Abstract
Purpose
Robotic inguinal hernia repair (RHR) is an evolving technique but is comparatively expensive and has yet to show superior outcomes versus open (OHR) or laparoscopic (LHR) approaches. The utilization and clinical outcomes of RHR have not been reported within the veterans affairs (VA) system. This study analyzes trends in utilization and 30-day post-operative outcomes between OHR, LHR, and RHR in veterans.
Methods
This is a retrospective review of patients that underwent inguinal herniorrhaphy using the Veterans Affairs Quality Improvement Program database. Multivariable analysis of outcomes was performed adjusting for pre-operative confounding covariates between OHR, LHR, and RHR. Trends in utilization, complication rates, and operative times were also reported.
Results
From 2008–2019, 124,978 cases of inguinal herniorrhaphy were identified: 100,880 (80.7%) OHR, 18,035 (14.4%) LHR, and 6063 (4.9%) RHR. Compared to LHR, RHR was associated with 4.94 times higher odds of complications, 100 min longer mean operative time, and 1.5 days longer median length of stay (LOS). Compared to OHR, RHR was associated with 5.92 times higher odds of complications, 57 min longer mean operative time, and 1.1 days longer median LOS. Utilization of RHR and LHR significantly increased over time. RHR complication rates decreased over time (2008: 20.8% to 2019: 3.2%) along with mean operative times (2008: 4.9 h to 2019: 2.8 h; p < 0.05).
Conclusion
While this study demonstrated inferior outcomes after RHR, the temporal trends are encouraging. This may be due to increased surgeon experience with robotics. Further prospective data will elucidate the role of RHR as this technique increases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inguinal hernia is commonly diagnosed in the United States and around the globe. Much more prevalent in men than women, it is estimated that one in three men will incur an inguinal hernia in their lifetime [1, 2]. Inguinal hernia repair (IHR), the definitive treatment, is one of the most commonly performed general surgery procedures, with over 800,000 cases performed every year in the United States alone [3, 4]. IHR represents a significant portion of healthcare costs, accounting for billions of dollars in annual expenditures [5, 6].
IHR has undergone numerous transformations over the past century. One of the most important developments was the creation of the Lichtenstein tension free repair with mesh, which today remains the most frequently practiced technique [4,5,6,7]. With the rise of minimally invasive surgery, several laparoscopic approaches were developed, such as the transabdominal preperitoneal (TAPP) and total extraperitoneal (TEP) techniques [8,9,10,11]. Comparative analysis of open inguinal hernia repair (OHR) and laparoscopic inguinal hernia repair (LHR) has been the subject of extensive research. The optimal surgical technique for inguinal herniorrhaphy remains controversial given the current data [12,13,14,15,16].
Robotic inguinal hernia repair (RHR) has emerged as an intriguing third option over the past few decades, given the enhanced three-dimensional visualization and precise instrumentation offered by the platform [17]. The current technique is derived from the laparoscopic TAPP repair. Over the past decade, utilization of RHR has rapidly expanded in the United States, and continued growth is projected [18]. At present, there is significant variability in the results of 30-day post-operative outcomes between these techniques, and RHR has not consistently shown superior clinical outcomes compared to OHR and LHR [19,20,21,22,23,24]. Moreover, RHR is significantly more expensive on a per-case basis, in addition to high installation costs [21, 25, 26]. Given these findings, the role of robotics in hernia surgery must be further elucidated.
While extensively studied in the civilian sector, comparisons of these herniorrhaphy techniques in veterans is lacking in the literature. To our knowledge, there are no existing studies analyzing trends in utilization and outcomes between the three IHR approaches on a national scale in the veterans affairs (VA) system. Veterans comprise a unique subset of the United States population. On average, veterans are older, predominantly male, with significantly higher rates of tobacco use and other comorbidities compared to national averages [27,28,29,30]. These factors are well-documented risks for development of an inguinal hernia, and in 2019, over 11,000 IHR were performed throughout the VA system [1, 2, 31]. The objective of this study is to analyze the trends in utilization and 30-day post-operative outcomes between open, laparoscopic, and robotic inguinal hernia repair in the veteran population.
Methods
Data collection
This was a retrospective review of Veterans Affairs Surgical Quality Improvement Project (VASQIP) data, a validated database prospectively maintained by specially trained clinicians that collates surgical outcomes from all participating VA hospitals [32]. Veteran patients at these hospitals between 2008 and 2019 that underwent inguinal herniorrhaphy were identified by current procedural terminology (CPT) codes for open inguinal hernia repair (OHR; CPT = 49,505, 49,507, 49,520, 49,521, 49,525) and laparoscopic inguinal hernia repair (LHR; CPT = 49,650, 49,651). Patients that underwent robotic inguinal hernia repair (RHR) during this time frame were identified using robotic modifier code “S2900”. Emergent cases, identified in VASQIP by American Society of Anesthesiology (ASA) classification, and recurrent hernia repairs were excluded from statistical analysis. Due to the retrospective nature of the study and utilization of de-identified data, Institutional Review Board (IRB) waiver of consent was applied and approved for this study (IRB-Exempt Protocol #01966).
Study variables
Clinical outcomes assessed in this analysis included 30-day mortality, operative time, length of post-operative hospital stay (LOS), incidence of unplanned return to operating room (OR) and incidence of post-operative complications. Post-operative complications were analyzed by relevant organ system, which included cardiac, pulmonary, renal, and infectious disease systems. Complications not pertaining to one system were assigned to a “systemic/other” category. Pre-operative demographics captured included age, gender, and race of each patient. Clinical characteristics and pre-operative comorbidities were analyzed, and organized by organ system: cardiovascular, pulmonary, metabolic, renal, and systemic/other.
Statistical analysis
All inguinal hernia repair cases were stratified into three comparison groups based on technique: robotic, laparoscopic, and open. Incidence of 30-day mortality, operative time, LOS, and incidence of post-operative complications were compared via multivariate analysis, adjusting for pre-operative confounders. Analysis of variance (ANOVA) and independent samples t test were used to compare parametric and continuous variables between each cohort. Wilcoxon rank sum/Mann–Whitney U test was used for nonparametric continuous variables. Normality of continuous variable distributions was performed with Kolmogorov–Smirnov test with respect to skew and kurtosis. Categorical variables of each cohort were compared with Fisher’s exact test or Chi-square for low cell counts (≥ 25% of cells with expected count ≤ 5) and adequate cell counts, respectively.
Patient demographics, clinical, and operative characteristics were assessed between all cohorts to locate potential confounding covariates. Multivariable logistic regression models compared categorical outcomes, reported as adjusted odds ratios with 95% confidence intervals. Corresponding p values were declared. Continuous variables were compared via multivariable generalized linear models. For continuous variables of interest, beta coefficient (β) with standard error (SE) was reported. LOS was skewed positively with kurtosis. Therefore, this metric was natural logarithm (ln) transformed to satisfy assumptions of normally distributed residuals, as well as homoscedasticity amongst variance of error terms within multivariable linear regression. Univariate patient demographics, clinical, and operative characteristics with overall corresponding univariate-test p value less than 0.2 were entered into multivariable models for adjustment and stay criteria for the selection procedure was α = 0.1.
Temporal trends in utilization of each technique were determined by percentage that each comparison group contributed to overall operative volume per year. Trends in complication rate and operative time were also compared amongst each cohort. In the RHR cohort, trends in specific complications such as surgical site infection, overall infectious-related complications (defined as superficial surgical site infection, deep wound infection, or urinary tract infection), and unplanned reoperation were tabulated individually. Spearman’s Rho correlation coefficient (ρ) was used for nonparametric measure of rank correlation. To correct for multiple comparisons and minimize Type I error conclusions, Bonferroni correction at the multivariable level for significance detection was used for our 30 outcomes of interest. Therefore, a p value of less than 0.0017 was considered statistically significant. All analysis was performed using SAS version 9.4 (SAS Institute Inc. Cary, NC).
Results
Overall
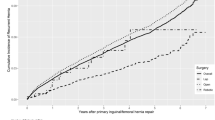
A total of 124,978 inguinal hernia repair cases were identified in VASQIP from 2008–2019 that met inclusion criteria. Of that total, there were 100,880 (80.7%) open inguinal hernia repair (OHR) cases, 18,035 (14.4%) laparoscopic inguinal hernia repair (LHR) cases, and 6,063 (4.9%) robotic inguinal hernia repair (RHR) cases. A comparison of pertinent clinical outcomes between each technique was presented in Fig. 1.
RHR versus LHR
Relative to LHR, RHR cases were significantly associated with higher body mass index (BMI), older age, and higher proportion of female gender and African American race. In addition, RHR was associated with higher incidence of: congestive heart failure (CHF), hypertension (HTN), peripheral artery disease (PAD), obstructive sleep apnea (OSA), diabetes mellitus (DM), pre-operative weight loss greater than 10% in 6 months, dependent functional status, and ASA class greater than III. Conversely, RHR was associated with lower incidence of smoking and alcohol use (Table 1). Univariate analysis of 30-day outcomes revealed that compared to LHR, RHR was significantly associated with higher complication rate, unplanned return to OR, longer operative time and longer LOS (Table 2).
Multivariate analysis, adjusting for confounding covariates showed that compared to LHR, RHR was significantly associated with increased adjusted odds of overall complication rate (aOR = 4.94; p < 0.001), pulmonary composite complication (aOR = 7.09; p < 0.001), renal composite complication (aOR = 4.54; p < 0.001), infectious composite complication (aOR = 4.65; p < 0.001), unplanned return to OR (aOR = 3.53; p < 0.001), as well as adjusted 100 ± 1 min longer operative time (p < 0.001)and an adjusted 94% ± 3% longer LOS(p < 0.001) (Table 3).
RHR versus OHR
Relative to OHR, RHR cases were significantly associated with higher BMI and younger relative age. In addition, RHR was associated with higher incidence of: CHF, HTN, PAD, OSA, DM, weight loss greater than 10% in 6 months. Conversely, RHR was associated with lower incidence of: prior MI, chronic obstructive pulmonary disorder (COPD), hemodialysis within two weeks of surgery, smoking, alcohol use, dependent functional status, and ascites (Table 1). Unadjusted relative to OHR, RHR was significantly associated with higher incidence of post-operative complications, longer operative times, and longer LOS (Table 2).
Multivariate analysis, adjusting for confounding covariates, showed that RHR was significantly associated with higher adjusted odds of: overall complications (aOR = 5.92; p < 0.001), cardiac composite complications (aOR = 4.22; p < 0.001), neurologic composite complications (aOR = 4.00; p = 0.002), pulmonary composite complications (aOR = 9.95; p < 0.001), renal composite complications (aOR = 11.49; p < 0.001), infectious composite complications (aOR = 4.35; p < 0.001), as well as adjusted 57 ± 1 min longer operative time (p < 0.001) and an adjusted 33% ± 1% longer LOS (p < 0.001) (Table 3).
LHR versus OHR
Relative to OHR, LHR was significantly associated with younger age. In addition, LHR was significantly associated with higher incidence of CHF, OSA, and smoking. Conversely, LHR was associated with lower incidence of: prior MI, HTN, COPD, DM, greater than 10% weight loss in 6 months, hemodialysis within two weeks of surgery, dependent functional status, ascites and ASA Class > 3 (Table 1). Unadjusted relative to OHR, LHR was significantly associated with less return to OR, superficial surgical site infection, as well as longer operative time and shorter LOS (Table 2).
Multivariate analysis with adjustment for confounding covariates, revealed that compared to OHR, LHR was significantly associated with increased adjusted odds of: overall complication (aOR = 1.22; p = 0.007), cardiac composite complication (aOR = 2.15; p = 0.002), renal composite complication (aOR = 2.05; p = 0.006), as well as adjusted 12 ± 1 min longer operative time (p < 0.001) and an adjusted 10% ± 3% shorter LOS (p < 0.001) (Table 3).
Temporal trends
Utilization of RHR increased from 0.24% in 2008 to 19.6% in 2019 of total operative volume. LHR utilization increased overall from 10.5% of operative volume in 2008 to 17.1% of cases in 2019. However, the peak of LHR operative volume was 18.0% in 2018. OHR utilization decreased over time from 89.3% in 2008 to 63.3% of total hernia repair volume in 2019 (Fig. 2).
RHR had a significant decrease in complication rate over time, from 20.8% in 2008 to 3.5% in 2019 (ρ = − 0.68; p value = 0.015). LHR complication rate decreased over time, with a peak of 1.7% in 2008 and a nadir of 0.9% in 2017 (ρ = − 0.69; p value = 0.014). OHR had a decrease in complication rate over time, from 1.5% in 2008 to 1.0% in 2019 (ρ = − 0.91; p value < 0.001) (Fig. 3). In the RHR cohort with respect to specific complications, infectious composite complication decreased from 20.8% (2008) to 1.77% (2019). Similarly, surgical site infection decreased from 12.5% in 2008 to 0.55% in 2019, while unplanned reoperation decreased from 12.5% (2008) to 1.83% (2019) (Fig. 4).
RHR had a significant decrease in mean operative time over the study period, from 4.9 ± 1.6 h in 2008 to 2.8 ± 1.6 h in 2019 (ρ = − 0.94; p < 0.001). LHR had a relatively constant average operative time over the study period, from 1.6 ± 0.7 h in 2008 to 1.6 ± 0.8 h in 2019 (ρ = 0.14; p value = 0.66). OHR cases had a slight increase in operative time over the study period, with nadir value 1.2 ± 0.6 h in 2009 and peak value of 1.3 ± 0.6 h in 2018 (ρ = 0.78; p value = 0.003) (Fig. 5).
Discussion
In review of inguinal hernia repair outcomes in the VA, OHR was found to comprise most of the operative volume. While this technique only offered modestly improved outcomes compared to laparoscopic approach, both OHR and LHR demonstrated significantly superior outcomes versus RHR. Despite these overall findings, RHR utilization greatly increased over the study period, while operative time and complication rate both significantly improved over time. The volume of robotic repairs in the setting of inguinal herniorrhaphy is projected to increase especially as clinical outcomes improve, and surgeons gain more experience with the platform.
In our comparison of LHR and RHR approaches, LHR was significantly associated with lower incidence of post-operative complication, shorter operative time, and shorter LOS. Since its inception, proponents of the robotic platform theorize that the technical advantages of robotic surgery would engender clinical benefits [17]. However in several studies, not only was there no clinical benefit derived from the robotic platform over traditional laparoscopic inguinal herniorrhaphy, in many instances it resulted in worse perioperative outcomes- particularly longer operative times and wound complications [19, 20, 25, 33]. In a retrospective review of these techniques, Khoracki et al. [21]. found that RHR had longer operative times, more clinically significant complications, higher readmission rates and substantially higher hospitals costs on a per-case basis without a significant difference in LOS. They inferred that the larger port sites (8 to 12 mm) in robotic surgery may predispose patients to higher likelihood of wound-related complications, or even port-site hernia requiring readmission and potential reoperation [21, 34]. Similarly, Charles et al. [19] compared RHR and LHR outcomes and found RHR to be associated with significantly longer operative times and higher incidence of surgical site infections. They surmised that the increased operative time in RHR contributed to more wound infections, which also may have led to increased costs compared to laparoscopic approach. Furthermore, the association between longer operative times and increased surgical site infections has been previously validated [35, 36]. In our sample, the RHR cohort not only had longer operative times and higher complication rates, but also had longer LOS, an outcome seen in few other studies. This finding of increased LOS was observed by Tatarian et al. [37] when comparing perioperative outcomes RHR to LHR; however, this association was not seen with application of their propensity-score model. This suggested that perhaps the longer LOS was secondary to increased incidence of cardiopulmonary comorbidities or other confounders in the RHR cohort [37]. A potential reason why our study demonstrated relatively higher LOS in the RHR cohort may due to the delayed incorporation of robotic surgery in the VA relative to civilian hospitals [18, 38]. With less longitudinal experience with the platform, VA surgeons may have been more reticent to discharge RHR patients on the same day, opting for overnight observation.
Although our analysis did not reveal any potential clinical benefits of RHR over LHR, the utilization and outcomes of RHR have trended in a positive direction. In the first year of our study period, RHR comprised a mere 0.24% of inguinal herniorrhaphy; but by 2019 this had expanded to just under 20% of operative volume, surpassing LHR that year. Yet, OHR remained the most utilized approach amongst veterans per year, with a nadir of 63% of operative volume in 2019. LHR experienced a slow upward trend from 10 to 18% until dropping slightly to 17% in 2019, indicating that RHR was mostly replacing open herniorrhaphy volume instead of laparoscopic volume. While the overall trend seems to be moving towards minimally invasive approach to IHR, the choice of robotic versus laparoscopic may be based on surgeon preference and experience. However, it is important to note that the availability of the robotic surgery platform will also dictate operative approach, as in the VA there are many centers without a dedicated robotics system in place. Sheetz et al. [18] described a comparable trend in which the robotic approach grew from 0.7% in 2012 to 28.8% of inguinal herniorrhaphy case volume in 2018. During that same time period, they observed that OHR proportional volume dropped from 74 to 60% [18].
The RHR cohort saw considerable improvements in several perioperative metrics over the study period in this analysis. These included operative time, overall complication rate, composite infectious complications, and wound-related complications. One potential reason for the trend of decreased operating time is the increasing familiarity with the robotic platform. In a single-surgeon review, Muysoms et al. [39] found that a cumulative operative volume of 50 RHR cases decreased mean operating time from 63 to 44 min, comparable to that same surgeon’s LHR mean operative time of 45 min. This demonstrates that RHR learning curve can be surmounted with a reasonable cumulative volume. Furthermore, Awad et al. [40] demonstrated the clinical benefit of increased RHR volume with a smaller cut-off of 20 cumulative cases. In their study, surgeons who performed more than 20 RHR had shorter operative times, lower complication rates, and lower per-case costs compared to surgeons with less than 20 RHR cases of experience. Given the relationship between shorter operating time and lower complication rates, it is plausible that increased surgeon and hospital experience with robotics in the VA validates the temporal trends that we observed. Moreover, this shows that outcomes may continue to improve with more operative volume experience. In addition, increased surgeon experience with the robotic console may augment the outcomes of other operations in general surgery that have trended towards a minimally invasive approach, such as ventral hernia repair or colectomy [18]. Nevertheless, it is important to note that even in the last year of our study period, the robotic perioperative outcomes remained inferior to the OHR and LHR cohorts.
OHR, the most utilized approach for herniorrhaphy in the VA, showed superior operative time, LOS, and complication rates in direct comparison with RHR. These two modalities have been thoroughly compared in the literature with mixed findings [19, 20, 22, 24, 41, 42]. Huerta et al. [24] reported that OHR had lower incidence of complications versus RHR or LHR. However, they did note that minimally invasive approaches were more likely to be utilized in complex hernias, such as recurrent, bilateral, or femoral which could partially explain their findings. In contrast with laparoscopic TAPP and RHR techniques, the open approach may carry a lower risk of complications given that it avoids entry into the abdomen as well as the physiologic effects of carbon dioxide insufflation. Select open inguinal herniorrhaphy cases can be performed under local sedation, which has been shown to decrease the risk of complications [43]. Notably, in our study population, 23.3% of the OHR cases were performed without the use of general anesthesia, which could certainly explain why composite pulmonary complications were lower in this group. OHR was also found to be associated with shorter operative time compared to laparoscopic and robotic approach in several studies [19, 22, 41]. The robotic approach requires additional time to position the console and arms, as well as safe entry into the abdominal cavity. Moreover, surgeon experience and case selection may contribute to the consistently reported shorter operative time of the open approach. Yet, some authors report RHR to have superior outcomes versus OHR in certain metrics [20, 42]. Janjua et al. [20] found the robotic approach to be associated with a shorter length of stay versus OHR, but conversely was associated with higher per-case costs. In a multi-institutional review, Gamagami et al. [42] reported a lower post-operative complication rate and longer operative time in RHR cases relative to OHR. With the current literature offering conflicting results, the role of robotic-assisted inguinal herniorrhaphy versus open and laparoscopic approaches will continue to be explored as more data emerges.
LHR was associated with a higher incidence of complications versus OHR cases, as well as longer mean operative time, but shorter LOS. Over the past few decades, these two techniques have been extensively compared, with differing findings [12,13,14,15,16, 44]. While some studies found the open approach to provide improved outcomes [24, 45, 46], other studies favor the laparoscopic technique [15, 16, 44]. Even still, some authors have found no significant differences between these approaches [12, 13, 23]. Despite the increased use of robotics, it is likely that laparoscopic and open techniques will continue to serve an important role in inguinal hernia management for the foreseeable future.
While the outcomes of the RHR improved over time, the profoundly inferior outcome of this cohort in the early part of the study period versus OHR and LHR was an unexpected finding. This is more likely a reflection of the relatively miniscule number of robotic cases that were recorded in those years, in which any difficult case with poor perioperative metrics would be disproportionately represented versus OHR and LHR. For example, in the RHR cohort there was less than 100 cases reported per year from 2008–2011, while there were over 1,000 cases per year during that time frame in the OHR and LHR cohorts.
The results of this study should be interpreted in the context of several key limitations. As a retrospective database review, there is the potential for confounding covariates that were not accounted for in our multivariate model that skewed our findings. In addition, with the immense sample size that VASQIP provided, especially with OHR cases, there is the potential for type I error. We attempted to account for this possibility with Bonferroni correction to raise our threshold for statistically significant findings. Our analysis model was dependent on accurate coding and data entry, but there were certain metrics we were unable to account for based on coding limitations. For example, there was no code in VASQIP specific to iatrogenic bowel injury that we could use in our analysis. In addition, within the laparoscopic cohort, we were unable to determine which cases were performed via TEP approach versus TAPP approach. Lastly, as VASQIP collates data up to 30 days into the post-operative period, we were unable to compare long-term outcomes, particularly hernia recurrence or chronic pain.
Conclusions
This study provided a nationwide analysis of the 30-day outcomes and utilization of open, laparoscopic, and robotic inguinal hernia repair in the VA. OHR was the most used approach, offering outcomes superior to both RHR and LHR. The robotic technique underwent massive expansion in utilization over the past decade with significant improvements in perioperative outcomes. Future studies should prospectively compare these techniques and explore long-term outcomes, such as hernia recurrence and reoperation rates. The well-established open and laparoscopic techniques for inguinal herniorrhaphy will continue to be valuable in general surgery practice in the appropriate setting. As the next generation of surgeons enters practice, robotics will continue to expand its role in the management of the inguinal hernia.
Data availability
Data were obtained from Veterans Affairs Surgical Quality Improvement Database and used only for the purposes of this research.
Code availability
Statistical analysis was conducted with SAS version 9.4.
References
Ruhl CE, Everhart JE (2007) Risk factors for inguinal hernia among adults in the US population. Am J Epidemiol 165(10):1154–1161
Berndsen MR, Gudbjartsson T, Berndsen FH (2019) Inguinal hernia—review. Laeknabladid 105(9):385–391. https://doi.org/10.17992/lbl.2019.09.247
Miller HJ (2018) Inguinal hernia: mastering the anatomy. Surg Clin North Am 98(3):607–621
Köckerling F, Simons MP (2018) Current concepts of inguinal hernia repair. Visc Med 34(2):145–150. https://doi.org/10.1159/000487278
Stroupe KT, Manheim LM, Luo P et al (2006) Tension-free repair versus watchful waiting for men with asymptomatic or minimally symptomatic inguinal hernias: a cost-effectiveness analysis. J Am Coll Surg 203(4):458–468
Ramanan B, Maloley BJ, Fitzgibbons RJ Jr (2014) Inguinal hernia: follow or repair? Adv Surg 48:1–11
Lichtenstein ME (1954) The custom-tailored inguinal hernia repair. J Okla State Med Assoc 47(8):222–224
Liem MS, van Vroonhoven TJ (1996) Laparoscopic inguinal hernia repair. Br J Surg 83(9):1197–1204
Wei FX, Zhang YC, Han W, Zhang YL, Shao Y, Ni R (2015) Transabdominal preperitoneal (TAPP) versus totally extraperitoneal (TEP) for laparoscopic hernia repair: a meta-analysis. Surg Laparosc Endosc Percutan Tech 25(5):375–383. https://doi.org/10.1097/SLE.0000000000000123
Birth M, Friedman RL, Melullis M, Weiser HF (1996) Laparoscopic transabdominal preperitoneal hernioplasty: results of 1000 consecutive cases. J Laparoendosc Surg 6(5):293–300. https://doi.org/10.1089/lps.1996.6.293
Wake BL, McCormack K, Fraser C, Vale L, Perez J, Grant AM (2005) Transabdominal pre-peritoneal (TAPP) vs totally extraperitoneal (TEP) laparoscopic techniques for inguinal hernia repair. Cochrane Database Syst Rev 1:CD004703
Tanphiphat C, Tanprayoon T, Sangsubhan C, Chatamra K (1998) Laparoscopic vs open inguinal hernia repair. A randomized, controlled trial. Surg Endosc. 12(6):846–851. https://doi.org/10.1007/s004649900727
McCormack K, Scott NW, Go PM, Ross S, Grant AM, EU Hernia Trialists Collaboration (2003) Laparoscopic techniques versus open techniques for inguinal hernia repair. Cochrane Database Syst Rev 1:CD001785
Trevisonno M, Kaneva P, Watanabe Y et al (2015) Current practices of laparoscopic inguinal hernia repair: a population-based analysis. Hernia 19(5):725–733. https://doi.org/10.1007/s10029-015-1358-5
Tadaki C, Lomelin D, Simorov A et al (2016) Perioperative outcomes and costs of laparoscopic versus open inguinal hernia repair. Hernia 20(3):399–404. https://doi.org/10.1007/s10029-016-1465-y
Perez AJ, Strassle PD, Sadava EE, Gaber C, Schlottmann F (2020) Nationwide analysis of inpatient laparoscopic versus open inguinal hernia repair. J Laparoendosc Adv Surg Tech A 30(3):292–298. https://doi.org/10.1089/lap.2019.0656
Tian W, Fei Y (2018) Application of da vinci robotic surgery to hernia repair. Zhonghua Wei Chang Wai Ke Za Zhi 21(7):740–743
Sheetz KH, Claflin J, Dimick JB (2020) Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open 3(1):e1918911. https://doi.org/10.1001/jamanetworkopen.2019.18911
Charles EJ, Mehaffey JH, Tache-Leon CA, Hallowell PT, Sawyer RG, Yang Z (2018) Inguinal hernia repair: is there a benefit to using the robot? Surg Endosc 32(4):2131–2136. https://doi.org/10.1007/s00464-017-5911-4
Janjua H, Cousin-Peterson E, Barry TM, Kuo MC, Baker MS, Kuo PC (2020) The paradox of the robotic approach to inguinal hernia repair in the inpatient setting. Am J Surg 219(3):497–501
Khoraki J, Gomez PP, Mazzini GS et al (2020) Perioperative outcomes and cost of robotic-assisted versus laparoscopic inguinal hernia repair. Surg Endosc 34(8):3496–3507. https://doi.org/10.1007/s00464-019-07128-8
Kakiashvili E, Bez M, Abu Shakra I et al (2021) Robotic inguinal hernia repair: is it a new era in the management of inguinal hernias? Asian J Surg. https://doi.org/10.1016/j.asjsur.2020.03.015
Aiolfi A, Cavalli M, Micheletto G et al (2019) Primary inguinal hernia: systematic review and bayesian network meta-analysis comparing open, laparoscopic transabdominal preperitoneal, totally extraperitoneal, and robotic preperitoneal repair. Hernia 23(3):473–484. https://doi.org/10.1007/s10029-019-01964-2
Huerta S, Timmerman C, Argo M et al (2019) Open, laparoscopic, and robotic inguinal hernia repair: outcomes and predictors of complications. J Surg Res 241:119–127
Abdelmoaty WF, Dunst CM, Neighorn C, Swanstrom LL, Hammill CW (2019) Robotic-assisted versus laparoscopic unilateral inguinal hernia repair: a comprehensive cost analysis. Surg Endosc 33(10):3436–3443. https://doi.org/10.1007/s00464-018-06606-9
Prabhu AS, Carbonell A, Hope W et al (2020) Robotic inguinal vs transabdominal laparoscopic inguinal hernia repair: the RIVAL randomized clinical trial. JAMA Surg 155(5):380–387. https://doi.org/10.1001/jamasurg.2020.0034
Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS (2018) Tobacco product use among military veterans—United States, 2010–2015. MMWR Morb Mortal Wkly Rep 67(1):7–12. https://doi.org/10.15585/mmwr.mm6701a2
Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM (2012) Health and health behavior differences: U.S. military, veteran, and civilian men. Am J Prev Med 43(5):483–489
Klevens RM, Giovino GA, Peddicord JP, Nelson DE, Mowery P, Grummer-Strawn L (1995) The association between veteran status and cigarette-smoking behaviors. Am J Prev Med 11(4):245–250
Eibner (2015) Current and projected characteristics and unique health care needs of the patient population served by the department of veterans affairs. RAND Corporation https://doi.org/10.7249/j.ctt19w735m
Oshinski R (2020) Annual surgery report 2019. National Surgery Office. Veterans Health Administration, Washington DC
Massarweh NN, Kaji AH, Itani KMF (2018) Practical guide to surgical data sets: veterans affairs surgical quality improvement program (VASQIP). JAMA Surg 153(8):768–769
Kudsi OY, McCarty JC, Paluvoi N, Mabardy AS (2017) Transition from laparoscopic totally extraperitoneal inguinal hernia repair to robotic transabdominal preperitoneal inguinal hernia repair: a retrospective review of a single surgeon’s experience. World J Surg 41(9):2251–2257. https://doi.org/10.1007/s00268-017-3998-3
Diez-Barroso R Jr, Palacio CH, Martinez JA et al (2018) Robotic port-site hernias after general surgical procedures. J Surg Res 230:7–12
Cheng H, Chen BP, Soleas IM, Ferko NC, Cameron CG, Hinoul P (2017) Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect (Larchmt) 18(6):722–735. https://doi.org/10.1089/sur.2017.089
Procter LD, Davenport DL, Bernard AC, Zwischenberger JB (2010) General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg 210(1):60–62. https://doi.org/10.1016/j.jamcollsurg.2009.09.034
Tatarian T, Nie L, McPartland C et al (2021) Comparative perioperative and 5-year outcomes of robotic and laparoscopic or open inguinal hernia repair: a study of 153,727 patients in the state of new york. Surg Endosc. https://doi.org/10.1007/s00464-020-08211-1
Napolitano MA, Skancke M, Walters J et al (2020) Outcomes and trends in colorectal surgery in U.S. veterans: a 10-year experience at a tertiary veterans affairs medical center. J Laparoendosc Adv Surg Tech A 30(4):378–382. https://doi.org/10.1089/lap.2019.0739
Muysoms F, Van Cleven S, Kyle-Leinhase I, Ballecer C, Ramaswamy A (2018) Robotic-assisted laparoscopic groin hernia repair: observational case-control study on the operative time during the learning curve. Surg Endosc 32(12):4850–4859. https://doi.org/10.1007/s00464-018-6236-7
Awad MA, Buzalewski J, Anderson C et al (2020) Robotic inguinal hernia repair outcomes: Operative time and cost analysis. J Soc Laparoendosc Surg. https://doi.org/10.4293/JSLS.2020.00058
Qabbani A, Aboumarzouk OM, ElBakry T, Al-Ansari A, Elakkad MS (2021) Robotic inguinal hernia repair: systematic review and meta-analysis. ANZ J Surg. https://doi.org/10.1111/ans.16505
Gamagami R, Dickens E, Gonzalez A et al (2018) Open versus robotic-assisted transabdominal preperitoneal (R-TAPP) inguinal hernia repair: a multicenter matched analysis of clinical outcomes. Hernia 22(5):827–836. https://doi.org/10.1007/s10029-018-1769-1
Balentine CJ, Meier J, Berger M et al (2020) Using local rather than general anesthesia for inguinal hernia repair is associated with shorter operative time and enhanced postoperative recovery. Am J Surg. https://doi.org/10.1016/j.amjsurg.2020.08.024
Patterson TJ, Beck J, Currie PJ, Spence RAJ, Spence G (2019) Meta-analysis of patient-reported outcomes after laparoscopic versus open inguinal hernia repair. Br J Surg 106(7):824–836. https://doi.org/10.1002/bjs.11139
Neumayer L, Giobbie-Hurder A, Jonasson O et al (2004) Open mesh versus laparoscopic mesh repair of inguinal hernia. N Engl J Med 350(18):1819–1827
Millat B, Fédération de Recherche EN CHirurgie (FRENCH) (2007) Inguinal hernia repair. A randomized multicentricstudy comparing laparoscopic and open surgical repair. J Chir (Paris) 144(2):119–124
Funding
No outside funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. Authors TH, MN, and AS contributed to the acquisition and analysis of data. All authors contributed to the interpretation of data. Authors TH, MN, AS, and FB contributed to drafting of the work while authors MG and JD made critical revisions. All authors read and approved the final manuscript and agree to be accountable for all aspects of work.
Corresponding author
Ethics declarations
Conflict of interest
Timothy Holleran, Michael Napolitano, Andrew Sparks, James Duncan, Meredith Garrett, and Fredrick Brody declare that they have no conflict of interest.
Ethical approval
Due to the retrospective nature of the study and utilization of de-identified data, Institutional Review Board (IRB) waiver of consent was applied and approved for this study (IRB-Exempt Protocol #01966).
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this review, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Holleran, T.J., Napolitano, M.A., Sparks, A.D. et al. Trends and outcomes of open, laparoscopic, and robotic inguinal hernia repair in the veterans affairs system. Hernia 26, 889–899 (2022). https://doi.org/10.1007/s10029-021-02419-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-021-02419-3