Abstract
Purpose
To concurrently evaluate the effect of half-fluence photodynamic therapy (hf PDT) on choriocapillaris (CC) perfusion and choroidal structure in chronic central serous chorioretinopathy (CSC).
Methods
This prospective study included 48 eyes of 41 patients with chronic CSC. Enhanced depth imaging optical coherence tomography and optical coherence tomography angiography (OCTA) images were analyzed. Choroidal area (CA), luminal area (LA), and stromal area (SA) were computed using Image J software.
Results
One month after hf-PDT, total CA decreased to 1.312 mm2 from 1.490 mm2 (p < 0.001), LA decreased to 0.981 mm2 from 1.097 mm2 (p < 0.001), and SA decreased to 0.331 mm2 from 0.393 mm2 (p < 0.001). In OCTA, the CC flow in the eyes with CSC (17.75 mm2) was statistically significantly lower than the fellow eyes (18.93 mm2) at the baseline visit (p < 0.001). After hf-PDT, the flow in the choriocapillaris statistically significantly increased to 18.81 mm2 at the first month (p = 0.02).
Conclusions
OCTA proves that after hf-PDT a significant increase in CC perfusion occurred at first month. The decrease of the luminal areas in enhanced depth imaging optical coherence tomography is mainly due to a decrease in large-caliber vessels, which indicates that hf-PDT has an effect on larger choroidal vessels and spares CC flow.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central serous chorioretinopathy (CSC) is a pathology with unknown mechanism characterized by detachment of the neurosensory retina due to the accumulation of serous fluid between the retinal pigment epithelium (RPE) and photoreceptor layers. Acute and chronic forms of the disease have been defined. Acute CSC is generally self-limited with spontaneous regression, and it results in minimal sequelae. In chronic CSC, the long-term persistence (longer than 6 months) of subretinal fluid (SRF) may cause atrophy of the RPE, choroidal neovascularization, cystoid retinal degeneration, and permanent vision loss [1, 2].
Treatment of chronic CSC includes various options, such as modification of the risk factors (discontinuation of steroids) [3], medical treatment (carbonic anhydrase inhibitors, oral eplerenone) [4, 5], conventional focal laser [6], micropulse laser, photodynamic therapy (PDT) [7, 8], and intravitreal injection of vascular endothelial growth factor inhibitors [7, 9]. Until now, the standard therapy for chronic CSC, which would prevent progressive pigment epithelium and photoreceptor damage resulting in persistent visual impairment, has not been discovered [10]. It has been reported that PDT with verteporfin induces the resorption of SRF by reducing choroidal vascular hyperpermeability [11, 12]. However, it has the potential for serious side effects, such as choroidal ischemia, RPE atrophy, and iatrogenic choroidal neovascularization [13, 14]. To avoid these complications, newer PDT protocols, including half-dose [15] and low-fluence [16] applications, have been developed.
The exact mechanism of the action of PDT on the choroid layer in chronic CSC remains unclear. There is a speculation that transient choriocapillaris (CC) occlusion leads to choroidal vascular remodeling and the reduction of choroidal exudation [7, 17, 18]. It has been reported that low-fluence PDT reduces the risk of PDT induced choroidal hypoperfusion without causing significant differences in treatment efficacy compared to standard fluence PDT [19].
Histopathological findings have demonstrated the vascular occlusion at the level of choriocapillaris 1 week after both 50 J/cm2 and 100 J/cm2 PDT [20]. Optical coherence tomography angiography (OCTA) is a novel imaging modality that enables choroidal vascular abnormalities to be detected and CSC to be diagnosed without the need for intravenous contrast injection [21,22,23]. It has been reported that OCTA is able to visualize enlarged CC vessels [22] and choroidal neovascularization (CNV), which is undetectable with other imaging modalities [24]. The correlation of the changes in the choriocapillaris and thickness of the choroidal area and vascular luminal area may enlighten us to understand what part of the choroid is affected the most after half-fluence PDT (hf-PDT) treatment. The aim of the present study is to define the choroidal alterations after hf-PDT in chronic CSC using OCTA and enhanced depth imaging optic coherence tomography (EDI-OCT), and to detect the changes in the luminal and stromal areas using Image J software.
Materials and methods
Patient selection
This prospective interventional study included 48 eyes of 41 patients with chronic CSC. The study was conducted at the Ophthalmology Department of the Ankara University School of Medicine from April 2016 to April 2018. Forty-one patients were enrolled in the study after the following criteria were excluded: symptom duration less than 3 months, previous PDT, evidence of choroidal neovascularization and polypoidal choroidal vasculopathy, high myopia defined as a refractive error (spherical equivalent) < −6.0 diopters or an axial length > 26.5 mm, patients under corticosteroid therapy, patients with other ocular diseases that may affect visual acuity or clinical findings, and patients who had undergone intraocular surgeries, including laser photocoagulation. The diagnosis was based on a fundoscopic examination, fluorescein angiography (FA), indocyanine green angiography (ICGA), EDI-OCT, and OCTA. Approval from the ethics committee of the Ankara University Faculty of Medicine was obtained (14-948-18). The protocol of this study conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all of the participants to perform PDT, take the measurements, and review their medical records.
The primary outcome measure of the study was a change in the choroidal microcirculation after hf-PDT, which was evaluated with OCTA by analyzing the flow area in the choriocapillaris layer. The secondary outcome measures were changes in the total choroidal area, stromal area, luminal area, and lumen to stroma ratio after hf-PDT, which were evaluated with Image J software integrated with SD-OCT.
Study protocol
Patients were examined during a baseline visit and 1, 3, and 6 months after undergoing PDT therapy. At each visit, each participant underwent a complete ophthalmic examination that included best corrected visual acuity (BCVA) with Early Treatment Diabetic Retinopathy Study (ETDRS) charts, slit-lamp biomicroscopy, and a dilated fundus examination. Spectral-domain optical coherence tomography (SD-OCT) with the enhanced depth imaging module (Spectralis®, Heidelberg Engineering Inc., Heidelberg, Germany) and OCTA (Avanti RT Vue XR® with AngioVue® software; Optovue Inc., Fremont, USA) were performed for all patients. All examinations were performed within the same time interval (between 11:00 am and 3:00 pm) to avoid diurnal variations of the choroidal structures. Six patients did not participate in the final visit.
The caliber tool of the Heidelberg Eye Examination software was used on EDI-OCT scans to calculate the inner foveal thickness (IFT; the distance between the internal limiting membrane [ILM] and the external limiting membrane [ELM]), which shows PDT influence on photoreceptors; choroidal thicknesses (distance between the outer border of the RPE and the chorioscleral interface); and the maximum height of the SRF (distance from the outer part of the external limiting membrane to the outer part of the RPE layer).
The binarization of the choroidal area was done with Image J software (version 1.50a; National Institutes of Health, Bethesda, MD, USA). A 3000-μm wide area with margins of 1500 μm nasal and 1500 μm temporal to the fovea was selected. The choroidal area defined from the RPE to the chorioscleral border, and the borders were set manually with the Image J ROI Manager. Three choroidal vessels with lumens > 100 μm were selected by the oval selection tool, and the average reflectivity of the luminal areas was determined. Niblack method was used for the binarization of the choroidal image. Then the image was converted to eight bits and adjusted by the Niblack auto local threshold. The luminal area was determined by using the threshold tool. After adding the distance between the pixels, the choroidal area, luminal area, and stromal area were calculated automatically. The light pixels were accepted as the stromal area, and the dark pixels were accepted as the luminal area.
All of the OCTA scans were performed with AngioVue software of the RTVue XR Avanti (Opto-Vue, Inc., Fremont, CA). This 70,000 Hz (840-nm wavelength) system obtained two repeated B-scans of the same 6 mm ×6 mm area centered on the fovea. The split spectrum amplitude-decorrelation angiography algorithm compares the amplitude fluctuation of the reflected light between the consecutive B-scans. The static background tissue has a low decorrelation value, whereas blood flow has a high decorrelation value, thus blood flow can be distinguished from background tissue. Several OCTA parameters, including the foveal avascular zone (FAZ) area and vessel density in both the superior capillary plexus (SCP) and deep capillary plexus (DCP), were also measured. The choriocapillary layer (from 30 to 60 μm beneath the upper boundary of the RPE) was evaluated by the enface OCT angiogram.
Photodynamic therapy
A half-fluence PDT protocol with Zeiss Visulas 690S PDT Laser System (Zeiss/Humphrey 2003, Jena, Germany) was applied. Verteporfin (Visudyne®; Novartis Europharm Ltd., Horsham, West Sussex, UK) was administrated intravenously at a dose of 6 mg/m2 over 10 min. Fifteen minutes after the start of the infusion, a contact lens (Volk® area centralis) was positioned on the affected eye, and a 689-nm laser was delivered for 83 s at a reduced light dose of 25 J/cm2 and an intensity of 300 mW/cm2. The area where the treatment was applied was chosen based on the hyperfluorescent areas in the midphase of the ICGA, corresponding to the SRF in the OCT and the hyperfluorescent “hot spots” of leakage in the midphase of FA. Greatest linear dimension was determined with reference to the area described above.
Statistical analysis
Descriptive statistics are expressed as mean ± standard deviation and median (min–max) for continuous variables. A Shapiro-Wilk test was performed for all variables to detect departures from a normal distribution. A paired-sample t test and Wilcoxon test were applied to compare the baseline values with each follow-up depending on the normality analyses. The level of significance was established at p < 0.05. The statistical analyses were performed using SPSS (Statistical Package for Social Sciences; version 15.0).
Results
Forty-eight eyes of 41 patients (20% female, 80% male) with a mean age of 46 ± 10.6 (34–74) were analyzed. The current systemic diseases of the patients were systemic hypertension in 7 cases, type A personality in 2 cases, hyperthyroidism in 1 case. The systemic blood pressure of the all hypertensive patients was under control with medical treatment.
The mean duration of symptoms before hf-PDT was 13 ± 6.8 months. Before hf-PDT, 13 patients had undergone anti-vascular endothelial growth factor injections (a mean of 4.3 ± 3.2 injections) and five eyes had received subthreshold micropulse yellow wavelength laser treatment. The last injection had been performed at least 3 months before hf-PDT. The mean hf-PDT spot size in the treated eyes was 3.8 ± 0.7 mm (range, 2.3–5.1). The mean logMAR BCVA before hf-PDT was 0.34 ± 0.30 and improved to 0.25 ± 0.29 at 1 month (p = 0.027), 0.26 ± 0.36 at 3 months (p = 0.081), and 0.27 ± 0.27 at 6 months after treatment (p = 0.017).
The statistical analysis including the data about the comparison of CSC eyes and fellow eyes was performed in 34 patients with unilateral involvement. In the structural OCT, when compared with the fellow eye, central choroidal thickness (CCT) (p = 0.001), total choroidal area (CA) (p = 0.006), luminal area (LA) (p = 0.009), and stromal area (SA) (p = 0.013) were greater, and inner foveal thickness (IFT) (p < 0.001) was lower in the CSC eyes (Table 1). The lumen to stroma ratio did not show any differences between the CSC eye (2.87 ± 0.4) and fellow eye (2.94 ± 0.5) (p = 0.68).
The changes in the structural OCT characteristics after hf-PDT throughout the follow-up period are summarized in Table 2. One month after hf-PDT, central choroidal thickness decreased to 428.29 ± 118.4 μm from those taken at baseline of 476.87 ± 112.9 μm (p < 0.001), total CA decreased to 1.312 ± 0.4 mm2 from 1.490 ± 0.42 (p < 0.001), LA decreased to 0.981 ± 0.3 mm2 from 1.097 ± 0.3 (p < 0.001), and SA decreased to 0.331 ± 0.1 mm2 from 0.393 ± 0.13 (p < 0.001) (Fig. 1). The lumen to stroma ratio (min, 2.83; max, 2.97) was almost the same throughout the entire follow-up period (p > 0.05).
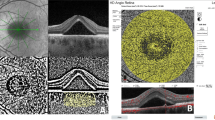
The change in total choroidal area (CA), stromal area (SA), and luminal area (LA) after half-fluence photodynamic therapy calculated with Image J software throughout follow-up period. a Baseline visit (CA = 1.52, SA = 0.43, LA = 1.09 mm2). b 1st month (CA = 1.41, SA = 0.37, LA = 1.04 mm2). c 3rd month (CA = 1.33, SA = 0.36, LA = 0.97 mm2). d 6th month (CA = 1.28, SA = 0.35, LA = 0.93 mm2)
In the OCTA images, when the mean values were compared with the mean values of the fellow eyes (18.93 ± 1.8 mm2), the flow in the choriocapillaris was lower in the eyes with CSC (17.75 ± 2.35 mm2) (p < 0.001) (Table 1). At the baseline visit, the mean values of superficial capillary plexus vessel density, deep capillary plexus vessel density, and foveal avascular zone area did not differ from the fellow eye (p > 0.05). The changes in the OCTA characteristics after hf-PDT throughout the follow-up period are summarized in Table 3. After hf-PDT, the flow in the choriocapillaris statistically significantly increased to 18.81 ± 1.9 mm2 after the first month (p = 0.02) and to 19.85 ± 1.6 mm2 after the sixth month of the therapy (p = 0.04) (Fig. 2). After hf-PDT, the statistical difference in CC flow between the fellow and CSC eye measurements disappeared throughout the follow-up visits (p = 0.47, p = 0.81, and p = 0.18, respectively). Throughout the entire follow-up, the OCTA parameters (except for CC flow) did not show any significant changes (p > 0.05). The boxplot graphics of the changes in total choroidal area, stromal area, luminal area, and CC flow throughout the follow-up period are shown in Fig. 3.
The change in choriocapillaris flow area (mm2) after half-fluence photodynamic therapy in a fovea-centered circle with 6-mm diameter calculated by optical coherence tomography angiography scans throughout follow-up period: a Fellow eye (21.326 mm2). b Baseline visit of CSC eye (21.180 mm2). c 1st month (21.204 mm2). d 6th month (21.920 mm2)
Discussion
To the best of our knowledge, this is the first study that evaluated the effect of hf-PDT on the vascular and structural parameters of the choroid using Image J and OCTA concurrently throughout a six-month period. Changes in the CC flow and the lumen-stroma ratio after hf-PDT treatment were evaluated using OCTA and Image J software, which was integrated on a cross-sectional B-scan of EDI-OCT. According to the results of this study, OCTA proves that hf-PDT does not decrease CC perfusion. The decrease of the luminal areas in EDI-OCT is mainly due to a decrease in large-caliber vessels, which indicates that PDT has an effect on larger choroidal vessels and spares CC flow.
Another controversy about PDT is presently debatable: that of whether or not it can cause severe complications such as RPE atrophy, choroidal neovascularization occurrence, and choroidal ischemia while maintaining positive effects for visual acuity, retinal sensitivity, the absorption of subretinal fluid, and the restoration of retinal architecture. In fact, it has been largely demonstrated that modified PDT protocols may be more effective in avoiding those severe complications [19, 25, 26]. Half-fluence PDT appears to be safe from many studies, but its real mechanism remains inconclusive [27, 28]. OCTA is a novel imaging technique that obtains high-quality images of the retinal and outer choroidal circulation of the choriocapillaris without the need for intravenous contrast injection [21]. This technique might provide additional insight regarding how choroidal vasculature is affected after hf-PDT. In addition, evaluation of the structural changes of the choroid such as the lumen and stroma ratio might bring additional knowledge to our understanding of the pathology of CSC and its treatment.
According to the results of the studies that investigated CC density as evaluated by OCTA in chronic CSC patients, the CC vessel density was reported to be lower in the treated eyes than the fellow eyes. Costanzo et al. reported dark areas corresponding rarefaction of choriocapillary vessel density probably caused by focal compression of choroid from enlarged vessels in the CSC eyes [29]. Rabiolo et al. found that structural and vascular parameters are correlated in CSC and they improve after different treatments [5]. Both hf-PDT and oral eplerenone do not permanently damage the choriocapillaris or other choroidal layers as evaluated by OCTA. Demircan et al. showed that in a limited number of CSC patients, choriocapillaris perfusion seemed to decrease during 3 days following hf-PDT and then returned to normal after 30 days of therapy [30]. Schlötzer-Schrehardt et al. demonstrated selective occlusion of the choriocapillary layer while the deeper and larger choroidal vessels appeared intact with open lumina in the 50 J/cm2 PDT areas after 1 week [20]. Nassisi et al. clearly showed that CC vessel density was significantly decreased after 1 week as compared with baseline, due to a possible short-term impact of PDT on CC perfusion [31]. After 1 month, they found that the CC vessel density was higher than the baseline value, probably because of CC recovery. They believed that even though the subretinal fluid level after 1 week and 1 month of PDT was not different, the CC vessel density was significantly different at that time point. Similar to this study, the CC flow area was not reduced after one, three, and 6 months of therapy compared to the pretreatment level in the present study. Since we did not have data regarding immediate post-treatment results, we could not claim that hf-PDT can decrease CC vessels after treatment and they become normal again. However, we can conclude that it might not cause a deleterious effect on the choriocapillaris even after 6 months of therapy.
Cheng et al. found that the choroidal perfusion (as reflected by the decrease of the ratio of fluorescence) seen in ICGA was significantly decreased at all post-PDT follow-up time points in both groups (P < 0.01) [26]. Similar to this result, even if OCTA proves that hf-PDT does not affect CC perfusion in the long term, the decrease of the luminal areas in EDI-OCT that is mainly due to a decrease in large-caliber vessels was consistent after 6 months of treatment. CCT may not be representative of the entire choroidal vascular network involved in the exudative processes of CSC. These findings could be explained by the decrease in total choroidal area, stromal area, and luminal area evaluated by the Image J software. Our findings indicate that PDT has an effect on larger choroidal vessels and spares CC flow. Bearing in mind that, in CSC, a decrease in the diameters of large dilated vessels need not always be associated with a reduction in choroidal thickness, we believe that further research is required to provide additional details on the correspondence between the variations in vascular choroidal permeability and changes in choroidal thickness.
In conclusion, the present study strengthens the diagnostic value of OCTA in evaluating the choriocapillaris flow of eyes with central serous chorioretinopathy. OCTA proved that after hf-PDT a significant increase in CC perfusion occurred at first month. This data may eliminate the most important concern about the hf-PDT that is the risk of the distortion in the choriocapillaris flow.
References
Piccolino FC, de la Longrais RR, Ravera G, Eandi CM, Ventre L, Abdollahi A, Manea M (2005) The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol 139:87–99. https://doi.org/10.1016/j.ajo.2004.08.037
Wang MS, Sander B, Larsen M (2002) Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol 133:787–793
Levy J, Marcus M, Belfair N, Klemperer I, Lifshitz T (2005) Central serous chorioretinopathy in patients receiving systemic corticosteroid therapy. Can J Ophthalmol 40:217–221. https://doi.org/10.1016/S0008-4182(05)80040-7
Pikkel J, Beiran I, Ophir A, Miller B (2002) Acetazolamide for central serous retinopathy. Ophthalmology 109:1723–1725
Rabiolo A, Zucchiatti I, Marchese A, Baldin G, Sacconi R, Montorio D, Cicinelli MV, Querques L, Bandello F, Querques G, Medscape (2018) Multimodal retinal imaging in central serous chorioretinopathy treated with oral eplerenone or photodynamic therapy. Eye (Lond) 32:55–66. https://doi.org/10.1038/eye.2017.290
Burumcek E, Mudun A, Karacorlu S, Arslan MO (1997) Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology 104:616–622
Bae SH, Heo JW, Kim C, Kim TW, Lee JY, Song SJ, Park TK, Moon SW, Chung H (2011) A randomized pilot study of low-fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol 152:784–792 e782. https://doi.org/10.1016/j.ajo.2011.04.008
Ozmert E, Batioglu F (2009) Fundus autofluorescence before and after photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmologica 223:263–268. https://doi.org/10.1159/000210386.000210386
Lim JW, Ryu SJ, Shin MC (2010) The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy. Korean J Ophthalmol 24:155–158. https://doi.org/10.3341/kjo.2010.24.3.155
Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P (2015) Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev CD011841. https://doi.org/10.1002/14651858.CD011841.pub2
Alkin Z, Perente I, Ozkaya A, Alp D, Agca A, Aygit ED, Korkmaz S, Yazici AT, Demirok A (2014) Comparison of efficacy between low-fluence and half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Clin Ophthalmol 8:685–690. https://doi.org/10.2147/OPTH.S58617.opth-8-685
Silva RM, Ruiz-Moreno JM, Gomez-Ulla F, Montero JA, Gregorio T, Cachulo ML, Pires IA, Cunha-Vaz JG, Murta JN (2013) Photodynamic therapy for chronic central serous chorioretinopathy: a 4-year follow-up study. Retina 33:309–315. https://doi.org/10.1097/IAE.0b013e3182670fbe
Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM (2003) Photodynamic therapy for chronic central serous chorioretinopathy. Retina 23:752–763
Colucciello M (2006) Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina 26:239–242
Chan WM, Lai TY, Lai RY, Liu DT, Lam DS (2008) Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology 115:1756–1765. https://doi.org/10.1016/j.ophtha.2008.04.014.S0161-6420(08)00373-4
Reibaldi M, Boscia F, Avitabile T, Russo A, Cannemi V, Uva MG, Reibaldi A (2009) Low-fluence photodynamic therapy in longstanding chronic central serous chorioretinopathy with foveal and gravitational atrophy. Eur J Ophthalmol 19:154–158
Chan WM, Lam DS, Lai TY, Tam BS, Liu DT, Chan CK (2003) Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 87:1453–1458
Taban M, Boyer DS, Thomas EL (2004) Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol 137:1073–1080. https://doi.org/10.1016/j.ajo.2004.01.043.S000293940400087X
Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S, Sanfilippo M, Russo A, Uva MG, Munno F, Cannemi V, Zagari M, Boscia F (2010) Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol 149:307–315 e302. https://doi.org/10.1016/j.ajo.2009.08.026
Schlotzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U (2002) Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol 240:748–757. https://doi.org/10.1007/s00417-002-0517-4
Bonini Filho MA, de Carlo TE, Ferrara D, Adhi M, Baumal CR, Witkin AJ, Reichel E, Duker JS, Waheed NK (2015) Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. JAMA Ophthalmol 133:899–906. https://doi.org/10.1001/jamaophthalmol.2015.1320
Chan SY, Wang Q, Wei WB, Jonas JB (2016) Optical coherence tomographic angiography in central serous chorioretinopathy. Retina 36:2051–2058. https://doi.org/10.1097/IAE.0000000000001064
Quaranta-El Maftouhi M, El Maftouhi A, Eandi CM (2015) Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. Am J Ophthalmol 160:581–587 e581. https://doi.org/10.1016/j.ajo.2015.06.016
Demirel S, Yanik O, Nalci H, Batioglu F, Ozmert E (2017) The use of optical coherence tomography angiography in pachychoroid spectrum diseases: a concurrent comparison with dye angiography. Graefes Arch Clin Exp Ophthalmol 255:2317–2324. https://doi.org/10.1007/s00417-017-3793-8
Chan WM, Lai TY, Lai RY, Tang EW, Liu DT, Lam DS (2008) Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina 28:85–93. https://doi.org/10.1097/IAE.0b013e318156777f
Cheng CK, Chang CK, Peng CH (2017) Comparison of photodynamic therapy using half-dose of verteporfin or half-fluence of laser light for the treatment of chronic central serous chorioretinopathy. Retina 37:325–333. https://doi.org/10.1097/IAE.0000000000001138
Nicolo M, Eandi CM, Alovisi C, Grignolo FM, Traverso CE, Musetti D, Cardillo Piccolino F (2014) Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol 157:1033–1037. https://doi.org/10.1016/j.ajo.2014.01.022
Ozmert E, Demirel S, Yanik O, Batioglu F (2016) Low-fluence photodynamic therapy versus subthreshold micropulse yellow wavelength laser in the treatment of chronic central serous chorioretinopathy. J Ophthalmol 2016:3513794. https://doi.org/10.1155/2016/3513794
Costanzo E, Cohen SY, Miere A, Querques G, Capuano V, Semoun O, El Ameen A, Oubraham H, Souied EH (2015) Optical coherence tomography angiography in central serous chorioretinopathy. J Ophthalmol 2015:134783. https://doi.org/10.1155/2015/134783
Demircan A, Yesilkaya C, Alkin Z (2018) Early choriocapillaris changes after half-fluence photodynamic therapy in chronic central serous chorioretinopathy evaluated by optical coherence tomography angiography: preliminary results. Photodiagn Photodyn Ther 21:375–378. https://doi.org/10.1016/j.pdpdt.2018.01.015
Nassisi M, Lavia C, Alovisi C, Musso L, Eandi CM (2017) Short-term choriocapillaris changes in patients with central serous chorioretinopathy after half-dose photodynamic therapy. Int J Mol Sci 18. https://doi.org/10.3390/ijms18112468
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demirel, S., Özcan, G., Yanık, Ö. et al. Vascular and structural alterations of the choroid evaluated by optical coherence tomography angiography and optical coherence tomography after half-fluence photodynamic therapy in chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 257, 905–912 (2019). https://doi.org/10.1007/s00417-018-04226-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-04226-6