Abstract
The phyto-synthesis of silver nanoparticles and cotton dyeing with natural colorants can reduce the environmental impact of the process considerably. In this study, the extraction of natural colorants from Achillea millefolium petals was optimized by ultrasound technique. The AMP extract was applied for synthesis of silver nanoparticles (Ag NPs) on the cotton fabrics. The dyeing, antibacterial and antioxidant characteristics of cotton samples were investigated to optimize the process and evaluate its efficiency. The AMP extract had good substantivity towards cotton fabrics and the presence of tannic acid, as an environmentally-friendly mordant, further improved the absorption of AMP dye. The antibacterial and antioxidant activities of the dyed samples with AMP extract of were 50%and 60%, respectively. The addition of TA and Ag enhanced the antibacterial and antioxidant activities on the cotton samples to over 99%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concerning the environmental impacts of textile wet processes, high water consumption, polluted wastewater, and the poor biodegradability of synthetic dyes, finding green and sustainable textile dyeing processes are invaluable (Okiyama et al. 2018; Yang et al. 2018). One attractive approach is to use natural dyes for textiles especially for natural fibers (İşmal and Yıldırım 2019; Liman et al. 2021). Despite several good advantages of natural dyes for dyeing cotton, there are also some shortcomings including the low affinity, weak interactions with fiber, and low colorfastness properties (Gorjanc et al. 2014; Yang and Park 2015; Pisitsak et al. 2016). Researchers have examined different methods and technologies to overcome these shortcomings and enhance the adsorption rates on cotton fibers including ultrasound irradiation, plasma/ozone treatment, enzymatic treatment, using biomordants, surface modification, pretreatment with biopolymers, and cross-linking agents (Toprak et al. 2018; Sadeghi-Kiakhani and Safapour 2015a, b).
Achillea is a kind of flowering plant that grows in Europe, North America, and moderate areas of Asia (Aburjai and Hudaib 2006) with beautiful white to white-yellow or white-purple flowers, and used topically for wound healing (Toncer et al. 2010; Konyalioglu and Karamenderes 2005). Compounds for instance phenolic acids, flavonoids, coumarins, terpenoids and sterols are responsible for the biological activity of the Achillea. In addition, Achillea contains flavonoids such as Luteolin, Quercetin, Rutine, Chlorogenic acid, caffeic acid and their isomers and Apigenin, which can be used for dyeing of textiles with yellow color (Demirci et al. 2009). Kiumarsi et al. used the dried Achillea powder for dyeing wool yarns mordanted with copper, aluminum, tin salts (Kiumarsi et al. 2009). The dyeing characteristics of the dyed samples with Achillea showed its good agronomic potential as a natural dye (Barani et al. 2017). Based on our literature survey, there are few studies using Achillea for textile coloration; thus, it is necessary to investigate this further and find its potential as a natural colorant in different applications including textile, food, and cosmetics (Taşkın et al. 2017).
The functionalization of fabrics with Ag NPs has received significant popularity, particularly for polyester fabrics (Hasan et al. 2019; Sadeghi-Kiakhani et al. 2019a; Arif et al. 2015). Silver-coated cotton fabrics have ultra-high electromagnetic interference shielding even after 20 washing cycles (Tan et al. 2018; Gao et al. 2021). Until now, several routes for synthesis of Ag NPs have been established (Zhang et al. 2016; Aryabadie et al. 2015; Shahidi and Moazzenchi 2019). Synthesis of Ag NPs often requires hazardous chemicals and yields toxic organic byproducts like citrate, borohydride, thioglycerol, and 2-mercaptoethanol. Many recent studies have attentive on the clean synthesis of Ag NPs and using them for textile coloration (Sadeghi-Kiakhani et al. 2019c). In this context, natural dyes may be a viable green stabilizing agent for Ag NPs with remarkable antimicrobial properties against bacteria (Vijayaraghavan et al. 2012; Sadeghi-Kiakhani et al. 2020). Natural dyes can bind to nanoparticles due to their functional groups (e.g., carbonyl and hydroxyl groups) as shown in Scheme 1 (Ferrero and Periolatto 2012; Patil et al. 2012). Therefore, natural dye-mediated Ag NPs could be a potential green coloration approach for textiles (El-Shishtawy et al. 2011; Velmurugan et al. 2015, 2017; Barani et al. 2017).
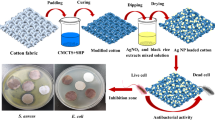
Despite some exhaustive review papers on the textile dyeing with natural dyes, few scientific papers are on the use of Achillea millefolium petals in the dyeing and antimicrobial treatment of cotton fabrics (Iqbal et al. 2008; Rajendran et al. 2013; Yusuf et al. 2017; Arora et al. 2012; Rehman et al. 2012). Hence, in the present study, the dried Achillea millefolium petals were extracted via conventional and ultrasound methods. The extract of AMP at different amounts of TA was used for dyeing of the cotton. The phyto-synthesis of silver NPs by AMP was also followed as a new green approach to render antibacterial and antioxidant properties on dyed samples. Moreover, the metallic mordants were used to achieve various hues on the dyed samples, and the colorimetric data and colorfastness characteristics of samples were analyzed.
Experimental
Materials
Achillea millefolium petals were obtained from Kerman- Iran. Each plant was dried in the shade, ground, and passed through a 60-mesh sieve to produce a dry powder. Cotton fabric with simple texture was provided from Mazandaran textile Company, Iran. Tannic acid with high purity (Sigma-Aldrich, USA) was used as a bio-mordanting. AgNO3 and metal mordants including copper (II) sulfate, aluminum (III) sulfate, and ferrous (II) sulfate heptahydrate were supplied by Merck Company, Germany. Antioxidant tests were performed by 1, 1-diphenyl-2-picrylhydrazyl (DPPH) (Merck, Germany).
Characterization
The double beam, Cecil 9200 spectrophotometer, UK, was used to determine the Ag NPs formation by the appropriate color change. The analyses of Ag NPs on the cotton samples were performed by apparatus such as Scanning Electron Microscopy (SEM)-Energy Dispersive Spectroscopy (EDS) and Fourier Transform Infrared (FTIR) spectra Nicolet Nexus 670 instrument. The Color eye 7000A, X-rite reflectance spectrophotometer with illuminant D65 and 10° standard observer was employed to obtain the reflectance and colorimetric data of dyed samples. The mechanical characteristics of dyed fabrics were determined by a MESDAN LAB instrument. The concentration of silver on cotton samples was measured by Inductively coupled plasma-optical emission spectrometry (ICP-OES) CCD simultaneous on Varian Vista Pro (argon plasma, Ag 328.068-nm excitation, Ag sensitivity 0.004 mg L−1), Australia.
Dye extraction
The extraction process of AMP was carried out via conventional water extraction method at high temperature and an ultrasonic-assisted extraction technique. The conventional extraction of AMP was performed by the dried plant powder: water (1 g: 50 mL) and boiled in water for 3 h. The solution was then filtered and freeze dried to obtain an extracted powder. We also studied the effect of sonication on the dye extraction efficiency at a fixed frequency rate of 35 MHz at various temperatures (30 °C, 50 °C, and 70 °C) for 5–30 min. The solid particles were filtered, and the solution under filter was concentrated using a rotary vacuum evaporator. The dried powder was obtained after placing the sample at 50 °C, and then weighed and stored in a desiccator to be used for the subsequent dyeing processes.
Phyto-synthesis of Silver nanoparticles
Silver nanoparticles (Ag NPs) was phyto-synthesized based on using these natural colorants as reducing and stabilizing agents. Silver nitrate (0.1–1%) was added to the extracted aqueous solution of the natural colorant (0.5–2%). The effect of different amounts of silver nitrate and extract was optimized and the ratios of MS extract: silver nitrate were 1:0.5, 1:1, 1:2, 1:4, 1:5, 2:1, and 4:1. The solution mixture was homogeneous with stirring at various conditions, so that the temperature was increased to 90 °C for 1 h.
Cotton preparation
The nonionic detergent (Lotensol, Hansa Co.) was used to scour the cotton fabric at 50 °C for 30 min. Mordanting of fabric samples was performed at 80 °C for 1 h, and the concentration of tannic acid was 5, 10, and 20% over the weight of fabric (%o.w.f.).
For benchmarking, the treated cotton fabrics with tannic acid were mordanted once again separately with Cu 3%, Al 5%, and Fe 3% o.w.f. at boiling for 60 min.
Dyeing procedure
The dyeing process was conducted using the dye powder (5–100% o.w.f.) and silver nitrate (0.25–1% o.w.f) with L.R: 40:1. The dyeing was performed by smart dyer rapid machine. The temperature was increased to 100 °C and continued for 90 min in the exhaust dyeing machine. Finally, the temperature of the dyeing machine was reduced and the dyed fabric was then removed and washed with water. All experiments were performed and measured in triplicate.
The color difference (ΔE) of dyed samples was determined by Eq. 1:
Color fastness
Standard ISO 105 C06:2010 was used for assessing the colorfastness properties of the fabrics against washing. The staining on adjacent fabrics and color change were evaluated by grayscale. The colorfastness to rub of the cotton was assessed through the standard ISO 105-X12:2016 method under wet and dry circumstances, and the staining on the adjacent cotton was measured by grayscale. The colorfastness to light of samples was assessed by xenon arc lamp as stated by ISO 105-C01:2006 standard test method. Standard blue scales were used to evaluate the fading as explained in more detail elsewhere (Sadeghi-Kiakhani and Safapour 2015a, b, c).
Antibacterial activity
The antibacterial test according to ASTM E2149-01 was performed on the cotton samples. Muller-Hinton broth was prepared and sterilized at 121 °C for 15 min. The cotton samples (2.0 × 2.0 cm2) were utilized for antibacterial tests against the prepared 106–107 CFU: colony-forming units.mL−1 of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) bacteria. The samples containing bacteria and cotton samples were incubated at 37 °C for 24 h. Then, 100 μL of the each samples was spread on the plate including agar, separately. The colonies numbers were counted after incubation at 37 °C for 24 h, and the antibacterial efficiency was determined using Eq. 2:
where, A and B represent the surviving bacterial cells on the treated and raw cotton fabric.
Antioxidant ability
The radical scavenging effect after the addition of DPPH was measured via the reduced speed of chemical response. Briefly, DPPH (0.15 mM) was added in methanol (40 mL), and all cotton samples (2.5 cm2) were soaked in the DPPH solution in dark condition for 30 min. The absorbance and maximum wavelength of each solution at λmax = 517 nm were determined, and the DPPH scavenging activity was specified using Eq. 3 (Sadeghi-Kiakhani et al. 2019b).
where, C and S are the absorbance amount of samples and control after 1 h in methanol including DPPH at dark condition, respectively.
Mechanical properties
The standard test method of ISO 5081 was used to evaluate the physical characteristics of dyed fabrics by a MESDAN LAB instrument. During the experiments, the constant cross-speed in the warp direction of fabrics was approximately 50 mm min−1. The measurements were carried out for four samples and average values were reported.
Washing durability of cotton samples
The ISO105-C10:2006(C)) was used to evaluate the laundering durability of cotton fabrics under condition 5 g L−1 soap solution, liquor ratio of 50:1, at 50 °C for 45 min. The samples were washed 10 cycles, and rinsed in cold distilled water and dried at room temperature. Finally, the antibacterial and antioxidant activities of washed samples were measured.
Results and discussion
Characterization of AMP extract and phyto-synthesized of AgNPs
The absorbance and maximum wavelength of the AMP extract solution under various conditions including temperature, time, and initial powder concentration are shown in Fig. 1. The maximum wavelengths of absorption (λmax) for Achillea millefolium was 370 nm. The absorbance of the solution is improved by increasing the initial powder concentration up to around 20 g/L which is self-explanatory and can be attributed to the higher gradient of concentration (Yusuf et al. 2017). However, the solvent has a certain capacity for dissolving the colorants and further increase of the plant powder in the solvent may not noticeably change the total extraction. The initial concentration of Achillea millefolium in water was set to 20 g/L for optimizing the rest of the parameters. When natural dyes are exposed to ultrasound waves, tiny bubbles are continuously produced and burst in the liquid. This phenomenon can increase the extraction rate and efficiency due to bursting bubbles with high temperature and pressure. The ultrasound-assisted method can then be performed at lower bath temperatures to protect temperature sensitive dye molecules (Zhu et al. 2014; Yusuf et al. 2017).
The absorbance UV–Vis spectra of the extracted dye solutions from AMP (a-d) under various conditions. The effects of various effective parameters (a) Conventional extraction at 100 °C and ultrasound extraction methods at 40 °C for 30 min, (b) The initial concentration of plants at 40 °C for 30 min, (c) The extraction temperature at the 20 g/L of AMP for 30 min, (d) The duration of extraction at the 20 g/L of AMP at 40 °C
The extracted dye concentration in water was noticeably raised by enhancing the temperature from 30 °C to 50 °C (Fig. 1c). The further temperature increase to 70 °C slightly enhanced the extraction; thus, the optimized temperature was considered as 50 °C. The temperature increment can: (a) increase the water solubility of extracted colorants, (b) enhance the extraction rate by increasing the kinetic energy of particles and better diffusion, (c) promote the extraction yield by opening the plant structure and pores, and (d) increase the hydrolysis of heat-sensitive colorants. The absorbance of the extracted solution was increased by prolonging the extraction duration up to 20 min. The extraction was enhanced slightly at a longer duration; thus, the duration of 20 min was considered as the optimum extraction time (Fig. 1d) (Zhu et al. 2014).
The UV–Vis spectra of the extracted colorants and 0.5% Ag ions solutions showed a strong bathochromic shift indicating strong intermolecular interactions between them (Fig. 2a). Owing to the surface plasmon vibration property of silver NPs formed, the λmax appeared around 430–450 nm for natural dye. The size and shape of Ag NPs are the effective parameters on the shifting of the surface plasmon bands at around λmax 430 to 450 nm (Velmurugan et al. 2015). The prediction of properties of AgNPs is relatively difficult; however, according to reports in this regard it seems that the size and shape of AgNPs maybe quasi-spherical shape and their size are around 10 to 80 nm (Fig. 2). The absorbance intensity also increased when the concentration of the natural dye increased, furthermore the functional groups in natural dye can support the more Ag ions reduced to Ag NPs (Scheme 1) (Ali et al. 2009; Omer et al. 2015; Iqbal et al. 2008; Rehman et al. 2012).
Figure 3a clearly shows that the λmax of the AMP extract changed from yellow (370 nm) to orange-brown (410 nm) with higher intensity, which it can be due to the formation of silver nanoparticles (Omer et al. 2015). When the reduction process is taking place, the λmax and absorbance intensities of Ag NPs increased. Results indicated that the heating could facilitate the reduction process of Ag ions by natural dye. Generally, the diversity in the type and concentration of natural dye and metal ions are the influence factors in phyto-synthesis of Ag NPs (Velmurugan et al. 2015).
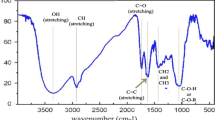
The infrared spectra of the extracted colorants from AMP and AMP + Ag NPs are given in Fig. 4. The stretching vibrations at 3414–3429 cm−1, 2924–2926 cm−1, 1727 cm−1 and 1633 cm−1, as well as at 1015 cm−1–600 cm−1 area can be related to the single bonding between elements such as O–H, C–H linkages in CH3 and CH2 groups, and C–O vibrations, respectively.
The FTIR spectra of flavonoids in natural dye and AgNPs reduced by natural dye demonstrated the absorptions of hydroxyl, methyl, and carbonyl groups. Generally, the extensive hydration of the hydrophilic sugar residues in the flavonoids in natural dyes is an important factor in the stabilization of the AgNPs (Barani et al. 2017; Shahidi and Moazzenchi 2019). Emerging peaks of the extracted colorants at the range of 1727–1633 cm−1 and below 1000 cm−1 in the presence of Ag show the successful phyto-synthesis of silver nanoparticles by AMP extract (Velmurugan et al. 2015). The shift in the above mentioned peaks after reaction with AgNO3 with Achillea dye signifying that -OH, -C = O and -NH-CO groups on the surface of Achillea. This dye is mainly composed of flavonoids compounds and the functional groups can provide the phyto-synthesis of Ag NPs (Omer et al. 2015).
Characterization of the phyto-synthesized AgNPs on the cotton
Figure 5 exhibits the SEM micrographs of the cotton fibers, the treatment with tannic acid, and TA + AMP + Ag NPs after five repeated washing cycles. Compared to the untreated sample with a smooth surface (Fig. 5a), the sample treated with TA (Fig. 5b) and TA + AMP + Ag NPs (Fig. 5c) showed a rough surface and the adsorbed nanoparticles (~ 60–70 nm) were visible on the surface (Fig. 5c). The corresponding EDS pattern proved the presence of Ag NPs on the cotton sample.
Dyeing properties
Tannic acid treatment
Untreated and tannic acid treated cotton samples were dyed with Achillea at 100 ºC for 90 min. The influence of TA concentration on the dye absorption of cotton fabric is shown in Table 1, as reflected by its colorimetric data (L×a×b×). The ΔE value among the cotton samples (untreated and treated by TA) was specified based on Eq. 1 and reported in the table for comparison. Based on the ΔE values of the treated samples, the tannic acid treatment showed a positive effect on cotton dyeability with the natural dye. This was much more pronounced in the case of Achillea + TA. This may be related to the new hydroxyl groups on the cotton (Mussak and Bechtold 2009). The chemical structure of TA contains several phenolic hydroxyl groups and can form hydrogen bonding and hydrophobic interactions to cellulose polymer chains. The same type of intermolecular interactions can occur between the colorants with cotton and cotton treated with TA (Lopes et al. 1999; Hemingway and Karchesy 2012). The more hydrogen bonding provides the better absorption and dye attachment on tannic acid-treated cotton samples (Ahmed et al. 2020; Sadeghi-Kiakhani et al. 2019c).
Effect of mordants
Metal mordants are commonly used for enhancing the dye adsorption and promoting the wash-fastness properties of organic colorants on cotton via complex formation. Untreated and tannic acid treated cotton samples were dyed with Achillea with and without pre-mordanting with alum, ferrous sulfate, and copper sulfate (Table 2). The presence of metal mordants changed and intensified the hue of samples (Vijayaraghavan et al. 2012). Metal mordants improve the dye uptake and dye-binding ability onto cellulose fibers via metal complex formation. Increased ∆E can be described by the enhanced affinity of natural dye toward fiber after mordanting with tannic acid and metallic mordants (Patil et al. 2012). The metal mordants bridge TA and colorants with the cotton surface via complexation which results in better and stronger dye adsorption and fixation. TA has a polyphenolic structure and the metal complexation of colorants with TA will also increase the molecular weight of the species. This can also explain a higher substantivity and adsorption rate of colorants on treated cotton.
Effect of Ag concentration
Influence of Ag amount on the enhancement AMP adsorption on the treated and non-treated cotton can be seen in Table 3. As an example, the color difference (∆E) of the samples dyed with Achillea + 10% TA increased from 4.14 to 19.08 by adding Ag NPs which is a great improvement and comparable to the corresponding values in the presence of Al, Fe, and Cu salts in Table 2. Thus, Ag NPs can also act as a metal mordant to bind the colorant + TA to the surface of cellulose polymer chains.
The absorption of AMP extract on the raw cotton fabric was lower than of all dyed samples with Ag ions. The samples dyed with AMP extract and Ag ions produced more functional groups than with untreated cotton. The Ag ions can reduce the AMP extract, and also can absorb on the cotton fabrics. Thus, more reactive sites are available on cotton fabrics that can attach to dye molecules as well as improve absorption of AMP extract (Barani et al. 2017). The size, shape, and dielectric properties of AgNPs can change the color from yellow to brown. It looks that loaded AgNPs on fabric forms a yellow-brownish color which enhances the absorption of AMP extract. Moreover, the ∆E values of dyed samples with Ag ions significantly increased, which can be due to the yellow brownish color appearing on the dyed samples due to synthesis of AgNPs (Barani et al. 2017).
Fastness properties
The colorfastness to wash of the cotton samples with natural extracts of Achillea was poor-moderate (2–4 out of 5). The addition of TA as a biomordant and metal (Al, Fe, Cu) mordants enhanced the wash-fastness properties noticeably to good-very good (3–5 out of 5) (Table 4). However, the colorfastness to light of all dyed samples were moderate-good (5 out of 8) and the metal complexation slightly enhanced the light-fastness of the samples. This fastness mainly depends on the photo-oxidation of colorants rather than the intermolecular interactions between the colorants and fiber (Yang and Park 2015; Pisitsak et al. 2016).
The impact of Ag concentration on the colorfastness characteristics of the cotton is shown in Table 5. The washing fastness properties, in terms of color change, increased up to 1/2–1 grade when TA and Ag NPs were used. The insoluble complexes between Ag and AMP extract on the cotton fabrics is a reason for improvement of color fastness ratings. The light-colorfastness of dyed cotton samples slightly improved perhaps due to dye-metal complexation and chromophore photo-stabilization.
The antibacterial efficiency
Table 6 shows the antibacterial efficiencies of the dyed samples with Achillea extracts. The cotton fabrics dyed with Achillea extracts showed 50% and 53% reduction of bacteria rate against S. aureus, and E. Coli, respectively. The antimicrobial components in Achillea extracts may include sesquiterpene lactones, flavonoids and tannic in Achillea (Ribitsch and Stana-Kleinscheck 1998; Sadeghi-Kiakhani et al. 2019b; Gyawali and Ibrahim 2014). The antibacterial properties of the cotton further improved to around 65% in the presence of TA.
The antibacterial and antioxidant durability of cotton samples against repeated washing was evaluated and results are given in Table 7. The antibacterial values were decreased by increasing the number of repeated washing cycles. However, the sample pre-mordanted with TA and Ag ions reserved over 91% antibacterial activity even after 10 washes. It was found that the pre-mordanting with TA should be performed in the presence of Ag ions for reaching a durable antibacterial activity on the cotton fabrics.
The antioxidant ability of dyed samples
Figure 6 exhibits the antioxidant potential of the cotton dyed with Achillea extracts. The fabrics dyed with Achillea extract without TA treatment showed around 60% antioxidant potential. The addition of TA to the recipe further enhanced the antioxidant properties of the samples over 97%, and negligible changes were observed with the additional Ag NPs. Thus, the natural colorants from Achillea and TA have noticeable antioxidant properties. The high antioxidant properties of TA had been previously mentioned in other research papers and were attributed to the hydrolysis of TA to several phenolic acid compounds (Lopes et al. 1999; Mussak and Bechtold 2009; Mussatto et al. 2011). Results clearly show that mordanting with TA is an important process to reach satisfactory antioxidant and antibacterial activities in the dyeing of cotton with Achillea extracts after several washing cycles.
Mechanical properties
The mechanical properties of treated and untreated were obtained and compared to each other (Table 8). It is clear that dyeing, mordanting with TA and dyeing in the presence of silver ions has negligible effect on the physical properties of cotton fabric, perhaps due to moderate dyeing and treatment conditions at neutral pH. So,cotton fabrics with high dye absorption, antibacterial and antioxidant activities can be obtained without adversely affecting their mechanical properties.
Silver concentration on the cotton
The concentration of the silver on cotton fabrics is given in Table 8, and results show that the concentration of silver on the dyed fabrics is much greater than untreated fabric. One can also observe that the samples dyed at 1%o.w.f. Ag have greater silver content than 0.5% o.w.f. Ag. It was found that the silver concentration of fabrics increased from 0.21 g kg−1 (untreated cotton) to 37.46 g kg−1 (fabric treated with 1%o.w.f. AgNO3). According to these results, the concentration of AgNO3 has a significant effect on the amount of AgNPs deposited on the fabrics. These results clearly show the significant effect of concentration of AgNO3 on increasing the amount of AgNPs deposited on cotton fabrics and are consistent with the SEM observations. Since the highest amount of antibacterial, antioxidant and dye absorption was observed in the 0.5% o.w.f. Ag, so this concentration was considered as the optimal concentration.
Conclusion
The in-situ synthesis of Ag NPs on the cotton fabrics using AMP extract was successfully performed. The results showed that AMP extracts have acceptable potential for reduction of silver ions in the solution and on the cotton fabrics. It was found that the mordanting with alum, ferrous sulfate, and copper sulfate increased the dyeing absorption and colorfastness characteristics of dyed samples. Furthermore, a green mordanting approach using TA + Ag NPs resulted in bathochromic shift, intensified color, enhanced fastness properties, and very high antibacterial and antioxidant properties on colored cotton samples. This environmentally-friendly approach can be proposed for the production of multifunctional cotton with natural colorants.
References
Aburjai T, Hudaib M (2006) Antiplatelet, antibacterial and antifungal activities of Achillea falcata extracts and evaluation of volatile oil composition. Pharmacogn Mag 2:191
Ahmed N, Nassar S, El-Shishtawy M (2020) Novel Green Coloration of Cotton Fabric. Part I Bio-mordanting and Dyeing Characteristics of Cotton Fabrics with Madder, Alkanet, Rhubarb and Curcumin Natural Dyes. Egypt J Chem 63:1605–1617
Ali S, Hussain T, Nawaz R (2009) Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. J Clean Prod 17:61–66
Arif D, Niazi MBK, Ul-Haq N et al (2015) Preparation of antibacterial cotton fabric using chitosan-silver nanoparticles. Fibers Polym 16:1519–1526
Arora A, Gupta D, Rastogi D, Gulrajani ML (2012) Kinetics and thermodynamics of dye extracted from Arnebia nobilis Rech f on wool. Indian J Fibre Tex Res 37(2):178–182
Aryabadie S, Sadeghi-Kiakhani M, Arami M (2015) Antimicrobial and Dyeing studies of treated cotton fabrics by prepared Chitosan-PAMAM Dendrimer/Ag Nano-emulsion. Fibers Polym 16:2529–2537
Barani H, Boroumand MN, Rafiei S (2017) Application of silver nanoparticles as an antibacterial mordant in wool natural dyeing: synthesis, antibacterial activity, and color characteristics. Fibers Polym 18:658–665
Demirci F, Demirci B, Gürbüz İ et al (2009) Characterization and biological activity of Achillea teretifolia Willd. and A. nobilis L subsp. neilreichii (Kerner) Formanek essential oils. Turkish J Biol. 33:129–136
El-Shishtawy RM, Asiri AM, Abdelwahed NAM, Al-Otaibi MM (2011) In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 18:75–82
Ferrero F, Periolatto M (2012) Antimicrobial finish of textiles by chitosan UV-curing. J Nanosci Nanotechnol 12:4803–4810
Gao YN, Wang Y, Yue TN, Weng YX, Wang M (2021) Multifunctional cotton non-woven fabrics coated with silver nanoparticles and polymers for antibacterial, superhydrophobic and high performance microwave shielding. J Colloid Interface Sci Part A 582:112–123
Gorjanc M, Jazbec K, Mozetič M, Kert M (2014) UV protective properties of cotton fabric treated with plasma, UV absorber, and reactive dye. Fibers Polym 15:2095–2104
Gyawali R, Ibrahim SA (2014) Natural products as antimicrobial agents. Food Control 46:412–429
Hasan KM, Pervez M, Talukder M et al (2019) A novel coloration of polyester fabric through green silver nanoparticles (G-AgNPs@ PET). Nanomaterials 9:569
Hemingway RW, Karchesy JJ (2012) Chemistry and significance of condensed tannins. Springer Science & Business Media
Iqbal J, Bhatti IA, Adeel S (2008) Effect of UV radiation on dyeing of cotton fabric with extracts of henna leaves. Indian J Fibre Text Res 33(2):157–162
İşmal ÖE, Yıldırım L (2019) Metal mordants and biomordants. In: The impact and prospects of green chemistry for textile technology. Elsevier..
Kiumarsi A, Abomahboub R, Rashedi SM, Parvinzadeh M (2009) Achillea millefolium, a new source of natural dye for wool dyeing. Prog Color Colorants Coat 2:87–93
Konyalioglu S, Karamenderes C (2005) The protective effects of Achillea L. species native in Turkey against H2O2-induced oxidative damage in human erythrocytes and leucocytes. J Ethnopharmacol. 102:221–227
Liman MLR, Islam MT, Hossain MM et al (2021) Coloration of cotton fabric using watermelon extract: mechanism of dye-fiber bonding and chromophore absorption. J Text Inst 112:243–254
Lopes GKB, Schulman HM, Hermes-Lima M (1999) Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta-General Subj 1472:142–152
Mussak RAM, Bechtold T (2009) Natural colorants in textile dyeing. Handb Nat Color, 315–335.
Mussatto SI, Ballesteros LF, Martins S, Teixeira JA (2011) Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep Purif Technol 83:173–179
Okiyama DCG, Soares ID, Cuevas MS et al (2018) Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res Int 114:20–29
Omer KA, Tao Z, Seedahmed AI (2015) New approach for dyeing and UV protection properties of cotton fabric using natural dye extracted from henna leaves. Fibres Text East Eur 23(5):60–65
Patil RS, Kokate MR, Kolekar SS (2012) Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 91:234–238
Pisitsak P, Hutakamol J, Thongcharoen R et al (2016) Improving the dyeability of cotton with tannin-rich natural dye through pretreatment with whey protein isolate. Ind Crops Prod 79:47–56
Rajendran R, Radhai R, Kotresh TM, Csiszar E (2013) Development of antimicrobial cotton fabrics using herb loaded nanoparticles. Carbohydr Polym 91:613–617
Rehman F, Adeel S, Qaiser S et al (2012) Dyeing behaviour of gamma irradiated cotton fabric using Lawson dye extracted from henna leaves (Lawsonia inermis). Radiat Phys Chem 81:1752–1756
Ribitsch V, Stana-Kleinscheck K (1998) Characterizing textile fiber surfaces with streaming potential measurements. Text Res J 68:701–707
Sadeghi-Kiakhani M, Safapour S (2015a) Eco-friendly dyeing of treated wool fabrics with reactive dyes using chitosanpoly (propylene imine) dendreimer hybrid. Clean Technol Environ Policy 17:1019–1027
Sadeghi-Kiakhani M, Safapour S (2015b) Salt-free reactive dyeing of the cotton fabric modified with chitosan-poly (propylene imine) dendrimer hybrid. Fibers Polym 16:1075–1081
Sadeghi-Kiakhani M, Safapour S (2015c) Improvement of the dyeing and fastness properties of a naphthalimide fluorescent dye using poly (amidoamine) dendrimer. Color Technol 131:142–148
Sadeghi-Kiakhani M, Hashemi E, Gharanjig K (2019a) Inorganic nanoparticles and natural dyes for production of antimicrobial and antioxidant wool fiber. 3 Biotech 9:1–16
Sadeghi-Kiakhani M, Safapour S, Ghanbari-Adivi F (2019b) Grafting of chitosan-acrylamide hybrid on the wool: characterization, reactive dyeing, antioxidant and antibacterial studies. Int J Biol Macromol 134:1170–1178
Sadeghi-Kiakhani M, Tehrani-Bagha AR, Gharanjig K, Hashemi E (2019c) Use of pomegranate peels and walnut green husks as the green antimicrobial agents to reduce the consumption of inorganic nanoparticles on wool yarns. J Clean Prod 231:1463–1473
Sadeghi-Kiakhani M, Hashemi E, Gharanjig K (2020) Treating wool fibers with chitosan-based nano-composites for enhancing the antimicrobial properties. Appl Nanosci 10:1219–1229
Shahidi S, Moazzenchi B (2019) The influence of dyeing on the adsorption of silver and copper particles as antibacterial agents on to cotton fabrics. J Nat Fibers 16:677–687
Tan YJ, Li J, Gao Y, Li J, Guo S, Wang M (2018) A facile approach to fabricating silver-coated cotton fiber non-woven fabrics for ultrahigh electromagnetic interference shielding. Appl. Surf. Sci. 458:236–244
Taşkın D, Alkaya DB, Dölen E (2017) Analysis of natural dyestuffs in Achillea grandifolia Friv Using HPLC-DAD and Q-TOF LC/MS. Indian J Traditional Knowledge. 16:83–88
Toncer O, Basbag S, Karaman S et al (2010) Chemical composition of the essential oils of some Achillea species growing wild in Turkey. Int J Agric Biol 12:527–530
Toprak T, Anis P, Kutlu E, Kara A (2018) Effect of chemical modification with 4-vinylpyridine on dyeing of cotton fabric with reactive dyestuff. Cellulose 25:6793–6809
Velmurugan P, Park J-H, Lee S-M et al (2015) Synthesis and characterization of nanosilver with antibacterial properties using Pinus densiflora young cone extract. J Photochem Photobiol B Biol 147:63–68
Velmurugan P, Kim J-I, Kim K et al (2017) Extraction of natural colorant from purple sweet potato and dyeing of fabrics with silver nanoparticles for augmented antibacterial activity against skin pathogens. J Photochem Photobiol B Biol 173:571–579
Vijayaraghavan K, Nalini SPK, Prakash NU, Madhankumar D (2012) Biomimetic synthesis of silver nanoparticles by aqueous extract of Syzygium aromaticum. Mater Lett 75:33–35
Yang H, Park Y (2015) Optimum dyeing condition of cotton by fermented grape by-products with degraded protein mordant. Text Color Finish 27:202–209
Yang TT, Guan JP, Tang RC, Chen G (2018) Condensed tannin from Dioscorea cirrhosa tuber as an eco-friendly and durable flame retardant for silk textile. Ind Crops Prod 115:16–25
Yusuf M, Shabbir M, Mohammad F (2017) Natural colorants: Historical, processing and sustainable prospects. Nat Products Bioprospect 7:123–145
Zhang X, Ren Y, Wang B (2016) Synthesis of Ag@poly Composites with Different Morphologies. Mater Manuf Processes 31:177
Zhu Z-Y, Pang W, Li Y-Y et al (2014) Effect of ultrasonic treatment on structure and antitumor activity of mycelial polysaccharides from Cordyceps gunnii. Carbohydr Polym 114:12–20
Acknowledgements
The researchers of this study express their special thanks for the financial support of the Institute for Color Science and Technology in the form of an international project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadeghi-Kiakhani, M., Tehrani-Bagha, A.R., Miri, F.S. et al. Application of Achillea millefolium extract as a reducing agent for synthesis of silver nanoparticles (AgNPs) on the cotton: antibacterial, antioxidant and dyeing studies. Biometals 35, 313–327 (2022). https://doi.org/10.1007/s10534-022-00366-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-022-00366-9