Abstract
Foliar and root applications of different silicon (Si)-based formulations were evaluated for their effects in reducing powdery mildew and promoting growth of wheat plants. X-ray microanalyses of treated plants revealed that root applications resulted in consistent deposition of Si in the leaves. In terms of powdery mildew control, root applications at 1.7 mM Si gave consistently the best results, reducing disease severity by as much as 80%, regardless of the product used. Although less effective than root applications, foliar treatments with both Si and nutrient salt solutions led to a significant reduction of powdery mildew on wheat plants. This suggests a direct effect of the products on powdery mildew rather than one mediated by the plant as in the case of root amendments. In our experiments, Si amendment, either through the roots or the leaves, did not increase plant growth. These results lead to the conclusion that Si is primarily, if not exclusively, absorbed by the root system and that such absorption by the roots is necessary for an optimal prophylactic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of soluble silicon (Si) to reduce the impact of plant diseases has been amply described in the case of powdery mildews and rice blast (Magnaporthe grisea) (Bélanger et al. 1995; Fawe et al. 2001; Rodrigues et al. 2003; Kim et al. 2002). For the prophylactic effect to be manifest, Si needs to be absorbed in the form of silicic acid [Si(OH)4], where along with water, it follows the transpiration stream to finally deposit as silica (Canny 1990; Sangster et al. 2001). Si absorption by roots can be active or passive and some plant species, like rice, have specific silicon transporters for active take-up (Ma et al. 2004, 2006).
While Si amendments to soils or nutrient solutions via silicate slags or liquid solutions have been the standard form of application, there is commercial interest to develop more user-friendly products for foliar applications. However, there is no strong evidence that Si can be absorbed by leaves and few scientific studies have confirmed the benefits of Si amendments through foliar applications. Some positive effects have been reported on cucumber, muskmelon, zucchini and grape to control powdery mildews (Bowen et al. 1992; Menzies et al. 1992). Nevertheless, for those studies, Si concentrations of 17 mM in the form of potassium silicate were reportedly used which far exceeds the standard 1.7 mM in root applications. As such it was not clear in those experiments if the observed prophylactic effects of foliar sprays were the result of Si or simply high potassium salts concentration reported for their activity against powdery mildew (Horst et al. 1992; Reuveni et al. 1995).
The nutritional properties of Si in plant growth are also not well established. The literature on Si in plants is replete with reports that the element promotes plant growth. In most cases however, it is uncertain whether growth stimulation is attributable to a nutritional effect or to the alleviation of biotic or abiotic stresses (Epstein 1994, 2001; Fauteux et al. 2006). In the midst of all these uncertainties, the search or development of more efficient Si-based products, for agricultural purposes, is perceived as the solution for greater efficacy of Si amendments. In this context, the objectives of this study were to evaluate: (1) the absorption; (2) the prophylactic role and (3) the effect on plant growth of different Si formulations. For this purpose, the wheat – Blumeria graminis f.sp. tritici (Bgt) interaction was selected because of the reported beneficial role of Si in this pathosystem (Bélanger et al. 2003; Rémus-Borel et al. 2005) and plants were submitted to both root and foliar applications.
Materials and methods
Plant material
The wheat cv. HY644 was used in all experiments because of its known susceptibility to Bgt (Bélanger et al. 2003). Seeds were sown in Premier Pro-Mix PGX (Premier Horticulture, Québec, Canada) in 15 cm plastic pots at a rate of five seeds per pot. The pots were incubated in a greenhouse maintained at 24/19°C with a relative humidity of 80% and a photoperiod of 16 h with a photosynthetic photon flux of ≈1,000 μmol m−2 s−1. The plants started receiving Si solution at the third-leaf stage.
Silicon amendments: root and foliar applications
Three Si-based products were used throughout the experiments: (1) a standard potassium silicate solution, (Kasil® 6, 26.5% SiO2, National Silicates, Toronto, Canada), (2) a potassium silicate solution formulated for foliar applications (Silamol, 2% SiO2, K2O, 46% PEG400, Label-Agro, Montreal, Canada) and (3) a volcanic rock dust, high in silicon content (MRD-250, 43.2% SiO2, IMRDL, London, UK). Silicon amendments, through root or foliar applications, were initiated one week before Bgt inoculation.
Root applications
There were four treatments for root applications. The control plants received: (1) standard Hoagland’s solution containing 2.1 mM NO3NH4, 2.6 mM K2SO4, 6.5 mM KNO3, 1.0 mM NH4H2PO4, 4.0 mM Ca(NO3)2.4H2O, 2.1 mM MgSO4, 0.5 mM Fe as Fe–EDTA, 0.45 mM H3BO3, 0.20 mM MnSO4.H2O, 0.004 mM CuSO4.5H2O, 0.008 mM ZnSO4.7H2O, 0.001 mM (NH4)6Mo7O24.4H2O, whereas the three silicon treatments consisted of application of the standard Hoagland’s nutrient solution amended with; (2) 1.7 mM Si in the form of Kasil # 6; (3) 1.7 mM Si, Silamol and (4) 20 g of MRD-250 powder suspended in 100 ml of water per pot which corresponded to 0.9 mM Si; attempts to obtain higher concentrations in solution were unsuccessful. Adjustments were made to the Hoagland’s solution to compensate for the additional input of K from potassium silicate solutions. Plants were treated weekly with 200 ml of the nutrient solutions amended or unamended with Si, and were watered on an as-needed basis; for all treatments, pots received the same volume of liquid. The experimental design was completely randomized within each compartment and there were three replications for each treatment. The experiment was repeated twice.
Foliar applications
There were six foliar treatments which consisted of: (1) control plants receiving no foliar spray; (2) deionized water; (3) Hoagland’s solution; (4) 1.7 mM Si, Kasil #6; (5) 0.9 mM Si, MRD-250 solution (20 g powder in 100 ml water extracted overnight and filtered) and (6) 0.35 mM Si, Silamol in water. The concentration of Silamol employed was based on the manufacturer’s recommendations. The pH of the suspensions was adjusted to 5.8–6.0. Plants were sprayed once a week and the base of the plant was covered to prevent run-off into the soil. Treated plants were fertilized weekly with Hoagland’s solution. Inoculated and non-inoculated plants were grown in separate compartments in a greenhouse. The experimental design was completely randomized within each compartment and there were three replications for each treatment. The experiment was repeated twice.
X-ray microanalysis of absorbed Si
Scanning electron microscopy, coupled to an energy dispersive X-ray microanalysis mapping, was used to determine Si absorption by the leaves of wheat plants under the different treatments. For each treatment the third leaf was examined. A minimum of three leaf samples, harvested after the third application of each treatment prior to pathogen inoculation, were prepared for X-ray microanalysis mapping and SEM analyses. Leaves were lyophilized and coated with gold and paladium to give conductivity to the samples. Samples were analyzed using a CAMECA SX-100 Universal EPMA microscope (Cameca instruments Inc., Trumbull, USA) operating at a voltage of 15 kV and a current of 20 nA.
Inoculation with Blumeria graminis f.sp tritici and disease severity
Wheat plants maintained in a growth cabinet and heavily infected with Bgt were shaken one day prior to inoculation to ensure that freshly formed conidia were available. Leaf segments bearing conidia were harvested, and one leaf segment per pot was used to inoculate the experimental plants (4-leaf stage) by gently brushing the infected leaf segment over the plants. Inoculated plants were observed daily for disease development. At the first visible signs of fungal colonies, disease was scored on the third leaf of a plant at the 5 to 6 leaf stage. A visual scale of 0–4 was used to measure disease severity where 0 = no visible colonization; 1 = 10%; 2 = 20%; 3 = 30%; 4 = >40% of leaf area covered (Bélanger et al. 2003). Disease severity was scored for 10 days, or less if a treatment was saturated with infection (i.e. 4 on the arbitrary scale). All five plants from each pot, assessed for disease reaction, represented one experimental unit.
Plant height and weight
At the end of the first experiments, infected and healthy plants were measured for height and fresh weight. Harvested plants were further measured for dry weight after being placed in a drying oven (70–110°C for 48 h). All five plants from each pot represented one experimental unit.
Data analysis
Data were subjected to analyses of variance with the software JMP6 (SAS Institute, Cary, NC). When F values were significant (P < 0.05), treatment means were compared by LS mean differences Student’s t. Similar results with the same statistical differences were obtained in all experiments. Therefore, data presented in Tables and Figs were taken from the first experiment of root and foliar applications where the best conditions for powdery mildew development prevailed.
Results
Si absorption
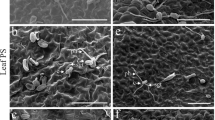
Wheat plants showed a different pattern of Si deposition among treatments based on X-ray microanalysis (Fig. 1). On the leaf surface, Si was concentrated along specific lines corresponding to silica cells. In control plants treated with water, a very faint Si deposit was observed specifically along trichomes of both foliar and root-treated plants (Fig. 1a,e). Plants fed with MRD-250 showed small concentrated niches of Si accumulation (Fig. 1b). Plants treated with Kasil and Silamol through root applications had the highest levels of Si deposition (Fig. 1c,d). By contrast, foliar-treated plants accumulated very little Si. In most cases, concentrations were comparable to that of control plants with only a few spots appearing above the background level (Fig. 1f–h).
X-ray microanalysis from wheat leaves infected by Blumeria graminis f. sp tritici showing the accumulation of silicon (Si) following treatment with: a, e water, b, f MRD-250, c, g Kasil and d, h Silamol. Leaf samples were taken for plants receiving Si through root or foliar applications. Silicon deposits are indicated by colour: red indicates the highest rate of Si whereas black represents no Si
Inoculation with Blumeria graminis f.sp tritici and disease assessment
Root applications
Treatment of wheat plants with the Kasil and Silamol solutions in the soil resulted in a significant reduction of powdery mildew severity compared to the MRD-250 and the control (Fig. 2). As expected, Kasil provided a good control of the disease at a time where control plants had high levels of infection. Interestingly, Silamol, a product formulated for foliar applications, also yielded excellent control of powdery mildew, comparable to that of Kasil, when used as a soil drench at 1.7 mM Si. The MRD-250 treatment, for which applied Si concentration was lower (0.9 mM vs 1.7 mM) than the other Si treatments because of solubility issues, gave a lower disease control than Kasil and Silamol but nevertheless resulted in a lower powdery mildew severity than the control (Fig. 2).
Severity of powdery mildew, caused by Blumeria graminis f. sp tritici on wheat plants fed with different silicon-based solutions amended to the soil. Silicon and formulation effects on powdery mildew severity were evaluated. Means followed by the same letter are not significantly different according to LS mean differences Student’s t (α = 0.05)
Foliar applications
Foliar applications of Si and control solutions gave varying levels of powdery mildew control on wheat plants (Fig. 3). From the onset, the application of foliar sprays caused a significant reduction of powdery mildew compared to plants receiving no foliar treatment. Hoagland’s solution resulted in a similar disease control as the Si-based products. In our experiments, all three Si-based products applied as a foliar solution performed equally well even though the concentration in Si varied among the products (Fig. 3). In fact, in preliminary trials, Silamol was as effective at 0.3 mM as at 1.7 mM in foliar sprays (results not shown). The water foliar spray was not significantly different from the MRD-250, but was significantly less effective than the other foliar sprays. The severity of powdery mildew on the MRD-250 foliar spray-treated plants was not significantly different to powdery mildew severity on plants receiving the other foliar spray treatments (Fig. 3).
Severity of powdery mildew, caused by Blumeria graminis f. sp tritici on wheat plants receiving foliar applications of different silicon-based solutions (Kasil, Silamol and MRD-250), Hoagland’s solution, water and a control treatment without foliar application. Means followed by the same letter are not significantly different according to LS mean differences Student’s t (α = 0.05)
Plant height and weight
For all treatments, for height and weight, there was no statistical difference when plants were subjected to foliar applications of Si or control solutions (results not shown). Similar results were obtained in non-infected plants subjected to root applications (Table 1). In infected plants, only the Silamol treatment resulted in significantly taller plants (Table 2).
Discussion
The results of this study present the first comparative data highlighting the differential and superior absorption of soluble Si by plants following root over foliar applications. These findings are particularly relevant in the context of assessing the attributes or benefits of emerging Si-based foliar fertilizers. From the start, absorption of Si by the plant is a pre-requisite for any positive effect associated with Si feeding (Epstein 1994). For this purpose, this study used scanning electron microscopy coupled to X-ray microanalysis to examine the absorption of Si-based products in wheat plants. Solid silica is deposited in plant tissues following the root uptake of monosilicic acid. Silicic acid will follow the transpiration stream and deposit mostly in the shoots (Sangster et al. 2001). On the adaxial leaf surface, Si will appear along the trichomes, sclerenchyma and silica cells while also being largely present in the apoplast (Sangster et al. 2001). Our results did confirm this pattern whereby Si was easily observed at the base of trichomes and in silica cells. However, Si deposition was most abundant in root applications following the recommended concentrations of 1.7 mM. Foliar applications led to negligible or inconsistent absorption. These results are somewhat surprising considering the current interest in Si-based foliar fertilizers by different fertiliser companies. On the other hand, no study has ever confirmed the absorption of Si in plant tissues following foliar sprays. In a recent study, Ma et al. (2006) reported that the presence of a gene for Si transport in rice was confined to the roots suggesting that root absorption is the optimal, if not the exclusive means by which a plant will take up Si.
Following the observations that root applications led to regular Si absorption, it was interesting to compare the relative impact of the two modes of application, root and foliar, on disease control. Both potassium silicate solutions had a good impact on disease control by root application. Kasil provided an excellent control of powdery mildew on wheat while Silamol, a formulation of potassium silicate chelated with PEG400 and branded for foliar use, was just as efficient when used as a soil amendment at an equal dose of 1.7 mM Si. The difficulty we had at solubilizing Si from MRD-250 probably accounts for its lesser effect on disease control. These results reinforce the concept that 1.7 mM Si is required for maximum effect (Bélanger et al. 1995; Epstein 1994).
Analysis of disease control following foliar application of Si-based products is more difficult to interpret. In our study, all these products conferred a certain level of disease repression compared to the control but so did Hoagland’s solution which is devoid of Si. Consequently, considering the limited absorption of Si with foliar applications, we can not conclude that the observed effect on powdery mildew reduction can be attributed exclusively or even in part to Si. As a first observation, foliar sprays alone can physically dislodge powdery mildew conidia and reduce disease incidence (Yarwood 1939) as exemplified by our results with water sprays. Secondly, salt sprays, including potassium salts such as potassium carbonates and potassium phosphates have been reported many times to reduce disease incidence with particular emphasis on powdery mildews (Reuveni and Reuveni 1995, Bélanger and Labbé 2002), an observation in line with our results with Hoagland’s solution. Liang et al. (2005), suggested that disease reduction caused by foliar sprays of potassium silicate was the result of an osmotic effect on spores germinating at the leaf surface. Alternatively, Reuveni et al. (1995) also proposed that salts could induce resistance. Based on this evidence, it appears that any foliar sprays of potassium salts, including potassium silicate, can afford a certain control of powdery mildew incidence as a result of a direct effect on the pathogen rather than one mitigated by the plant.
In our study, wheat plants fed with Si through soil amendments did not register an increased height or weight over control plants; only Bgt-inoculated plants treated with Silamol showed an increase in height. Whether this difference is attributable to the prophylactic effect of the treatment is uncertain considering that Kasil-treated plants were equally resistant to powdery mildew and did not register better height and weight within the scope of our experiments. In recent work, Fauteux et al. (2006) analyzed the effect of Si feeding on some 30,000 Arabidopsis genes and observed differences only if the plants were stressed. It might thus be interesting to evaluate the benefits of Silamol in stressed plants over longer periods.
In conclusion, this work presents the first comprehensive comparison of the effect of Si-based products on powdery mildew control and on plant height and weight under two different forms of application. Root applications of potassium silicate at a concentration of 1.7 mM gave the best results in terms of Si absorption and powdery mildew control regardless of the source. Foliar applications of Si did not lead to a consistent absorption of the element by the plant, and their observed effect on powdery mildew was likely caused by a direct deleterious action on the fungus. While Si feeding did not yield a direct measurable effect on plant growth, its positive role on disease suppression may lead to better plant productivity.
Abbreviations
- Bgt :
-
Blumeria graminis f.sp. tritici
- SEM:
-
scanning electron microscopy
- Si:
-
silicon
References
Bélanger, R. R., & Labbé, C. (2002). Control powdery mildews without chemicals: prophylactic and biological alternatives for horticultural crops. In R. R Bélanger, W. R. Bushnell, A. J. Dik, & T. L. W. Carver (eds.), The powdery mildews: A comprehensive treatise (pp 256–267). St. Paul: APS Press.
Bélanger, R. R., Benhamou, N., & Menzies, J. G. (2003). Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici). Phytopathology, 93, 402–412.
Bélanger, R. R., Bowen, P. A., Ehret, D. L., & Menzies, J. G. (1995). Soluble silicon: its role in crop and disease management of greenhouse crops. Plant Disease, 79, 329–336.
Bowen, P. A., Menzies, J. G., & Ehret, D. L. (1992). Soluble silicon sprays inhibit powdery mildew development on grape leaves. Journal of the American Society for Horticultural Science, 117, 906–912.
Canny, M. J. (1990). What becomes of the transpiration stream? New Phytologist, 114, 341–368.
Epstein, E. (1994). The anomaly of silicon in plant biology. Proceedings of the National Academy of Sciences of the USA, 91, 11–17.
Epstein, E. (2001). Silicon in plants: Facts vs. concepts. In L. E. Datnoff, G. H. Snyder, & Korndörfer, G. H. (Eds.), Silicon in agriculture (pp 1–15). Amsterdam: Elsevier.
Fauteux, F., Chain, F., Belzile, F., Menzies, J. G., & Bélanger, R. R. (2006). The protective role of silicon in the Arabidopsis-powdery mildew pathosystem. Proceedings of the National Academy of Sciences (USA), 103, 17554–17559.
Fawe, A., Menzies, J. G., Chérif, M., & Bélanger, R. R. (2001). Silicon and disease resistance in dicotyledons. In L. E. Datnoff, G. H. Snyder, & G. H. Korndörfer (eds.), Silicon in Agriculture (pp 159–169). Amsterdam: Elsevier.
Horst, R. K., Kawamoto, S. O., & Porter, L. L. (1992). Effect of sodium bicarbonate and oils on the control of powdery mildew and black spot of roses. Plant Disease, 76, 247–251.
Kim, S. G., Kim, K. W., Park, E. W., & Choi, D. (2002). Silicon-induced cell wall fortification of rice leaves: A possible cellular mechanism of enhanced host resistance to blast. Phytopathology, 92, 1095–1103.
Liang, Y. C., Sun, W. C., Si, J., & Römheld, V. (2005). Effects of foliar and root applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathology, 54, 678–685.
Ma, J. F., Mitani, N., Nagao, S., Konishi, S., Tamai, K., Iwashita, T., et al. (2004). Characterization of the silicon uptake system and molecular mapping of the silicon transporter gene in rice. Plant Physiology, 136, 3284–3289.
Ma, J. F., Tamai, K., Yamaji, N., Mitani, N., Konishi, S., Katsuhara, M. et al. (2006). A silicon transporter in rice. Nature, 440, 688–691.
Menzies, J. G., Bowen, P. A., Ehret, D. L., & Glass, A. D. M. (1992). Foliar applications of potassium silicate reduce severity of powdery mildew on cucumber, muskmelon, and zucchini squash. Journal of the American Society for Horticultural Science, 112, 902–905.
Rodrigues, F. A., Benhamou, N., Datnoff, J. B., Jones, B., & Bélanger, R. R. (2003). Ultrastructural and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology, 93, 535–546.
Rémus-Borel, W., Menzies, J. G., & Bélanger, R. R. (2005). Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiological and Molecular Plant Pathology, 66, 108–115.
Reuveni, M., Agapov, V., & Reuveni, R. (1995). Suppression of cucumber powdery mildew (Sphaerotheca fuliginea) by foliar sprays of phosphate and potassium salts. Plant Pathology, 44, 31–39.
Reuveni, M., & Reuveni, R. (1995). Efficacy of foliar sprays of phosphates in controlling powdery mildews in field-grown nectarine, mango trees and grapevine. Crop Protection, 14, 311–314.
Sangster, A. G., Hodson, M. J., & Tubb, H. J. (2001). Silicon deposition in higher plants. In L. E. Datnoff, G. H. Snyder, & G. H. Korndörfer (eds.), Silicon in agriculture (pp 85–114). Amsterdam: Elsevier.
Yarwood, C. E. (1939). Control of powdery mildews with a water spray. Phytopathology, 29, 288–290.
Acknowledgments
The authors would like to thank Caroline Labbé for technical assistance and statistical analyses and Florian Chain for critical review of the manuscript. This work was supported by grants from the Natural Sciences and Engineering Research Coucil of Canada (NSERC) in collaboration with Label Agro Inc (Montreal, Canada), and the Canada Research Chairs Programme to R.R. Bélanger.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guével, MH., Menzies, J.G. & Bélanger, R.R. Effect of root and foliar applications of soluble silicon on powdery mildew control and growth of wheat plants. Eur J Plant Pathol 119, 429–436 (2007). https://doi.org/10.1007/s10658-007-9181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-007-9181-1