacy (Shakya et al. 2008). CARB is proposed as an anthropogenic marker in water bodies (Clara et al. 2004). CARB concentration up to 1,075 ng L−1 has been reported in surface water (Heberer et al. 2002).
The environmental persistence, rate of spread, and bioaccumulation of biologically active substances differ depending on their chemical properties and on the environmental conditions. The continuous input of these compounds into the environment may lead to ecotoxicological effects (Iglesias et al. 2013). Overall, the widespread environmental detection of pharmaceuticals and their potential ecotoxicological significance had engendered regulatory and scientific concern (Monteiro and Boxall 2010; Al-Rajab et al. 2010). The increased presence of these compounds in environment raises concern about their propensity to select for resistant bacteria and facilitate the establishment and amplification of pathogenic reservoir that threaten public health (Luo et al. 2010). Yet, the environmental factors that determine the reach and attenuation patterns of pharmaceuticals at the regional scale are not fully understood, and limited data exist on the spatial and temporal variability of pharmaceuticals in the environment, which underscores the need for complimentary regional studies (Luo et al. 2011). Quantitative evaluation of the fate of the pharmaceuticals in the aquatic environment, proper risk assessment, and improvement of the efficiency of sewage treatment plants needs sensitive and reliable methods. In India, where hospital standards are reportedly inconsistent and drug prescription pattern is irrational (Ganguly et al. 2011), it would be a subject of interest to assess the concentration levels of pharmaceuticals in the Indian wastewaters. There is no data available regarding pollution with pharmaceutical residues in north Indian environment.
Model compounds were selected among the pharmaceuticals, which predominate in the analysis of environmental samples, as well as on the list compiled from prescription data. Most of these pharmaceuticals belong to the class of analgesics (NSAIDs), anti-biotics, anti-hypertensives, diuretics, anti-epileptic, and anti-microbials (Kümmerer 2001). For this reason, the following four pharmaceuticals from the class of NSAIDs (IBF, KTF, NPX, DCF), one each from the anti-epileptic (CARB), and anti-microbials (trimethioprim, TRIM) were chosen as model compounds. The selected pharmaceutical compounds were analyzed in wastewater samples by solid phase extraction high-performance liquid chromatography (SPE-HPLC) method validated according to the International Conference on Harmonization guidelines (ICH 1995; ICH 2009).
In addition, the ecological risk associated with the occurrence of most of the pharmaceuticals in the environment is not sufficiently described and could be crucial in these natural protected areas, which have been established as one of the most useful tools for preserving large pools of biodiversity (Vazquez-Roig et al. 2012). The European Medicine Agency (EMEA), following the European Directive 2001/83/EC, has elaborated a guideline for the environmental risk assessment (ERA) of new and existing medicinal products in the environment (De Lange et al. 2006). Several studies have tested this approach in waste and surface waters describing its advantages and disadvantages (Hernando et al. 2006; Ginebreda et al. 2010; Gros et al. 2010). This ERA approach is based on short-term ecotoxicological laboratory determination which is valuable since it provides an a priori identification (and quantification) of risk. However, it does not necessarily reflect the real ecosystem situation, since many additional concurrent factors (stressors) can be equally present (Ginebreda et al. 2010). Our study also attempts to provide a preliminary risk assessment of the hazard that the detected concentration of pharmaceuticals has in the aquatic environment of north India.
The present study aims to generate baseline database on the levels of selected NSAIDs (IBF, KTF, NPX, DCF), anti-epileptic (CARB), and anti-microbial (TRIM) in wastewater of two north Indian cities of Lucknow and Ghaziabad with a view to develop national guidelines on these contaminants. The detected concentrations were then compared with known toxicity data to evaluate the potential risk posed by these compounds to aquatic life.
Materials and method
Study area
Here, Ghaziabad (north latitude 28.67′, east longitude 77.42′) and Lucknow (north latitude 28.847′, east longitude 80.947′) cities were selected for investigating the occurrence of pharmaceuticals in wastewater (Fig. 1). Ghaziabad, in the northern Indian terrain, is a part of the Indo-Gangetic alluvial plain. Ghaziabad receives an average annual rainfall of 732 mm, and the temperature soars up to 45 °C in summer season and may drop to 2 °C in winter season. The Hindon River drains the city which later on merges with the Yamuna. The Cis Hindon (10,340 ha) and the Trans Hindon (5,500 ha) areas together house a population of greater than two million inhabitants. Ghaziabad is an important industrial hub with around 300 miscellaneous manufacturing units dispersed within the city (Suthar et al. 2010). These industries utilize the water from the Hindon during manufacturing process and discard the untreated wastewater back into the river. Regarding consumption of human pharmaceuticals in Ghaziabad, Goel et al. (2013) reported the most commonly prescribed drugs in fixed dose combinations to be anti-inflammatory analgesic, anti-microbials, and cold and cough remedies followed by multivitamins preparation at a health care facility in Ghaziabad.
Lucknow, the capital city of Uttar Pradesh State, is spread over a total geographical area of about 253 km2 with a population of more than four million inhabitants. The city receives an average rainfall of 896.2 mm and is characterized by warm humid subtropical climate with the maximum temperature during summer months reaching 40–45 °C while minimum temperature during winter months falls to 3–7 °C. Located in the central Indo-Gangetic plain, the city is drained by the Gomti River which flows through the heart of the city. Ki et al. (2008) reported that the drug prescription pattern was irrational in public health care facilities of Lucknow, and the most prescribed drugs were vitamins (25.6 %), anti-biotics, anti-infectives (20.6 %), and NSAIDs/anti-pyretics (17.7 %).
Chemicals and materials
Standards (99.8 % purity) of ibuprofen, naproxen, ketoprofen, diclofenac, carbamazepine, and trimethoprim were obtained from Sigma-Aldrich, USA. The chemical structures of the selected drug molecules are shown in Fig. 2, and their physicochemical properties are presented in Table 1. Analytical grade chemicals (trifluoroacetic acid, potassium dihydrogen phosphate) and HPLC grade solvents (methanol, acetonitrile, ethyl acetate, n-hexane) were purchased from Merck, India. Milli-Q water was prepared using a purification system (Millipore, Milford, MA, USA). Stock solutions of all compounds were prepared in methanol. Working standard solutions were prepared each week by dilution of the stock solution in methanol–water (25:75 %, v/v). A working mixed solution of labeled standards was prepared by the dilution of individual stock solutions with methanol and stored at −20 °C. All solvents for HPLC application were filtered (0.45 μm membrane filter) and degassed by ultrasonication (PCi Analytics) for 30 min prior to use. Hydrophilic–lipophilic-balanced reversed phase sorbent cartridges (Supelco hydrophilic–lipophilic balanced (HLB) 500 mg sorbent/12 ml from Supelco, USA) and Supelco VisiprepTM SPE vacuum manifold (Supelco, USA) were used in SPE.
Sample collection and preparation
Wastewater samples were collected from sewage drains (at discharge points to the main sewer) in Ghaziabad (28 nos.) and Lucknow (26 nos.) during January 2010. Four-hourly grab samples were collected over a period of 24 h from each sampling point, and a representative sample was drawn after mixing them. Two samples of influent streams of sewage treatment plants (STPs) from both the cities were also collected following the same sampling procedure. All the wastewater samples were collected in 2.5-L amber glass bottles rinsed with site water prior to collection of final wastewater sample. All samples were transported to the laboratory in hermetic boxes refrigerated with ice (at 4 °C). Upon arrival at the laboratory, the samples were vacuum filtered through 0.45-μm membrane filter (MF-Millipore TM) to remove the suspended matter (prevents clogging of cartridges) and stored at 4 °C for extraction and analysis carried within the next 24 h.
Analytical methods
Wastewater samples were processed to extract the targeted pharmaceuticals. The drugs in wastewater were concentrated through a SPE by an HLB cartridge. HLB cartridges contain the lipophilic divinylbenzene units and hydrophilic N-vinylpyrrolidone units which facilitate retention of polar and nonpolar analytes at a wide pH range (1–14) (Seifrtová et al. 2009). The pH of the filtered water samples was adjusted to 2 with 1 N HCl using a pH meter (Metrohm 744). During the process of HLB extraction, the best recoveries were achieved at low pH, which could keep typical carboxylic group in the quinolone structure in its dissociation form and strengthen the interaction between the carboxylic group and the hydrophobic structure of HLB (Ye et al. 2007). The extraction procedures adopted for IBF, KTF, NPX, DCF, and CARB were similar but slightly different for TRIM. In case of the first five pharmaceuticals, the HLB cartridges were preconditioned sequentially with three fractions of ethyl acetate, methanol, and Milli-Q water at a flow rate of 3 ml min−1. Wastewater samples were loaded onto the cartridges using a vacuum manifold at a flow rate of 15 ml min−1. The loaded cartridges were rinsed with 3 ml of methanol/water (5:95 (v/v)) and 3 ml of n-hexane (to avoid elution of any impurity with the desirable analytes) and air-dried on the manifold for a minimum of 30 min, until visibly dry. The analytes were eluted with 3 ml aliquots of ethyl acetate, and the collected extracts were concentrated under a stream of nitrogen gas. The concentrated extracts were reconstituted with 0.5 ml of methanol for chromatographic analysis. For trimethoprim, conditioning of cartridges was done sequentially by 2 ml methanol, 3 ml of acetonitrile, and 5 ml of Milli-Q water and elution was performed by 6 ml of acetonitrile at 1–2 ml min−1 flow rate.
The extracted pharmaceuticals were analyzed using HPLC (Shimadzu, Japan) equipped with quaternary pumps (LC-20AJ) and UV detector (SPD-M20A). The chromatographic separation of the targeted drugs was performed on a chromolith RP18e column (100 × 4.6 mm I.D., 5 μm, Merck, Germany) and protected by an RP18 guard column (4.0 × 3.0 mm I.D., 5 μm, Phenomenex, USA). The injection volume of each sample was 50 μl. The detection wavelengths were set at 220, 255, 230, 220, 285, and 220 nm for IBF, KTF, NPX, DCF, CARB, and TRIM, respectively. Methanol (solvent A), acetonitrile (solvent B), and 50 mM potassium dihydrogen phosphate solution (solvent C) were used as mobile phase at a total flow rate of 1 ml min−1, with the following gradients: 15 % A, 0 % B, and 85 % C for 4.5 min; 0 % A, 20 % B, and 80 % C for 8.0 min; 0 % A, 25 % B, and 75 % C for 5.5 min; 0 % A, 45 % B, and 55 % C for 9 min; and 0 % A, 45 % B, and 55 % C for 18 min. The run was completed in 45 min. In the case of TRIM, the mobile phases were (A) 0.01 % trifluoroacetic acid (TFA) in Milli-Q water and (B) 0.01 % trifluoroacetic acid in acetonitrile. The elution was achieved by adjusting flow conditions as 0 % B (3 min), 0–37 % B (5 min), and 37 % B (5 min), and the run was completed in 13 min. Compounds of interest were identified in the chromatograms comparing the retention time (RT) of the peaks with that of the corresponding compound in the standard solution. To confirm the identification of the pharmaceutical compounds, the UV spectra of the peaks in the sample and in the standard solution chromatogram were compared. Chromatograms of standard solutions containing 140 μg L−1 of CARB, KTF, NPX, DCF, and IBF and 200 μg L−1 of TRIM obtained with PDA detector are shown in Fig. 3.
Method validation and quality control
The method was validated according to the International Conference on Harmonization (ICH) guidelines (ICH 1995), addressing the method linearity, recovery, accuracy, precision, limit of detection, and quantitation (Santos et al. 2005; Mostafavi et al. 2009; Madureira et al. 2010). The linearity was tested using a five-point calibration curve in the concentration range of 0.960–960 μg L−1 for each of the compound and calculating the corresponding linear regression parameters. The reliability and suitability of the method were studied using recovery experiments. The Milli-Q water and wastewater samples were spiked with different amount of each analyte yielding three different concentration levels (2.0, 6.0, and 10.0 μg L−1) in triplicate for each one, and unspiked samples of both matrices were retained as respective blanks. Measured values were compared with the theoretical concentration and recovery for each analyte was determined. The precision of the method was assessed in terms of repeatability and intermediate precision by analyzing three replicate standard samples at 0.96, 480, and 960 μg L−1 levels that cover the calibration range for each analyte. The percent variation in peak area and corresponding relative standard deviation (RSD) values of the results were expressed for intra-day precision and on 3 days for intermediate (inter-day) precision. The limit of detection (LOD) and limit of quantitation (LOQ) for the procedure were calculated by using a signal-to-noise ratio of 3 and 10, respectively (the ratio between peak intensity and intensity of the noise was used). Blank samples were derived from Milli-Q water and were used to determine whether sampling procedures, sampling equipment, field conditions, or sample shipment procedures introduce the target analytes to environmental samples (field blank) or to assess the potential for sample contamination in the laboratory (laboratory blanks).
Statistical analysis of data
The nonparametric Kolmogorov-Smirnov (K-S) and Kruskal-Wallis (K-W) tests were used to analyze the data. The K-S statistics was used to test the normality of sample distributions. It computes the maximum vertical distance between F 1(u 1) and F 2(u 2), the empirical distributions of u for groups 1 and 2, respectively. This statistics, by construction, takes values between 0 and 1, and a high value of this statistic is indicative of significant differences in inefficiency between the two groups. The K-S test, denoted as D, is also derived from inefficiency variable u m. For any real value Z, D is distributed as (Smirnov 1939):
where P is the probability, and N 1 and N 2 are the data points. Here, K-S test was performed on the raw and transformed (logarithmic and Box-Cox) data. Box-Cox transformation renders the data scaled so that it follows normal distribution. The Box-Cox transformation is given by (Osborne 2010):
where y is the transformed value, and x is the value to be transformed. For a given dataset, (x 1, x 2, …,x n ), the parameter λ is estimated based on the assumption that the transformed value (y 1, y 2, …, y n ) are normally distributed. When λ = 0, the transformation becomes the logarithmic transformation
The K-W test compares the residual value of each analyte among aquatic sources and between different geographical locations with the significance level set at p < 0.05, which is suitable for comparison between different datasets with uneven sample numbers (Zhao et al. 2010). The K-W test does not rely on the data normality assumption. The K-W test statistic can be computed as (Tcheslavski and (Louis) Beex 2010):
where N i is the sample size of each m groups, R i is the sum of the ranks of the ith group, and \( {N}_{{\displaystyle \sum }} = {\displaystyle \sum_{i=1}^m}{N}_i \) is the total number of samples considered in the experiment.
Further, in conventional statistics, a normal distribution for the variables under study is desirable. Any significant differences among the mean concentrations of various analytes in surface and wastewater of two study areas were evaluated using the two-tailed t test statistics. The t test assumes that samples are randomly drawn from normally distributed populations with unknown population variances. A two-tailed t test statistics can be computed as:
where \( {\overline{x}}_{1\ } \) and \( {\overline{x}}_{2\ } \) are the means of two different populations, s is the pooled standard deviation, and n 1 and n 2 are the respective sample sizes. The calculated t statistics is compared with the critical value of t corresponding to the number of degrees of freedom at certain confidence level.
To better quantify the relationships among the selected drugs under study, principal components analysis (PCA) was applied. PCA is a pattern recognition technique that attempts to explain the variance of a large set of intercorrelated variables. It indicates association between variables. PCA extracts the eigenvalues and eigenvectors from the covariance matrix of original variables (Singh et al. 2008) as:
where X is the matrix of measurements, A is the matrix of scores, B is the matrix of loadings, and E is the error matrix containing the variance not explained by the model defined by principal components (PCs) described in A and B. The PCs are the uncorrelated (orthogonal) variables, obtained by multiplying the original correlated variables with the eigenvector (loadings). The eigenvalues of the PCs are the measure of their associated variance, the participation of the original variables in the PCs is given by the loadings, and the individual transformed observations are called scores (Singh et al. 2005).
Environmental risk assessment (ERA)
The wastewater in treated or untreated form is commonly discharged into proximal water bodies in real scenario. The flora and fauna of the recipient aquatic environment are most vulnerable to the toxic effects of the wastewater constituents such as pharmaceuticals. Hence, it is imperative to evaluate the ecological risks posed by the pharmaceuticals in wastewater at their measured environmental concentrations. According to the European Agency for the Evaluation of Medicinal Products (EMEA 2012), the potential risk of each pharmaceutical is assessed by calculating the risk quotient (RQ) as the ratio between maximum measured environmental concentration (MEC) and its predicted no-effect concentration (PNEC) for a particular test organism.
where RQ < 0.1 implies low risk, 0.1 ≤ RQ ≤ 1, medium risk, and RQ ≥ 1 high risk (Hernando et al. 2006). According to the Technical Guidance Document (TGD), when only short-term toxicity data (LC50, EC50) are available, the calculation of PNEC is obtained from LC50 (or EC50) divided by an assessment factor of 1,000. Once long-term no observed effect concentration (NOEC) values for one, two, or three trophic levels are available, an assessment factor of 100, 50, or 10 is used (Yang et al. 2011). The PNEC values are obtained from acute ecotoxicity test data for standard test organisms such as bacteria, duckweed, algae, daphnid, rotifers, and fish in order to represent different trophic levels. It is worth noting that when more than one EC50 value was found, the lowest value was taken into consideration. When no experimental values were available, we used EC50 value estimated with ECOSAR (Sanderson et al. 2003). The data from the literature about the toxicity of six detected drugs to sensitive test organisms of different trophic levels are given in Table 2.
Results and discussion
Selected characteristic properties (Table 1) suggest that these drugs are highly water soluble and persistent in aqueous medium. The bioconcentration factor (BCF) of these molecules ranges between 1 and 63.28. The BCF serves as a measure of the chemical concentration in the organism concurrent with ambient water concentration under steady-state conditions and thus indicates bioaccumulation potential of the chemical. Octanol–water partition coefficient (expressed in logarithmic form as log P) describes the solute lipophilicity and indicates the ability of solute to penetrate to lipid-rich zones from aqueous solution. This is a very important feature of any anti-biotic that is administered orally and is supposed to pass the gastrointestinal equilibrium. Here, the log P values of the studied drugs ranged between 0.79 (TRIM) and 4.55 (DCF). The half-life of the targeted drugs in aqueous medium ranged between 360 and 1,440 h. The polar surface area (PSA) of the molecule ranged between 37.30 Å2 (IBF) and 105.51 Å2 (TRIM). It affects molecular transportation through membranes and therefore allows an estimation of the apparent volume of distribution in the body (Fatemi and Ghorbannezhad 2011). All these drugs show single pKa value, except TRIM, which has two pKa values.
Method validation parameters
The linearity and range of application were established by the calibration curves in the concentration ranges given in Table 3 with squared coefficients of correlation (R 2) values ranging between 0.991 and 0.999. The RSD (%) of each calibration standard (n = 3) varied from 0.13 to 3.17. The recovery percentages were established by three replicates for each of the three concentration levels in Milli-Q water and wastewater samples. Recoveries obtained for all target compounds ranged from 84.13 to 97.86 % in Milli-Q water and 74.52–98.57 % in wastewater, with respective RSD (%) values between 1.84–5.25 and 2.64–7.83 (Table 4). The extraction efficiencies were acceptable for all compounds analyzed at three different concentration levels. It was observed that the recovery values obtained for the Milli-Q water and wastewater were in some cases significantly distinct, but there was no direct correlation between the matrix influences. Precision of the method (peak area and retention time) was established across the analytical range of selected analytes. The intra- and inter-day precisions were calculated from the standard solutions. Repeatability (intra-day precision) of the analytical method (peak area of standards) was found to be reliable with RSD (%) values ranging between 0.05 and 1.86. Intermediate precision was determined on different days and evaluating the peak area data at three different concentrations of each compound that cover the assay method range. The RSD (%) values were 0.05–3.88 and illustrated the good precision for the analytical method (ICH 2009). The intra- and inter-day variations in RT of peaks for all the compounds were within 0.40, and the RSD (%) values obtained as the result of precision estimation of RT between the standard and spiked matrix were lower than 0.36 (n = 10). The LOD and LOQ of the considered compounds ranged from 20.0–185.0 to 66.7–616.7 ng/L, respectively. These values were used as a reference for the measurements to be carried out in the wastewater samples.
Statistical analysis
The descriptive statistics of these drugs in wastewater of Lucknow and Ghaziabad are summarized in Tables 5 and 6. Concentration of different NSAIDs, anti-epileptic, and anti-microbial drugs for both the regions showed large variations between the samples, with a high coefficient of variation (CoV). The CoV, a measure of statistical dispersion of data, is the mean normalized standard deviation of the given data set. The KTF (303.77 %) in Ghaziabad and TRIM (459.44 %) in Lucknow wastewater exhibited highest CoV, whereas DCF showed lowest CoV both in Ghaziabad (162.38 %) and Lucknow (112.90 %). The large variations in concentration of these drugs correspond to the degree of heterogeneity in the nature and types of sources distributed in the study regions with diverse anthropogenic activities. The low CoV values indicated spatially homogeneous distribution of these compounds.
The parameters of skewness, kurtosis, and the significance level of K-S test for normality (K-S p) are shown in Table 7. The skewness and kurtosis values for different drugs varied between 3.34–4.69 and 13.64–23.18 in Ghaziabad and 1.20–5.26, 0.61–27.75 in Lucknow. Skewness and kurtosis describe the shape and symmetry of distribution of drug concentration data in the study areas. Concentration values of all the drugs in both the study areas are strongly skewed, with skewness higher than 0, implying that there are some extremely higher values in the datasets. The kurtoses are also very sharp, caused by the fact that majority of samples are clustered at relatively low values. The unsymmetrical distribution of drug concentrations in the study areas suggests for uneven and nonhomogenous distribution of sources. The results of the K-S test (p < 0.05) implied that concentrations of all the drugs in both in wastewater of both the cities were not normally distributed. In such cases, the geometric mean or the transformed mean (log transformed or Box-Cox transformed) is used to describe average concentrations (McGrath et al. 2004). In our case, both the logarithmic and Box-Cox transformations resulted in smaller skewness and kurtosis of the variables. Table 8 summarizes the results of K-W test. It tests differences in residue levels of six drugs in wastewaters of two study areas. The results suggest that concentrations of IBF and KTF in two wastewaters are not different at p < 0.05. The mean rank values suggest that DCF in Ghaziabad and CARB in Lucknow wastewater exhibited highest residue levels. For all the anti-biotics (except IBF and KTF), the K-W test results were below 0.05, indicating that the statistically significant differences indeed existed for each of these compounds within locations (Xie et al. 2012). It further suggested a spatial heterogeneity of the residue levels of these compounds in wastewaters of two study areas. The t test statistics revealed that the mean concentrations of the selected drugs (except DCF) were not significantly different in these two wastewaters.
Concentrations of selected drugs in wastewater of Ghaziabad and Lucknow
The concentration levels of the six pharmaceuticals in wastewater of two study areas are provided in Tables 5 and 6. The maximum concentration level of these drugs in Ghaziabad and Lucknow wastewaters ranged between below detection limit (BDL) 51.82 and BDL 12.36 μg L−1, respectively. TRIM registered highest concentration level in Ghaziabad (51.82 μg L−1), whereas CARB in Lucknow (12.36 μg L−1) was lowest. Ramaswamy et al. (2011) reported CARB concentration of 28.3 ng L−1 in Kaveri river water (India). Concentrations of CARB in Lucknow and Ghaziabad wastewater are lower than that reported for influent municipal streams (21.6 μg L−1) in South Korea (Sim et al. 2011). Comparatively, TRIM concentrations found in Ghaziabad and Lucknow wastewater are higher than in case of municipal wastewater (1.51 μg L−1) in the above-mentioned study. CARB (5H-dibenzo(b,f) azepine-5-carboxamide) is an anti-epileptic drug and is the most preferred anti-convulsant for pediatric seizure disorders. It is also used in treatment of trigeminal neuralgia, schizophrenia, restless leg syndrome, diabetes insipidus, and bipolar depression (Miao and Metcalfe 2003; Mohapatra et al. 2013). Owing to its high persistence in the environment, CARB has been noted as an anthropogenic marker of wastewater contamination in water bodies and has the potential to bioaccumulate in the organisms of aquatic food chain (Vernouillet et al. 2010). TRIM (2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine)) is the earliest anti-bacterial diaminopyrimidine used to treat human and animal diseases (Liguoro et al. 2012). TRIM is used in combination with sulfonamides with its therapeutic action consisting of inhibiting an enzyme (dihydroflate reductase) active in the folic acid metabolism in bacteria. The TRIM concentration levels in wastewater of both cities under the present study are higher than in domestic wastewaters of other countries including the USA (1.30 μg L−1; Karthikeyan and Meyer 2006), China (0.162 μg L−1; Zhou et al. 2012; <0.30 μg L−1; Peng et al. 2008), Japan (0.042 μg L−1; Chang et al. 2008), Australia (0.858 μg L−1; Le-Minh et al. 2010), and Korea (0.496 μg L−1; Choi et al. 2008). High concentration level for TRIM could be attributed to its low hydrophobic partition coefficient (Table 1), which enhances its detection in the aqueous phase. CARB concentrations in present study are also higher than those in wastewaters of Sweden (1.68 μg L−1; Bendz et al. 2005), Italy (0.265 μg L−1; Aukidy et al. 2012), Japan (0.270 μg L−1; Nakada et al. 2006), Korea (0.451 μg L−1; Choi et al. 2008), and Spain (2.15 μg L−1; Santos et al. 2007). Such higher levels of CARB may be due to its persistent nature as it was reported to be resistant towards natural attenuation in surface water (Tixier et al. 2003).
In terms of the detection frequencies (DF, %), DCF was the most consistently detected drug in wastewater of both the cities (Ghaziabad: max 25.68 μg L−1, DF 66.67 %; Lucknow: max 1.43 μg L−1, DF 46.43 %). DCF concentrations in the range of not detected (ND) –103 ng L−1 have been reported in the water of Kaveri, Vellar, and Tamiraparani rivers in India (Shanmugam et al. 2014). The ubiquitous presence of DCF (2-[2,6-dichlorophenyl)-amino]benzeneacetic acid) in the wastewaters can be accounted to its use as human and veterinary medicine in every possible route of administration from oral to intramuscular (Buser et al. 1998). Moreover, direct photolysis was identified as the possible mode of DCF transformation in surface water (Tixier et al. 2003). In relevance to the wastewaters sampled in the present study, the sewage drains were mostly covered eliminating the chances of photo-transformation of DCF. DCF concentrations are higher than those reported in wastewater treatment plant (WWTP) effluent in Italy (0.8 μg L−1) (Aukidy et al. 2012) and in municipal (0.523 μg L−1) wastewaters in Korea (Sim et al. 2011). In another study, Behera et al. (2011) reported DCF levels in WWTP influent (0.243 μg L−1) of South Korea lower than those found in the present study. Lower levels of this drug were also reported in WWTP influents in the USA (0.33–0.49 μg L−1; Thomas and Foster 2005). However, the DCF concentrations observed in the present study are higher than those reported in STP effluent in Switzerland (0.6 μg L−1) (Soulet et al. 2010) and Greece (0.186 μg L−1) (Samaras et al. 2010).
IBF was detected at maximum concentration of 26.45 μg L−1 (DF 36.67 %) in Ghaziabad and 10.02 μg L−1 (DF 39.29 %) in Lucknow wastewaters. IBF concentrations in the range of ND – 200 ng L−1 have been reported in the water of Kaveri, Vellar, and Tamiraparani rivers in India (Shanmugam et al. 2014). IBF (2-(4-isobutylphenyl)propanoic acid) is one of the core medicines (Heckmann et al. 2007) and finds applications in combating musculature pain, inflammatory disorders, and fever. IBF was detected at a higher concentration range of 12.1–373.1 μg L−1 in influent streams of four WWTPs in Spain (Santos et al. 2007) but at lower level in effluent samples in Canada (5.2 μg L−1) (Crouse et al. 2012) and influent sample in South Korea (2.853 μg L−1) (Behera et al. 2011). The IBF concentrations in Ghaziabad wastewater are found higher than that reported for WWTP in the USA where the concentration ranged from 9.5 to 14.7 μg L−1 (Thomas and Foster 2005) and much higher than in the STP wastewaters in Switzerland (influent 1–4.6 μg L−1; effluent 0.01–0.3 μg L−1) (Tauxe-Wuersch et al. 2005; Soulet et al. 2010) and Greece (influent 0.096–0.403 μg L−1; effluent 0.005–0.262 μg L−1) (Samaras et al. 2010). IBF (0.28 μg L−1), DCF (0.46 μg L−1), and NPX (0.24 μg L−1) were also detected in Poland WWTPs (Migowska et al. 2012).
NPX and KTF were present at maximum concentrations of 1.65 μg L−1 (DF: 20 %) and 16.21 μg L−1 (DF 30 %), respectively, in Ghaziabad wastewater. In case of Lucknow, NPX was not detected in any of the wastewater samples, but KTF was detected at a maximum concentration of 1.69 μg L−1 (DF 25 %). Shanmugam et al. (2014) reported the NPX and KTF concentrations in Indian river water in the range of ND 28 and 0.60–660 ng L−1, respectively. NPX (6-methoxy-α-methyl-2-naphthalene acetic acid) is used to reduce mild to moderate pain, fever, inflammation, stiffness, and in treatment of osteoporosis, rheumatoid arthritis, menstruation, and headaches (Ceurda-Correa et al. 2010; Ziylan and Ince 2011), whereas KTF (2-(3-benzoylphenyl) propanoic acid) possesses analgesic, anti-pyretic, and anti-inflammatory properties and acts by inhibition of prostaglandin synthesis (Ceurda-Correa et al. 2010). The drug is used to relieve pain associated with sciatica, neuralgia, arthritis, and other inflammatory disorders. According to Tixier et al. (2003), NPX was susceptible to biodegradation and may be considered as the possible elimination mechanism for the drug. NPX and KTF have been reported in wastewater in Italy (Aukidy et al. 2012) at a maximum concentration of 0.021 μg L−1 each, but KTF was at higher DF (67 %) than NPX (33 %) similar to the pattern observed herein. However, higher concentrations have been reported for NPX (5.033 μg L−1) than KTF (0.286 μg L−1) in WWTP influents of South Korea (Behera et al. 2011). NPX was detected at higher range of 10.3–12.8 μg L−1 while KTF was at lower range of 0.41–0.52 μg L−1 in WWTP of the USA (Thomas and Foster 2005). Higher concentrations of NPX have been reported in untreated municipal wastewaters (12.5 μg L−1) in South Korea (Sim et al. 2011). In another study, Samaras et al. (2010) reported NPX and KTF concentrations in the respective ranges of 0.219–0.302 and 0.036–0.097 μg L−1 in Swiss WWTP influents. KTF and NPX in wastewaters in Japan (Nakada et al. 2006) were detected with lower concentrations (0.108–0.369 and 0.038–0.230 μg L−1) as compared to those in the present study. Both NPX and KTF have been reported at higher levels in wastewaters of Spain (27.40 and 2.50 μg L−1) (Santos et al. 2007). Higher concentration of NPX (3.2 μg L−1) has also been reported in Canadian STP effluent (Crouse et al. 2012).
Principal component analysis
Separate PCAs were performed on two datasets of Ghaziabad and Lucknow. Based on the eigenvalues being greater than 1, three PCs were chosen both for the Ghaziabad and Lucknow data, which captured 80.6 and 70.0 % of the data variance. Table 9 shows the respective PC loadings for two study areas. For the Ghaziabad data, the PC1 (33.6 %) showed high positive loadings (>0.7) on NPX and CARB. PC2 (27.4 %) exhibited high positive loadings on KTF and DCF and moderate (>0.5) positive loadings on IBF. PC3 (19.6 %) had high negative loadings on TRIM. In the case of Lucknow, PC1 capturing 27.1 % variance showed high positive loadings on DCF and high negative loadings on KTF, whereas PC2 explaining 20.8 % variance had high negative loadings on CARB. PC3 (22.1 %) exhibited high negative loadings on IBF and moderate positive loadings on TRIM. A different PCA loading pattern of various drugs in two study areas suggests for different usage and combinations of these drugs in these regions.
Environmental risk assessment
The potential environmental risk of the detected drugs in wastewater was assessed on the basis of RQ, according to the European Technical Guidance Document on Risk Assessment (EC 2003). Here, the RQs of different detected drugs were computed for six different bioassays, such as bacteria, duckweed, algae, daphnia, rotifers, and fish (Fig. 4). As expected, since the respective toxicities are different for different species, each one shows its own sensitivity respect to various drug molecules. Thus, for the bacteria-based bioassay, IBF, KTF, and DCF exhibited RQ > 1 in Ghaziabad wastewater, whereas these drugs showed moderate risk in Lucknow wastewater. Although drugs at low concentration have little toxic effects on aquatic organisms, long-term exposure of bacteria to trace level of drugs may increase anti-biotic resistance in the environment, and their resistance genes could be transferred between bacteria through plasmids, transposons, and integrons (Pruden et al. 2006). In Spain, the RQs of KTF and NPX in bacteria were 0.23 and 1.28 in influent and 0.10 and 0.20 in effluent samples have been reported (Santos et al. 2007). In duckweed bioassay, none of the six drugs showed RQ value greater than 1; however, the IBF both in Ghaziadad and Lucknow and DCF only in Ghaziabad wastewater exhibited moderate risk. The algae and daphnia bioassays revealed that KTF showed high risk in Ghaziabad, and DCF and TRIM have moderate risk. In Lucknow wastewater, KTF and CARB showed moderate risk to both the algae and daphnia. Higher RQ values of 41 and 5.30 towards daphnid have been reported for IBF in influent and effluent sewage samples of Spain (Santos et al. 2007). Aukidy et al. (2012) reported RQ values for DCF (0.05–0.08), KTF (0.001), and TRIM (0.015) for algae in WWTP effluents in Italy.
NPX and CARB exhibited high risk in rotifers in Ghaziabad as well as the later one in Lucknow. Among all the bioassay species considered here, fish belongs to the highest trophic level. In wastewater of both the study areas, IBF exhibited high risk to fish. IBF and other NSAIDs are known to inhibit ovulation in mammals, including humans (Hernando et al. 2006). IBF exposure of more than 6 weeks resulted in changes in the pattern of reproduction of Japanese Medaka fishes (Flippin et al. 2007). IBF manifests acute and chronic toxicity and has been tested positive to cause endocrine disruption in nontarget aquatic organisms (Han et al. 2010). Bioassay tests on aquatic organisms revealed NPX photoderivatives to elucidate higher toxicity than the parent compound under both acute and chronic conditions (Isidori et al. 2005). Fent et al. (2006) reviewed that DCF had the highest acute toxicity on aquatic organisms among all the NSAIDs. DCF was also found to cause cytological alterations in livers, kidneys, and gills of rainbow trout at lowest observed effect concentration of 1 μg L−1. The drug was responsible for population decline of vultures in Asia (renal lesions and visceral gout formation) which fed upon the carcass of animals treated with diclofenac (Oaks et al. 2004) and was banned for manufacture in India, Nepal, and Pakistan (Taggart et al. 2009). The RQ values of rest of the drugs were no more than 0.01 for all the bioassays, suggesting that these molecules are unlikely to cause adverse toxic effects on aquatic organisms. However, this approach may underestimate the risk associated with pharmaceuticals present in combinations which in real scenario are present in mixtures and rarely occur singly.
Conclusions
This work shows a predominant presence of NSAIDs (IBF, KTF, NPX, DCF), anti-epileptic (CARB), and anti-microbial (TRIM) in the urban wastewater of two north Indian cities. Levels of all these detected drugs were relatively higher in wastewater of Ghaziabad as compared to those of Lucknow. DCF was the most frequently detected drug in wastewater of both the cities which suggests for anthropogenic sources in the study regions. Highly skewed distribution of the concentration data for all the detected drugs in two cities suggests for heterogeneous nature of the sources. Statistical analysis of the data revealed that the two populations are significantly different (p < 0.05). The environmental risk assessment of the target drugs in six bioassays at different trophic levels revealed that NSAIDs pose highest risk to all the test species in both the study areas. Since that toxicity of a single drug might be enhanced by the occurrence of other drugs with similar activity, the overall risk of these could be significant. Nevertheless, the presence of these drugs in the environment is not limited only to an ecological problem since contamination also affects drinking water, being a potential to human health. The present study may provide baseline drug levels for invoking further investigations and most importantly may contribute to the formulation of guidelines or control measures for regulation of such emerging contaminants in the environment.
References
Al-Rajab, A. J., Sabourin, L., Lapen, D. R., & Topp, E. (2010). The non-steroidal anti-inflammatory drug diclofenac is readily biodegradable in agricultural soils. Science of the Total Environment, 409, 78–82.
Ascar, L., Ahumada, I., Lopez, A., Quintanilla, F., & Leiva, K. (2013). Nonsteriodal anti-inflammatory drug determination in water sample by HPLC-DAD under isocratic conditions. Journal of the Brazilian Chemical Society, 24, 1160–1166.
Aukidy, M. A., Verlichhi, P., Jelic, A., Petrović, M., & Barceló, D. (2012). Monitoring release of pharmaceutical compounds: occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po valley, Italy. Science of the Total Environment, 438, 15–25.
Babic, S., Horvat, A. J. M., Pavlovic, D. M., & Kastelan-Macan, M. (2007). Determination of pKa values of active pharmaceutical ingredients. Trends in Analytical Chemistry, 26, 1043–1061.
Behera, S. K., Kim, H. W., Oh, J. E., & Park, H. S. (2011). Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Science of the Total Environment, 409, 4351–4360.
Bendz, D., Paxéus, N. A., Ginn, T. R., & Loge, F. J. (2005). Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. Journal of Hazardous Materials, 122, 195–204.
Buser, H. R., Poiger, T., & Muller, M. D. (1998). Occurrence and fate of the pharmaceutical drug diclofenac in surface waters: rapid photodegradation in a lake. Environmental Science and Technology, 33, 2529–2535.
Ceurda-Correa, E. M., Domínguez-Vargas, J. R., Olivares-Marín, F. J., & de Beltrán, H. J. (2010). On the use of carbon blacks as potential low-cost adsorbents for the removal of non-steroidal anti-inflammatory drugs from river water. Journal of Hazardous Materials, 177, 1046–1053.
Chang, H., Hu, J., Asami, M., & Kunikane, S. (2008). Simultaneous analysis of 16 sulfonamide and trimethoprim antibiotics in environmental waters by liquid chromatography-electrospray tandem mass spectrometry. Journal of Chromatography A, 1190, 390–393.
Choi, K., Kim, Y., Park, J., Park, C. K., Kim, M., Kim, H. S., et al. (2008). Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Science of the Total Environment, 405, 120–128.
Clara, M., Strenn, B., & Kreuzinger, N. (2004). Carbamazepine as a possible anthropogenic marker in the aquatic environment: investigations on the behaviour of carbamazepine in wastewater treatment and during groundwater infiltration. Water Research, 38, 947–954.
Cleuvers, M. (2003). Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicology Letters, 142, 185–194.
Cleuvers, M. (2004). Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicology and Environmental Safety, 5, 309–315.
Crouse, B. A., Ghoshdastidar, A. J., & Tong, A. Z. (2012). The presence of acidic and neutral drugs in treated sewage effluents and receiving waters in the Cornwallis and Annapolis River watersheds and the Mill Cove sewage treatment plant in Nova Scotia, Canada. Environmental Research, 112, 92–99.
De Lange, H. J., Noordoven, W., Murk, A. J., Larling, M., & Peeters, E. T. H. M. (2006). Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquatic Toxicology, 78, 209–216.
De Liguoro, M., Fioretto, B., Poltronieri, C., & Gallina, G. (2009). The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim. Chemosphere, 75, 1519–1524.
Derle, D. V., Gujar, K. N., & Sagar, B. S. H. (2006). Adverse effects associated with the use of non steroidal anti-inflammatory drugs: an overview. Indian Journal of Pharmaceutical Sciences, 68, 409–414.
Eguchi, K., Nagase, H., Ozawa, M., Endohi, Y. S., Goto, K., Hirata, K., et al. (2004). Evaluation of antimicrobial agents for veterinary use in the ecotoxicity test using microalgae. Chemosphere, 57, 1733–1738.
EMEA (2012) Guideline on the environmental risk assessment of medicinal products for human use. Committee for medicinal products of human use (CHMP). Brussels: EMEA.
European Commission (EC) (2003) The Technical Guidance Document (TGD) on risk assessment in support of Commissions Directive 93/67/EEC on risk assessment for new notified substances and Commissions Regulation (EC) No. 1488/94 on risk assessment for existing substances. European Chemicals Bureau, 2nd Edition, Ispra Italy.
Farré, M. L., Ferrer, I., Ginebreda, A., Figueras, M., Olivella, L., Tirapu, L., et al. (2001). Determination of drugs in surface water and wastewater samples by liquid chromatography–mass spectrometry: methods and preliminary results including toxicity studies with Vibro fisheri. Journal of Chromatography A, 938, 187–197.
Fatemi, M. H., & Ghorbannezhad, Z. (2011). Estimation of the volume of distribution of some pharmacologically important compounds from their structural descriptors. Journal of the Serbian Chemical Society, 76, 1003–1014.
Fent, K., Weston, A. A., & Caminada, D. (2006). Review ecotoxicology of human pharmaceuticals. Aquatic Toxicology, 76, 122–159.
Ferrari, B., Paxeus, N., Giudice, R. L., Pollio, A., & Garric, J. (2003). Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicology and Environmental Safety, 55, 359–370.
Flippin, J. L., Huggett, D., & Foran, C. M. (2007). Changes in the timing of reproduction following chronic exposure to ibuprofen in Japanese medaka Oryzias latipes. Aquatic Toxicology, 81, 73–78.
Ganguly, N. K., Arora, N. K., Chandy, S. J., Gill, J. P. S., Gupta, U., Joglekar, S., et al. (2011). Rationalizing antibiotic use to limit antibiotic resistance in India. Indian Journal of Medical Research, 134, 281–294.
Ginebreda, A., Muñoz, I., de Alda, M. L., Brix, R., López-Doval, J., & Barcelo, D. (2010). Environmental risk assessment of pharmaceuticals in rivers: relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain). Environmental International, 36, 153–162.
Goel, R. K., Bhati, Y., Dutt, H. K., & Chopra, V. S. (2013). Prescribing pattern of drugs in the outpatient department of a tertiary care teaching hospital in Ghaziabad, Uttar Pradesh. Journal of Applied Pharmaceutical Science, 3, S48–S51.
Gros, M., Petrović, M., Ginebreda, A., & Barceló, D. (2010). Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environmental International, 36, 15–26.
Hallare, A. V., Köhler, H. R., & Triebskorn, R. (2004). Developmental toxicity and stress protein responses in zebrafish embryos after exposure to diclofenac and its solvent, DMSO. Chemosphere, 56, 659–666.
Han, S., Choi, K., Kim, J., Ji, K., Kim, S., Ahn, B., et al. (2010). Endocrine disruption and consequences of chronic exposure to ibuprofen in Japanese medaka (Oryzias latipes) and freshwater cladocerance Daphina magna and Moina macrocarpa. Aquatic Toxicology, 98, 256–264.
Harada, K., Komori, N., Nakada, K., & Kitamura, S. Y. (2008). Biological effects of PPCPs on aquatic lives and evaluation of river waters. Water Science and Technology, 58, 1541–1546.
Heberer, T., Reddersen, K., & Mechlinski, A. (2002). From municipal sewage to drinking water: fate and removal of pharmaceutical residues in the aquatic environment in urban areas. Water Science and Technology, 46, 81–88.
Heckmann, L. H., Callaghan, A., Hooper, H. L., Connon, R., Hutchinson, T. H., Maund, S. J., et al. (2007). Chronic toxicity of ibuprofen to Daphnia magna: effects on life history traits and population dynamics. Toxicology Letters, 172, 137–145.
Hernando, M. D., Mezcua, M., Fernandez-Alba, A. R., & Barceló, D. (2006). Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta, 69, 334–342.
ICH. (1995). Q2B, validation of analytical procedures: methodology. London: International Conference on harmonization.
ICH (2009) Q2(R1), Validation of analytical procedures: text and methodology, International Conference on harmonization. http://www.ich.org/LOB/media/Media417.pdf (accessed June 2009).
Iglesias, A., Nebot, C., Miranda, J. M., Vázquez, B. I., Abuín, C. M. F., & Cepeda, A. (2013). Determination of the presence of three antimicrobials in surface water collected from urban and rural areas. Antibiotics, 2, 46–57.
Isidori, M., Lavorgna, T. M., Nardelli, A., Parrella, A., Previtera, L., & Rubino, M. (2005). Ecotoxicity of naproxen and its phototransformation products. Science of the Total Environment, 348, 93–101.
Karthikeyan, K. G., & Meyer, M. T. (2006). Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Science of the Total Environment, 361, 196–207.
Ki, S., Chandy, S. J., Jeyasedan, L., Kumar, R., & Suresh, S. (2008). Antimicrobial prescription patterns for common acute infections in some rural and urban health facilities of India. Indian Journal of Medical Research, 128, 165–171.
Kim, Y., Choi, K., Jung, J., Park, S., Kim, P. G., & Park, J. (2007). Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environmental International, 33, 370–375.
Kleywegt, S., Pileggi, V., Yang, P., Hao, C., Zhao, X., Rocks, C., et al. (2011). Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—occurrence and treatment efficiency. Science of the Total Environment, 409, 1481–1488.
Kolpin, D. W., Furlong, E. T., Meyer, M. T., Thurman, E. M., Zuagg, S. T., Barber, L. B., et al. (2002). Pharmaceuticals, hormones and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environmental Science and Technology, 36, 1202–1211.
Kosjek, T., Heath, E., & Krbavčič, A. (2005). Determination of non-steroidal anti-inflammatory drug (NSAIDs) residues in water samples. Environmental International, 31, 679–685.
Kümmerer, K. (2001). Pharmaceuticals in the environment: sources, fate, effects and risks. Berlin: Springer.
Latorre, A., Lacorte, S., & Barcelo, D. (2003). Presence of nonylphenol, octyphenol and bisphenol a in two aquifers close to agricultural, industrial and urban areas. Chromatographia, 57, 111–116.
Le-Minh, N., Khan, S. J., Drewes, J. E., & Steutz, R. M. (2010). Fate of antibiotics during municipal water recycling treatment processes. Water Research, 44, 4295–4323.
Liguoro, M. D., Leva, V. D., Bona, M. D., Merlanti, R., Caporale, G., & Radaelli, G. (2012). Sublethal effects of trimethoprim on four fresh water organisms. Ecotoxicology and Environmental Safety, 82, 114–121.
Luo, Y., Mao, D. Q., Rysz, M., Zhang, H. J., Xu, L., & Alvarez, P. J. J. (2010). Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environmental Science and Technology, 44, 7220–7225.
Luo, Y., Xu, L., Rysz, M., Wang, Y., Zhang, H., & Alvarez, P. J. J. (2011). Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe river basin, China. Environmental Science and Technology, 45, 1827–1833.
Madureira, T. V., Rocha, M. J., Cass, Q. B., & Tiritan, M. E. (2010). Development and optimization of a HPLC-DAD method for the determination of diverse pharmaceuticals in estuarine surface waters. Journal of Chromatographic Science, 48, 176–182.
McGrath, D., Zhang, C., & Carton, O. T. (2004). Geostatistical analyses and hazard assessment on soil lead in silvermines area, Ireland. Environmental Pollution, 127, 239–248.
Miao, X. S., & Metcalfe, C. D. (2003). Determination of carbamazepine and its metabolites in aqueous samples using liquid chromatography-electrospray tandem mass spectrometry. Analytical Chemistry, 75, 3731–3738.
Migowska, N., Caban, M., Stepnowski, P., & Kumirska, J. (2012). Simultaneous analysis of non steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography–mass spectrometry and gas chromatography with electron capture detection. Science of the Total Environment, 441, 77–88.
Mohapatra, D. P., Brar, S. K., Tyagi, R. D., Picard, P., & Surampalli, R. Y. (2013). A comparative study of ultrasonication, Fenton’s oxidation and ferro-sonication treatment for degradation of carbamazepine from wastewater and toxicity test by yeast estrogen screen (YES) assay. Science of the Total Environment, 447, 280–285.
Monteiro, S. C., & Boxall, A. B. (2010). Occurrence and fate of human pharmaceuticals in the environment. Reviews of Environmental Contamination and Toxicology, 202, 53–154.
Mostafavi, A., Abedi, G., Jamshidi, A., Afzali, D., & Talebi, M. (2009). Development and validation of a HPLC method for the determination of buprenorphine hydrochloride, naloxone hydrochloride and noroxymorphone in a tablet formulation. Talanta, 77, 1415–1419.
Nakada, N., Tanishima, T., Shinohara, H., Kiri, K., & Takada, H. (2006). Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Research, 40, 3297–3303.
Oaks, J. L., Gilbert, M., Virani, M. Z., Watson, R. T., Meteyer, C. U., Rideout, B. A., et al. (2004). Diclofenac residues as the cause of vulture population decline in Pakistan. Nature, 427, 630–633.
Oggier D (2011) Trimethoprim, growth inhibition test with Lemna minor. BMG A11-00372; BMG Engineering, on behalf of F. Hoffmann-La Roche Ltd: Schlieren, Switzerland.
Osborne, J. W. (2010). Improving your data transformations: applying the Box-Cox transformation. Practical assessment, research & evaluation, 15, 1–9.
Peng, X., Tan, J., Tang, C., Yu, Y., & Wang, Z. (2008). Multiresidue determination of fluoroquinolone, sulfonamide, trimethoprim, and chloramphenicol antibiotics in urban waters in China. Environmental Toxicology and Chemistry, 27, 73–79.
Pomati, F., Netting, A. G., Calamari, D., & Neilan, B. A. (2004). Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquatic Toxicology, 67, 387–396.
Pruden, A., Pei, R., Storteboom, H., & Carlson, K. H. (2006). Antibiotic resistance genes as emerging contaminants: studies in northern Colorado. Environmental Science and Technology, 40, 7445–7450.
Ramaswamy, B. R., Shanmugam, G., Velu, G., Rengarajan, B., & Larsson, D. G. (2011). GC- MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. Journal of Hazardous Materials, 186, 1586–1593.
Rhee, J. S., Jeong, C. B., Kim, B. M., & Lee, J. S. (2012). P-glycoprotein (P-gp) in the monogonont rotifer, Brachionus koreanus: molecular characterization and expression in response to pharmaceuticals. Aquatic Toxicology, 114–115, 104–118.
Samaras, V. G., Thomaidis, N. S., Stasinakis, A. S., Gatidou, G., & Lekkas, T. D. (2010). Determination of selected nonsteroidal anti-inflammatory drugs in wastewater by gas chromatography mass spectrometry. International Journal of Environmental Analytical Chemistry, 90, 219–229.
Samaras, V. G., Thomaidis, N. S., Stasinakis, A. S., & Lekkas, T. D. (2011). An analytical method for the simultaneous trace determination of acidic pharmaceuticals and phenolic endocrine disrupting chemicals in wastewater and sewage sludge by gas chromatography–mass spectrometry. Analytical and Bioanalytical Chemistry, 399, 2549–2561.
Sanderson, H., Johnson, D. J., Wilson, C. J., Brain, R. A., & Solomon, K. R. (2003). Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicology Letters, 144, 383–395.
Santos, J. L., Aparicio, I., Alonso, E., & Callejon, M. (2005). Simultaneous determination of pharmaceutically active compounds in wastewater samples by solid-phase extraction and high-performance liquid chromatography with diode array and fluorescence detectors. Analytica Chimica Acta, 550, 116–122.
Santos, J. L., Aparicio, I., & Alonso, E. (2007). Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environmental International, 33, 596–601.
Seifrtová, M., Nováková, L., Lino, C., Pena, A., & Solich, P. (2009). An overview of analytical methodologies for the determination of antibiotics in environmental waters. Analytica Chimica Acta, 649, 158–179.
Shakya, G., Malla, S., Shakya, K. N., & Shrestha, R. (2008). Therapeutic drug monitoring of antiepileptic drugs. Journal of Nepal Medical Association, 47, 94–97.
Shanmugam, G., Sampath, S., Selvaraj, K. K., Larsson, D. G., & Ramaswamy, B. R. (2014). Non-steroidal anti-inflammatory drugs in Indian rivers. Environmental Science and Pollution Research International, 21, 921–931.
Sim, W., Lee, J., Lee, E., Shin, S., Hwang, S., & Oh, J. (2011). Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere, 82, 179–186.
Singh, K. P., Malik, A., Singh, V. K., Mohan, D., & Sinha, S. (2005). Chemometric analysis of groundwater quality data of alluvial aquifer of Gangetic plain, North India. Analytica Chimica Acta, 550, 82–91.
Singh, K. P., Malik, A., Kumar, R., Saxena, P., & Sinha, S. (2008). Receptor modeling for source apportionment of polycyclic aromatic hydrocarbons in urban atmosphere. Environmental Monitoring and Assessment, 136, 183–196.
Smirnov NV (1939) On the estimation of the discrepancy between empirical curves of distribution for two independent samples. (Russian) Bull Moscow Univ 2, 3–16.
Soulet, B., Tauxe, A., & Taradellas, J. (2010). Analysis of acidic drugs in Swiss wastewaters. International Journal of Environmental and Analytical Chemistry, 82, 659–667.
Stasinakis, S., Gatidou, G., Mamais, D., Thomaidis, N. S., & Lekkas, T. D. (2008). Occurrence and fate of endocrine disrupters in Greek sewage treatment plants. Water Research, 42, 1796–1804.
Suthar, S., Sharma, J., Chabukdhara, M., & Nema, A. K. (2010). Water quality assessment of river Hindon at Ghaziabad, India: impact of industrial and urban wastewater. Environmental Monitoring and Assessment, 165, 103–112.
Taggart, M., Senacha, K., Green, R., Curthberth, R., Jhala, Y. V., Meharg, A., et al. (2009). Analysis of nine NSAIDs in ungulate tissues available to critically endangered vultures in India. Environmental Science and Technology, 43, 4561–4566.
Tauxe-Wuersch, A., Alencastro, L. F. D., Grandjean, D., & Tarradellas, J. (2005). Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Research, 39, 1761–1772.
Tcheslavski, G., & (Louis) Beex, A. A. (2010). Effects of smoking, schizotypy, and eyes open/closed conditions on the γ1 rhythm phase synchrony of the electroencephalogram. Biomedical signal processing, 5, 164–173.
Thiele-Bruhn, S. (2003). Pharmaceutical antibiotic compounds in soils—a review. Journal of Plant Nutrition and Soil Science, 166, 145–167.
Thomas, P. M., & Foster, D. G. (2005). Tracking acidic pharmaceuticals, caffeine, and triclosan through the wastewater treatment process. Environmental Toxicology and Chemistry, 24, 25–30.
Tixier, C., Singer, H. P., Oellers, S., & Muller, S. R. (2003). Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environmental Science and Technology, 37, 1061–1068.
Vazquez-Roig, P., Andreu, V., Blasco, C., & Picó, Y. (2012). Risk assessment on the presence of pharmaceuticals in sediments, soils and waters of the Pego–Oliva Marshlands (Valencia, eastern Spain). Science of the Total Environment, 440, 24–32.
Vernouillet, G., Eullaffroy, P., Lajeunesse, A., Blaise, C., Gagné, F., & Juneau, P. (2010). Toxic effects and bioaccumulation of carbamazepine evaluated by biomarkers measured in organisms of different trophic levels. Chemosphere, 80, 1062–1066.
Vieno, N. M., Harkki, H., Tuhkanen, T., & Kronberg, L. (2007). Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environmental Science and Technology, 41, 5077–5084.
Wiegel, S., Aulinger, A., Brockmeyer, R., Harms, H., Loffler, J., Reincke, H., et al. (2004). Pharmaceuticals in the river Elbe and its tributaries. Chemosphere, 57, 107–126.
Xie, Y. F., Li, X. W., Wang, J. F., Christakos, G., Hu, M. G., An, L. H., et al. (2012). Spatial estimation of antibiotic residues in surface soils in a typical intensive vegetable cultivation area in China. Science of the Total Environment, 430, 126–131.
Yang, J. F., Ying, G. G., Zhao, J. L., Tao, R., Su, H. C., & Liu, Y. S. (2011). Spatial and seasonal distribution of selected antibiotics in surface waters of the Pearl Rivers, China. Journal of Environmental Science and Health. Part. B, 46, 272–280.
Ye, Z., Weinberg, H. S., & Meyer, M. T. (2007). Trace analysis of trimethoprim and sulfonamide, macrolide, quinolone, and tetracycline antibiotics in chlorinated drinking water using liquid chromatography electronspray tandem mass spectrometry. Analytical Chemistry, 79, 1135–1114.
Zhao, L., Dong, Y. H., & Wang, H. (2010). Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Science of the Total Environment, 408, 1069–1075.
Zhou, L., Ying, G., Liu, S., Zhao, J., Chen, F., Zhang, R., et al. (2012). Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography-electrospray ionization tandem mass spectrometry. Journal of Chromatography, A1244, 123–138.
Ziylan, A., & Ince, N. H. (2011). The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: treatability by conventional and non-conventional processes. Journal of Hazardous Materials, 187, 24–36.
Acknowledgments
The authors thank the Director, CSIR-Indian Institute of Toxicology Research, Lucknow (India) for his keen interest in this work and providing all necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, K.P., Rai, P., Singh, A.K. et al. Occurrence of pharmaceuticals in urban wastewater of north Indian cities and risk assessment. Environ Monit Assess 186, 6663–6682 (2014). https://doi.org/10.1007/s10661-014-3881-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3881-8