Abstract
Purpose
Neuroblastoma is a biological, genetic and morphological heterogeneous tumor with a variable clinical course. NCAM is a cell adhesion molecule belonging to the immunoglobulin superfamily with structural similarities to cell adhesion molecule L1. The aim of this study was to determine the expression of NCAM in neuroblastoma and to compare the results to the findings of a previous study which examined L1 expression in the same group of patients.
Materials and methods
NCAM expression was investigated on a tissue array with 66 surgically resected neuroblastoma samples by immunohistochemistry with a monoclonal antibody clone 1B6 and peroxidase method.
Results
Strong expression of NCAM was detected in all of the 66 (100%) neuroblastoma tumors in contrast to L1 which was not expressed in all tumors.
Conclusion
In contrast to L1, which was found to predict favorable outcome, NCAM is universally expressed in neuroblastoma. Therefore NCAM represents a marker for neuroblastomas irrespectively of their stages whereas L1 as an indicator for developing neuronal cells seems to identify more mature stages of this tumor. The high grade of NCAM expression might present a prerequisite for establishment of antibody-based therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
NCAM (CD56) is a cell adhesion molecule structurally belonging to the immunoglobulin superfamily. The extracellular part of NCAM consists of five Ig like domains and two fibronectin type III like domains [1]. NCAM is expressed on nearly 100% of rhabdomyosarcoma, small cell lung cancer and brain tumors [2–4]. Therefore subsequent observations in various human cancers as well as in several experimental models have led to the hypothesis that NCAM plays also an important role in tumor progression.
In neuroblastoma, which is a tumor with origin from neural crest cells, known as the most common solid tumor in childhood outside of the central nervous system, there are also reports describing a high expression of NCAM in neuroblastoma cell lines [5, 6]. Neuroblastoma exhibits high expression of NCAM that is frequently associated with cancer progression. In this tumor type the role of NCAM would be consistent with that of a tumor-promoting factor [7].
Furthermore, it was described that a peculiar property of NCAM in neuroblastoma cells is the inhibition of tumor cell adhesion to the endothelium [8]. This plays a role in the acquisition of chemoresistance by neuroblastoma cells and the ability of these cells to disseminate to distant organs [9].
Another member of the immunoglobulin superfamily with similarities to the molecular structure of NCAM is L1 (CD171). Several studies have also shown a high expression of L1 in different tumor types [10, 11]. The presence of L1 was found to be associated with poor prognosis [12]. Remarkably on neural cells, the L1 cell adhesion molecule is found to be co-expressed with NCAM. Also in neuroblastic cells these neural cell adhesions molecules have been found to interact functionally [17].
The aim of our study was to evaluate the expression of NCAM in neuroblastomas by immunohistochemistry on a multi-tissue-array and compare the pattern of expression to previously described data of L1 expression in the same tissue array.
Materials and methods
Children and clinical data
The Ethics Committee of the Chamber of Physicians in Hamburg, Germany approved the study. Written informed consent was obtained from all parents for the use of the resected samples from their children for research purposes. A total of 66 surgically resected neuroblastomas treated at the University Medical Center Hamburg Eppendorf between November 1999 and October 2004 were chosen retrospectively. No preselection was performed. All data including the International Neuroblastoma Staging System (INSS), stage, histological grade (according to Hughes), age at diagnosis, Myc-n amplification and loss of heterozygosity chromosome 1p (LOH p1) were obtained from the clinical and pathological records.
Tissue microarray and NCAM immunohistochemistry
Resected neuroblastoma tissues were fixed and embedded in paraffin. After preselection, tissue cylinders with a diameter of 600 μm were punched out of the native paraffin tumor block and arrayed on a new paraffin block with a precision instrument.
For immunohistochemical staining, 3-μm thick sections were placed on precoated slides with 3-triethoxysilylpropylamin (Merck, Darmstadt Germany), deparaffinized in xylol for 1 h and washed in series of decreasing alcohol concentrations. For heat induced epitope retrieval, sections were heated in a microwave oven for 20 min using citrate buffer (pH 6.0). The sections were then incubated with the monoclonal antibody CD 56 (clone 1B6, 1:50 Novocastra®) in a DAKO autostainer using a Dako envision kit (goat anti-mouse/rabbitK5007, Dako, Hamburg, Germany) for 25 min. Antigen localization was performed with the avidin biotin method and 3,3′-diaminobenzidine (DAB) as chromogen. Sections were then counterstained with hematoxylin and dehydrated with concentrations. For each sample one slide, a control section, was incubated with irrelevant murine monoclonal IgG1 (MOPC21; Sigma) as a negative control to determine unspecific binding. Staining Specimens were considered immunopositive for CD 56 when >20% of the tumor cells had clear evidence of immunostaining. Immunohistochemical analysis and scoring was performed by three independent investigators (T. R., J. T. K. and R. W.).
Results
Characteristics of the patients
A total of 66 children suffering from neuroblastoma were included in this study. Median follow-up time of all children included for survival analysis (n = 66) was 72 month. Of the 66 children included retrospectively in our study, 12 (18%) experienced relapse and 8 (12%) children died as a result of tumor disease.
Median age at diagnosis was 30 month. A total of 25 children (38%) were younger than 1 year at diagnosis and 41 children (62%) were older than 1 year at diagnosis. Characteristics were summarized in Table 1. MYC-N amplification was positive in nine cases (14%). LOH p1 was observed in 12 children (21%). A total of 37 (57%) were located adrenal, and 28 (43%) occurred on other locations.
NCAM expression in neuroblastoma
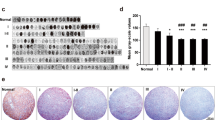
Of the 66 neuroblastomas all tumors (100%) showed intense staining with the CD 56 antibody. As control staining indicated positive results in brain tissue whereas in placenta no staining was observed (data not shown). The NCAM expression patterns of neuroblastoma are presented in Fig. 1. Due to the unique intense staining pattern no correlation between NCAM expression and clinicopathological characteristics of the children was found.
Discussion
NCAMs are a family of closely related glycoproteins described as being involved in cell to cell interactions during growth and development of the nervous system [13].
Based on our finding on a neuroblastoma tissue micro-array we could show a universal NCAM expression. Due to this strong expression NCAM may establish to an interesting candidate in the development of an antibody based therapy for neuroblastoma. Our finding is much similar to that reported by Winter et al. [14] who also examined NCAM protein expression in a neuroblastic tumor tissue micro-array. Due to this, our finding confirms other studies, which detected high degree of NCAM expressions in neuroblastic cell lines [5]. The fact that all tumors in this series were characterized by strong NCAM expression indicated that NCAM might be a promising diagnostic marker for neuroblastoma irrespectively of its clinical features.
These results are in contrast to a former reported study of L1 expression assessed to the same tumor series. In correlation to existing clinical data of the children we observed that L1 expression was associated with favorable outcome [15]. Interestingly L1 (CD 171) is also a member of the immunoglobulin superfamily with similarities of the molecular structure [16]. Furthermore in neural cells, L1 is generally found to be co-expressed with N-CAM [17].
Based on our observations of the different expression patterns of these cell adhesion molecules in this limited number of tumors NCAM and L1 might play different roles in this tumor biology. Hypothetically, L1 seems to be a marker for developing neuroblastic cells to identify more mature stages of neuroblastic cells and is associated with less aggressive tumor cell behavior whereas NCAM might play a role as universal marker of neuroblastomas due to its strong and common expression in this tumor.
In summary, our finding shows that NCAM is highly expressed in neuroblastoma irrespectively of its biological behavior. This stresses the importance of this adhesion molecule as a diagnostic marker for detection of neuroblastic cells. Furthermore, the strong expression is a prerequisite for the establishment of antibody-based immunotherapies in neuroblastoma.
References
Prag S, Lepekhin EA, Kolkova K, Hartmann-Petersen R, Kawa A, Walmod PS, Belman V, Gallagher HC, Berezin V, Bock E, Petersen N (2002) NCAM regulates cell motility. J Cell Sci 115:283–292
Bourne SP, Patel K, Walsh F, Popham CJ, Coakaham HB, Kemshead JT (1991) A monoclonal antibody raised against retinoblastoma, that recognizes the neural cell adhesion molecule (NCAM) expressed on brain and tumours arising from the neuroectoderm. J Neurooncol 10:111–119
Hirano T, Hirohashi S, Kunii T, Noguchi M, Shimosato Y, Hayata Y (1989) Quantitative distribution of cluster 1 small cell lung cancer antigen in cancerous and non cancerous tissues, cultured cells and sera. Jpn J Cancer Res 80:348–355
Molenaar WM, Muntinghe FL (1998) Expression of neural cell adhesions molecules and neurofilament protein isoforms in skeletal muscle tumors. Hum Pathol 29:1290–1293
Phimister E, Kiely F, Kemshead JT, Patel K (1991) Expression of neural cell adhesion isoforms in neuroblastoma. J Clin Pathol 44:580–585
Mechtersheimer G, Staudter M, Moller P (1991) Expression of the natural killer cell-associated antigens CD 56 and CD 57 in human neural and striated muscle cells and in their tumors. Cancer Res 51:1300–1307
Zecchini S and Cavallaro U (2008) Neural cell adhesion molecule in cancer: expression and mechanisms. Neurochem Res Mar 6
Blaheta RA, Hundemer M, Mayer G, Vogel JU, Kornhuber B, Cinatl J, Markus BH, Driever PH, Cinatl J Jr (2002) Expression level of neural cell adhesion molecule (NCAM) inversely correlates with the ability of neuroblastoma cells to adhere to endothelium in vitro. Cell Commun Adhes 9:131–147
Blaheta RA, Daher FH, Michaelis M, Hasenberg C, Weich EM, Jonas D, Kotchetkov R, Doerr HW, Cinatl J Jr (2006) Chemoresistance induces enhanced adhesion and transendothelial penetration of neuroblastoma cells by down-regulating NCAM surface expression. BMC Cancer 6:294
Huszar M, Moldenhauer G, Gschwend V, Ben-Arie A, Altevogt P, Fogel M (2006) Expression profile analysis in multiple human tumors indentifies L1 as a molecular marker for differential diagnosis and targeted therapy. Hum Pathol 37:1000–1008
Kaifi J, Reichelt U, Quaas A, Schurr P, Wachowiak R, Yekebas E, Strate T, Pantel K, Schachner M, Sauter G, Izbicki JR (2007) L1 is associated with micrometastatic spread and poor outcome in colorectal carcinoma. Mod Pathol 20:1183–1190
Vogel M, Gutwein P, Merchtesheimer SRiedle S, Stoeck A, Smirnov A, Edler L, Ben Arie A, Huszar M, Altevogt P (2003) L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 362:869–875
Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM (1987) Neural cell adhesion molecule structure, immunoglobuline-like domains, cell surface modulation, and alternative RNA splicing. Science 236:799–806
Winter C, Pawel B, Seiser E, Huaqing Z, Raabe E, Wang Q, Judkins AR, Attiyeh E, Maris MJ (2008) Neural cell adhesion molecule (NCAM) isoforms expression is associated with neuroblastoma differentiation status. Pediatr Blood Cancer 51:10–16
Wachowiak R, Fiegel H, Kaifi J, Quaas A, Krickhahn A, Schurr P, Ertmann R, Schachner M, Kluth D, Sauter G, Izbicki JR (2007) L1 is associated with favourable outcome in neuroblastomas in contrast to adult tumors. Ann Surg Oncol 14:3575–3580
Moos M, Tacke R, Scherer H, Teplow D, Früh K, Schachner M (1988) Neural adhesion molecule L1 as a member of the immunglobulin superfamily with binding domains similar to fibronectin. Nature 334:701–703
Kadmon G, Kowitz A, Altevogt P, Schachner M (1990) The neural Cell adhesion molecule N-CAM enhances L1-dependent cell–cell interactions. J Cell Biol 110:193–208
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wachowiak, R., Rawnaq, T., Metzger, R. et al. Universal expression of cell adhesion molecule NCAM in neuroblastoma in contrast to L1: implications for different roles in tumor biology of neuroblastoma?. Pediatr Surg Int 24, 1361–1364 (2008). https://doi.org/10.1007/s00383-008-2264-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-008-2264-z