Abstract
Purpose
The aim of this study was to determine the changes in the luminal and stromal areas of the choroid in eyes with Vogt-Koyanagi-Harada disease by optical coherence tomography (OCT).
Methods
A retrospective observational study. Choroidal images were recorded by enhanced depth imaging (EDI-OCT) at the baseline, and at 1 week and 1 month after initiating steroid therapy. The EDI-OCT images were converted to binarized images, and the luminal areas and the stromal areas were measured separately.
Results
Thirty-two eyes of 16 patients were enrolled, and 16 eyes of 10 patients had suitable images for the binarization analyses. The ratio of the luminal areas to the choroidal areas was 0.60 ± 0.03 at the baseline, 0.67 ± 0.04 at 1 week, and 0.66 ± 0.04 at 1 month. There was a significant increase from the baseline at 1 week (P < 0.01) but not from 1 week to 1 month. Although both the stromal and luminal areas were reduced, the percent reduction of the stromal areas (56.5 ± 7.2 %) was significantly greater than that of the luminal areas (42.5 ± 12.6 %) at 1 week (P < 0.01).
Conclusions
A significant decrease of the choroidal area was detected in eyes with Vogt-Koyanagi-Harada disease at 1 week after beginning steroid therapy. The decrease was more evident in the stromal area than in the luminal area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vogt-Koyanagi-Harada disease (VKH) is a bilateral, diffuse granulomatous uveitis associated with poliosis, vitiligo, alopecia, and pathological alterations in the central nervous system and auditory system [1]. The incidence and the manifestations of VKH disease vary among different ethnic groups, and it is one of the most common type of uveitis in the Asian populations [1–3]. Although the general prognosis of VKH disease is fair, some of the patients develop complications such as cataracts, glaucoma, and retinal neovascularization due to the disease and/or its treatment [1–10]. Therefore, selecting the optimal treatment is important for the recovery of good vision.
The development of optical coherence tomography (OCT) has enabled clinicians to examine the retinal microstructures in situ. This also holds for the choroid especially after the introduction of enhanced depth imaging OCT (EDI-OCT) [11]. With this technique, it was found that the choroid of eyes with active VKH was markedly thickened, and the thickness decreases rapidly after corticosteroid therapy [12–16]. Thus, the choroidal thickness can be an important marker for the general status of the eye, and it can also act as a marker for the effectiveness of treatment. However, it would be better if data other than the thickness that are quantifiable by OCT could be collected to determine which actual structures are affected by the steroid therapy.
The retina has a well-organized architecture such that it is relatively easy to detect pathologic changes in the retina in the OCT images. The choroid is composed of blood vessels and stroma without a uniform or organized architecture, and it is quite difficult to differentiate structural changes. To overcome this difficulty, we have developed a new method to examine the luminal and stromal areas of the choroid in the OCT images quantitatively [17, 18]. Because this method can be done by an open access software, this technique can be used by any clinician or researcher.
Thus, the purpose of this study was to determine the changes in the luminal and stromal components of the choroid after steroid therapy. To accomplish this, we used a binarization technique and studied the changes in the choroidal structure during the recovery phase of VKH disease. Here, we shall show that while both the luminal and stromal areas were reduced after the steroid therapy, the reduction was greater in the stromal area. The ability to quantify the changes in the choroid should be helpful in determining the effectiveness of a treatment.
Subjects and methods
The procedures of this retrospective study conformed to the tenets of the Declaration of Helsinki. This study was approved by the Ethics Committee of Kagoshima University Hospital (Kagoshima, Japan) and Tokyo Women’s Medical University Hospital, and registered with the University Hospital Medical Network (UMIN) Clinical Trials Registry (Trial No. UMIN000012310; Title: Choroidal structure on OCT images). A detailed protocol is available at; https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000014386&type=summary&language=E. For this type of study, a formal consent was not required. Eyes with VKH disease were selected from the medical records of Kagoshima University Hospital and Tokyo Women’s Medical University Hospital that were examined between January 2012 and December 2013.
The diagnosis of VKH disease was based on the results of our previous studies and published diagnostic criteria [1, 7, 9, 14, 19]. The diagnosis criteria include the presence of bilateral uveitis associated with exudative retinal detachment. In all patients, multiple secondary leaks from the level of the retinal pigment epithelium (RPE) were seen during fluorescein angiography. We excluded patients with lymphoma, other forms of uveitis, and trauma.
The subfoveal choroidal images were obtained by a Heidelberg Spectralis OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany) with the EDI mode. The scans were seven horizontal lines of 30° × 10° that passed through the center of the fovea. The OCT images were collected before, and 1 week and 1 month after initiating steroid therapy. Twenty scans were averaged to improve the signal-to-noise ratio. The subfoveal choroidal thickness (SFCT) was defined as the distance between the outer border of the hyperreflective retinal pigment epithelium (RPE) and the outer border of the choroid beneath the center of the fovea. When the choroidal thickness was greater than 1,000 μm, they were recorded as 1,000 μm that occurred in eyes where the inner scleral border could not be detected in the EDI-OCT images [13].
We describe the procedures of binarization of the choroidal image as below. Briefly, after recording the EDI-OCT images, the best image was selected and displayed on a computer screen. The selection and analyses were made by two masked graders independently (HK and SS). When the two graders determined that the subfoveal choroidal image was clearly distinguishable, the image was deemed acceptable and used for the following analyses. If the determination was split, the images were excluded from the analysis. Then, the binarization of the subfoveal choroidal area in the OCT image was done by a modified Niblack method as previously reported in detail [17, 18]. Next, the OCT image was analyzed by ImageJ (version 1.47; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/). The examined area was selected to be 7,500-μm-wide, and extended from the transitional point of the RPE and disc to the temporal side across the fovea. The light pixels were defined as the interstitial choroid or choroidal stroma, and the dark pixels were defined as the luminal area (Fig. 1).
Enhanced depth imaging optical coherence tomographic (EDI-OCT) image (a) and converted binary image (b) of an eye with Vogt-Koyanagi-Harada disease. The luminal areas (dark areas, asterisk) and stromal areas (sharp) can be seen. The area surrounded by a yellow line was excised, and the dark areas were traced by the Niblack method (c)
The average brightness was set as the minimum value to minimize the noise in the OCT image. Then the image was converted to 8 bits and adjusted by an auto local threshold of Niblack. The binarized image was converted to an RGB image again, and the luminal area was determined using the threshold tool. After adding the data of the distance of each pixel, the total choroidal area, luminal area, and interstitial or stromal area were automatically calculated. The light pixels were defined as the interstitial choroid or choroidal stroma and the dark pixels were defined as the luminal area. A more detailed protocol is described in our earlier report [17, 18].
Statistical analyses was performed on one eye from each patient. When a clear binarized image was obtained from only one eye, it was selected for the analyses. When a clear image was obtained from both eyes, the right eye was selected for the analyses.
Statistical analyses
All statistical analyses were performed with a commercial analytical package (SPSS Statistics 22 for Windows; SPSS, Inc., IBM, Somers, NY, USA). The changes of the visual acuity before and after treatment were compared by Wilcoxon signed-rank test. The differences between the groups of eyes in which the image processing could be done and those that could not be done were compared by Mann-Whitney’s U test or Wilcoxon signed-rank test. The changes in the size of the luminal and stromal areas was compared by Friedman's test followed by repeated measures analysis of variance (ANOVA) followed by Tukey’s post-hoc test. A P value < 0.05 was considered to be statistically significant.
Results
Thirty-two eyes of 16 treatment-naïve VKH patients were studied. The mean age of the patients was 41.4 ± 16.4 years; two were men and 14 were women. The mean visual acuity (VA) at baseline was 0.34 logMAR units. All patients were treated with 16 mg intravenous prednisolone sodium succinate with tapering for about 10 days, or 1,000 mg intravenous methylprednisolone for 3 days followed by oral prednisolone. One month after the treatment, the serous retinal detachment had resolved in all eyes, and the mean visual acuity was significantly improved to -0.04 logMAR units (P < 0.01, Wilcoxon signed-rank test). A small amount of subfoveal fluid, which was detectable by OCT, was noted in 1/16 eyes (6.3 %).
In our study, 16 eyes of 10 cases were judged suitable for the image processing analyses. In the remaining eyes, 16 eyes of 10 cases were judged unsuitable, because the chorioscleral border could not be clearly identified. These unsuitable eyes were used to determine the factors related to the incompletion of binarization. Detailed data of the patients are shown in Table 1.
For the eyes in the binarized group, the total choroidal area was 472 ± 63 × 104 μm2 at the baseline, which was reduced significantly to 242 ± 44 × 104 μm2 at 1 week (P < 0.01 vs baseline) and 217 ± 48 × 104 μm2 at 1 month (P = 0.25 vs 1 week; repeated measures ANOVA followed by Tukey’s post-hoc test; Fig. 2a). The luminal area was 285 ± 32 × 104 μm2 at the baseline, which was reduced significantly to 163 ± 34 × 104 μm2 at 1 week (P < 0.01 vs baseline) and 143 ± 35 × 104 μm2 at 1 month (P = 0.18 vs 1 week; repeated measures ANOVA followed by Tukey’s post-hoc test; Fig. 2b). The stromal area was 188 ± 34 × 104 μm2 at the baseline, which was reduced significantly to 80 ± 13 × 104 μm2 at 1 week (P < 0.01 vs baseline) and 73 ± 14 × 104 μm2 at 1 month (P = 0.61 vs 1 week; repeated measures ANOVA followed by Tukey’s post-hoc test; Fig. 2c).
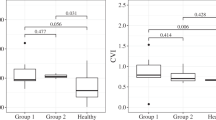
The ratio of the luminal area to the choroidal area was 0.60 ± 0.03 at the baseline, 0.67 ± 0.04 at 1 week, and 0.66 ± 0.04 at 1 month. There was a significant increase in the ratio from the baseline to 1 week (P < 0.01; repeated measures ANOVA followed by Tukey’s post-hoc test; Fig. 3a), but not from 1 week to 1 month. Thus, the ratio of the stromal area was significantly reduced from baseline only at 1 week. For the analysis of one eye, 10 eyes were studied. Eight were right eyes and the average age was 41.0 ± 17.7 years (one man and 9 women). Because the statistical result in the one eye analysis was almost the same as analysis of the results of all eyes, the followings results were those obtained from all the eyes. Detailed data are shown in the Supplementary Data File.
Ratio of luminal to choroidal area of EDI-OCT images after steroid therapy and comparisons of the percent reduction of the luminal and stromal areas relative to the baseline. a The ratio of the luminal to choroidal area increased significantly from the baseline after steroid therapy (**; P < 0.01, repeated measures ANOVA followed by Tukey’s post-hoc test). b The difference in the percentage reduction of the luminal and stromal areas is significant at 1 week and 1 month. (**; P < 0.01, Wilcoxon signed-rank test). Lu = Luminal area; St = Stromal area
The percent reduction of the luminal area from baseline at 1 week was 42.5 ± 12.6 % and that of the stromal area was 56.5 ± 7.2 %. The percentage reductions of the stromal area at 1 week and 1 month were significantly greater than that of the luminal area (P < 0.01, Wilcoxon signed-rank test; Fig. 3b). The percentage reduction of the luminal area from the baseline at 1 month was 48.9 ± 14.5 %, and that of stromal area was 59.7 ± 10.4 %, and this difference was also significant (P < 0.01, Wilcoxon signed-rank test; Fig. 3b).
Examination in the binarized group and the non-binarized group showed that the choroidal thickness at the baseline in the binarized group was 678.8 ± 150.2 μm and that of non-binarized group was 881.5 ± 116.8 μm. The difference was significant (P < 0.01; Mann-Whitney’s U test). On the other hand, the visual acuity at the baseline of the binarized group was 0.22 ± 0.28 logMAR units and that of the non-binarized group was 0.47 ± 0.59 logMAR units (P = 0.69; Mann-Whitney’s U test). The visual acuity at 1 month of the binarized group was -0.03 ± 0.11 logMAR units and that of the non-binarized group was -0.05 ± 0.10 logMAR units (P = 0.76; Mann-Whitney’s U test).
The choroidal thickness of the binarized group was 678.8 ± 150.2 μm at the baseline, which was reduced significantly to 363.3 ± 74.3 μm at 1 week and 307.8 ± 61.3 μm at 1 month (P < 0.01, at 1 week; P = 0.24 at 1 month; repeated measures ANOVA followed by Tukey’s post-hoc test).
Discussion
Our results showed that both the luminal and the stromal areas were increased at the baseline and decreased after the steroid treatment. Earlier histological studies showed that substantial numbers of inflammatory cells had infiltrated into the stroma diffusely in eyes with VKH disease [5, 6, 8]. These infiltrating cells secreted different types of inflammatory molecules such as tumor necrosis factor-α and vascular endothelial growth factor, which then caused stromal edema leading to a thickening of the choroid [20]. Our observations are consistent with these finding of stromal thickening. On the other hand, these cytokines can also upregulate endothelial nitric oxide synthase (eNOS), which can dilate the choroidal vessels [21, 22]. This is also consistent with the enlargement of luminal area in our patients with VKH disease.
At the baseline, the luminal area was about 60 % of the total choroidal cross-sectional area, and it recovered to about 65 % after the treatment. This indicated a substantial enlargement of the choroidal stroma in eyes with VKH disease before the treatment. Our previous study showed that the luminal area was about 65 % of the choroidal cross-sectional area in normal eye so that the ratio of luminal area to the stromal area returned to approximately the normal level after 1 week of steroid treatment [17, 18]. Importantly, the decrease in the stromal area was greater and more rapid than the luminal area from the results of the percent reduction at 1 week compared to baseline. These findings might reflect the structural changes that were previously reported after histological studies [4–6]. However, we do not have any data to prove their correlations. Further study is needed.
Although the OCT findings can provide chronological information of the choroidal changes, only limited evaluations can be made with only the choroidal thickness. The present method is superior because it can provide more qualitative information on the individual elements of the choroid [17, 18].
Histologically, the choroid is composed of blood vessels and stromal tissues. The stromal tissues include pigment cells, smooth muscles, neurons, vascular walls, inflammatory cells, and connective tissue. Unfortunately, they cannot be differentiated even with the most advanced OCT. Therefore, we used the binarization technique to differentiate the vascular (luminal) from the stromal areas. In our previous study, we found that the Niblack was the most suitable method for differentiating the luminal areas and the stromal areas in the EDI-OCT images of the choroid with high reproducibility [17, 18]. Although we do not have definitive evidence that the dark areas represent the vascular areas and the light areas the stromal tissues, the findings of our earlier studies and those of numerous empirical observations strongly suggest that the dark areas were the vascular areas [23]. In addition, a comparison of the original EDI-OCT images to the binary images showed that the dark areas corresponded with the vascular components of the choroid, especially the larger choroidal vessels. A better validation of our binarization technique will require further studies.
We have also shown that the binarization had high reproducibility and repeatability [17, 18]. Because manual segmentation can introduce significant artifacts, the present automatic binarization process seems better. Of note is that the present method does not require any custom-made software, but rather an open-access software, ImageJ. Thus, any researcher can repeat our method, which is a strong advantage of the present method.
There are several limitations of this study. The small numbers of non-randomized cases cannot be free from biases. Above all, the success rate of binarization of EDI-OCT (50 %) at baseline was comparatively low, all of which had highly thickened choroids. The highly thickened choroid was defined as measuring at least 1,000 μm or thicker. In these cases, the outer choroid was not clearly identifiable. Spaide et al. described the maximal reliable choroidal thickness on OCT images as less than 1,000 μm and others cited it as less than 800 μm [13, 14]. Given the fact that most eyes with VKH have a thickened choroid, this would be a technical limitation of this method for VKH eyes. In addition, histological findings of VKH eyes showed many inflammatory cells invading throughout the choroid, and the vascular lumens were occasionally unidentifiable [5, 6, 8]. Although this may be consistent with the present OCT images, these inflammatory cells may cause unknown artifacts in the OCT images. This should be remembered when interpreting the present data.
In conclusion, the EDI-OCT image results showed that the cross-sectional area of the choroid of eyes with VKH disease decreased significantly one week after treatment with steroids. This was most evident in the stromal area. This method is noninvasive and does not require any specific software. A detailed comparison of choroidal findings and clinical observation will be helpful for developing better treatments for VKH.
References
Moorthy RS, Inomata H, Rao NA (1995) Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol 39:265–292
Ohno S (1981) Immunological aspects of Behcet’s and Vogt-Koyanagi Harada’s diseases. Trans Ophthalmol Soc U K 101:335–341
Weisz JM, Holland GN, Roer LN et al (1995) Association between Vogt-Koyanagi-Harada syndrome and HLA-DR1 and -DR4 in Hispanic patients living in southern California. Ophthalmology 102:1012–1015
Ohno S, Char DH, Kimura SJ, O’Connor GR (1977) Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 83:735–740
Inomata H, Sakamoto T (1990) Immunohistochemical studies of Vogt-Koyanagi-Harada disease with sunset sky fundus. Curr Eye Res 9(Suppl):35–40
Sakamoto T, Murata T, Inomata H (1991) Class II major histocompatibility complex on melanocytes of Vogt-Koyanagi-Harada disease. Arch Ophthalmol 109:1270–1274
Sonoda S, Nakao K, Ohba N (1999) Extensive chorioretinal atrophy in Vogt Koyanagi-Harada disease. Jpn J Ophthalmol 43:113–119
Rao NA (2007) Pathology of Vogt-Koyanagi-Harada disease. Int Ophthalmol 27:81–85
Nakao K, Mizushima Y, Abematsu N et al (2009) Anterior ischemic optic neuropathy associated with Vogt-Koyanagi-Harada disease. Graefes Arch Clin Exp Ophthalmol 24:1417–1425
Nakao K, Abematsu N, Mizushima Y, Sakamoto T (2012) Optic disc swelling in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci 53:1917–1922
Margolis R, Spaide RF (2009) A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 147:811–815
Fong AH, Li KK, Wong D (2011) Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi- Harada disease. Reina 31:502–509
Maruko I, Iida T, Sugano Y et al (2011) Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina 31:510–517
Nakai K, Gomi F, Ikuno Y et al (2012) Choroidal observations in Vogt-Koyanagi Harada disease using high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 250:1089–1095
Nakayama M, Keino H, Okada AA et al (2012) Enhanced depth imaging optical coherence tomography of the choroid in Vogt-Koyanagi-Harada disease. Retina 32:2061–2069
da Silva FT, Sakata VM, Nakashima A et al (2013) Enhanced depth imaging optical coherence tomography in long-standing Vogt-Koyanagi-Harada disease. Br J Ophthalmol 97:70–74
Sonoda S, Sakamoto T, Yamashita T et al (2014) Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci 55:3893–3899
Sonoda S, Sakamoto T, Yamashita T et al (2015) Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol 159:1123–1131
Read RW, Holland GN, Rao NA et al (2001) Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: Report of an international committee on nomenclature. Am J Ophthalmol 131:647–652
Oh H, Takagi H, Takagi C et al (1999) The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci 40:1891–1898
Hattenbach LO, Falk B, Nurnberger F et al (2002) Detection of inducible nitric oxide synthase and vascular endothelial growth factor in choroidal neovascular membranes. Ophthalmologica 216:209–214
Ando A, Yang A, Mori K et al (2002) Nitric oxide is proangiogenic in the retina and choroid. J Cell Physiol 191:116–124
Spaide RF (2009) Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol 147:644–652
Acknowledgments
The authors thank Prof. Duco Hamasaki, Miami University, for his critical discussion and editing of the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers’ bureaus, membership, employment, consultancies, stock ownership, or other equity interest, and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Kawano, H., Sonoda, S., Yamashita, T. et al. Relative changes in luminal and stromal areas of choroid determined by binarization of EDI-OCT images in eyes with Vogt-Koyanagi-Harada disease after treatment. Graefes Arch Clin Exp Ophthalmol 254, 421–426 (2016). https://doi.org/10.1007/s00417-016-3283-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3283-4