Abstract
Background
Laparoscopic cholecystectomy (LC) is the most common elective abdominal surgery in the USA, with over 750,000 performed annually. Fluorescent cholangiography (FC) using indocyanine green dye (ICG) permits identification of extrahepatic biliary structures to facilitate dissection without requiring cystic duct cannulation. Achieving the “critical view of safety” with assistance of ICG cholangiogram may support identification of anatomy, safely reduce conversion to open procedures, and decrease operative time. We assess the utility of FC with respect to anatomic visualization during LC and its effects on patient outcomes.
Methods
A retrospective review of a prospectively maintained database identified patients undergoing laparoscopic cholecystectomy at a single academic center from 2013 to 2019. Exclusion criteria were primary open and single incision cholecystectomy. Patient factors included age, sex, BMI, and Charlson Comorbidity Index. Outcomes included operative time, conversion to open procedure, length of stay (LOS), mortality rate, and 30-day complications. A multivariable logistic regression was performed to determine independent predictors for open conversion.
Results
A total of 1389 patients underwent laparoscopic cholecystectomy. 69.8% were female; mean age 48.6 years (range 15–94), average BMI 29.4 kg/m2 (13.3–55.6). 989 patients (71.2%) underwent LC without fluorescence and 400 (28.8%) underwent FC with ICG. 30-day mortality detected 2 cases in the non-ICG group and zero with ICG. ICG reduced operative time by 26.47 min per case (p < 0.0001). For patients with BMI ≥ 30 kg/m2, operative duration for ICG vs non-ICG groups was 75.57 vs 104.9 min respectively (p < 0.0001). ICG required conversion to open at a rate of 1.5%, while non-ICG converted at a rate of 8.5% (p < 0.0001). Conversion rate remained significant with multivariable analysis (OR 0.212, p = 0.001). A total of 19 cases were aborted (1.35%), 8 in the ICG group (1.96%) and 11 in the non-ICG group (1.10%), these cases were not included in LC totals. Average LOS was 0.69 vs 1.54 days in the ICG compared to non-ICG LCs (p < 0.0001), respectively. Injuries were more common in the non-ICG group, with 9 patients sustaining Strasberg class A injuries in the non-ICG group and 2 in the ICG group. 1 CBDI occurred in the non-ICG group. There was no significant difference in 30-day complication rates between groups.
Conclusion
ICG cholangiography is a non-invasive adjunct to laparoscopic cholecystectomy, leading to improved patient outcomes with respect to operative times, decreased conversion to open procedures, and shorter length of hospitalization. Fluorescence cholangiography improves visualization of biliary anatomy, thereby decreasing rate of CBDI, Strasberg A injuries, and mortality. These findings support ICG as standard of care during laparoscopic cholecystectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cholecystectomy is the most commonly performed elective abdominal procedure in the USA, with over 750,000 performed annually [1]. Since the introduction of laparoscopy in the 1980s, laparoscopic cholecystectomy (LC) has become the standard of care for benign pathology of the gallbladder. Demonstrated benefits include improved cosmesis, shorter length of stay (LOS), earlier oral intake, and decreased post-operative pain [2,3,4]. Despite widespread implementation of the procedure, serious complications such as common bile duct injury (CBDI) and conversion to open continue to be ongoing occurrences [3, 5]. Though infrequent, CBDI is a significant source of patient morbidity as serious injuries often require at least one surgical repair, often a complex reconstructive surgery, with variability in long term outcomes [1]. Furthermore, CBDI has a negative impact on overall survival and quality of life, even 10 years after the event [6]. Reported rates of conversion to open cholecystectomy range from 5 to 20% in the literature. Conversion is shown to be associated with increased morbidity, risk of surgical site infections, increased pulmonary complications, and prolonged LOS [7, 8].
Though sources cite variable rates of CBDI in LC, the rate is generally acknowledged to be higher in LC as compared to OC. The reported incidence of CBDI during LC ranges from 0.03% to 2.6%, with significant heterogeneity across studies [2, 9, 10]. While the initial increase in rate of CBDI was attributed to the learning curve with the laparoscopic approach, CBDI continues to occur at a non-zero rate despite improved quality of equipment and increased surgeon experience with the technique [9, 11, 12]. In response to increasing complication rates, Strasberg et al. introduced the Critical View of Safety (CVS) in 1995, as a preventative measure to decrease the risk of injury to extrahepatic bile ducts [13]. Literature, however, continues to indicate the primary cause of CBDI is an error in visual perception of the anatomy in 71–97% of cases. Factors increasing the potential for distorted anatomy include obesity, past or ongoing inflammation, variant ductal anatomy, and limited surgical experience [10, 14]. The use of intraoperative cholangiography (IOC) has been recommended to help prevent misinterpretation of biliary anatomy, though its routine use is debated [1, 15, 16].
A recent alternative to routine IOC for anatomic delineation is the use of fluorescent cholangiography (FC). Indocyanine green dye (ICG) is injected via the intravenous route preoperatively and is excreted exclusively into the bile; protein-bound ICG fluoresces when illuminated with near-infrared (NIR) light [15]. FC offers potential detailed anatomical mapping of extrahepatic biliary structures, and may be a useful adjunct to the CVS. With the platform currently in use at our institution, the surgeon can switch between bright light mode and NIR fluoroscopy using a button on the laparoscopic camera. Through constant reassessment of the anatomy with NIR imaging, surgeons may continuously identify the position of critical biliary structures. Furthermore, the technology incorporates smoothly into the operation without increased need for staffing or additional supplies in the operative theater, beyond the addition of a NIR-capable laparoscope. In contrast, IOC can be time consuming and involves exposure of the patient and ancillary staff to radiation, with associated increases in cost [15, 16]. A 2014 study reported that the mean cost of surgery generated by IOC (U.S. $778.43) was greater than the cost with FC ($14.10) [16].
No absolute contraindications exist for the administration of ICG dye, and ICG has been used safely in patients with documented iodine allergy [16,17,18]. Risk of an adverse event with ICG is low. Anaphylactic reaction is reported at a rate of 0.003%, with an 0.34% overall incidence of adverse reaction (i.e., hypotension, urticaria), the majority of which are mild [16, 18]. Our group avoids the use of ICG dye in patients with a history of anaphylactic reaction to shellfish or iodine out of an abundance of caution. Anecdotally, we have not experienced any adverse reactions with the use of ICG. A potential advantage of ICG cholangiography over IOC is the ability to delineate biliary anatomy prior to commencing dissection of the triangle of Calot [15]. This study aims to evaluate the safety, utility, and effectiveness of FC in identification of biliary anatomy with an analysis of patient outcomes.
Materials and methods
A retrospective review of a prospectively maintained IRB-approved database was performed to identify patients undergoing cholecystectomy at two hospitals (Hillcrest and La Jolla locations) within a single academic institution, from October 2013 to June 2019. Patient characteristics included age, sex, BMI, Charlson Comorbidity Index (CCI), and indication for cholecystectomy. Perioperative data included elective versus non-elective procedure, operative time (skin incision to skin closure), intraoperative drain placement, estimated blood loss (EBL), and requirement for conversion from a laparoscopic to an open approach. Procedures were performed at a teaching institution, thus presence or absence of a surgical resident or fellow during the case was included. Exclusion criteria were open cholecystectomy, single site cholecystectomy, transvaginal cholecystectomy, oncologic resections, and complex biliary reconstructive procedures. Of note, patients undergoing a concomitant procedure during LC (i.e., esophagogastroduodenoscopy, liver biopsy, lap band removal, etc.) were excluded from the operative time analysis.
Postsurgical data included 30-day morbidity, rate of CBDI, rate of cystic duct stump leak and biloma, mortality rate, 30-day readmissions, and 30-day emergency department (ED) visits. Further subgroup analysis was performed, stratifying indication for laparoscopic cholecystectomy into inflamed versus non-inflamed disease processes of the gallbladder. This was based on initial clinical presentation, pre-operative laboratory tests, and imaging findings, with final determination of disease grouping based on intraoperative findings as documented in operative note. Inflammatory pathologies included acute cholecystitis, chronic cholecystitis, gangrenous/perforated/hemorrhagic cholecystitis, acalculous cholecystitis, ascending cholangitis, Mirizzi syndrome, and acute-on-chronic cholecystitis. Non-inflammatory disease processes included biliary colic, gallstone pancreatitis, choledocholithiasis, gallbladder polyp, biliary dyskinesia, gallbladder mass, adenomyomatosis, porcelain gallbladder, and cholesterolosis. Final pathology was collected and analyzed; however, only 5% of patients (72/1389) were found to have a non-inflamed process according to final pathologic report. Given the fact that technical difficulty of peri-cholecystic dissection is more pertinent to patient safety and outcomes, stratification into inflamed versus non-inflamed disease of the gallbladder was based on intraoperative findings (i.e., evidence of surrounding adhesions and inflammation).
The use of FC was implemented in April 2014 and has since, in conjunction with the CVS, become standard of care during laparoscopic cholecystectomy at one hospital in our health system. FC is selectively used at other facilities and this variation in approach to laparoscopic cholecystectomy within our institution permitted comparison of the procedure with and without ICG cholangiography. For the remainder of this paper, bright light laparoscopic cholecystectomy without ICG will be referred to as LC, while laparoscopic cholecystectomy using ICG cholangiography will be referred to as FC.
The routine protocol for ICG administration was for 7.5 mg (3 ml of a 25 mg/10 ml solution) to be administered intravenously in the pre-operative holding area at least 45 min prior to surgical intervention. An Indocyanine Green for Injection, USP (HUB Pharmaceuticals, LLC, Rancho Cucamonga, CA) 25 mg vial is reconstituted using 10 ml of sterile water, immediately prior to injection.
Surgical approach
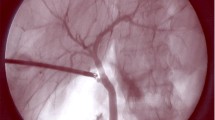
For patients who underwent FC, laparoscopic imaging was performed using either the Stryker 1588 AIM camera system, or the Stryker 1688 AIM 4K platform through a standard 5 mm or 12-mm umbilical trocar port. Current platforms permit the capability to switch from bright light to fluorescent mode without changing the camera or light cord. FC is performed throughout the course of the procedure, in particular at the following time points: prior to peritoneal dissection (Fig. 1), during the course of skeletonizing the cystic duct (Fig. 2), prior to clipping the cystic duct (Fig. 3), after clipping but prior to transection of the cystic duct (Fig. 4), post-cystic duct transection (Fig. 5), and immediately after removal of the gallbladder but prior to closure.
Statistical methods
Descriptive analysis
Descriptive statistics on patient, surgeon, and intraoperative data were compared for patients with and without use of FC. Categorical data were presented as count (frequency) and analyzed with Fisher’s exact test for discrete variables. Continuous, normally distributed variables were analyzed using Student’s t-test.
Multivariable analyses
In order to determine independent predictors for conversion to open, we performed a univariable logistic regression with clinically relevant predictors, including patient, surgeon, and intraoperative characteristics. Then, we performed a multivariable logistic regression with inclusion criteria set at p-value < 0.20. A similar subgroup analysis was also performed for patients with inflammatory indications for LC. All analyses were performed using IBM SPSS Statistics (IBM Corporation, Version 25, Armonk, NY) and Prism 8 (GraphPad Prism, Version 8.4.0, La Jolla, CA). Confidence intervals were set at 95%. All statistical tests were 2-sided with p < 0.05 considered to be statistically significant.
Results
Patient characteristics
A total of 1461 patients were identified. 1389 met inclusion criteria for the study, and underwent laparoscopic cholecystectomy. 989 (71.2%) patients underwent standard bright light LC; 400 (28.8%) patients underwent FC. Mean age was 47.4 years (range 15–94) in the LC group and 51.5 years (range 18–86) in the FC group (p < 0.0001). 68.6% versus 72.5% of patients were female in the LC versus FC groups, respectively (p = 0.1757). Average BMI in the LC group was 29.85 kg/m2, while average BMI in the LC group was 28.41 kg/m2 (p = 0.0002). Demographic data for all laparoscopic cholecystectomy patients as a whole, and for FC and LC groupings are shown in Table 1. Patients presented with a variety of indications for cholecystectomy: 38.8% with biliary colic, 15% with acute cholecystitis, and 14.4% with gallstone pancreatitis. The remainder of clinical indications for cholecystectomy is listed in Table 1.
Intraoperative data
FC had a mean operative time of 72.53 min (range 17–268), as compared to 99 min (range 23–435) for LC; resulting in an average decrease of 26.47 min per case (p < 0.0001) (Table 2). In patients with BMI ≥ 30 kg/m2, operative duration for FC versus LC was 75.57 versus 104.9 min, respectively, for an average operative time reduction of 29.33 min (p < 0.0001), excluding concomitant procedures (Table 2). EBL and drain placement were both decreased in the FC compared to the LC group (p < 0.0001) (Table 2). In the inflamed gallbladder pathology subgroup, operative time in the FC versus LC group was 83.6 versus 117.2 min, respectively (p < 0.0001), and in patients with BMI ≥ 30 in the inflamed FC versus LC groups was 90.38 versus 122.0 min (p = 0.0006). Similar results were noted in the non-inflamed pathology subgroups as well (Table 3). FC required conversion to open at a rate of 1.5%, while LC converted at a rate of 8.5% (p < 0.0001), which held true across subgroups (Table 2). While rate of conversion to open was reduced in the inflamed pathology with BMI ≥ 30 subgroup with FC versus LC, 4.88% versus 12.68%, respectively, this was not statistically significant (p = 0.25) (Table 3).
After controlling for clinically relevant predictors via multivariable logistic regression, FC was associated with a 78.8% odds reduction for conversion to an open procedure (p = 0.001, OR 0.212, Table 4). Subgroup analysis of only patients with inflamed gallbladder pathology also showed an 84.5% odds reduction in conversion with FC (p = 0.002, OR 0.155, Table 5) in multivariable analysis. In the multivariable logistic regression for both the overall cohort and the inflamed gallbladder subgroup, female patients had a 69.2% and 48.5% odds reduction of conversion to open, respectively (Tables 4, 5).
A total of 19 patients underwent diagnostic laparoscopy and aborted cholecystectomy, 8 patients received pre-operative ICG, and 11 were in the non-ICG group; no significant difference was noted in aborted case rate. Average age for aborted cholecystectomy patients was 58.9 (range 31–78), 57.9% of patients were male, with an average BMI of 28.6 kg/m2 (range 20–36.82 kg/m2). Within the ICG diagnostic laparoscopy group, 7 cases were aborted due to significant inflammation and adhesions in the peri-cholecystic region precluding identification of anatomy and a safe operation. Two of these seven patients were additionally noted to have previously undiagnosed macronodular cirrhosis. One was canceled for a hemangioma overlying the gallbladder. In the non-ICG diagnostic laparoscopy group, 10 LC were aborted for significant inflammation and adhesions, and one was aborted due to pneumoperitoneum-induced asystole with subsequent return of spontaneous circulation after CPR.
In the ICG aborted cholecystectomy group, 5 patients subsequently underwent cholecystectomy: 4 via laparoscopic approach, 1 via laparoscopic converted to open. 4 of these patients were referred to a hepatobiliary surgeon for cholecystectomy. In the non-ICG group, 7 patients subsequently underwent cholecystectomy: 2 OC, 3 LC, and one laparoscopic converted to open approach. 3 of these patients were referred to a hepatobiliary surgeon. Of the patients who did not undergo subsequent cholecystectomy, three were lost to follow up, for the other two a decision was made not to offer intervention in the absence of symptoms as risk was thought to outweigh benefit.
Post-operative outcomes
LOS was reduced in the FC cohort: average LOS was 0.69 days in the FC group and 1.54 days in the LC group (p < 0.0001) (Table 2). LOS in the inflamed and non-inflamed groups remained lower on average in the FC cohort across subgroups; with LOS 0.87 versus 2.3 days for FC versus LC in the inflamed subset (p < 0.0001), and 0.59 versus 1.18 days for FC versus LC in the non-inflamed subset (p < 0.0001) (Table 3). No statistical significance was seen in the rate of CBDI or Strasberg A injury between groups. One CBDI occurred in the LC group (0.1%); zero in the FC group (Table 2). In total, 11 patients presented with post-operative biloma or bile leak; 9 (0.91%) in the LC group and 2 (0.5%) in the FC group (p = 0.74); these were classified as Strasberg A injuries. For Strasberg A injuries, patients were managed with a combination of conservative therapy, percutaneous drain placement, or ERCP with transampullary stenting depending on the volume of leak. Of the 19 patients with CBDI, post-operative biloma, or bile leak, 2 (18.2%) had a prior cholecystostomy tube in place, 7 (63.6%) underwent non-elective cholecystectomy, 5 (45.5%) required conversion from a laparoscopic to an open approach, and all patients (100%) had an inflammatory disease process on final pathology (either acute or chronic cholecystitis). Two inpatient mortalities occurred within the LC group. One patient sustained an aspiration event leading to acute hypoxia and cardiovascular collapse on post-operative day 4 from a laparoscopic converted to open cholecystectomy for gallstone pancreatitis. The second mortality occurred as a result of intraoperative hemorrhage secondary to severe vasculobiliary injury after attempted fundus-down laparoscopic cholecystectomy in an inflamed gallbladder, with subsequent conversion to open cholecystectomy and cardiac arrest. No differences were observed in 30-day morbidity, mortality, readmissions, or ED visits between groups.
Discussion
Despite the widespread use of laparoscopic cholecystectomy, ICG fluorescent cholangiography has not yet become standard of care as an adjunct to the critical view of safety. We show that the routine use of FC reduces operative time as well as conversion to open procedures regardless of diagnosis and BMI. Overall CBDI and Strasberg A injuries were decreased in the FC as compared to LC groups, suggesting that improved visualization of the biliary tree via ICG as standard of care during laparoscopic cholecystectomy may decrease rate of iatrogenic injury. However, we showed no significant difference between LC and FC, owing credence to CVS as well as lack of sample size to detect potential small differences between the techniques.
The incorporation of ICG cholangiography into LC has the potential to provide real-time visualization of the extrahepatic biliary tree prior to commencing dissection within Calot’s Triangle [15]. Anecdotally, we find the biliary anatomy can be identified in this manner in most patients, although there are certainly cases where further dissection is needed before ICG is useful as an adjunct for visualization. Improved anatomical identification allows for targeted dissection of the cystic duct while utilizing the CVS technique, and helps to confirm cystic artery versus duct during dissection. With this targeted dissection and assistance with identifying complex (inflamed, aberrant) biliary anatomy, we have shown a decrease in operative times and conversion to open surgery. Increased operative duration has been associated with increased LOS with general surgical procedures. Procter et al. reported that LOS increases geometrically with increasing operative duration, after adjusting for procedural and patient risk factors, at a rate of 6% per hour [19]. Our data suggest that use of this minimally invasive adjunct also led to decreased LOS across subgroups, perhaps secondary to operative time decreases.
The benefits of ICG cholangiography have been described in the literature. In one of the earliest series on ICG cholangiography, Ishizawa et al. identified the cystic duct in 100% of patients prior to dissecting the triangle of Calot [15]. Prior studies have postulated that rapid identification of the cystic duct-common hepatic duct junction with FC shortens both the dissection phase and operative time [20]. We provide the largest cohort comparing the outcomes of standard bright light LC to FC in current literature. Additionally, our study provides results on objective measures that show improved outcomes in FC compared to LC. Due to sample size, we are able to identify significant differences across subgroups. While Buchs et al. suggested that quality of NIR-assisted biliary tract visualization is diminished with increased BMI [20], we were able to demonstrate statistically significant decreases in operative time in patients with BMI ≥ 30. In further subgroup analysis, we looked at patients with inflamed gallbladder pathology and noted a statistically significant decrease in operative time within the FC group as compared to conventional LC overall, and in patients with BMI ≥ 30. Even in the setting of excess adipose tissue overlying Calot’s triangle, the use of FC may orient the surgeon to the location of biliary structures during the dissection [14, 16].
Studies focusing on the effect of inflammation on NIR visualization have tended to be small, with inconsistent definitions on criteria for uncomplicated versus complicated disease, and have largely pooled all benign biliary diseases together [21, 22]. In contrast, our study encompassed 455 patients with inflamed etiology, with 137 in the FC group and 318 in the LC group. Complications during LC have been reported to be higher with obese patients, anatomic abnormalities of the gallbladder, and in cases with recurrent inflammatory episodes resulting in significant adhesions and distorted anatomy [23]. Despite these risks, we found our operative times were decreased with the addition of FC even in patients with inflammatory gallbladder disease.
While our LC conversion rates were concordant with reported rates, we noted a statistically significant reduction in conversion rates with FC, maintained across subgroups and after controlling for covariates via logistic regression. Conversion from laparoscopic to open cholecystectomy is sometimes mandatory, often due to inability to identify anatomy, need to avoid or repair injury, or insurance of patient safety [4, 7, 8]. It is possible that easier definition of anatomy with FC contributed to decreased rates of conversion to open. Notably, male sex was a statistically significant risk factor for conversion to open in both univariable and multivariable analyses. This trend has been demonstrated in previous studies, and has been attributed to increased severity of gallstone disease in males, and the tendency for males to accumulate higher amounts of visceral adipose tissue [7, 24,25,26]. Given the increased risk of morbidity and prolonged LOS associated with conversion to open, and our demonstrated reduction in conversion rates with the use of FC, routine use of this adjunct may improve overall patient outcomes during LC if conversion to open may be avoided more often.
The majority of CBDI occur as a result of misidentification of biliary anatomy. In the “classical injury,” the CBD is identified as the CD and divided [27]. While implementation of the CVS has undoubtedly improved the safety of LC [14, 28], our data suggest that routine use of ICG cholangiography may be the logical adjunct to the CVS. Within our cohort, we observed a decreased rate of Strasberg type A injuries and CBDI within the FC as compared to LC groups overall; this was not statistically significant due to sample size. Decreases in operative time and conversions were significant, and show excellent outcomes with FC. NIR fluoroscopy has the potential to increase both patient safety and procedural efficiency via enhancement and optimization of tissue visualization [29]. Particularly in the setting of inflammation or aberrant anatomy, FC as an adjunct to CVS may improve both LC safety and outcomes.
Limitations of this study include restriction to a single institution and inadequate sample size to identify any differences in CBDI between techniques, although these are expected to be minimal due to the use of CVS in both LC and FC. Moving forward, larger, prospective trials are indicated. A study looking at specific time in minutes to identification of critical anatomy, and whether this time period was decreased on account of FC would be beneficial. While it would be ideal to define whether the use of FC reduces risk of CBDI, there are several challenges inherent to the structure of this hypothetical study. With the low incidence of CBDI, the event rate is approximately 3 per 1000 LC performed in the USA. Strasberg et al. highlighted these challenges, stating that a randomized trial would be exceedingly challenging to perform with this low rate, and would require approximately 4500 patients per arm [28]. Large observational studies may be the only realistic approach to assess the relationship between use of routine FC and CBDI. Another significant limitation is the retrospective nature of our study. As a result, we were unable to collect data on time to identification of critical anatomical structures. We also lack data on incidence of adequate visualization of biliary anatomy prior to commencing peritoneal dissection. Future studies could examine these crucial time points with respect to identification of anatomy, and could focus specifically on the effect of BMI, visceral fat, and inflamed versus non-inflamed disease processes.
With noted improvements in operative time and conversion to open surgery, our group has adopted routine FC in addition to CVS as standard of care. ICG cholangiography should be standard of care as an adjunct to potentially help identify anatomy, and expedite safe dissection during laparoscopic cholecystectomy.
Conclusions
Fluorescence cholangiography provides a non-invasive confirmation of the extrahepatic biliary anatomy during laparoscopic cholecystectomy, reducing operative time, LOS, and rate of conversion to open surgery. Although not statistically significant, common bile duct injury occurred less with fluorescence cholangiography, and is a potential future topic of study. This real-time approach to expedite identification of crucial structures supports the use of fluorescence cholangiography as standard of care.
References
Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T (2003) Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. J Am Med Assoc. https://doi.org/10.1001/jama.289.13.1639
Kohn JF, Trenk A, Kuchta K et al (2018) Characterization of common bile duct injury after laparoscopic cholecystectomy in a high-volume hospital system. Surg Endosc. https://doi.org/10.1007/s00464-017-5790-8
van de Graaf FW, Zaïmi I, Stassen LPS, Lange JF (2018) Safe laparoscopic cholecystectomy: a systematic review of bile duct injury prevention. Int J Surg. https://doi.org/10.1016/j.ijsu.2018.11.006
Tayeb M, Raza SA, Khan MR, Azami R (2005) Conversion from laparoscopic to open cholecystectomy: multivariate analysis of preoperative risk factors. J Postgrad Med 51(1):17–20
Zroback C, Chow G, Meneghetti A et al (2016) Fluorescent cholangiography in laparoscopic cholecystectomy: the initial Canadian experience. Am J Surg. https://doi.org/10.1016/j.amjsurg.2016.01.013
de Reuver PR, Sprangers MAG, Rauws EAJ et al (2008) Impact of bile duct injury after laparoscopic cholecystectomy on quality of life: a longitudinal study after multidisciplinary treatment. Endoscopy. https://doi.org/10.1055/s-2008-1077444
Lipman JM, Claridge JA, Haridas M et al (2007) Preoperative findings predict conversion from laparoscopic to open cholecystectomy. Surgery. https://doi.org/10.1016/j.surg.2007.07.010
Shah AA, Bhatti UF, Petrosyan M et al (2019) The heavy price of conversion from laparoscopic to open procedures for emergent cholecystectomies. Am J Surg. https://doi.org/10.1016/j.amjsurg.2018.12.038
Stefanidis D, Chintalapudi N, Anderson-Montoya B, Oommen B, Tobben D, Pimentel M (2017) How often do surgeons obtain the critical view of safety during laparoscopic cholecystectomy? Surg Endosc. https://doi.org/10.1007/s00464-016-4943-5
Aziz H, Pandit V, Joseph B, Jie T, Ong E (2015) Age and obesity are independent predictors of bile duct injuries in patients undergoing laparoscopic cholecystectomy. World J Surg. https://doi.org/10.1007/s00268-015-3010-z
Keus F, De Jong JAF, Gooszen HG, Van Laarhoven CJHM (2006) Laparoscopic versus small-incision cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006229
Keus F, De Jong JAF, Gooszen HG, Van Laarhoven CJHM (2006) Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006231
Strasberg SM, Hertl M, Soper NJ (1995) An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180(1):101–125
Buddingh KT, Nieuwenhuijs VB, Van Buuren L, Hulscher JBF, De Jong JS, Van Dam GM (2011) Intraoperative assessment of biliary anatomy for prevention of bile duct injury: a review of current and future patient safety interventions. Surg Endosc. https://doi.org/10.1007/s00464-011-1639-8
Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N (2010) Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. https://doi.org/10.1002/bjs.7125
Dip FD, Asbun D, Rosales-Velderrain A et al (2014) Cost analysis and effectiveness comparing the routine use of intraoperative fluorescent cholangiography with fluoroscopic cholangiogram in patients undergoing laparoscopic cholecystectomy. Surg Endosc. https://doi.org/10.1007/s00464-013-3394-5
Bjerregaard J, Pandia MP, Jaffe RA (2013) Occurrence of severe hypotension after indocyanine green injection during the intraoperative period. A A Case Rep. https://doi.org/10.1097/acc.0b013e3182933c12
Chu W, Chennamsetty A, Toroussian R, Lau C (2017) Anaphylactic shock after intravenous administration of indocyanine green during robotic partial nephrectomy. Urol Case Rep. https://doi.org/10.1016/j.eucr.2017.02.006
Procter LD, Davenport DL, Bernard AC, Zwischenberger JB (2010) General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg. https://doi.org/10.1016/j.jamcollsurg.2009.09.034
Buchs NC, Pugin F, Azagury DE et al (2013) Real-time near-infrared fluorescent cholangiography could shorten operative time during robotic single-site cholecystectomy. Surg Endosc. https://doi.org/10.1007/s00464-013-3005-5
Boni L, David G, Mangano A et al (2015) Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. https://doi.org/10.1007/s00464-014-3895-x
Vlek SL, van Dam DA, Rubinstein SM et al (2017) Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc. https://doi.org/10.1007/s00464-016-5318-7
Esposito C, Corcione F, Settimi A et al (2019) Twenty-five year experience with laparoscopic cholecystectomy in the pediatric population—from 10 mm clips to indocyanine green fluorescence technology: long-term results and technical considerations. J Laparoendosc Adv Surg Tech. https://doi.org/10.1089/lap.2019.0254
Ballal M, David G, Willmott S, Corless DJ, Deakin M, Slavin JP (2009) Conversion after laparoscopic cholecystectomy in England. Surg Endosc. https://doi.org/10.1007/s00464-009-0338-1
Genc V, Sulaimanov M, Cipe G et al (2011) What necessitates the conversion to open cholecystectomy? A retrospective analysis of 5164 consecutive laparoscopic operations. Clinics. https://doi.org/10.1590/S1807-59322011000300009
Frank AP, De Souza SR, Palmer BF, Clegg DJ (2019) Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. https://doi.org/10.1194/jlr.R086975
Strasberg SM, Brunt LM (2017) The critical view of safety. Ann Surg. https://doi.org/10.1097/SLA.0000000000002054
Strasberg SM, Brunt LM (2010) Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. https://doi.org/10.1016/j.jamcollsurg.2010.02.053
Schols RM, Connell NJ, Stassen LPS (2015) Near-infrared fluorescence imaging for real-time intraoperative anatomical guidance in minimally invasive surgery: a systematic review of the literature. World J Surg. https://doi.org/10.1007/s00268-014-2911-6
Funding
Funding was provided via a grant from Stryker Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
This study was conducted with a grant from Stryker Corporation. Dr. Santiago Horgan is a consultant for Stryker Corporation, Intuitive Surgical, Fortimedix Surgical, and Medtronic. Dr. Jacobsen receives a teaching honorarium from Gore Medical. Dr. Sandler is a consultant for Intuitive Surgical and Boston Scientific. Drs. Doucet, Broderick, Lee, Zhao, Blitzer, Patel, and Cheverie have no conflicts of interest or financial ties to disclose. Ms. Soltero has no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ryan C. Broderick and Arielle M. Lee have shared co-first authorship.
Rights and permissions
About this article
Cite this article
Broderick, R.C., Lee, A.M., Cheverie, J.N. et al. Fluorescent cholangiography significantly improves patient outcomes for laparoscopic cholecystectomy. Surg Endosc 35, 5729–5739 (2021). https://doi.org/10.1007/s00464-020-08045-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08045-x