Abstract
Neural cell adhesion molecule, an integrated molecule of immunoglobulin protein superfamily involved in cell-cell adhesion, undergoes various structural modifications through numerous temporal-spatial regulations that generously alter their expressions on cell surfaces. These varied expression patterns are mostly envisioned in the morphogenesis and innervations of different human organs and systems. The considerable role of NCAM in neurite growth, brain development and etc. and its altered expression of NCAM in proliferating tumour cells and metastasis of various human melanomas clearly substantiate its appropriateness as a cell surface marker for diagnosis and potential target for several therapeutic moieties. This characteristic behaviour of NCAM is confined to its novel biochemistry, structural properties, signalling interactions and polysialylation. In particular, the characteristic expressions of NCAM are mainly attributed by its polysialylation, a post-translational modification that attaches polysialyl groups to the NCAM. The altered expression of NCAM on cell surface develops curiosity amidst pharmaceutical scientists, which drives them to understand its role of such expressions in various human melanomas and to elucidate the promising therapeutic strategies that are currently available to target NCAM appositely. Therefore, this review article is articulated with an insight on the altered expressions of NCAM, the clinical significances and the consequences of such atypical expression patterns in various human organs and systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cell adhesion molecule (CAM) belongs to either of the two major families, viz., cadherin family or immunoglobulin (Ig) superfamily. The neural cell adhesion molecule (NCAM), a member of the Ig family, finds clinical significance as a stem cell marker to detect various human melanomas [1]. NCAM or CD56 is recognized either as a homophilic, Ca2+-independent, or a heterophilic, Ca2+-dependent membrane-bound glycoprotein [2]. NCAM activates the G-protein in neural tissues and opens the voltage-dependent calcium channel that eventuates in an increased intracellular calcium concentration [3]. NCAM-mediated cellular interactions were either a homophilic or heterophilic interaction, depending on the intracellular calcium concentration. Homophilic interaction is a divalent cation-dependent cellular interaction that involves binding of NCAM with another NCAM present on the opposing cell surface. Heterophilic interaction is a divalent cation-dependent cellular interaction that involves the binding of NCAM with other molecules such as fibroblasts present on the cell surface [4]. The adhesion of cell-to-cell and cell-to-matrix components was regulated through the polysialylation of NCAM, wherein the polysialylation highly alters the binding ability of NCAM.
Depending upon the alternate splicing in the C-terminal domains, the NCAM isoforms were grouped under three main classes: a glycosylphosphatidyl inositol linked Mr 120,000 (NCAM 120), transmembrane Mr 140,000 (NCAM 140) and transmembrane Mr 180,000 (NCAM 180). Among these isoforms, NCAM 140 and NCAM 180 are expressed in neural and non-neural tissues, while NCAM 120 was expressed by glial cells in various tissues of adults. Apart from these three important isoforms, NCAM also exists either as the soluble form or as the form cleaved by metalloproteinases [5]. Extracellularly, the NCAM isoforms comprise five N-terminal Ig-like domains and two fibronectins type III (FN III) repetitive, followed by a transmembrane domain in NCAM 140, NCAM 180 isoforms and a glycol-phosphatidyl inositol (GPI)-linked cell membrane in NCAM 120. A unique post-translational modification of NCAM is enabled through polysialylation that adds the polysialic acid, an α-2,8-linked sialic acid homopolymer linearly at the fifth Ig-like domain of NCAM, [6]. The first Ig-like CAM isolated and characterized was NCAM, whose functional significance yet remains unclear [7, 8]. In normal humans, NCAM and its polysialylated form (PSA–NCAM) play a significant role in the architect of the developing tissues, neurite outgrowth, axon guidance, intracellular signal transductions, cell migration and stabilization of synaptic contacts, and in the contact-dependent inhibition of cell growth Guan and etc. [8, 9]. In the adult human brain, the neuronal plasticity associated with learning and memory was mainly attributed by the downregulation of the elevated PSA–NCAM expression on the cell surfaces of the adult brain cells [4, 10]. Though NCAM is prominently expressed in the central nervous system of the mammalian, they are also expressed on the epithelial cells of various organs, muscles, pancreatic β cells and some non-neuronal cells [11]. These NCAM and PSA–NCAM expressions were controlled by regulating the interaction of NCAM/PSA–NCAM with the fibroblast growth factor receptor (FGFR) Raf1 kinase; Src family tyrosine kinases Fyn, FAK [12,13,14] and numerous neurotrophic receptors, viz., brain-derived, glial cell line-derived and platelet-derived neurotrophic factors [15,16,17]. The levels of NCAM and PSA–NCAM expression on the cell surface should be strictly regulated so as to maintain them in a balanced state. The balanced levels of NCAM and PSA–NCAM expressions on the cell surface must be conserved, as they usually function opposite [18]. In the remodelling of neuronal circuits, NCAM functions to stabilize the intracellular signal transduction and synaptic contacts, while the polysialylated NCAM functions oppositely, i.e. destabilize the intracellular signal transduction and synaptic contacts [10].
The NCAM1 gene was localized in chromosome 11 of humans, chromosome 9 of mice and chromosome 8 of rats. The NCAM1 gene in mice comprises 20 major exons and 6 small exons. In various carcinoma cells, NCAM expression results in the transformation of Mr 120,000 isoform to Mr 140,000 and Mr 180,000 isoforms [19]. Considering to this perspective of NCAM expression in cancer cells, the NCAM expression in colon cancer, pancreatic cancer and astrocytoma was downregulated, while they were upregulated in neuroblastoma and in certain neuroendocrine tumours that are characterized with an extensive polysialylated NCAM [20]. The soluble form of NCAM is produced as a result of the enzymatic processing of the extracellular domain at the cell membrane and detachment of NCAM containing membrane fragments. The progression or clinical state of many diseases depends on the levels of soluble NCAM, which highly varies in the brain tissue, cerebrospinal fluid and serum of a diseased patient. Patients with haematopoietic disorders were characterized with an elevated level of soluble NCAM in their cerebrospinal fluid samples [21, 22].
NCAM undergoes an extensive altered expression in different regions of human system, and these atypical expressions bring forth numerous significant biological events that promote neurological development, tumorigenesis and other related disorders [23]. A detailed review of NCAM expression with a thorough elucidation on the significance and consequences of such varied expression pattern will definitely throw limelight in the efforts of pharmaceutical scientists in devising a successful pharmacological strategy to target the NCAM effectively in the alleviation of cancer and many other related diseases. The review further simplifies the drug design and drug discovery process by providing the quite essential knowledge required in the development of novel therapeutic entities to target NCAM appropriately.
NCAM and PSA–NCAM’s Normal Physiological Role in the Human System
NCAM, a homophilic cell adhesion molecule, plays a prominent role in neurogenesis and is widely expressed in the developing myotubes, motor axons, Schwann cells and both pre-synaptic and post-synaptic regions of a neuromuscular junction [24]. In foetal kidneys, the metanephrogenic mesenchyme cell prominently expressed the NCAM and in the adult kidneys, only the nerves and none of the other cells expressed the NCAM. Immuno-fluorescent staining technique substantiates the prominent expression of NCAM in the undifferentiated mesenchyme cells of the kidney during the tubule formation and a complete loss of NCAM expression in the fully differentiated and polarized cells [25]. In smooth muscles, the NCAM expressions were transient and the monoclonal antibody 3F4-based staining technique detected, an elevated level of NCAM expressions on the entire surface of the cultured aortic smooth muscle lines, compared to their expression in the cardiac and skeletal muscle [26]. The expression of NCAM-140 isoform on the embryonic lens tissue differentiating cells marks to be an essential event in the development of eye [27]. NCAM not only recognizes the appropriate interacting cells based on their adhesive preferences but also regulates the bio-communications between the cell membranes. The level of expression of NCAM on the nerve and muscle cells determines the nerve–nerve and nerve–muscle interactions. NCAM-mediated myoblast interactions were envisioned as a result of the adhesion of NCAM with other molecules such as N-cadherin, in the muscles, during the formation of multi-nucleated cells [28]. The varied expression patterns along with the functional role of NCAM are given in Fig. 1.
The immunohistochemical localization of NCAM in the pontine migratory stream located beneath the pia mater was examined in the brain of foetal rats using the specific monoclonal antibody for the rat NCAM, MAb-AF11 [29]. NCAM was expressed at a weak gradient mode in the pontine migratory stream, as evinced by greater NCAM-immunoreactivity in the anterior part where the neuron resides than in the posterior part. This weak NCAM gradient favours the formation of the pontine cell strand and the basal pontine grey matter. In the normal human colon muscles, the NCAM expression pattern was observed in the inner border of the circular muscle [30].
The surgical transection of the hypophyseal stalk revealed the disappearance of PSA rather than the NCAM in the pituicytes, the resident astrocytes of the neurohypophysis confirming the role of PSA–NCAM in the cell plasticity. The polysialylated form of NCAM, PSA–NCAM, plays a significant role in the hippocampus plasticity and the presence of PSA, and protects the cells against kainite-induced cell death due to heat shock. PSA–NCAM assists in heat shock preconditioning-induced neuroprotection, and hyperthermia upregulates the polysialylation of NCAM which induces complex molecular cascade that contributes neuroprotection [31].
NCAM widely involves in the development of brain, neurocognitive functions of the brain and development of adult inner ears. The peripheral fibres of the auditory epithelium expressed the polysialylated form of NCAM more extensively. The polysialylated form of NCAM plays a major role in the processing of auditory information and in the maintenance of neuronal plasticity [32].
NCAM plays a pivotal role in the axonal growth and fasciculation of the hippocampus in the aged brains. The neuronal plasticity of an adult human brain is significantly conserved by the NCAM. Generally, the NCAM expression was elevated in the developing brain of infants, whereas a 20-fold reduction in the expression of NCAM was ensued in a well-developed adult brain. The muscle differentiation and their repair mechanisms largely depend upon the level of NCAM expression [33]. NCAM mechanistically controls the μ-opioid receptor of the embryonic carcinoma cells. NCAM activates the genes involved in the development of the mammalian nervous system and the activation occurs via the pathway that involves phospholipase C-arachidonic acid-calcium channel/calmodulin kinase II [34].
NCAM/PSA–NCAM play an active role in the process of learning and memory during stress. NCAM and PSA–NCAM expressions were critically involved in the mechanism of learning and their expression patterns were highly modulated during stress. The effects of stress were facilitated at an elevated level and were impaired at reduced levels of the NCAM/PSA–NCAM expressions [35].
In the adult brains, the expression of NCAM disappears but an extensively polysialylated form of NCAM occurs in the superficial laminae of the dorsal horn and in the lateral spinal nucleus. In the adult brain, the extensively polysialylated form of NCAM, PSA–NCAM, was deployed during the processing of somatic and visceral information. The pathology and other related consequences in the brain were mainly associated with either the downregulation or the upregulation of NCAM expression. In Alzheimer’s disease, the olfactory bulb and mossy fibre system of the hippocampus were formed, as a consequence of an enhanced synaptic remodelling and neuronal sprouting in the brain due to the increased NCAM [36]. Cellular therapy targeting the mesenchymal stem cells was facilitated via NCAM-mediated migration and the mechanism leading such migrations depended on the interplay between NCAM and FGFR that induces activation of MAPK/ERK signalling pathway [37].
In the new-born mice, the polysialylated NCAM in the rostral migratory stream facilitated the tangential cell migration of the sub-ependymal cells. The NCAM expression favours an activity-induced plasticity with a simultaneous regulation and maintenance of the mossy fibre system in the hippocampus [38]. The corticosterone hormone involves in the upregulation of the PSA–NCAM expression and as a facilitator in the attachment of PSA with NCAM. During the early stage of angiogenesis in the developing quail brain embryo, the PSA–NCAM-expressed pericytes were found around the capillaries in the parenchyma and these pericytes were assumed to be derived from the middle and posterior cerebral artery [39].
After a severe injury in the brain and spinal cord, an upregulated NCAM expression was observed in the innervations of the brain and sciatic nerves. Post-injury, a decreasing gradient of NCAM expression levels was ensued at the transaction sites, with an increased level in the brain followed by a decreased level in the spinal cord, substantiating an over-expression of NCAM at the injured site that plays crucial role in the endogenous process of spinal cord regeneration. The presence of PSA–NCAM expressions in the medial prefrontal cortex of the rat confirmed that the sites exhibiting PSA–NCAM expressions in the central nervous system were mainly involved in the structural remodelling of the hypothalamus, regulation of the neurite outgrowth and regulation of the synaptogenesis [40].
The atypical bio-physical properties of PSA regulate the cell interactions in an unconstructive way. Throughout the embryonic and adult neurogenesis, various events such as axonal growth, neurite outgrowth and fasciculation, cell migration, synaptic activity-induced plasticity and neuronal glial-induced plasticity, facilitating the neuronal plasticity of the developing nervous system, were all regulated through the modified PSA–NCAM expressions. During the embryonic to adult neurogenesis, an altered structural plasticity that benefits the developing nerve to accustom with the non-permissive environment of the matured nervous tissue was attained due to the diverse morphological and molecular changes undergone at the neurogenic sites; and the extent of these variations was analogous to the extent of variations expressed by the PSA–NCAM [41]. PSA–NCAM expression in the embryonic and adult neurogenesis of rat thalamus was investigated using the light and microscopic immuno-cytochemical technique. The study reports confirmed the selective expression of PSA–NCAM in the neurons of the developing thalamus, its participation in the cell migration and synaptogenesis in the cerebral cortex. At all the examined ages, the immuno-cytochemical technique labelled with cytoskeletal and membrane markers identified the presence of PSA–NCAM staining mainly along the neuronal plasma membrane and the absence of such staining in the astrocytes of the developing rat thalamus. Immunoreactivity was dense and homogeneously distributed during the prenatal period that gradually and selectively decreased throughout the adulthood that finally confines only to the reticular nucleus, ventral lateral geniculate nucleus and midline including the intralaminar nuclei. Neurons containing distinct calcium binding proteins expressed PSA–NCAM differently with the PSA–NCAM labelling being more intense around calretinin-positive neurons and of lesser in few of the calbindin-immunoreactive sites [42].
Post-natal neurogenesis predicted a decreasing gradient of PSA–NCAM expression in several regions of rat telencephalons throughout their embryonic to adulthood, authenticating its involvement in the maintenance of neuronal structural plasticity. The PSA–NCAM expressions were downregulated in the dentate gyrus and piriform cortex layer II during the ageing of adult rats [43]. At the initial stage of angiogenesis, PSA–NCAM-expressed pericytes were found around the capillaries and in the parenchymatous tissue of the developing quail brain embryo and their origin was believed the middle and posterior cerebral artery [38, 39]. The opposing functional nature of PSA and NCAM plays a pivotal role in the rapid or chronic neurotransmission while NCAM-180 isoform plays an essential role in the controlled and repetitive release of neuro-transmitters [44].
The post-mortem analysis of the dissected amygdala of the Schizophrenia, bipolar and highly depressed patients showed a reduced level of PSA–NCAM expression in the basolateral and biomedial amygdala of depressed patients and an elevated level, in the lateral nucleus of bipolar patients [43]. The extra-synaptic N-methyl-D-aspartate receptors (NMDARs), an inhibitor that involves in the development of long-term potentiating neuro degeneration, were suppressed by the PSA–NCAM expressions. The reelin molecule secreted by Cajal-Retzius cells in the marginal zone of the prenatal neocortex activates the extra-synaptic NMDARs that significantly induces the synaptic plasticity and cell survival [45].
Expression of NCAM in Various Cancer Types
Gastrointestinal Cancer
The expression patterns of NCAM in gastric cancer, colorectal cancer, pancreatic cancer, chronic pancreatitis and colon adenoma were compared, and the study infers a non-correlation between the tumour stage and NCAM levels in colorectal and pancreatic cancer [2] (Fig. 2).
The existence of NCAM-180 in the colon serves to be a good prognostic factor for colorectal carcinoma (CRC). NCAM-180 expressions were prominent in the epithelium of normal colon and benign colon tumours but were completely absent in aggressive colon carcinoma. NCAM-180 expressions were completely absent in the epithelial villous tips of the colon of a CRC patient [46]. The absence of NCAM-180 expression in aggressive CRC cells resulted in impaired intracellular adhesion. NCAM-180’s potential as a tumour suppressor in CRC was confirmed because, thanks to its cellular adhesive property, it reinforces and maintains the epithelial integrity of colon cells, thereby protecting them against tumour cell invasion and metastasis. Numerous molecular biologists have articulated many comprehensive reviews that illustrate the phenomenal role of NCAM in the oesophageal, pancreatic and colon cancer.
Oesophageal Cancer
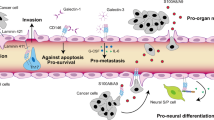
The unique components of tumorogenetic microenvironment (TME) such as proliferative tumour cells, tumour stroma, blood vessels, infiltrating inflammatory cells, and various associated tissue cells interact with the hosts and result in various cellular and molecular interactions that promotes the spread of tumour cells in the proximal tissues. In solid tumours, the most abundant immune cells of the TME, macrophage, actively participate in the angiogenesis, tumour cell invasion and intravasation at the primary site. At metastatic site, macrophage creates a supportive metastatic niche either through the blocking of immune-mediated clearance or by prompting the pro-survival signalling pathways, which promotes the extravasations and survival of aggressive tumour cells [47].
The development of novel agents to dysfunction the macrophages at their primary and metastatic sites evolves out to be a promising strategy that could improve the survival rate in cancer patients. The two distinct macrophage phenotypes M1 and M2 were termed based on their response to interferon gamma (IFNγ) and lipo-polysaccharides (LPS) or to IL-4 and IL-13, respectively. M1 and M2 were opposite in its functions; M1 phenotype is associated with production of pro-inflammatory cytokines, viz., IL-12, IFNγ and TNFα, antigen presentation, generation of reactive oxygen species and its ability to eliminate pathogens and cells while M2 phenotype is associated with the production of anti-inflammatory cytokines, viz., IL-10, upregulation of scavenging receptors and tissue remodelling. Macrophages propitiating in the surrounding TME, termed as tumour-associated macrophages (TAM), induce metastasis prominently in the TME. Many novel therapeutic agents that target TAM to prevent tumour progression and metastasis were in discovery pipeline. Tumour-associated macrophages are characterized with an elevated interleukin IL-10, elevated expression of CD-163, CD-204 and a reduced IL-12. A high degree of TAM infiltration in tumour tissues has been associated with poor prognosis in many cancers [48].
Many scientific reports cited the infiltrating CD-204 macrophage in the oesophageal squamous cell carcinoma (ESCC) tissue as a significant clinical pathological factor, which ensured its suitability as a prognostic marker for the oesophageal cancer [49]. In vitro experimental studies conducted on conditioned media of ESCC cell lines in bone marrow-derived peripheral blood monocyte-derived macrophages induced greatly M2-like genes than the macrophage-like cells, viz., IL-10, CD163, CD204, VEGFA, MMP2 and MMP9. A cDNA microarray between macrophage-like cells treated with and without TECM analysed the upregulated genes including cysteine-rich angiogenic inducer 61 (Cyr 61) and growth differentiation factor 15 (GDF15); the analysis authenticated the existence of a significant positive correlation between the upregulated genes Cyr61 and GDF 15 with infiltrating CD204 TAMs in human ESCC samples [50].
An elevated level of NCAM in TE-9 suggests the involvement of NCAM in the migration of TAM-like cells and macrophage-like cells. The TE series of human ESCC cell line (TE-8, TE-9 and TE-15) significantly induced NCAM mRNA and protein expression than the macrophage-like cells and these expressions are morphologically characterized in the segment of lamellipodia that comprise branched F-actin (phalloidin) filaments. The migration and survival of TAM-like cell TE-9 were significantly decreased due to NCAM silencing. The modulation in the phosphorylated protein levels due to NCAM knock-down was assessed through phosphor-kinase antibody array. NCAM silencing in TAM-like cells suppressed phosphokinase-Akt (Thr308) and p70S6K (Thr421/Ser424) which ensue in the inhibition of the P13K-Akt signalling pathway in macrophage-like cells. NCAM mRNA and protein expression induced by the TE series-human ESCC cell line (TE-8, TE-9 and TE-15) activates the PI3K-Akt signalling pathway in the macrophage-like cells which promoted the cellular invasion and metastasis of the melanoma cells [51]. In the macrophage-like cells, the macrophage colony-stimulating factor (M-CSF) was stimulated to induce the formation of lamellipodia, which enhances the cell migration and chemotaxis.
NCAM, a non-conventional ligand for FGFR1, interacts with FGFR1 which eventually recycles the FGFR1 for NCAM-induced sustained activation of various effectors that prevents the ubiquitination of proteins and protein degradation. The tumour progression was enhanced by activating the downstream signalling factors such as P13K-Akt and ERk1/2, via the NCAM-mediated FGFR1 cellular interactions. In TAM-like cells, the NCAM expressions synergized with the FGFR1 expressions and its phosphorylation. The upregulation of NCAM and FGFR1 expressions induced the macrophages to acquire M2-like characters and supported the migration and survival of TAM-like cells in the TME of ESCC. FGF-2 or basic fibroblast growth factor, a member of FGF family, is expressed in normal cells as well as in macrophages (Fig. 3A). Among the four FGF-2 isoforms, the low molecular weight isoform was characterized with autocrine, paracrine and intracrine effects. FGF-2 facilitates the healing of wound by stimulating the chemotaxis which in turn triggers the proliferation of endothelial cells via Erk1/2 signal. The autocrine, paracrine and intracrine growth factor FGF-2-induced FGFR1 signalling was confirmed in various types of tumours. Advanced, relapsed or refractory cancers were better subdued by inhibiting the FGF-2-mediated FGFR1 signal. The FGF-2/FGFR1 intracrine and paracrine loops in TAM are activated by NCAM expression generated by FGFR1 and upregulation of FGF-2 in stromal cells, which quickly disseminates the cancer cells [52].
Varied expression patterns of NCAM causing cancer progression in oesophageal, pancreatic and colon cancer types. A In non-cancerous oesophagus, there is no significant expression of NCAM; this inhibits P13K-Akt signalling pathway which is important in promoting the metastasis of melanoma cells. However, upregulation of NCAM at cancerous stage is induced by variety of humoral factors, leading to the action of P13K-Akt signalling pathway. This eventually increases the survival and migration rate of TAMs, causing the advanced stage of oesophageal cancer. B In normal pancreatic cells, NCAM is upregulated. Mutation in K-Ras leads to the expression of PSA-NCAM which defines them as highly malignant and reasons out for 90% of pancreatic adenocarcinoma. Thus, an extremely low-level expression of soluble NCAM resulting in increased migration of the carcinoma cells is the characteristic feature of pancreatic cancer. C NCAM is upregulated in normal colonic cells. Impaired alternative mRNA splicing i.e. defect in the splice site that exists between the two FN III domains of NCAM leads to the attachment of PSA. This results in the downregulation of soluble form of NCAM and secondary invasion of cancerous cells. Complete loss of NCAM leads to malignant form of colon cancer
Pancreatic Cancer
Pancreatic cancer or pancreatic ductal adenocarcinoma ranks to be the fourth most common lethal cancer in the USA [53]. Molecular-based characterization of pancreatic cancer yet remains unclear [54], whereas an unambiguous characterization is achieved by studies that are based on the genetic and protein expression profiles of the samples withdrawn from the pancreatic cancer patients. The highly vulnerable population who easily contracts to pancreatic cancer were the smokers, aged people, male gender, obese men and men with familial history of pancreatic cancer and diabetics. CD44+, CD24+, CD133+, epithelial surface antigens and etc. were used as cell surface marker or stem cell marker to detect pancreatic cancer and CD56, a cell surface marker, also aids in the diagnosis of pancreatic cancer.
In chronic pancreatitis and pancreatic cancer, the NCAM expression was significantly lesser, compared to its expression in other cancers. An extremely low-level expression of the soluble NCAM form was characterized in both patients, manifested with either pancreatic neoplasm or non-carcinoma pancreatic diseases. The main source of serum NCAM being the pancreas; in pancreatic cancer, the serum NCAM was significantly low that prompted the destruction of the nearby normal tissues [2]. In pancreatic cancer, the serum NCAM concentration was less compared to that predicted in control subjects, lung adenocarcinoma, chronic pancreatitis and extra pancreatic diseases. Patients manifested with stages III and IV pancreatic cancer were characterized with a decreased serum NCAM concentration compared to that of patients who were in their early stage of pancreatic cancer. Thus, the poor survival of patients at advanced stage of pancreatic cancer can be corroborated to the decreased serum NCAM concentration.
Neurotropism, the important characteristic of pancreatic cancer, mainly associates with the neural invasion. The homophilic adhesion molecules of NCAM were expressed on nerve cells as a result of neurotropism. In a study, 66.7% pancreatic cancer patients expressed NCAM, and the level of expression of the polysialylated form of NCAM, PSA–NCAM, defines them as highly malignant [21].
The mutation of K-Ras (the individual Ras has three isoforms: K, H and N form, found in the epithelial-mesenchymal transition and is poorly characterized in E-cadherin-mediated cellular adhesion) leads to the expression of PSA–NCAM and reduced E-cadherin-mediated cellular adhesion that reasons out for 90% pancreatic adenocarcinoma. The structural resemblance of PSA–NCAM and E-cadherin adhesion complex causes decreased cell-to-cell aggregation that enhanced the tumour cell migration in pancreatic carcinoma. An increase in the E-cadherin-mediated cell–cell aggregation and reduced cell migration of pancreatic carcinoma cells was facilitated by removing PSA from NCAM or by reducing the expressions of PSA–NCAM and E-cadherin-mediated cell adhesions (Fig. 3B) [55].
Colon Cancer
Globally, colon cancer or colorectal cancer (CRC) or bowel cancer accounts to be the second most widely spread melanoma among women (614,000 cases per annum) and the third most among men (746,000 cases per annum). Investigational reports have affirmed the high incidence of CRC in well-developed nations (737,000 cases per year) than that of the developing nations (624,000 cases per year). On gender basis, CRC was found to be highly prevalent among the males than the females [56].
The role of NCAM expressions in normal human colon cells and its participation as tumour suppressor in CRC needs to be investigated and established. In normal humans, NCAM-180 isoform is expressed at the basolateral surface membrane of the colonic epithelial cell villous tips and is mostly confined to the simple tubular glands of the colonic upper villous and crypts. The apical portion of the colon cells was characterized with colonic cell epithelial adhesion (CEA) molecules. NCAM structurally resembles to the tumour suppressor, deleted in colon carcinoma (DCC) that are located at the basolateral surface membrane of the goblet cells of the colon. The tumour progression that sequences from adenoma to carcinoma envisages the loss of the adhesion molecule DCC. The homophilic interactions of NCAM involve the binding of colon cells among themselves and/or with NCAM. The binding of NCAM with the matrix molecule heparin/heparin sulphate reinforces the docking of colon cells with the basal lamina. NCAM-180 expression was prominent in benign tumour, which gradually decreases in basolateral distribution and becomes totally absent in aggressive CRC, and this sequence of NCAM-180 distribution evinces the role of NCAM-180 as a potent tumour suppressor. The interaction of NCAM 180 with an actin cross-linking protein spectrin supports the plasma membrane by strongly binding the cells to their proximal cells and/or to the cells of basal lamina. The invasion, detachment, dissemination, implantation and secondary invasion of carcinoma cells were all promoted due to the reduced NCAM expression. The reduced NCAM expression enhances the cell migration by disrupting the homophilic binding and the tissue architecture [57].
In malignant tumours, the loss of NCAM expression is ensued due to impaired alternative mRNA splicing, which suggests both the alternative mRNA splicing and NCAM are inter-related functionally. In tumours, the normal alternative splicing mechanism is disrupted, i.e. intron exsiccation becomes defective, and also the selected splice site gets altered (Fig. 3C). In malignant tumours, the loss of NCAM was due to the defect in the splice site, i.e. the space between exon 12 and 13 that exists between the two FN III domains [58]. The expression rate of NCAM in different cancer types is given in Fig. 4.
The dissemination of colon carcinoma cells was facilitated by reducing the tumour cell adhesions through the polysialylation of NCAM. The onco-developing antigen, PSA–NCAM, facilitates the detachment of carcinoma cells from the primary tumour site, which promoted the spread of malignant cells and metastasis. The non-polysialylated NCAM diminishes tumour progression and the malignancy of tumour is inversely correlated to the levels of expression of non-polysialylated NCAM. NCAM-180 expressions were comparatively less in aggressive CRC than that expressed in the epithelial villous tips of normal human colon. Based on all of the above discussions, we can concur that “the down-regulation in NCAM molecule, serves to be poor prognosis of colorectal cancer” [59]. A recent study reported the bioconjugation of CD56-targeting antibody to monomethyl auristatin E, a cytotoxic drug. The results revealed a significant reduction of tumour growth in an MCC mouse model and proving its potential as a therapeutic target [60].
Thyroid Cancer
Thyroid cancer, a common endocrine malignancy, is the fifth most common cancer among the women in the USA [61, 62]. In Thiruvananthapuram (India), the highest frequency of thyroid cancer was reported in 5.71% females and 1.99% males. The incidence of thyroid cancer is three times greater in female population than that in the male population. The exact cause for thyroid cancer is unknown, but certain risk factors associated are high exposure to radiation and the familial thyroid syndrome. Primary thyroid cancer originates from the thyroid follicular cells, and the mechanism of tumorigenesis in thyroid remains subtle due to genetic alteration or environmental factors. Cancer stem cell for thyroid cancer do exists and their characteristics were of mostly unknown [63].
NCAM or CD56, a multi-valent adhesion molecule, mediates cell–cell and cell–matrix component adhesions via homophilic binding and via numerous heterophilic interactions with other molecules respectively. In cancer patients, the tumour progression was potentiated by the reduced NCAM expressions. Immunohistological studies of normal thyroid glands authenticated the presence of NCAM in the thyroid follicular cells. In benign thyroid tumour, the absence of NCAM expression facilitated the tumour metastasis [64]. In vascular system, the circulating cancer cell stimulates the growth factor that alters the angiogenesis and lymphangiogenesis (lymphatic vessel growth) which in consequence, promotes cell migration and metastasis of tumours. In a recent study, researchers reported the binging of Natein, a novel peptide ligand to human CD56 using T7 phage display technology. The binding of Natein to natural killer (NK) cells and CD56-positive (CD56+) cancer cells was demonstrated using biotinylated Natein-conjugated microbeads. The results revealed the potential of Natein as alternative to CD56 antibody in peptide-based cell isolation and diagnosis [65]. Another study reported on anti-CD56 antibody-conjugated Fe3O4 nanoparticles revealed the target-specific binding with NK-92 cells in vivo under external magnetic field guidance [66].
The tumour cells in a mouse model simulated with loss of NCAM produced an increased VEGF-C and VEGF-D that favours peri-tumoral neolymphangiogenesis and metastasis of the tumour-drained lymph node. The less dense tumour-drained lymphatic vessels expressed NCAM at reduced level that consequently decreased the production of VEGF-D. Distant metastasis in well-differentiated thyroid carcinoma remarked as the most unfavourable complication of thyroid cancer, which accounts to be a major cause of morbidity and mortality in such patients. The distant metastasis comprises of primary tumour size, extra thyroidal extension and nodal metastasis. Currently, no valid molecular predictor of metastasis of thyroid carcinoma exists in clinical practices. Reduced NCAM expression is frequently diagnosed in malignant thyroid tumours especially in papillary carcinoma [67]. In the NCAM-silenced TPC-1 of thyroid papillary carcinoma cell line, a significant decrease in the expression of both VEGF-C and VEGF-D mRNAs was accounted due to the downregulation of NCAM expressions (Fig. 5A). The NCAM-silenced TPC-1 cells were highly adhesive to different extracellular matrix components that accounts for its poor role in the migration and invasion of tumour cells [68].
Varied expression patterns of NCAM causing cancer progression in thyroid cancer, Wilm’s tumour and ovarian cancer. A Normal thyroid glands contain NCAM in the thyroid follicular cells. In cancer patients, tumour progression is potentiated by reduced NCAM expression, which leads to increased production of VEGF-C and VEGF-D. This resulted in distant metastasis, the most unfavourable complication of thyroid cancer and a major cause for morbidity. B Wilm’s tumour originated from the remnants of immature kidney tissue. During nephrogenesis of an embryonic kidney, the presence of PSA-NCAM is found. However, it is completely absent in an adult kidney and re-expression of PSA-NCAM is observed on the surface of Wilm’s tumour cells. C The expression of NCAM is completely absent in healthy ovarian epithelial surface. But during the onset and late stage of epithelial ovarian cancer, NCAM is upregulated through FGFR signalling. This interaction resulting in the post-translational modification of NCAM is resulting in the formation of PSA-NCAM, reducing the hemophilic cellular adhesion properties of NCAM. Thus, the NCAM/FGFR interplay is causing cancer cell migration and invasion
Neuroblastoma
Neuroblastoma, an extra-cranial solid tumour, was progressive in the undeveloped cells of the sympathetic nerve ganglia and is envisioned as invading cancerous cells into the regions innervated with sympathetic nervous system. The neural crest cell lineages of the neurons, Schwann cells, melanocytes and osteoblasts act as a precursor cell for neuroblastoma. The malignant embryonic neuroblastoma most commonly occurs in children aged below 15 [69]. Among all paediatric tumours prevailing, 8–10% of the reported cases were of neuroblastoma and the fatality encountered due to neuroblastoma was reported to be of 15%.
The significant role of NCAM in neuroblastoma was substantiated based on the investigational reports of PSA–NCAM expressions examined at various histological grades and clinical stages of both childhood ganglioneuroma and neuroblastoma. During embryogenesis, the neuronal development was significantly regulated by the PSA–NCAM expression and during post-natal development, the regulation of PSA–NCAM expression was confined to few regions that are actively involved in the development of neuroplasticity and neural tissue regeneration. The PSA–NCAM expression was elevated in undifferentiated neuroblastoma or in aggressive cancers and is either reduced or completely lost in differentiated tumours and at the early clinical stages of neuroblastoma [70].
The polysialyl transferase expression pattern was studied using human neuroblastoma cell line, SH-SY5Y. The polysialyl transferase expression pattern depicts attachment of two dissimilar polysialyltransferases, ST8SiaII and ST8SiaIV, to the polysialylated NCAM and these two polysialyltransferases synthesize the PSA that attaches with NCAM [71].
In neuroblastoma, NCAM inhibits the tumour cell adhesion with endothelium, which enables the neuroblastoma cells to acquire chemo-resistance that favours the dissemination of neuroblastoma cells to distal organs. The strong NCAM expression in neuroblastoma cells appropriates NCAM as universal marker and as tumour-promoting factor for neuroblastoma [72].
Various adhesion molecules, viz., cadherins, integrins, selectins and several cell adhesion molecules, play an important role in cancer metastasis. Researchers focused on NCAM because it is homologous to the tumour suppressor IGSF4 among the different adhesion molecules. Neuroblastoma patients are diagnosed with haematogenous and lymphatic metastasis. The therapeutic cure rate of neuroblastoma directly relates to the occurrence/lack of the haematogenous metastasis and on the efficacy of the cytoreductive therapy that are capable to lessen the metastasis. A real-time RT-PCR quantitative test was done to investigate the NCAM mRNA developmental expression pattern in the human brain of different ages. The researchers detected an elevated level of expression of NCAM-120 mRNA isoform in immature foetal brain cells and a comparatively lesser level in matured adult brain cells. NCAM-120 KDa isoform was highly expressed in ganglioneuroblastoma and NCAM-180 KDa isoform was highly expressed in neuroblastoma while neither the ganglioneuroblastoma nor the neuroblastoma expressed NCAM-140 KDa isoform. The invasion of the neuroblastoma cells was facilitated by the post-translational modification of the GPI content in the NCAM and its interaction with other factors in the extracellular matrix [69].
Wilm’s Tumour
Wilm’s tumour or nephroblastoma is a paediatric renal malignancy that is initiated in the kidneys of children mostly [73] and is supposed to originate from the remnants of an immature kidney tissue. The histology of Wilm’s tumour indicated cells to be of metanephrogenic origin, as their structure resembled to the metanephron developed during the embryogenesis of kidney. Few of the differentiation stage involved in the development of metanephron had shown the presence of PSA–NCAM. The PSA–NCAM expression was prominent during the nephrogenesis of an embryonic kidney and was completely absent in an adult kidney (Fig. 5B). Re-expressions of PSA–NCAM were observed on the surface of Wilm’s tumour cells [74].
The three major types of Wilm’s tumour (WT) were categorized based on their gene expression: blastemal, epithelial and stromal. Immunodeficit mice propagated with WT patient-derived xenografts show WT, which closely resembled to the blastemal type at its advanced clinical stage. Localization of WT cancer stem cells in the blastema and its association with the tumour malignancy corroborates for a higher blastemal type of WT, which might ensue due to the loss of well-differentiated tubular epithelial structure and/or because of an increased NCAM+ ALDH+ cancer stem cell marker in the blastemal subsets [75].
The role of renal stem cells as progenitor and their task in maintaining the cancer cells were proven by the succeeding findings. Cancer stem cells (CSC) are highly differentiating and self-renewing cells, which propagates at an increased capacity to form metastases that are highly resistant to chemotherapy. These cancer stem cells reside as renal stem cells inside the nephrogenic blastema. The fluorescence-activated cell sorting (FACS) analysis test depicted the presence of various surface antigens in the stem cells, viz., haematopoietic, CD133, CD34, c-Kit; mesenchymal, CD105, CD90, CD44; cancer, CD133, MDR1; hESC, CD24; and putative renal cadherin 11. The different levels of expressions of these stem cells were detected and the expressions of CD133, FZD7 and NCAM were identified in the stem cell fractions. The FACS study data authenticated that the CSC expressions were due to NCAM, CD133 and the cell death solely due to FZD7. The RT-PCR analytical reports predicted an increased level of NCAM expression in the CSC than in any other adhesion molecules studied. The study further confirmed the association of NCAM in the over-expression of both WT stemness gene and the topoisomerase (TOP2A). TOP2A is employed as a tumour marker in detecting the WT [76].
NCAM acts as a specific biomarker to target the CSC population. A retrospective cross-sectional study was done to validate NCAM as specific biomarker to target the CSC population. Forty-six patients experiencing WT were diagnosed for CD133 and CD56 for a period of 17 years (i.e. from 1999 to 2015). The study data predicts that over 34 patients (73.9%) were positive for CD133 and 39 patients (84.8%) were positive for CD56. The survival chance and the clinical stages of WT patients were predicted by employing CD133, CD56, as prognostic factors. The therapy based on CD133, CD56 as targets completely destroyed WT cells in CSC population and also minimized the chance of recurrent tumours [77].
Gall Bladder Cancer
Gall bladder cancer (GBC), a rare biliary tract malignancy, was ranked as the sixth most lethal melanoma among all the other prevailing gastrointestinal cancers. Globally, the incident rate of GBC was determined to be as less than 2 individuals per 100,000 populations. Currently, no scientific data exists that discusses about the targeted therapies, adapted to treat this orphan disease [78], since their incidence happens to be a most uncommon one. Perineural invasion (PNI) substantiates as one of the major prognostic factors in several malignant tumours. The significance of PNI and its correlation to NCAM expression was studied and the reports indicated a less common PNI in GBC, compared to that of a bile duct cancer. Based on the study report, it was concluded that PNI does not have any significant role in the dissemination and in the prognosis of GBC. In gall bladder cancer, the direct invasion of cancerous cells into the lymphatic and veins was mainly considered instead of PNI even though the perineural invasion had been specifically influenced by the NCAM expression. As of now, no active research works were pursued to establish the role of NCAM in GBC; therefore, it is necessary to pursue an erudite, comprehensive research work that establishes a successful NCAM-based targeted therapeutic approach in the alleviation of GBC.
Ovarian Cancer
Among the various ovarian cancers that prevail, the epithelial ovarian cancer (EOC) accounts to be the most lethal gynaecological malignancy in women. NCAM expressions were completely absent in the normal ovarian epithelial cell surfaces, whereas in the EOC cells, a significant level of NCAM expression was reported. The mechanism of proliferation of tumour cells in EOC via the FGFR signalling moiety was investigated [79]. The study affirmed NCAM expression in the early stage of EOC was more specific to the transformed ovarian epithelial cell surfaces and not to that of pre-neoplastic lesions. NCAM expressions in the advanced stage of EOC play an essential role in the promotion of cell migration and in the invasion of the phenotype EOC cells. The NCAM expression was upregulated at the invasive front of neoplastic lesion in EOC cells and was reduced in pancreatic cancer and CRC; the overall reduced expression of NCAM is correlated to the higher malignancy rate [80]. The interplay of NCAM/FGFR in Schwann cell growth and their deployment in tumour cell migration and invasions was studied [24, 81]. The post-translational modification of NCAM isoforms by the addition of polysialylic acid decreased the homophilic cellular interactions of NCAM and results in reduced cell adhesive properties that promotes the cell migration and invasion [24]. The study clearly instigates on the NCAM-mediated stimulation of FGFR function being responsible for enhanced cell migration and invasion in aggressive EOC (Fig. 5C). Bombardelli et al. have described the deployment of immunoglobulin-like cell adhesion molecules as signalling molecules in the progression of EOC [82]. The FGFR-mediated cell migration was confirmed in many non-neural cells, albeit several study reports authenticate the neuronal development via the combined interactions of L1 CAM and NCAM with FGFR. An increase in the progression of EOC malignancy was indicated by the combined involvement of EGFR and FGFR [83].
Small Lung Cell Cancer
The WHO reports of 2018 proclaim lung cancer as the most common cancer, with 20% of the prevalence being the small cell lung cancer (SCLC) and the remaining 80% prevalence being the non-small cell lung cancer (NSCLC). The potential role of NCAM in the early metastasis of SCLC was well documented in previous research publications. The SCLC cell line NCI-H446 grown as floating clusters expressed a higher surface expression of NCAM, while the same cell line grown as adherent cultures expressed comparatively lesser surface expression of NCAM.
The response of SCLC patients characterized with varying NCAM expressions to chemotherapy was evaluated. SCLC patients characterized with an elevated NCAM expression responded poorly to chemotherapy and were also manifested with distant metastasis of liver, bone and bone marrow. The treatment outcome of chemotherapy in SCLC patients advocates the suitability of NCAM as a diagnostic marker to detect SCLC. A similar study was done to evaluate the varied expression of NCAM and PSA–NCAM in neuroendocrine lung tumours. The study was based on the hypothesis, PSA–NCAM due to its reduced adhesion properties favours the metastasis in the embryonic state of neuroendocrine lung tumours. Comparative to the typical and atypical carcinoids, a significant and more frequent PSA–NCAM expression (70%) was observed in aggressive neuroendocrine tumour [84, 85].
The NCAM expressions were prominent in almost all the cases (100%) reported of small cell lung cancers [86] and about 80% of the test samples detected NCAM expressions positively. Immunotoxins are the conjugated product of toxins with cell binding antibodies that are intended to target-specific cells. Immunotoxins are the commonly employed active substance in anti-NCAM immunotoxin therapy. N901-br immunotoxin was prepared by conjugating the monoclonal antibody N901 with the blocked ricin B toxin. N901-br immunotoxin reduces the non-specific binding of NCAM with the endothelial cells through the chemical modifications ensued at the galactose binding site by blocking the ricin B toxin. The humanized variant of murine N901 antibody was developed to reduce the immunogenicity in humans. N901-br immunotoxins were modified as huN901-DMI by replacing ricin B with a better cytotoxic drug mayatansine. The novel anti-NCAM-mayatansine huN901-DMI was found to be more specific in reducing the NCAM expression in SCLC.
Borght et al. studied the specific expression of exon 18 of NCAM in both SCLC and normal healthy subjects. The study predicted the specific expression of exon 18 of NCAM in 80% of the SCLC biopsy samples and none in the normal healthy controls. This prediction substantiates the potentiality of NCAM exon 18 as a tumour marker and their suitability as diagnostic aid in the detection of SCLC [87].
Uveal Melanoma
Uveal melanoma, a rare and deadly intra-ocular tumour in adults, was primarily reported in the Caucasian population. Eighty-three percent of ocular melanomas rise from the uvea with choroid as the most common site [88]. Antibody-based immunohistochemical staining of the NCAM was done to examine the distribution of all three NCAM isoforms in the primary and metastatic stages of uveal melanoma. NCAM was stained using a polyclonal antibody that recognizes all three NCAM isoforms and a monoclonal antibody MAb Leu-7 to recognize the HNk-1 epitope of the cell binding domain of few NCAM isoforms [89]. An elevated level of NCAM expression was reported in an aggressive, rapid metastatic tumour. The study report further confirmed the involvement of NCAM isoforms that lacks HNk-1 epitope in the organ-specific metastatic behaviour of uveal melanomas.
Salivary Gland Cancer
Around 10–15% of the primary salivary gland malignancies were characterized to be of adenoid cystic carcinoma. Several research works authenticating the role of NCAM in numerous melanomas including pancreatic cancer and gall bladder cancer had been well documented, but only very less number of research works relating to salivary gland cancer is published until date. Shang et al. evaluated the potentiality of NCAM as a prognostic biomarker and also detected the correlation of NCAM with PNI of salivary gland in adenoid cystic carcinoma. The study identified 86% of positive NCAM patients with PNI, which proves the involvement of NCAM expression in the potential spread of adenoid cystic carcinoma along the nerves. Furthermore, the study strongly indicated the existence of novel relationship between the lymph node metastases and NCAM expression with PNI [90].
Ewing Sarcoma
Ewing’s sarcoma (ES), a systemic disease, characterized as progressive tumours occurs most commonly in the bone marrow and less often in the soft tissues of the chest, abdomen, limbs or other locations. Ewing’s sarcoma is the second most common bone cancer that occurs in children and in young adolescents. Poor survival rate of ES patients was mainly due to the poor prognosis and failed outcome in the preliminary diagnosis for the disease, while the relapse conditions was accounted as a less common factor. ES, a highly malignant tumour, is picturized as minute circular cells of neuroectodermal origin that affects the soft tissue and bone. Tumour metastasis is manifested as one of the major prognostic factors in ES patients [91]. Nearly 25% of ES patients studied were characterized with a major adverse effect, tumour metastasis. The other additional adverse effects characterized included poor response, i.e. less than 90% necrosis in the definitive surgery cases. In spite of multimodal therapy, the tumour recurred in one-third of the affected population. Many of the early-stage ES patients indicated micro-metastasis [92]. The circulating cancerous cells of the bone marrow and the malignant cells in peripheral blood samples collected were effectively detected even at traceable levels using the reverse transcriptase-polymerase chain reactor technique (RT-PCR) and/or a highly sensitive technique, the flow cytometry. The flow cytometry was highly reliable and sensitive in detecting even the minimal residual diseased cells of haematopoietic neoplasm. The levels of NCAM expression were significantly related to the progression of disease. Negative or reduced NCAM expressions were reported in the surviving patients of non-progressive tumours. CD56 expression helps in the tailoring of personalized therapy to patients manifested of ES detected through excellent prognosis or ES patients predisposed with relapse.
Ameloblastoma
The NCAM expression of odontogenic cells in ameloblastoma was analysed by an indirect immunoperoxidase and immuno-fluorescent techniques [93]. The histopathological observation of the tumour nests, i.e. the irregular ameloblastoma cell islands, depicted denser central cells alike stellate reticulum, surrounded peripherally by columnar cells alike normal ameloblasts. The CD56 antigens are well expressed in the non-collagenous stromal component, peripheral to the tumour nests, rather than the central stellate reticulum cells. Few investigators identified that the basic structure of teeth and periodontal tissues was remarkably influenced by the altered expressions of NCAM occurring at various stages of tooth development.
Astrocytic Tumours
Sasaki et al. examined the expression profile of NCAM in the brain of normal human, as well as in the patients with astrocytic brain tumour, and this comparative study established an inverse correlation of NCAM expression with the malignancy of astrocytic tumours. The reports of the comparative study further authenticated the downregulation of NCAM in the development of malignant astrocytic tumours [94].
Perineural Invasion of Head and Neck Cancer
The head and neck cancers were commonly manifested with squamous cell carcinoma (SCC) that is associated with high perineural invasion (PNI). The NCAM expression aids as a significant tool in predicting the PNI of head and neck cancers. Vural et al. confirmed the NCAM expression in 93% of cases with PNI and 36% of cases without PNI [95]. The presence of NCAM was preferably identified by the monoclonal IgG antibody-based immunoperoxidase staining rather than the haematoxylin and eosin-based histopathological examination. The NCAM expressions on Schwann cells and neurons were highly prominent in PNI cases than without PNI cases [96]. An elucidative study to understand the underlying mechanism of NCAM expression on Schwann cells in PNI would be of immense value in clarifying the role of NCAM in squamous cell carcinoma [97].
Malignant Mesothelioma
Neural cell adhesion molecules (NCAM) are often expressed in malignant mesotheliomas (MM) cells, which are of epithelioid type. 123C3 antibody experiment was conducted to assess the NCAM expression in both normal mesothelium and malignant mesothelioma cells. The NCAM expression was reported in 19 of 26 malignant mesothelioma cells assessed. The experimental outcome clearly established the participation of NCAM in the development of malignancy with NCAM expression being significant in mesoderm during the embryonic MM and constant in biphasic tumours. The data further instigates that the elevated expression of NCAM in MM exceeded the expression levels observed in lung adenomas [98].
Pituitary Tumour
The reduced expression of NCAM in prolactinomas, in comparison to their expression in other hypophyseal tumours, advocates their suitability as a diagnostic tool in the prognosis of pituitary tumour. Immuno-blotting technique established the complete absence of NCAM140 isoform in prolactinomas and the prominent presence of NCAM140 isoform in normal human adenohypophysis, growth hormone adenomas and inactive adenomas [99]. The NCAM1 gene was prominently expressed in GH-secreting adenomas, while the prolactin-secreting tumours and normal pituitary tissues expressed them at a reduced level [100]. The invasive nature of neural cell adhesion molecule (NCAM) and their role in the progression of pituitary adenomas are yet to be resolved.
Pharmacological Strategies to Target NCAM in Alleviating Cancer
Neural cell adhesion molecule (NCAM) and the polysialylated form of NCAM and PSA–NCAM were of research quest, since many of the pathological conditions in the human system rely on the levels of NCAM and PSA–NCAM expressions on the cell surfaces. An efficacious and apposite targeting of the NCAM, and their regulation in various disorders, is enabled through many novel therapeutic moieties developed such as NCAM-derived peptides. NCAM-derived peptides mimic the functions of NCAM and are categorized as NCAM binding peptides, viz., peptides with NCAM homophilic binding sites and peptides with NCAM heterophilic binding sites (Table 1).
Wilm’s Tumour
Successful cancer therapy is attained by adopting novel strategies to actively target the NCAM expression in various melanomas. The continuous advancements in technology, ensued the development of nanomedicine to target NCAM expression in Wilm’s tumour, comprise an admixture of NCAM-derived polypeptide and paclitaxel, as nano-sized conjugates, which is coated by a biodegradable polymer, polyglutamic acid. The formulated nanomedicine produces a hydrodynamic radius of approximately 10 nm, in the WT cell culture developed from WT patients, and is quite sufficient to attain enhanced permeability and retention (EPR) that yields better cytotoxicity with repressed proliferation of WT cells. Generally, free drug is very much active than the conjugated one, as free drug easily traverses the cell membrane by diffusion, but with respect to the safety and activity prospects, conjugated drug fares perfect than free drug, with an improved anti-tumour activity due to its preferential attachment with NCAM-expressing cells, facilitating for an effective accumulation and internalization of tumour cells [101]. NCAM expression was found on human umbilical vein endothelial cells (HUVEC) only during the capillary-like tube formation process, and these endothelial cells transiently acquire NCAM, when organized in the form of vessel-like structures. The developed polymeric nanomedicine was demonstrated as effective, in inhibiting the tumour cell proliferation and the HUVEC organization into capillary-like tubes.
Small Cell Lung Cancer and Neuroblastoma
Scientific researchers have well established that polysialylation of NCAM highly favours the invasiveness of SCLC. Extensive in vitro methods were conducted to determine the different affinities of the novel monoclonal antibody MAb123C for SCLC between natural killer (NK) cells and tumour tissues, and reported that the antibody is preferably internalized in the SCLC of tumour tissues, than the NK cells which highly desired for NCAM. MAbs, viz., MAb123C3, targets the SCLC preferably at the FN-III-like immunological domain, proximal to the NCAM [102]. The phase II/III clinical trials of AZD4547, a tyrosine kinase inhibitor, confirmed them as a potential target of FGFR1-3 in lung carcinoma [103]. The covalent linkage existing between the monoclonal antibody (MAb) N901 with cytotoxic effector, blocked ricin was established by tissue specificity analysis of anti-NCAM immunotoxin N901-blocked ricin in NCAM-positive tumours of both SCLC and neuroblastoma cells. The study results conferred that N901-br effectively targets CD56, i.e. NCAM. Treatment of the neuroblastoma and SCLC cells with N901-br, yielded better results, i.e. were highly effective with more specific elimination and do not provoked any negative impact on normal haematopoietic progenitors [104].
Acrylamide on NCAM-expressing neuroblastoma cells yielded decreased NCAM expression. The inhibitory effect of acrylamide on NCAM-expressing neuroblastoma was investigated and the study conferred that acrylamide diminished the Ikaros DNA binding activity via CK2 pathway, eventually provoking the suppression of NCAM [105].
Researchers developed a drug delivery system to target NCAM that effectively treats neuroblastoma. A novel formulation composing NCAM targeting peptide (NTP) with paclitaxel (PTX) as nano-sized conjugate, enclosed in the coat of a biodegradable polymer, PGA, was developed. The immunogenic elicited a best-humanized NCAM targeting antibody, huN901-DMI (IMGN901), which had been conjugated with the cytotoxic drug maytansine. The phase II trials of the conjugate yielded insignificant results, compared to the existing etoposide and carboplatin-based therapy for SCLC treatment, and the study data enforced the withdrawal of the conjugate. A novel C3 peptide which inhibited both tumour proliferation and HUVEC organization into capillary-like tubes was incorporated to effectively target the NCAM. Furthermore, scientific studies concluded the strong expression of NCAM in tumour-derived endothelial cells rather than in normal cancer cells, and reinforced the deliberation of NCAM to target the tumour vasculature. The study results demonstrated the dual effect of NCAM, i.e. inhibiting tumour vasculature formation and cancer cell proliferation [73].
Researchers succeeded a PEGylated dendritic polyglycerol (PG) conjugate, to target NCAM. They developed a novel formulation composing PTX, a mitotic inhibitor admixed with NCAM targeting peptide (NTP) and this conjugate was proven to inhibit the proliferating endothelial cell’s migration, confirming their potentiality in inhibiting the tumour angiogenesis [1].
Salivary Gland Carcinoma
The inhibitory effect of cimetidine, a histamine type II receptor antagonist, against several tumours was investigated. Earlier studies conceded the inhibitory effect of cimetidine against the proliferation of glandular tumours including colorectal cancer and gastric tumours, though the mechanism being not completely understood. NCAM was spontaneously expressed in malignant salivary gland tumour, i.e. in adenoid cystic carcinoma. In malignant tumours, the NCAM is associated with perineural invasion (PNI). Homophilic binding of NCAM (NCAM–NCAM) regulated the proliferation of the tumour cells and the adhesion of cancer cells on tumour cells is predominant in cancer invasion and metastasis. The study was the first of its kind to report the effect of cimetidine, i.e. blocking the adhesion of salivary gland tumour cells with the monolayers of neural cells. Their investigation also confirmed the inhibitory effect of cimetidine on NCAM and its ability to induce apoptosis in cancer cells. The apoptotic effect might be due to the downregulation of NCAM expression, eventually suppressing NF-κB, a transcriptional activator, that regulates the NCAM gene expression [106].
Kaposi’s Sarcoma
Kaposi’s sarcoma, an angiogenic tumour, is a serious condition that occurs due to an impaired immune system, and is manifested often in HIV or in immune-deficient patients. Grange et al. developed a drug delivery strategy to target NCAM along with imaging techniques. Liposome loaded with doxorubicin and lipophilic gadolinium (Gd) was developed, and this liposome is conjugated with NCAM binding peptide to specifically target the NCAM-expressing tumours. The clinical efficacy of the formulated liposome was assessed using the severe combined immunodeficiency (SCID) mouse model manifested with Kaposi’s sarcoma of NCAM-positive expression. The developed liposome exhibited improved internalization, enhanced cell necrosis, enhanced apoptosis and a significant decline in the tumour vasculature as well as the tumour mass. The increased internalization confirmed the interaction between NCAM and anti-NCAM peptideC3d. Comparing to the non-targeted drugs, the targeted moieties are localized in the cytoplasm of tumour cells, while the non-targeted drugs were localized in the extracellular space around the tumours [107].
Multiple Myeloma
Multiple myeloma (MM) survives as an incurable disease, amidst several technological advancements. Multiple myeloma is prevalent mainly as malignancy of bone marrow and in lesser cases as malignancy of haematopoietic system. The most aggressive myeloma was reported in patients who had a medical history of relapsed/refractory multiple myeloma. The safety and pharmacokinetic profile of Lorvotuzumab mertansine (IMGN901) were elucidated in patients with NCAM-expressed multiple myeloma. The stage I clinical trial, i.e. dose-escalation phase followed by an expansion phase, of Lorvotuzumab mertansine, a unique antibody-drug conjugate (ADC) targeted against the most frequently expressed NCAM, in multiple myeloma patients, established the existence of disulphide linkage between the anti-NCAM antibody huN901 and DM 1, a cytotoxic maytansinoid effector molecule; and the study confirmed their anti-myeloma effects at a maximum tolerated dose (MTD) of 112 mg/m2 [108]. Cottini et al. demonstrated the use of CD56 as a predictive biomarker for multiple myeloma therapies [109].
Chu et al. reviewed on the role of NCAM mimetic peptides as potential therapeutic targets for neurological disorders. The review authenticated the involvement of NCAM mimetic peptides C3, FGL and Plannexin in neural differentiation and also explored their underlying mechanisms which surged for an expanded exploration of their potential as regenerative medicine to alleviate neurological disorders. These synthetic peptides also play a pivotal role in promoting neurogenesis, neuroprotection and synaptic plasticity and modulation under both in vitro and in vivo conditions [110].
Ovarian Cancer
Kim et al. declared an abnormal signalling of FGFR1 gene amplification in lung adenocarcinoma, based on the perception that various human melanomas are characterized with significant alterations of FGFR, the subtype of tyrosine kinase during the tumorigenesis. The interplay between NCAM–FGFR in the malignancy of ovarian cancer corroborated that more selective inhibitors can directly target FGFR, effectively [79]. Nintedanib and pazopanib, approved as FGFR inhibitors, were targeted for the tyrosine activity instead of FGFR. SU4984 and SU5402, the arylidenyl indolin-2-ones derivatives, were described as inhibitors of tyrosine kinase activity of FGFR1. The inhibitory effect of FGFR was improved in SU4984, by incorporating numerous aromatic rings at fifth position of oxindole core, by modifying and replacing the terminal formyl groups in piperazine rings with numerous six-membered aromatic rings possessing hydrogen bonding acceptor and/or donor groups that favours hydrogen bonding between amino acid residues of ATP binding sites of FGFRs. Among the p-amidophenyl derivatives, compounds with C4 piperazine groups exhibited potent inhibitory effect of FGFR1. The initial decade of the twenty-first century is envisioned as the era of anti-angiogenic therapy. Neoangiogenesis accelerates the proliferation and invasion of tumour cells. The progression of EOC is promoted by an important proangiogenic factor, vascular endothelial growth factor (VEGF). Nintedanib was proven as a potent oral inhibitor of the VEGFR-1,-2 and -3, fibroblast growth factor receptors (FGFRs), platelet-derived growth factor, PDGFR-α and –β [111].
Micro RNAs (miRNAs) vitalize various cellular processes, and their aberrant expressions lead several malignancies in humans, suggesting their role as therapeutic targets, oncogenes or tumour suppressors, depending upon their expression pattern and function in tumorigenesis. Downregulation of miR-99a, in several human malignancies, suggests the potentiality of miR-99a as a tumour suppressor. Jiang et al. authenticated the involvement of miRNA in the tumorigenesis of EOC; computational algorithms predicted FGFR3 as a target gene of miRNA, and asFGFR3 is highly expressed in EOC cells. Dual luciferase assay validated FGFR3 as a direct target of miR-99a. Expression level of miR-99a is inversely correlated with FGFR3 expression levels, implies considerable repression of FGFR3 expressions with an increase in the expression of miR-99a and eventually leads in an inhibited EOC cell proliferation, with reduced EOC cell growth. Extreme downregulation of miR-99a in serums, cells and tissues represses the proliferation of EOC cells by targeting FGFR3, and designates miR-99a as a prospective prognosis marker and potential target in EOC therapeutics [112]. Earlier studies of Nam et al. also declared 90% of downregulation of miRNA-99a in 20 serous ovarian cancer patients [113].
Colon Cancer
Drug delivery system to target NCAM using chitosan, which competitively binds to PSA than NCAM [114], was devised based on the rationale “Polysialylation down-regulates the expression of NCAM in aggressive colon carcinoma” [59]. A polymeric-coated nanoformulation of the drug thymoquinone (TQ) was developed to target colon cancer. The physicochemical profile of TQ depicts a low bio-availability, higher plasma binding, shorter half-life, rapid elimination rate and high sensitivity towards pH, thermal and photo stimuli. The developed polymeric nanoformulation called ECT (Eudragit L100–Chitosan–TQ) comprises a polymer coat of Eudragit L100 and chitosan (a medium molecular weight polymer) coated over the core TQ, which was successful in targeting colon carcinoma, amidst the TQ physicochemical challenges.
The mechanism of drug release from ECT depends upon the pH and colonic microbial flora. The outer coat of ECT, Eudragit L100, dissolves at pH greater than 7 or is digested by the anaerobic bacteria present in the cecal fluid that comprises hydrolytic and reductive enzymes, viz., nitro-reductase, urea-hydroxylase and azo-reductase. These enzymes hydrolyse the polysaccharide (chitosan) with 93% of drug release at the specific site.
In silico cum in vitro sialic acid binding assay confirmed the competitive binding of chitosan in formulation composing PSA, which ensures the inhibition of PSA binding with NCAM. The binding energy of PSA–chitosan binding 3.0 Kcal/mol is comparatively lesser to 3.5 Kcal/mol, the binding energy required for PSA–NCAM binding. Dynamic light scattering (DLS) technique and UV–visible spectrophotometric analysis confirmed the chitosan–sialic acid complex formation. The experimental result of the chitosan-based formulation substantiates the blockade of PSA and authenticates the potential role of the novel formulation in the successful targeting of NCAM to treat the colon carcinoma [114].
Role of NCAM in Other Diseases and Disorders of the Human System
The involvement of NCAM in cellular bio-communications and cellular recognitions relies mainly on its invasive character. The composite role of NCAM in many diseases depends on the cellular recognitions of NCAM, persisting on the membrane surfaces of the cells. In tumorigenesis, the NCAM expression varies largely that brings in an elevated expression, a downregulated expression and re-expressions of NCAM respectively during an embryogenesis, at differentiation of cells and in the proliferation of tumour cells. The metastatic spread is determined based on the adhesion of cancer cells with endothelium. NCAM plays an essential role in tumour angiogenesis, which involves the organization of endothelial cells into capillary tube-like structures. NCAM is well expressed in immature phenotype endothelial cells and in the renal tumour-derived endothelial cells (TEC) while the NCAM expression is absent in normal endothelial cells (HMEC). The renal embryonic transcription factor PAX2 controls the NCAM expression, whereas introduction of PAX2 antisense, deliberately in to TEC, repealed the expression of NCAM. Thus, NCAM suits to be emphasized as a target to localize the tumour-derived endothelial cells.
Human Chronic Myocardial Ischemia
The CD56 antigen was over-expressed in human chronic myocardial ischemia than in the normal heart and was slightly upregulated in few cardiac diseases such as congestive cardiomyopathy, myocarditis and sarcoidosis. In ischemic cardiomyopathy, NCAM expressions were specific that were regulated by the isoform RUNX1 (AML1) [115].
Hirschsprung’s Disease
NCAM expression was downregulated during the embryogenesis of human skeletal and cardiac muscles; NCAM was re-expressed in the matured muscle during the denervation or paralysis of muscle and in myopathies. The neurogenetic disorder, Hirschsprung’s disease, a congenital aganglionosis, is associated with the neuromuscular dysfunction within the affected colon. Romanska et al. elucidated the presence of NCAM in the nerve fibres and muscles of an infant bowel, and also ascertained the altered expression of NCAM in an aganglionic bowel. The immuno-cytochemistry study confirmed a weaker immunoreactivity in the inner border of the circular muscle and a denser immunoreactivity in ganglion cells and nerve fibres of the entire gut wall in the colon of normal humans, whereas an aganglionic bowel displayed an elevated NCAM expression in the muscularis mucosac and a denser immunoreactivity in the hypertrophied nerve bundles of the intermuscular zone and submucosa [30].
Schizophrenia
Dysregulation of polysialylated NCAM as well as an altered immunoreactivity at the hippocampus and dorsal prefrontal cortex were apparent in schizophrenia (SZ). The post-mortem and genetic studies of schizophrenic patients confirmed the prominent role of dysregulation of polysialylated NCAM in SZ [116]. The neurocognitive functions were primarily attributed by an undeniable gene candidate, NCAM1 molecule and in schizophrenic patients, a decreased level of polysialylated NCAM1 isoform was identified in the hilar region of the hippocampus [117].
Psychiatric Disorder
The neuroleptics administered were capable of affecting the expression of NCAM and PSA–NCAM protein in the prefrontal cortex. D2 receptor induces dopaminergic signals to regulate the NCAM and PSA–NCAM protein expression in the medial prefrontal cortex. The above-stated mechanism controls the synaptic activity appropriately, which had been disrupted in the medial prefrontal cortex of a schizophrenic or depressive patient. Thus, NCAM and PSA–NCAM protein is concluded as one of the bio-chemical factors envisioned in the neuropsychiatric disorders, viz., schizophrenia and depression [39].
Alzheimer and Parkinson Disease
The comparative study of PSA–NCAM distribution in Alzheimer’s and Parkinson’s disease indicated that polysialylated NCAM were highly expressed in embryonic and juvenile mammalian brains while they are heavily downregulated in the adult human brain. The PSA–NCAM were commonly expressed at two neurogenic niches of an adult human brain, i.e. in the sub granular zone of the dentate gyrus of the hippocampus and in the sub ventricular zone of the lateral walls of the telencephalic ventricles, while their distribution is less evident in other regions of the human brain. Conversely, the PSA–NCAM expression is completely altered in neurodegenerative diseases such as Alzheimer and Parkinson diseases. The structural plasticity mediated by PSA–NCAM not only confines to those two neurogenic niches cited above, but also is well conserved in all other regions of the aged brains. Alzheimer disease severely affects the entorhinal cortex of the brain, which results in decreased PSA–NCAM expression [4].
Hereditary Inclusion Body Myopathy
Hereditary inclusion body myopathies (HIBM) or GNE myopathy, a group of autosomal recessive or dominant inheritance disorder, manifested as slowly progressing muscle weakness and atrophy in young adults. Muscle fibres with rimmed vacuoles and assorted tubulofilaments without any inflammations were revealed in the histopathology of HIBM muscle biopsy. GNE gene encodes as a bi-functional enzyme, uridine-diphospho-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE/MNK). Mutations in the GNE gene impaired the enzyme activity, which decreased the sialic acid biosynthesis. The abnormal sialylation of glycoprotein results in degeneration of muscle fibres. The serum NCAM sialylation in the collected serum samples of numerous HIBM patients was detected by Western blot. The serum extracts of HIBM patients indicated the existence of a marked difference between the polysialylated and hyposialylated form of NCAM, and also predicted that NCAM was mostly in the hyposialylated form in HIBM serum samples [118]. The experimental outcome authenticated the perspective of hyposialylated NCAM as serum biomarker to diagnose GNE myopathy, and this finding reduced the necessity of serial muscle biopsies in the HIBM treatment trials. The hyposialylated NCAM as potential serum biomarker in HIBM should be validated by assessing their specificity, reliability and robustness in meeting their intended purpose.
Conclusion
NCAM plays a prolific role in multicellular processes and this review emphasizes on the expression and signalling aspects of several classes of NCAM in numerous diseases and disorders. It has been illustrious that there is a great physiological crosstalk between NCAM and different systems of cell communication and is evident that NCAM signal integration with extracellular and intracellular physiological proteins is much more ubiquitous than what was assumed. This review also divulges the evolving arena of NCAM in cancer therapy, immunotherapy and drug targeting therapy along with its indications and limitations like difficulty in the prediction of protein-ligand interaction during in silico/in vivo studies. It should be noted that there is a greater linkage between the versatile NCAM and other physiological and pathological gene-mediated intercellular communications. However, the effect and mechanisms of action of NCAM in cancers and drug targeting approaches including its proliferation, expression, apoptosis, autophagy and cellular transition are still warranted further analysis and are evolving fields in the future. The molecular screening and preclinical and clinical investigations on the functions and mechanisms of NCAM need proper assessment of its role and are the need of the hour. This definitely helps to find new insights leading to novel therapeutic approaches in the management of various pathological conditions and to be a good platform for advanced drug delivery. This review consolidated copious in vitro experimental settings for NCAMs that give promising anticipations for future targets, but it is evident that the clinical settings in vivo are still challenging and remain as controversial.
References
Vossen LI, Markovsky E, Eldar-Boock A et al (2018) PEGylated dendritic polyglycerol conjugate targeting NCAM-expressing neuroblastoma: limitations and challenges. Nanomed: Nanotechnol Biol Med 14:1169–1179. https://doi.org/10.1016/J.NANO.2018.02.009
Fogar P, Basso D, Pasquali C et al (1997) Neural cell adhesion molecule (N-CAM) in gastrointestinal neoplasias. Anticancer Res 17:1227–1230
Kozlova I, Sah S, Keable R et al (2020) Cell adhesion molecules and protein synthesis regulation in neurons. Front Mol Neurosci 13:208. https://doi.org/10.3389/FNMOL.2020.592126/BIBTEX
Murray HC, Low VF, Swanson MEV et al (2016) Distribution of PSA-NCAM in normal, Alzheimer’s and Parkinson’s disease human brain. Neuroscience 330:359–375. https://doi.org/10.1016/J.NEUROSCIENCE.2016.06.003
Secher T (2010) Soluble NCAM. In: Berezin V (ed) Structure and function of the neural cell adhesion molecule NCAM. Springer, New York, pp. 227–242
Hoffman S, Sorkin BC, White PC et al (1982) Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem 257:7720–7729
Aonurm-Helm A, Jaako K, Jürgenson M, Zharkovsky A (2016) Pharmacological approach for targeting dysfunctional brain plasticity: focus on neural cell adhesion molecule (NCAM). Pharmacol Res 113:731–738. https://doi.org/10.1016/J.PHRS.2016.04.011
el Maarouf A, Petridis AK, Rutishauser U (2006) Use of polysialic acid in repair of the central nervous system. Proc Natl Acad Sci USA 103:16989–16994. https://doi.org/10.1073/PNAS.0608036103
Guan F, Wang X, He F (2015) Promotion of cell migration by neural cell adhesion molecule (NCAM) is enhanced by PSA in a polysialyltransferase-specific manner. PLoS One 10:e0124237. https://doi.org/10.1371/JOURNAL.PONE.0124237
Crnic I, Strittmatter K, Cavallaro U et al (2004) Loss of neural cell adhesion molecule induces tumor metastasis by up-regulating lymphangiogenesis. Cancer Res 64:8630–8638. https://doi.org/10.1158/0008-5472.CAN-04-2523
Miyahara R, Tanaka F, Nakagawa T et al (2001) Expression of neural cell adhesion molecules (polysialylated form of neural cell adhesion molecule and L1-cell adhesion molecule) on resected small cell lung cancer specimens: in relation to proliferation state. J Surg Oncol 77:49–54. https://doi.org/10.1002/JSO.1065
Kiselyov VV, Skladchikova G, Hinsby AM et al (2003) Structural basis for a direct interaction between FGFR1 and NCAM and evidence for a regulatory role of ATP. Structure 11:691–701. https://doi.org/10.1016/S0969-2126(03)00096-0
Bodrikov V, Leshchyns’ka I, Sytnyk V et al (2005) RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation. J Cell Biol 168:127–139. https://doi.org/10.1083/JCB.200405073
Weledji EP (2018) The neural cell adhesion molecule (NCAM): from memory formation to cancer. Arch Clin Pathol J 1:1000104
Vutskits L, Djebbara-Hannas Z, Zhang H et al (2001) PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur J Neurosci 13:1391–1402. https://doi.org/10.1046/J.0953-816X.2001.01516.X
Paratcha G, Ledda F, Ibáñez CF (2003) The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113:867–879. https://doi.org/10.1016/S0092-8674(03)00435-5
Zhang H, Vutskits L, Calaora V et al (2004) A role for the polysialic acid-neural cell adhesion molecule in PDGF-induced chemotaxis of oligodendrocyte precursor cells. J Cell Sci 117:93–103. https://doi.org/10.1242/JCS.00827
Johnson CP, Fujimoto I, Rutishauser U, Leckband DE (2005) Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J Biol Chem 280:137–145. https://doi.org/10.1074/JBC.M410216200
Kolkova K (2010) Biosynthesis of NCAM. Adv Exp Med Biol 663:213–225. https://doi.org/10.1007/978-1-4419-1170-4_14
Ames HM, Yuan M, Vizcaíno MA et al (2017) MicroRNA profiling of low grade glial and glioneuronal tumors shows an independent role for cluster 14q32.31 member miR-487b. Mod Pathol 30:204–2016. https://doi.org/10.1038/MODPATHOL.2016.177
Kameda K, Shimada H, Ishikawa T et al (1999) Expression of highly polysialylated neural cell adhesion molecule in pancreatic cancer neural invasive lesion. Cancer Lett 137:201–207. https://doi.org/10.1016/S0304-3835(98)00359-0
Mehrabian M, Hildebrandt H, Schmitt-Ulms G (2016) NCAM1 polysialylation: the prion protein’s elusive reason for being? ASN Neuro 8:1–25. https://doi.org/10.1177/1759091416679074
Parcerisas A, Ortega-Gascó A, Pujadas L, Soriano E (2021) The hidden side of NCAM family: NCAM2, a key cytoskeleton organization molecule regulating multiple neural functions. Int J Mol Sci 22:10021. https://doi.org/10.3390/IJMS221810021
Weledji EP, Assob JC (2014) The ubiquitous neural cell adhesion molecule (N-CAM). Ann Med Surg 3:77–81. https://doi.org/10.1016/J.AMSU.2014.06.014
Klein G, Langegger M, Goridis C, Ekblom P (1988) Neural cell adhesion molecules during embryonic induction and development of the kidney. Development 102:749–761
Adhikari UK, Sakiz E, Zhou X et al (2021) Cross-linking cellular prion protein induces neuronal type 2-like hypersensitivity. Front Immunol 12:639008. https://doi.org/10.3389/FIMMU.2021.639008/BIBTEX
Watanabe M, Kobayashi H, Rutishauser U et al (1989) NCAM in the differentiation of embryonic lens tissue. Dev Biol 135:414–423. https://doi.org/10.1016/0012-1606(89)90190-5
Knudsen KA, McElwee SA, Myers L (1990) A role for the neural cell adhesion molecule, NCAM, in myoblast interaction during myogenesis. Dev Biol 138:159–168. https://doi.org/10.1016/0012-1606(90)90185-L
Siegenthaler JA, Miller MW (2004) Transforming growth factor β1 modulates cell migration in rat cortex: effects of ethanol. Cortex July 14:791–802. https://doi.org/10.1093/cercor/bhh039
Romanska HM, Bishop AE, Moscoso G et al (1993) Immunohistochemical study of NCAM expression in the developing human large bowel. Regul Peptides 47:109. https://doi.org/10.1016/0167-0115(93)90316-Z
Amoureux MC, Coulibaly B, Chinot O et al (2010) Polysialic acid neural cell adhesion molecule (psa-ncam) is an adverse prognosis factor in glioblastoma, and regulates olig2 expression in glioma cell lines. BMC Cancer 10:91. https://doi.org/10.1186/1471-2407-10-91/FIGURES/4
Kajikawa H, Umemoto M, Mishiro Y et al (1997) Expression of highly polysialylated NCAM (NCAM-H) in developing and adult chicken auditory organ. Hear Res 103:123–130. https://doi.org/10.1016/S0378-5955(96)00171-2
Charlton CA, Mohler WA, Blau HM (2000) Neural cell adhesion molecule (NCAM) and myoblast fusion. Dev Biol 221:112–119. https://doi.org/10.1006/DBIO.2000.9654
Chen HC, Loh HH (2001) mu-Opioid receptor gene expression: the role of NCAM. Neuroscience 108:7–15. https://doi.org/10.1016/S0306-4522(01)00397-9
Bisaz R, Conboy L, Sandi C (2009) Learning under stress: a role for the neural cell adhesion molecule NCAM. Neurobiol Learn Mem 91:333–342. https://doi.org/10.1016/J.NLM.2008.11.003
Gnanapavan S, Giovannoni G (2013) Neural cell adhesion molecules in brain plasticity and disease. Mult Scler Relat Disord 2:13–20. https://doi.org/10.1016/J.MSARD.2012.08.002
Shi Y, Xia YY, Wang L et al (2012) Neural cell adhesion molecule modulates mesenchymal stromal cell migration via activation of MAPK/ERK signaling. Exp Cell Res 318:2257–2267. https://doi.org/10.1016/J.YEXCR.2012.05.029
Cremer H, Chazal G, Lledo PM et al (2000) PSA-NCAM: an important regulator of hippocampal plasticity. Int J Dev Neurosci 18:213–220. https://doi.org/10.1016/S0736-5748(99)00090-8
Miyakawa M, Seki T, Uchiyama Y (2010) Origin of PSA-NCAM expressing blood vessels in the developing forebrain of avian embryo. Neurosci Res 68:e370. https://doi.org/10.1016/J.NEURES.2010.07.1640
Varea E, Nácher J, Blasco-Ibáñez JM et al (2005) PSA-NCAM expression in the rat medial prefrontal cortex. Neuroscience 136:435–443. https://doi.org/10.1016/J.NEUROSCIENCE.2005.08.009
Bonfanti L (2006) PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol 80:129–164. https://doi.org/10.1016/J.PNEUROBIO.2006.08.003
Mazzetti S, Ortino B, Inverardi F et al (2007) PSA-NCAM in the developing and mature thalamus. Brain Res Bull 71:578–586. https://doi.org/10.1016/J.BRAINRESBULL.2006.11.015
Varea E, Castillo-Gómez E, Gómez-Climent MÁ et al (2009) Differential evolution of PSA-NCAM expression during aging of the rat telencephalon. Neurobiol Aging 30:808–818. https://doi.org/10.1016/J.NEUROBIOLAGING.2007.08.016
el Maarouf A, Kolesnikov Y, Pasternak G, Rutishauser U (2012) Neural cell adhesion molecule and its polysialic acid moiety exhibit opposing and linked effects on neuropathic hyperalgesia. Exp Neurol 233:866–870. https://doi.org/10.1016/J.EXPNEUROL.2011.12.017
Varbanov H, Dityatev A (2017) Regulation of extrasynaptic signaling by polysialylated NCAM: impact for synaptic plasticity and cognitive functions. Mol Cell Neurosci 81:12–21. https://doi.org/10.1016/J.MCN.2016.11.005
Tascilar O, Cakmak GK, Tekin IO et al (2007) Neural cell adhesion molecule-180 expression as a prognostic criterion in colorectal carcinoma: feasible or not? World J Gastroenterol 13:5476. https://doi.org/10.3748/WJG.V13.I41.5476
Trovato R, Canè S, Petrova V et al (2020) The engagement between MDSCs and metastases: partners in crime. Fronti Oncol 10:165. https://doi.org/10.3389/FONC.2020.00165
Orecchioni M, Ghosheh Y, Pramod AB, Ley K (2019) Macrophage polarization: different gene signatures in M1(Lps+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front Immunol 10:1084. https://doi.org/10.3389/FIMMU.2019.01084/BIBTEX
Ichihara Y, Yokozaki H (2021) Close association of intraepithelial accumulation of M2-skewed macrophages with neoplastic epithelia of the esophagus. Kobe J Med Sci 67:E18–E33. https://doi.org/10.1111/pin.12674
Urakawa N, Utsunomiya S, Nishio M et al (2015) GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab Invest 95:491–503. https://doi.org/10.1038/labinvest.2015.36
Kodama T, Ichiro KY, Arai N et al (2020) CCL3–CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab Invest 100:1140–1157. https://doi.org/10.1038/S41374-020-0441-4
Takase N, Ichiro KY, Urakawa N et al (2016) NCAM- and FGF-2-mediated FGFR1 signaling in the tumor microenvironment of esophageal cancer regulates the survival and migration of tumor-associated macrophages and cancer cells. Cancer Lett 380:47–58. https://doi.org/10.1016/J.CANLET.2016.06.009
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362:1605–1617. https://doi.org/10.1056/NEJMRA0901557
Li C, Heidt DG, Dalerba P et al (2007) Identification of pancreatic cancer stem cells. Cancer Res 67:1030–1037. https://doi.org/10.1158/0008-5472.CAN-06-2030
Schreiber SC, Giehl K, Kastilan C et al (2008) Polysialylated NCAM represses E-cadherin-mediated cell-cell adhesion in pancreatic tumor cells. Gastroenterology 134:1555–1566. https://doi.org/10.1053/J.GASTRO.2008.02.023
Tariq K, Ghias K (2016) Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med 13:120–135. https://doi.org/10.28092/J.ISSN.2095-3941.2015.0103
Roesler J, Srivatsan E, Moatamed F et al (1997) Tumor suppressor activity of neural cell adhesion molecule in colon carcinoma. Am J Surg 174:251–257. https://doi.org/10.1016/S0002-9610(97)00142-6
Huerta S, Srivatsan ES, Venkatasan N, Livingston EH (2001) Human colon cancer cells deficient in DCC produce abnormal transcripts in progression of carcinogenesis. Dig Dis Sci 46:1884–1891. https://doi.org/10.1023/A:1010626929411
Fernández-Briera A, García-Parceiro I, Cuevas E, Gil-Martín E (2010) Effect of human colorectal carcinogenesis on the neural cell adhesion molecule expression and polysialylation. Oncology 78:196–204. https://doi.org/10.1159/000313699
Esnault C, Leblond V, Martin C et al (2022) Adcitmer ® , a new CD56-targeting monomethyl auristatin E-conjugated antibody, is a potential therapeutic approach in Merkel cell carcinoma. Br J Dermatol 186:295–306. https://doi.org/10.1111/BJD.20770
Xing M (2013) Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13:184–199. https://doi.org/10.1038/NRC3431
Cabanillas ME, McFadden DG, Durante C (2016) Thyroid cancer. Lancet 388:2783–2795. https://doi.org/10.1016/S0140-6736(16)30172-6
Zhang P, Zuo H, Ozaki T et al (2006) Cancer stem cell hypothesis in thyroid cancer. Pathol Int 56:485–489. https://doi.org/10.1111/J.1440-1827.2006.01995.X
Krashin E, Piekiełko-Witkowska A, Ellis M, Ashur-Fabian O (2019) Thyroid hormones and cancer: a comprehensive review of preclinical and clinical studies. Front Endocrinol 10:59. https://doi.org/10.3389/FENDO.2019.00059/FULL
Huang H, Liu Y, Ouyang X et al (2020) Identification of a peptide targeting CD56. Immunobiology 225:151982. https://doi.org/10.1016/J.IMBIO.2020.151982
Zhao S, Duan J, Lou Y et al (2021) Surface specifically modified NK-92 cells with CD56 antibody conjugated superparamagnetic Fe3O4 nanoparticles for magnetic targeting immunotherapy of solid tumors. Nanoscale 13:19109–19122. https://doi.org/10.1039/D1NR03329H
Scarpino S, di Napoli A, Melotti F et al (2007) Papillary carcinoma of the thyroid: low expression of NCAM (CD56) is associated with downregulation of VEGF-D production by tumour cells. J Pathol 212:411–419. https://doi.org/10.1002/PATH.2183
Yang AH, Chen JY, Lee CH, Chen JY (2012) Expression of NCAM and OCIAD1 in well-differentiated thyroid carcinoma: correlation with the risk of distant metastasis. J Clin Pathol 65:206–212. https://doi.org/10.1136/JCLINPATH-2011-200416
Winter C, Pawel B, Seiser E et al (2008) Neural cell adhesion molecule (NCAM) isoform expression is associated with neuroblastoma differentiation status. Pediatr Blood Cancer 51:10–16. https://doi.org/10.1002/PBC.21475
Glüer S, Zense M, Radtke E, von Schweinitz D (1998) Polysialylated neural cell adhesion molecule in childhood ganglioneuroma and neuroblastoma of different histological grade and clinical stage. Langenbecks Arch Surg 383:340–344. https://doi.org/10.1007/S004230050145
Hildebrandt H, Becker C, Glüer S et al (1998) Polysialic acid on the neural cell adhesion molecule correlates with expression of polysialyltransferases and promotes neuroblastoma cell growth. Cancer Res 58:779–784
Wachowiak R, Rawnaq T, Metzger R et al (2008) Universal expression of cell adhesion molecule NCAM in neuroblastoma in contrast to L1: implications for different roles in tumor biology of neuroblastoma? Pediatr Surg Int 24:1361–1364. https://doi.org/10.1007/S00383-008-2264-Z
Markovsky E, Eldar-Boock A, Ben-Shushan D et al (2017) Targeting NCAM-expressing neuroblastoma with polymeric precision nanomedicine. J Control Release 249:162–172. https://doi.org/10.1016/J.JCONREL.2017.01.044
Roth J, Zuber C, Wagner P et al (1988) Presence of the long chain form of polysialic acid of the neural cell adhesion molecule in Wilms’ tumor. Identification of a cell adhesion molecule as an oncodevelopmental antigen and implications for tumor histogenesis. Am J Pathol 133:227–240
Raved D, Tokatly-Latzer I, Anafi L et al (2019) Blastemal NCAM + ALDH1 + Wilms’ tumor cancer stem cells correlate with disease progression and poor clinical outcome: a pilot study. Pathol Res Pract 215:152491. https://doi.org/10.1016/J.PRP.2019.152491
Pode-Shakked N, Metsuyanim S, Rom-Gross E et al (2009) Developmental tumourigenesis: NCAM as a putative marker for the malignant renal stem/progenitor cell population. J Cell Molec Med 13:1792–1808. https://doi.org/10.1111/J.1582-4934.2008.00607.X
Jafarian AH, Zabolinejad N, Roshan NM et al (2020) Evaluation of CD133 and CD56/NCAM expression in Wilms tumor and their association with prognostic factors. Iran J Basic. Med Sci 23:853–857. https://doi.org/10.22038/IJBMS.2020.41468.9804
Nemunaitis JM, Brown-Glabeman U, Soares H et al (2018) Gallbladder cancer: review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer 18:665. https://doi.org/10.1186/S12885-018-4575-3
Zecchini S, Bombardelli L, Decio A et al (2011) The adhesion molecule NCAM promotes ovarian cancer progression via FGFR signalling. EMBO Mol Med 3:480–494. https://doi.org/10.1002/EMMM.201100152
Cavallaro U, Niedermeyer J, Fuxa M, Christofori G (2001) N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol 3:650–657. https://doi.org/10.1038/35083041
Mehanna A, Mishra B, Kurschat N et al (2009) Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice. Brain 132:1449–1462. https://doi.org/10.1093/BRAIN/AWP128
Bombardelli L, Cavallaro U (2010) Immunoglobulin-like cell adhesion molecules: novel signaling players in epithelial ovarian cancer. Int J Biochem Cell Biol 42:590–594. https://doi.org/10.1016/J.BIOCEL.2010.01.017
de Cecco L, Marchionni L, Gariboldi M et al (2004) Gene expression profiling of advanced ovarian cancer: characterization of a molecular signature involving fibroblast growth factor 2. Oncogene 23:8171–8183. https://doi.org/10.1038/SJ.ONC.1207979
Segawa Y, Ohnoshi T, Hiraki S et al (1994) Neural cell adhesion molecule (NCAM) expression and clinical features in small cell lung cancer (SCLC), with reference to treatment outcome. Lung Cancer 11:26. https://doi.org/10.1016/0169-5002(94)93871-7
Lantuejoul S, Moro D, Michalides RJAM et al (1998) Neural cell adhesion molecules (NCAM) and NCAM-PSA expression in neuroendocrine lung tumors. Am J Surg Pathol 22:1267–1276. https://doi.org/10.1097/00000478-199810000-00012
Jensen M, Berthold F (2007) Targeting the neural cell adhesion molecule in cancer. Cancer Lett 258:9–21. https://doi.org/10.1016/J.CANLET.2007.09.004
van der Borght A, Duysinx M, Broers JLV et al (2018) The 180 splice variant of NCAM-containing exon 18-is specifically expressed in small cell lung cancer cells. Transl Lung. Cancer Res 7:376–388. https://doi.org/10.21037/TLCR.2018.03.03
Kaliki S, Shields CL (2017) Uveal melanoma: relatively rare but deadly cancer. Eye 31:241–257. https://doi.org/10.1038/EYE.2016.275
Mooy CM, Luyten GPM, de Jong PTVM et al (1995) Neural cell adhesion molecule distribution in primary and metastatic uveal melanoma. Hum Pathol 26:1185–1190. https://doi.org/10.1016/0046-8177(95)90191-4
Shang J, Sheng L, Wang K et al (2007) Expression of neural cell adhesion molecule in salivary adenoid cystic carcinoma and its correlation with perineural invasion. Oncol Rep 18:1413–1416. https://doi.org/10.3892/OR.18.6.1413/HTML
Ohali A, Avigad S, Zaizov R et al (2004) Prediction of high risk Ewing’s sarcoma by gene expression profiling. Oncogene 23:8997–9006. https://doi.org/10.1038/SJ.ONC.1208060
Durer S, Shaikh H (2022) Ewing sarcoma. StatPearls Publishing
Er N, Daǧdeviren A, Taşman F, Zeybek D (2001) Neural cell adhesion molecule and neurothelin expression in human ameloblastoma. J Oral Maxillofacial Surg 59:900–903. https://doi.org/10.1053/JOMS.2001.25025
Sasaki H, Yoshida K, Ikeda E et al (1998) Expression of the neural cell adhesion molecule in astrocytic tumors: an inverse correlation with malignancy. Cancer 82:1921–1931. https://doi.org/10.1002/(sici)1097-0142(19980515)82:10<1921::aid-cncr16>3.0.co;2-v
Vural E, Hutcheson J, Korourian S et al (2016) Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg 122:717–720. https://doi.org/10.1067/MHN.2000.105057
Bakst RL, Wong RJ (2016) Mechanisms of perineural invasion. J Neurol Surg B Skull Base 77:96–106. https://doi.org/10.1055/S-0036-1571835
Deborde S, Omelchenko T, Lyubchik A et al (2016) Schwann cells induce cancer cell dispersion and invasion. J Clin Invest 126:1538–1554. https://doi.org/10.1172/JCI82658
Lantuéjoul S, Laverriére MH, Sturm N et al (2000) NCAM (neural cell adhesion molecules)expression in malignant mesotheliomas. Human Pathol 31:415–421. https://doi.org/10.1053/HP.2000.6552
Aletsee-Ufrecht MC, Langley K, Gratzl O, Gratzl M (1990) Differential expression of the neural cell adhesion molecule NCAM 140 in human pituitary tumors. FEBS Lett 272:45–49. https://doi.org/10.1016/0014-5793(90)80445-O
Mendes GA, Haag T, Trott G et al (2017) Expression of E-cadherin, Slug and NCAM and its relationship to tumor invasiveness in patients with acromegaly. Braz J Med Biol Res 51:e6808. https://doi.org/10.1590/1414-431X20176808
Markovsky E, Vax E, Ben-Shushan D et al (2017) Wilms tumor NCAM-expressing cancer stem cells as potential therapeutic target for polymeric nanomedicine. Mol Cancer Ther 16:2462–2472. https://doi.org/10.1158/1535-7163.MCT-17-0184
Patel K, Moore SE, Dickson G et al (1989) Neural cell adhesion molecule (NCAM) is the antigen recognized by monoclonal antibodies of similar specificity in small-cell lung carcinoma and neuroblastoma. Int J Cancer 44:573–578. https://doi.org/10.1002/IJC.2910440402
Hallinan N, Finn S, Cuffe S et al (2016) Targeting the fibroblast growth factor receptor family in cancer. Cancer Treat Rev 46:51–62. https://doi.org/10.1016/J.CTRV.2016.03.015
Roy DC, Ouellet S, le Houillier C et al (1996) Elimination of neuroblastoma and small-cell lung cancer cells with an anti-neural cell adhesion molecule immunotoxin. J Natl Cancer Inst 88:1136–1145. https://doi.org/10.1093/JNCI/88.16.1136
Lee HR, Cho SJ, Park HJ et al (2010) The inhibitory effect of acrylamide on NCAM expression in human neuroblastoma cells: involvement of CK2/Ikaros signaling pathway. Toxicol In Vitro 24:1946–1952. https://doi.org/10.1016/J.TIV.2010.08.004
Fukuda M, Kusama K, Sakashita H (2008) Cimetidine inhibits salivary gland tumor cell adhesion to neural cells and induces apoptosis by blocking NCAM expression. BMC Cancer 8:376. https://doi.org/10.1186/1471-2407-8-376
Grange C, Geninatti-Crich S, Esposito G et al (2010) Combined delivery and magnetic resonance imaging of neural cell adhesion molecule-targeted doxorubicin-containing liposomes in experimentally induced Kaposi’s sarcoma. Cancer Res 70:2180–2190. https://doi.org/10.1158/0008-5472.CAN-09-2821
Ailawadhi S, Kelly KR, Vescio RA et al (2019) A phase I study to assess the safety and pharmacokinetics of single-agent lorvotuzumab mertansine (IMGN901) in patients with relapsed and/or refractory CD-56-positive multiple myeloma. Clin Lymphoma Myeloma Leuk 19:29–34. https://doi.org/10.1016/J.CLML.2018.08.018
Cottini F, Rodriguez J, Hughes T et al (2022) Redefining CD56 as a biomarker and therapeutic target in multiple myeloma. Mol Cancer Res:OF1–OF13. https://doi.org/10.1158/1541-7786.MCR-21-0828
Chu C, Gao Y, Lan X et al (2018) NCAM mimetic peptides: potential therapeutic target for neurological disorders. Neurochem Res 43:1714–1722. https://doi.org/10.1007/S11064-018-2594-8
Tsibulak I, Zeimet AG, Marth C (2019) Hopes and failures in front-line ovarian cancer therapy. Crit Rev Oncol Hematol 143:14–19. https://doi.org/10.1016/J.CRITREVONC.2019.08.002
Jiang H, Qu L, Wang Y et al (2014) miR-99a promotes proliferation targeting FGFR3 in human epithelial ovarian cancer cells. Biomed Pharmacother 68:163–169. https://doi.org/10.1016/J.BIOPHA.2013.12.001
Nam EJ, Yoon H, Kim SW et al (2008) MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res 14:2690–2695. https://doi.org/10.1158/1078-0432.CCR-07-1731
Pushpa Sweety J, Sowparani S, Mahalakshmi P et al (2020) Fabrication of stimuli gated nanoformulation for site-specific delivery of thymoquinone for colon cancer treatment – insight into thymoquinone’s improved physicochemical properties. J Drug Deliv Sci Technol 55:101334. https://doi.org/10.1016/J.JDDST.2019.101334
Gattenlöhner S, Waller C, Ertl G et al (2004) Strong overexpression of NCAM(CD56) is specific for human ischemic cardiomyopathy and might be regulated by two novel isoforms of RUNX1(AML1). Pathol Res Pract 200:290. https://doi.org/10.1016/S0344-0338(04)80537-5
Piras F, Schiff M, Chiapponi C et al (2015) Brain structure, cognition and negative symptoms in schizophrenia are associated with serum levels of polysialic acid-modified NCAM. Transl Psychiatry 5:e658. https://doi.org/10.1038/TP.2015.156
Sullivan PF, Keefe RSE, Lange LA et al (2007) NCAM1 and neurocognition in schizophrenia. Biol Psychiatry 61:902–910. https://doi.org/10.1016/J.BIOPSYCH.2006.07.036
Valles-Ayoub Y, Esfandiarifard S, Sinai P et al (2012) Serum neural cell adhesion molecule is hyposialylated in hereditary inclusion body myopathy. Genet Test Mol Biomarkers 16:313–317. https://doi.org/10.1089/GTMB.2011.0146
Acknowledgements
The authors JPS and NS acknowledge the support by Centre for Excellence in Nanobio Translational Research, Department of Pharmaceutical Technology, Anna University, BIT Campus, Tiruchirappalli
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by N. Selvasudha and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sowparani, S., Mahalakshmi, P., Sweety, J.P. et al. Ubiquitous Neural Cell Adhesion Molecule (NCAM): Potential Mechanism and Valorisation in Cancer Pathophysiology, Drug Targeting and Molecular Transductions. Mol Neurobiol 59, 5902–5924 (2022). https://doi.org/10.1007/s12035-022-02954-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02954-9