Abstract
Low-level laser therapy (LLLT) is a biophysical form of intervention in the fracture-repair process, which, through several mechanisms, accelerates the healing of fractures and enhances callus formation. The effect of laser on fracture healing is controversial. Some authors affirm that LLLT can accelerate bone formation by increasing osteoblastic activity. The objective of our study was to evaluate the effect of laser therapy on fracture healing. Thirty rabbits were subjected to tibial bone open osteotomies that were stabilized with external fixators. The animals were divided into two study groups: laser group and control group. Callus development and bone mineral density were quantitatively evaluated by CT; the animals were then killed and the fractures were assessed for biomechanical properties. The results demonstrated that the increasing rate of bone mineral density was higher in the laser (L) group than in the control (C) group. CT at 5 weeks revealed a mean callus density of 297 Hounsfield units (HU) for the control group and 691 HU for the L group, which was statistically significant (P = 0.001). In the L group, the mean recorded fracture tension was 190.5 N and 359.3 N for healed and intact bones, respectively, which was statistically significant (P < 0.001). The result of the study showed that the use of laser could enhance callus development in the early stage of the healing process, with doubtful improvement in biomechanical properties of the healing bone; therefore, laser therapy may be recommended as an additional treatment in non-union fractures in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Millions of fractures occur every year worldwide. Even though treatment methods have improved over the past few decades, 5–10% of fractures still show delayed healing [1]. One of the fundamental concepts in orthopedics is the understanding that the mechanical environment at the site of a fracture influences the pattern of fracture repair [2, 3]. Fracture healing may be modified by several factors, such as hormones, vitamins, minerals, local vascularity, weight bearing, protein diet, ultrasound and electrical stimuli [4–8].
Laser light irradiation has been applied in the medical field and possesses biostimulatory effects on wound healing, collagen synthesis [9], and fibroblast proliferation [10]. In addition, laser light appears to increase mitochondrial respiration and adenosine triphosphate (ATP) synthesis [11, 12]. Low-level laser therapy (LLLT) is less frequent in clinical attention [13]. Laser irradiation is a form of electromagnetic field that can elevate the structural stiffness of bone callus. Some authors affirm that LLLT can accelerate bone formation by increasing osteoblastic activity, vascularization, organization of collagen fibers and ATP levels [14–16].
The aim of this study was to evaluate the effect of LLLT on the bone healing process in respect of callus formation rate and biomechanical properties of healed bones.
Methods and materials
Thirty female New Zealand white rabbits were used in the study. All animals were skeletally mature and weighed between 2,500 g and 3,000 g, so that the osteotomy could be better performed. We selected an animal model because we needed the bones to be extracted for resistance testing after therapy. Prior to its selection, each rabbit was examined by a veterinarian so that those with co-morbidity could be excluded. Osteotomy for each right tibial bone was performed under aseptic conditions, and general anesthesia was achieved with subcutaneous injection of ketamine (50 mg/kg) and xylazine (10 mg/kg). Cefazolin (16 mg/kg, three times a day) was administered 30 min before the operation and for 48 h after, for infection prophylaxis.
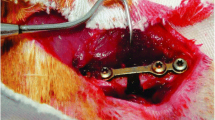
Under aseptic conditions, four Steinmann transfixation pins (two proximal and two distal to the osteotomy site) were positioned in the frontal plane of the right tibial bone with a surgical pin driver by an expert orthopedic surgeon. After skin incision in the middle of the front leg, osteotomy was performed with an oscillating power saw. Fixation was provided by double-bar, uniplanar, external fixators into which pins were incorporated to create a 3 mm gap between the bone fragments. Stable bone fixation with a 3 mm gap eliminates the effects of bone contact stress between the two fragments. Irrigation was performed so that no tissue or foreign body was left between the two parts of the gap (Fig. 1). Then, the soft tissue and the skin were approximated. The pin tracts were cleaned daily with Betadine solution. Radiographic examination (anteroposterior view) was performed post-operatively to confirm good alignment of bone fragments. After surgery, the animals had free activity in their own standard laboratory cage; an adequate analgesia (morphine, 5 mg/kg) was administered for 2 days post-operatively.
The animals were divided into two equal groups of 15. The laser group (L) was subjected to LLLT and the control group (C) was given sham treatment. In both groups treatment was started on the 4th post-operative day for a 4-week period.
In group L, LLLT [gallium–aluminum–arsenide (Ga-As-Al) laser, 4 J/cm, 780 nm, 5 min/day] was administered on the osteotomy site on the anterior aspect with a Endolaser 476, Enraf Nonius device. All treatment was administered by an expert physical therapist.
Callus development was evaluated qualitatively by CT using a multidetector (64MD) Siemens scanner. Callus bone density was determined at the 2nd, 5th and 8th post-operative weeks under general anesthesia. The 64MD CT scanner allowed three-dimensional evaluation with very thin slices for thorough evaluation of the callus area. All calluses were observed by an expert radiologist, and callus densities were reported in Hounsfield units (HU). The radiologist was unaware of the group division.
At the end of the 12th post-operative week, all the rabbits were euthanized and both tibial bones (intact and healed) of each animal were retrieved and immediately examined in a three-point lateral bending force setting by a Hounsfield H5KS instrument, which evaluated the transverse strength in the bones. For this purpose, each end of the bones was supported and a load was applied on the middle part of the bone with a simple beam (Fig. 2). The maximum force (in newtons) that the bones could resist was recorded for evaluation of their biomechanical properties. The examiner was unaware of the group identities.
For statistical analysis, Student’s t-test was used for comparison between groups, and a P value of less than 0.05 was considered significant. The study protocol was approved by the Institutional Animal Care Committee of Tabriz University of Medical Sciences.
Results
Two rabbits were excluded from the study, one animal from each group. Both animals were excluded due to tibial fracture at the pin site. Therefore, in each group, 14 rabbits remained in the study. No infection was detected in the post-surgery phase. Callus formation and callus density were measured at the 2nd, 5th and 8th post-operative weeks (Table 1).
In the 2nd week, group L had the greatest mean callus density (172 ± 200 HU versus 38.3 ± 105 HU, P < 0.001). At the 5th post-operative week, development of callus was more prominent in group L than in the control group (691 ± 143 HU versus 297 ± 337 HU, P < 0.001). The mean callus density at the 8th post-operative week showed that group L had greater callus density (1,202 ± 162 HU) than that of group C (940 ± 303 HU, P = 0.01).
We also compared the mean difference between callus densities at the 2nd, 5th and 8th weeks to evaluate the callus formation rate (Table 2). After 12 weeks, all tibial bones, both intact and healed, of each rabbit were extracted and were examined for resistance by tensiometry testing (Table 3). Data were analyzed either in healed bones between groups and between healed or intact bones inside each group.
In the control group the mean recorded fracture tension was 359 ± 274 N and 311 ± 78 N for intact and healed bones, respectively, which was statistically insignificant; P = 0.14. In group L the maximum resistance force in healed bones (190 ± 135 N) was lower than that in group C (359 ± 274 N); P = 0.021. Similarly, in group L, the maximum resistance force of healed bone (190 ± 135 N) was lower than that of intact bone (342 ± 91 N); P = 0.002. Therefore, the calluses of healed bones in group L were not only weaker than those of intact bones of the same group but also of those in the control group.
Discussion
Several studies have compared the effect of laser irradiation on bone healing in regard to histochemical and biomechanical properties. However, the exact mechanism of laser in bone healing is unclear. It has recently been found that, at low radiation doses, the light energy is absorbed by intracellular chromophores such as porphyrins and cytochromes and is converted to metabolic energy involving the respiratory chain via production of a transmembrane electrochemical proton gradient [8, 17].
We applied Ga-As-Al laser in this study. Conventional laser systems for bone formation consist of helium–neon (He-Ne), Ga-As-Al, semiconductors and others in a continuous wave, an interrupted wave or pulsed lights [12]. He-Ne laser irradiation, which has a wavelength of 632.8 nm, promotes proliferation and differentiation of human osteoblasts in vitro, and low-power light with Ga-As-Al laser, which has a wavelength of 830 nm, has a positive effect on osteoblast proliferation [18]. David et al. concluded that He-Ne laser irradiation did not affect bone healing in rats [19], but Luger et al. concluded that He-Ne laser at 632.8 nm and 35 mW may play a role in enhancing bone healing [8]. McDavid and co-workers also showed that the osseous healing response was severely delayed by laser irradiation of bone [20].
Wavelength is an important factor, which relates to penetration of laser light through biological tissue [12]. Interrupted laser light has been reported to be more effective for nodule formation than continuous laser light [21] and nanosecond pulsed stress waves in tissue by rapid heating [22]. Nissan et al. compared laser irradiation at 4 mW/cm2 and 22.4 mW/cm2 and concluded that a 4 mW/cm2 power density significantly increased radio-calcium accumulation 2 weeks after surgery, whereas 22.4 mW/cm2 had no effect [23], but Silva and colleagues showed opposite results and concluded that greater volume of newly formed bone was observed in irradiation using 10.2 J/cm2 in comparison with 5.1 J/cm2 [24]. Fukuhara et al. demonstrated that Ga-As-Al laser induced not only acceleration of bone formation but also initial mitosis (G2/M phase) arrest, which may cause wound healing-like tissue repair [25]. The application of low power Ga-As-Al laser is effective in the bone healing process because it affects calcium transport during new bone formation [23]. Our study revealed that laser irradiation enhances bone formation in the initial stages of the healing process, which is similar to the findings of the study by da Silva et al. [24]. Several other studies also support our study with respect to the positive effect of laser on bone formation [8, 15, 16, 18, 24, 25].
We found weaker bones in group L than in the control group in the assessment of mechanical properties. Luger et al. [8] revealed that He-Ne lasers at 632.8 nm and 35 mW elevated the maximal load at failure and structural stiffness, whereas the extension maximum load was reduced. Additionally, Otermski et al. [26] showed that, although histologically laser therapy was thought to induce a slight delay in fracture healing, mechanical testing showed that there is no evidence that the strength of the underlying fracture will be enhanced. Therefore, our study and other studies proved that laser irradiation could enhance bone formation, but its effect on biomechanical properties is yet controversial and further studies seem to be warranted.
Conclusion
Our study shows that low level laser therapy on small animals can facilitate fracture healing in the early stages of fracture, however, with weak biomechanical properties. Hence, we recommend the use of this type of laser therapy for humans only in cases of poor bone formation, such as in non-union fractures. Owing to weak biomechanical properties, further research ought to be carried out into this, and the use of laser therapy for the fractures is not recommended as a conventional treatment.
References
Claes L, Willie B (2007) The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol 93:384–398. doi:10.1016/j.pbiomolbio.2006.07.021
Claes LE, Heigele CA, Neidlinger-Wilke C et al (1998) Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res 355:S132–S147. doi:10.1097/00003086-199810001-00015
Wu J, Shyr HS, Chao EY, Kelly PJ (1984) Comparison of osteotomy healing under external fixation devices with different stiffness characteristics. J Bone Joint Surg Am 66:1258–1264
Buckwalter JA, Einhorn TA, Bolander JA, Cruess RL (1996) Healing of musculoskeletal tissues. In: Rockwood CA, Green DP, Bucholz RW, Heckman JD (eds) Rockwood’s and Green’s fracture in adults, Lippincott, Philadelphia, pp 261–304
Dekel S, Salama R, Edelstein S (1983) The effect of vitamin D and its metabolites on fracture repair in chicks. Clin Sci 65:429–436
Scott G, King JB (1994) A prospective, double-blind trial of electrical capacitive coupling in the treatment of non-union of long bones. J Bone Joint Surg Am 76:820–826
Heckman JD, Rayby JP, McCabe J, Frey JJ, Kilcoyne RF (1994) Acceleration of tibial fracture-healing by non-invasive, low intensity pulsed ultrasound. J Bone Joint Surg Am 76:26–34
Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102. doi:10.1002/(SICI)1096-9101(1998)22:2<97::AID-LSM5>3.0.CO;2-R
Conlan MJ, Rapley JW, Cobb CM (1996) Biostimulation of wound healing by low-energy laser irradiation. J Clin Periodontol 23:492–496. doi:10.1111/j.1600-051X.1996.tb00580.x
Pourzarandian A, Watanabe H, Ruwanpura SM, Aoki A, Ishikawa I (2005) Effect of low-level Er:YAG laser irradiation on cultured human gingival fibroblasts. J Periodontol 76:187–193. doi:10.1902/jop. 2005.76.2.187
Morimoto Y, Arai T, Kikuchi M, Nakajima S, Nakamura H (1994) Effect of low-intensity argon laser irradiation on mitochondrial respiration. Lasers Surg Med 15:191–199. doi:10.1002/lsm.1900150207
Ninomiya T, Hosoya A, Nakamura H, Sano K, Nishisaka T, Ozawa H (2007) Increase of bone volume by a nanosecond pulsed laser irradiation is caused by a decreased osteoclast number and an activated osteoblasts. Bone 40:140–148. doi:10.1016/j.bone.2006.07.026
Guerino MR, Baranauskas V, Guerino AC, Parizotto N (2000) Laser treatment of experimentally induced chronic arthritis. Appl Surf Sci 154–155:561–564. doi:10.1016/S0169-4332(99)00460-2
Usuba M, Akai M, Shirasaki Y (1998) Effect of low level laser therapy (LLLT) on viscoelasticity of the contracted knee joint: comparison with whirlpool treatment in rats. Lasers Surg Med 22:81–85. doi:10.1002/(SICI)1096-9101(1998)22:2<81::AID-LSM3>3.0.CO;2-S
Lirani-Galvão AP Jorgetti V, Lopes da Silva O (2006) Comparative study of how low-level laser therapy and low-intensity pulsed ultrasound affect bone repair in rats. Photomed Laser Surg 24:735–740
Karu T, Pyatibrat L, Kalendo G (1995) Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 27:219–223. doi:10.1016/1011-1344(94)07078-3
Rochkind S, Ouaknine GE (1992) New trend in neuroscience: low power laser effect on peripheral and central nervous system (basic science, preclinical and clinical studies). Neurol Res 14:2–11, review 87–95
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166. doi:10.1089/pho.2005.23.161
David R, Nissan M, Cohen I, Soudry M (1996) Effect of lowpower He-Ne laser on fracture healing in rats. Lasers Surg Med 19:458–464. doi:10.1002/(SICI)1096-9101(1996)19:4<458::AID-LSM12>3.0.CO;2-Z
McDavid VG, Cobb CM, Rapley JW, Glaros AG, Spencer P (2001) Laser irradiation of bone: III. Long-term healing following treatment by CO2 and Nd:YAG lasers. J Periodontol 72:174–182. doi:10.1902/jop.2001.72.2.174
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22:347–354. doi:10.1016/S8756-3282(97)00294-9
Cleary SF (1977) Laser pulses and the generation of acoustic transients in biological materials. In: Wolbarsht ML (ed) Laser applications in medicine and biology, vol 3, Plenum, New York pp 175–219
Nissan J, Assif D, Gross MD, Yaffe A, Binderman I (2006) Effect of low intensity laser irradiation on surgically created bony defects in rats. J Oral Rehabil 33:619–924. doi:10.1111/j.1365-2842.2006.01601.x
Silva RV, Camilli JA, Bertran CA, Moreira NH (2005) The use of hydroxyapatite and autogenous cancellous bone grafts to repair bone defects in rats. Int J Oral Maxillofac Surg 34:178–184. doi:10.1016/j.ijom.2004.06.005
Fukuhara E, Goto T, Matayoshi T, Kobayashi S, Takahashi T (2006) Optimal low-energy laser irradiation causes temporal G2/M arrest on rat calvarial osteoblasts. Calcif Tissue Int 79:443–450. doi:10.1007/s00223-006-0072-9
Otremski I, Irga D, Edelstein S, Ornoy A, Newman R (2004) Does laser irradiation effect fracture healing? Med Laser Appl 19:146–149. doi:10.1078/1615-1615-00136
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazem Shakouri, S., Soleimanpour, J., Salekzamani, Y. et al. Effect of low-level laser therapy on the fracture healing process. Lasers Med Sci 25, 73–77 (2010). https://doi.org/10.1007/s10103-009-0670-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-009-0670-7